Isolation and Characterization of an Anti-Osteoporotic Compound from Melia toosendan Fructus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
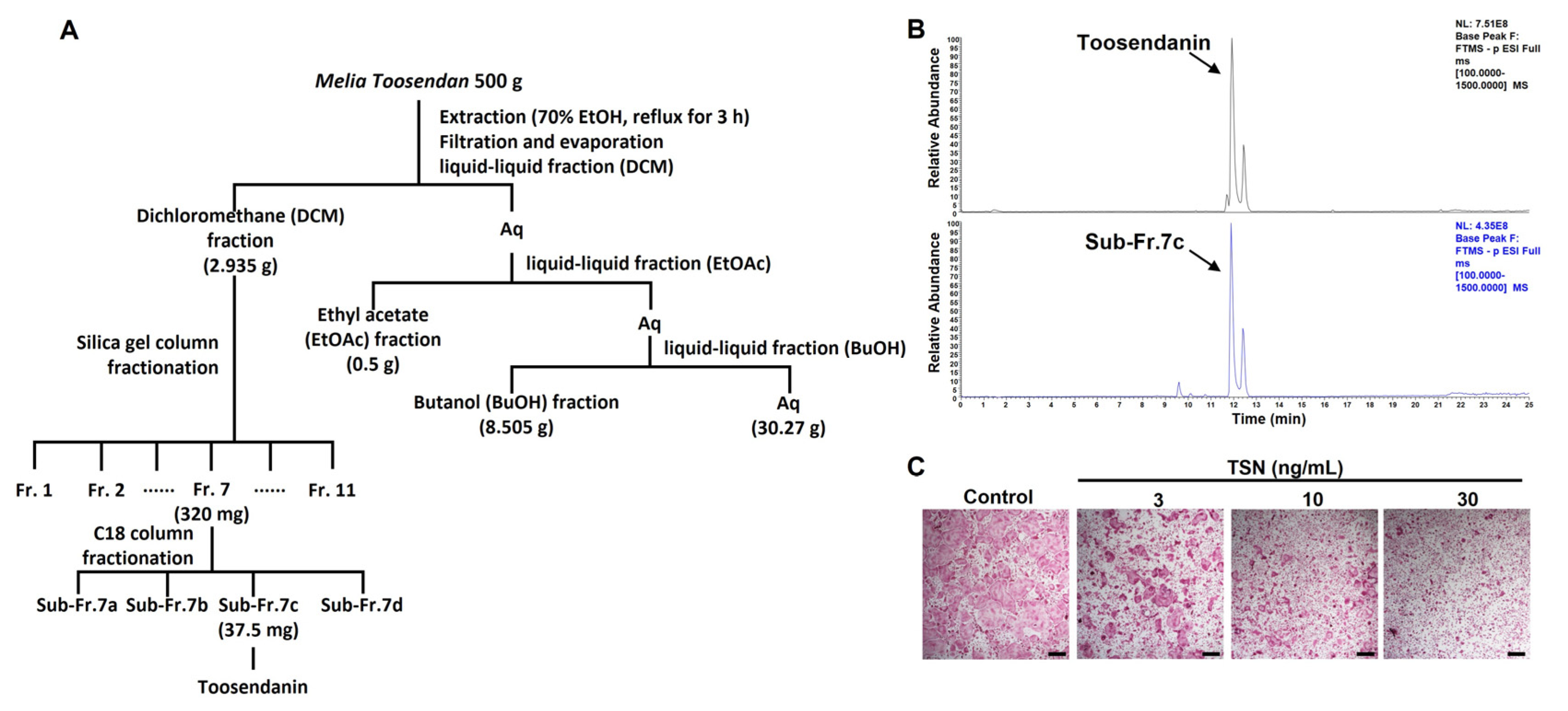
2.2. Extraction and Fractionation of MTE
2.3. UHPLC-MS/MS Analysis
2.4. BMM Culture and Cell Viability Assay
2.5. Osteoclast Differentiation Assay
2.6. Actin Ring and Acridine Oragne Staining
2.7. Osteoclast Activity Assay
2.8. Western Blot Anlysis
2.9. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.10. Animal Experiment
2.11. Statistical Analysis
3. Results
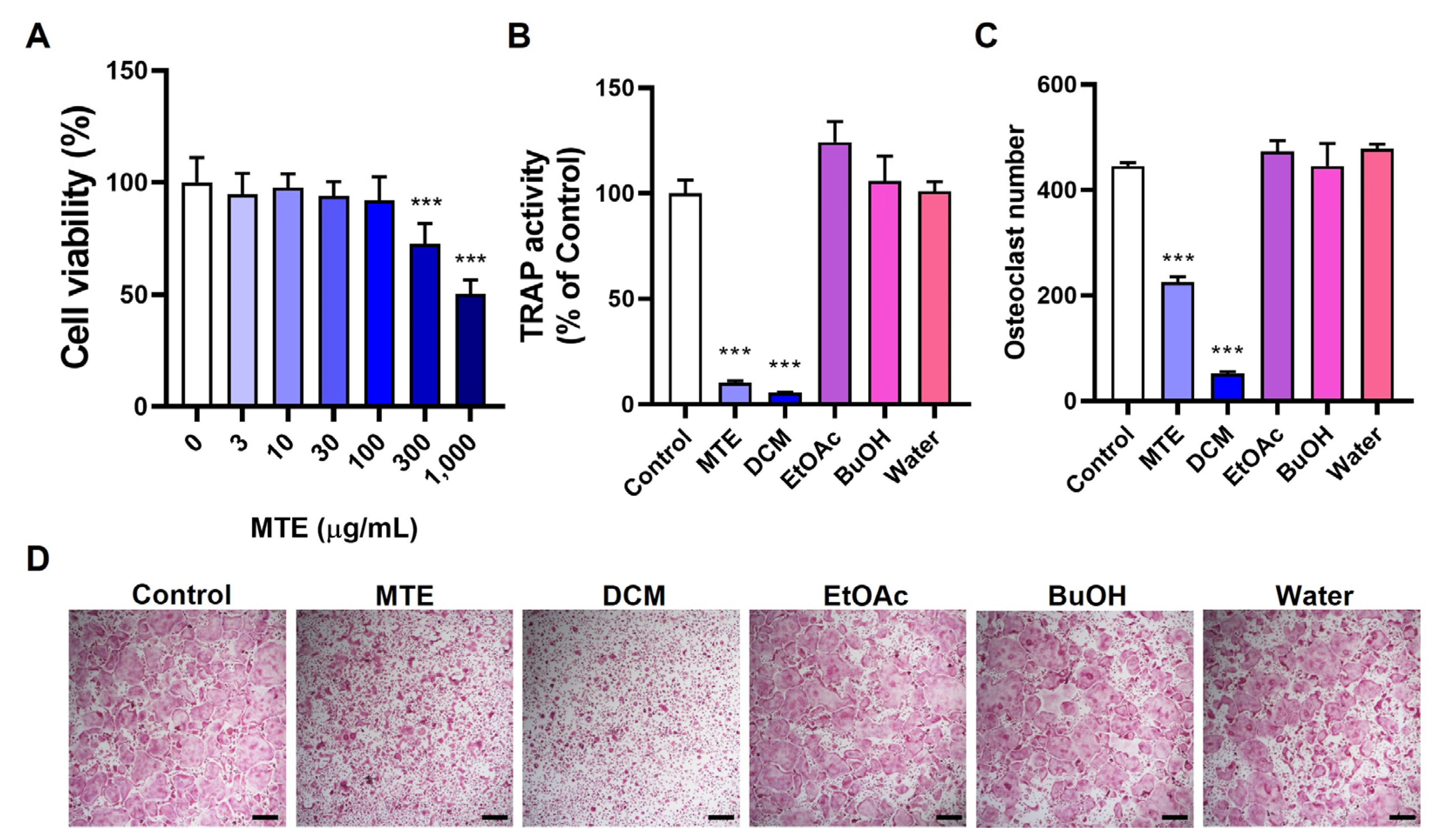
3.1. Inhibitory Effects of MTE and Its Fractions on Osteoclast Differentiation
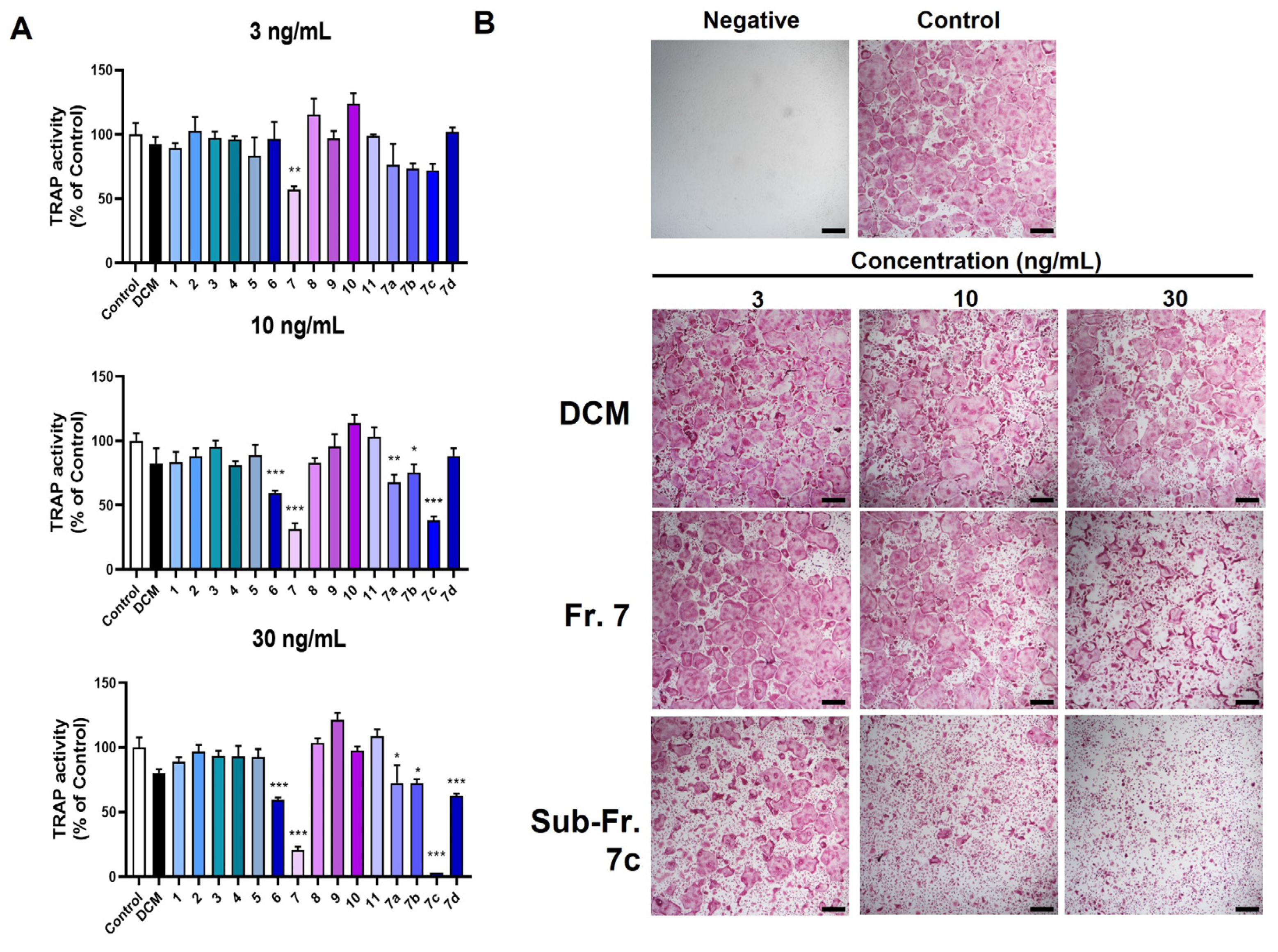
3.2. Bioassay-Guided Isolation of Bioactive Compound TSN from MTE
3.3. Mechanistic Insights into TSN’s Inhibition of Osteoclast Differentiation
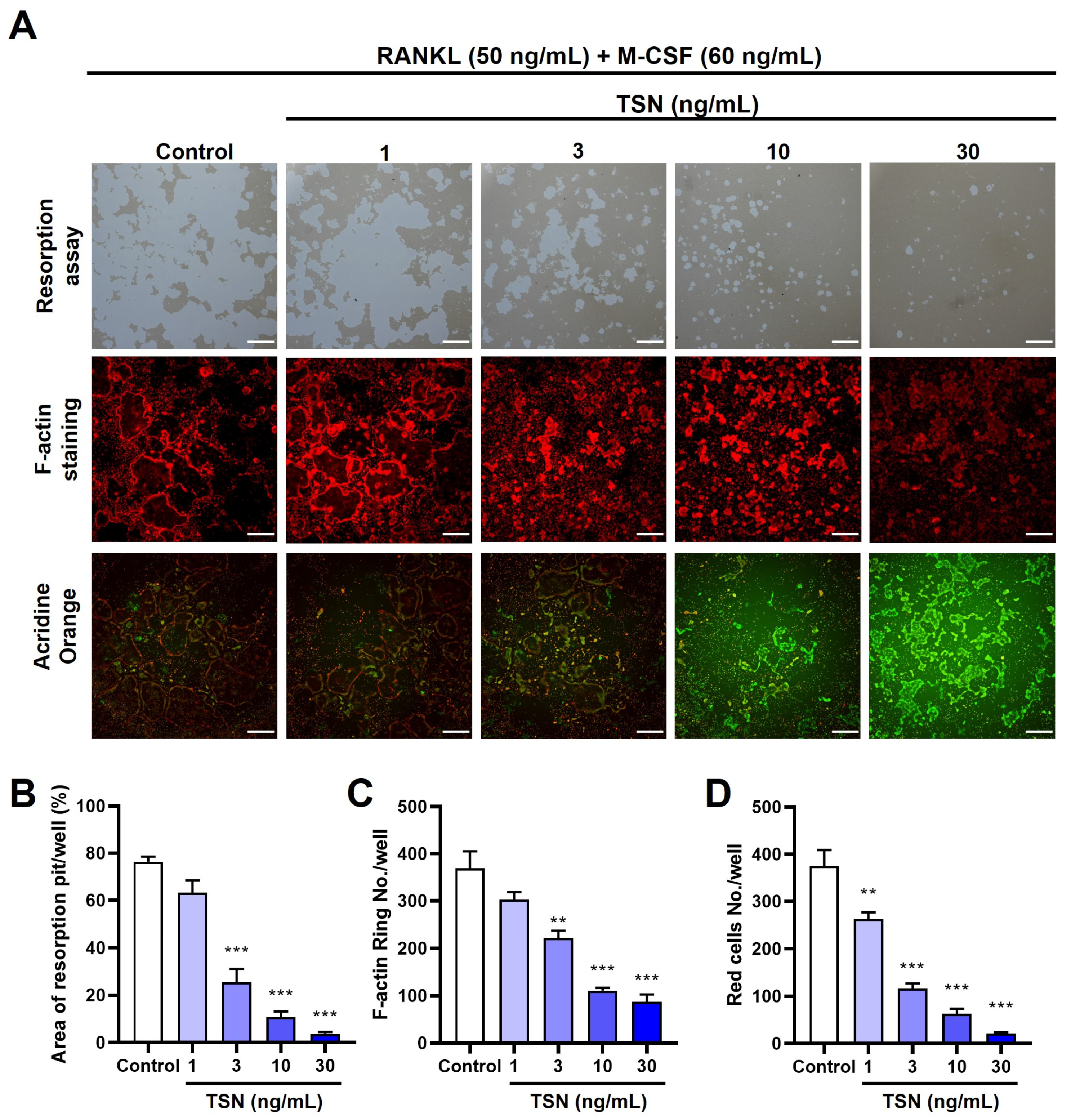
3.4. Impact of TSN on the Resorptive Function of Mature Osteoclasts
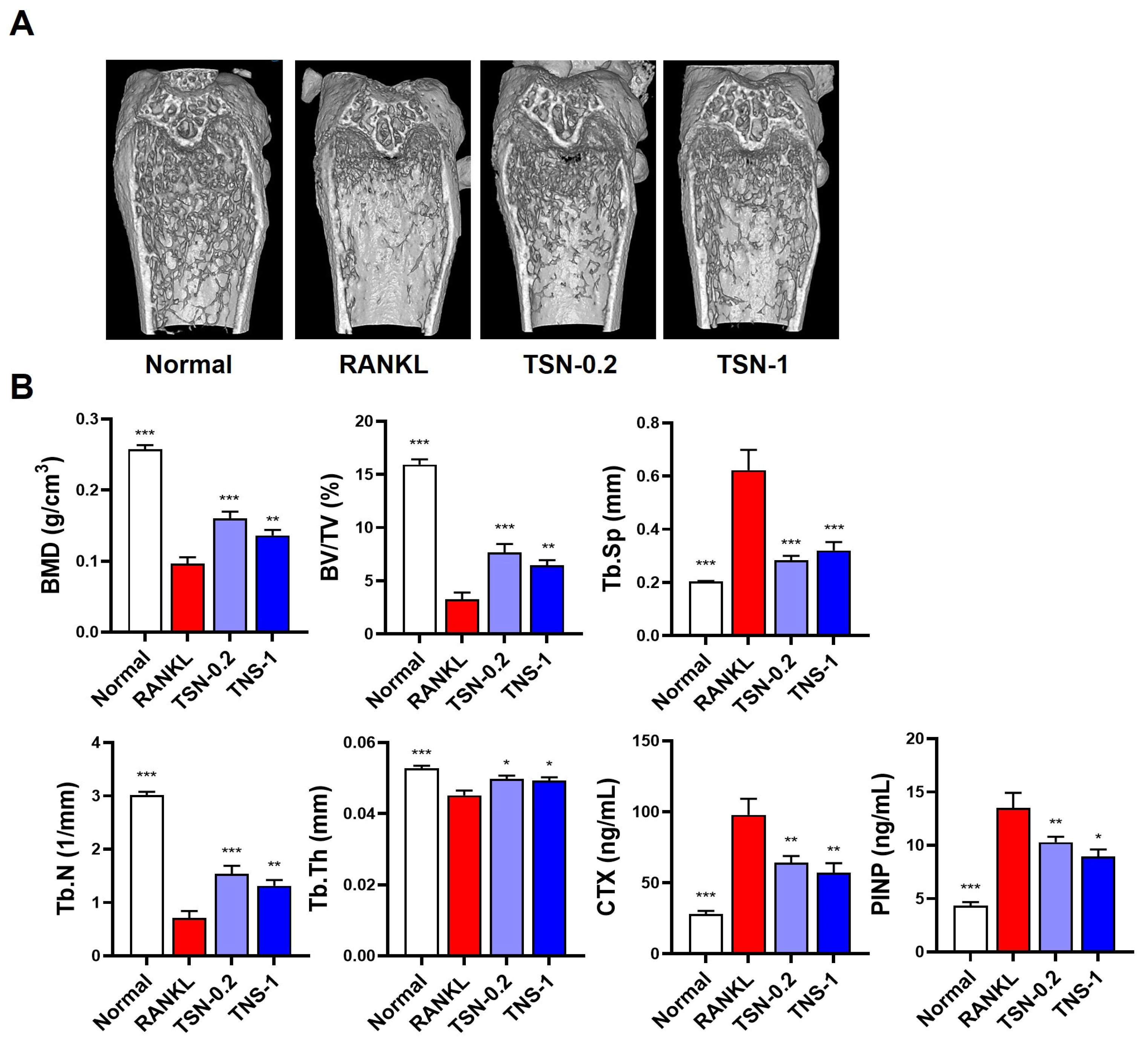
3.5. Protective Effect of TSN on RANKL-Induced Bone Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. Embo J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef]
- Pang, M.; Rodríguez-Gonzalez, M.; Hernandez, M.; Recinos, C.C.; Seldeen, K.L.; Troen, B.R. AP-1 and Mitf interact with NFATc1 to stimulate cathepsin K promoter activity in osteoclast precursors. J. Cell Biochem. 2019, 120, 12382–12392. [Google Scholar] [CrossRef]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef]
- Guo, S.; Gu, J.; Ma, J.; Xu, R.; Wu, Q.; Meng, L.; Liu, H.; Li, L.; Xu, Y. GATA4-driven miR-206-3p signatures control orofacial bone development by regulating osteogenic and osteoclastic activity. Theranostics 2021, 11, 8379–8395. [Google Scholar] [CrossRef]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef]
- Chang, H.; Wang, C.; Gong, L.; Zhang, Y.; Liang, C.; Liu, H. An overview of Fructus Meliae Toosendan: Botany, traditional uses, phytochemistry, pharmacology and toxicology. Biomed. Pharmacother. 2023, 157, 113795. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, H.; Zhang, L.; Wang, Y.L.; Wang, J.; Wang, P.; He, X.; He, Y.J. Anticancer effects of crude extract from Melia toosendan Sieb. et Zucc on hepatocellular carcinoma in vitro and in vivo. Chin. J. Integr. Med. 2016, 22, 362–369. [Google Scholar] [CrossRef]
- Baek, S.-H.; Han, D.S.; Yook, C.N.; Kim, Y.C.; Kwak, J.S. Synthesis and Antitumor Activity of Cannabigerol. Arch. Pharmacal Res. 1996, 19, 228–230. [Google Scholar] [CrossRef]
- Nakai, Y.; Pellett, S.; Tepp, W.H.; Johnson, E.A.; Janda, K.D. Toosendanin: Synthesis of the AB-ring and investigations of its anti-botulinum properties (Part II). Bioorg Med. Chem. 2010, 18, 1280–1287. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Zhang, C.F.; Wang, Z.T.; Yu, B.Y.; Kou, J.P. Anti-inflammatory and analgesic activities of ethanolic extract and two limonoids from Melia toosendan fruit. J. Ethnopharmacol. 2008, 117, 463–466. [Google Scholar] [CrossRef]
- Xiang, X.X.; Tang, D.X.; Xiong, J.Y.; Liang, Y.J.; Mu, D.H.; Yang, X.W.; Hang, M.; Tan, Z.H. Effect of meliae toosendan fructus on nerves system and its mechanism. Zhong Yao Cai 2013, 36, 767–771. [Google Scholar]
- Jin, Y.H.; Choi, J.G.; Cho, W.K.; Ma, J.Y. Ethanolic Extract of Melia Fructus Has Anti-influenza A Virus Activity by Affecting Viral Entry and Viral RNA Polymerase. Front. Microbiol. 2017, 8, 476. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Liu, X.T.; Liang, J.Y.; Ip, N.Y.; Min, Z.D. Minor limonoids from Melia toosendan and their antibacterial activity. Planta Med. 2007, 73, 1298–1303. [Google Scholar] [CrossRef]
- Putnam, S.E.; Scutt, A.M.; Bicknell, K.; Priestley, C.M.; Williamson, E.M. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. PTR 2007, 21, 99–112. [Google Scholar] [CrossRef]
- Che, C.T.; Wong, M.S.; Lam, C.W. Natural Products from Chinese Medicines with Potential Benefits to Bone Health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef]
- Gu, D.R.; Yang, H.; Kim, S.C.; Hwang, Y.H.; Ha, H. Water Extract of Piper longum Linn Ameliorates Ovariectomy-Induced Bone Loss by Inhibiting Osteoclast Differentiation. Nutrients 2022, 14, 3667. [Google Scholar] [CrossRef]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Kook, H.; Kim, K.K.; Lee, S.Y.; Kim, N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007, 109, 3253–3259. [Google Scholar] [CrossRef]
- Zhao, B.; Takami, M.; Yamada, A.; Wang, X.; Koga, T.; Hu, X.; Tamura, T.; Ozato, K.; Choi, Y.; Ivashkiv, L.B.; et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat. Med. 2009, 15, 1066–1071. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kato, S.; Kodama, T.; Takahashi, S.; Calame, K.; Takayanagi, H. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 3117–3122. [Google Scholar] [CrossRef]
- Tomimori, Y.; Mori, K.; Koide, M.; Nakamichi, Y.; Ninomiya, T.; Udagawa, N.; Yasuda, H. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J. Bone Miner. Res. 2009, 24, 1194–1205. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, L.; Wang, Z.R.; Li, Z.; Ma, J. Anti-cancer effect of toosendanin and its underlying mechanisms. J. Asian Nat. Prod. Res. 2019, 21, 270–283. [Google Scholar] [CrossRef]
- Chen, T.X.; Cheng, X.Y.; Wang, Y.; Yin, W. Toosendanin inhibits adipogenesis by activating Wnt/β-catenin signaling. Sci. Rep. 2018, 8, 4626. [Google Scholar] [CrossRef]
- Jin, Y.H.; Kwon, S.; Choi, J.G.; Cho, W.K.; Lee, B.; Ma, J.Y. Toosendanin From Melia Fructus Suppresses Influenza A Virus Infection by Altering Nuclear Localization of Viral Polymerase PA Protein. Front. Pharmacol. 2019, 10, 1025. [Google Scholar] [CrossRef]
- Fan, H.; Chen, W.; Zhu, J.; Zhang, J.; Peng, S. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int. Immunopharmacol. 2019, 76, 105909. [Google Scholar] [CrossRef]
- Tan, T.; Li, T.; Xiang, C.; Ouyang, Z. Toosendanin inhibits osteoclast formation and alleviate postmenopausal osteoporosis by regulating the p38 signaling pathway. Int. Immunopharmacol. 2023, 116, 109745. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, S.; Han, S.; Luo, L.; Shen, F.; Huang, Z. Toosendanin induced hepatotoxicity via triggering PERK-eIF2α-ATF4 mediated ferroptosis. Toxicol. Lett. 2023, 377, 51–61. [Google Scholar] [CrossRef]
- Lu, X.; Ji, C.; Tong, W.; Lian, X.; Wu, Y.; Fan, X.; Gao, Y. Integrated analysis of microRNA and mRNA expression profiles highlights the complex and dynamic behavior of toosendanin-induced liver injury in mice. Sci. Rep. 2016, 6, 34225. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, H.; Liu, Z.; Lai, J.; Zhan, Z.; Chen, Y.; Huang, H. Mechanisms involved in the anti-tumor effects of Toosendanin in glioma cells. Cancer Cell Int. 2021, 21, 492. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, Z.L.; Li, L.; Yang, Y.; Wang, C.H.; Wang, Z.T.; Ji, L.L. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol. Sin. 2019, 40, 75–85. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.C.; Gu, D.R.; Yang, H.; Lee, S.-J.; Ryuk, J.A.; Ha, H. Isolation and Characterization of an Anti-Osteoporotic Compound from Melia toosendan Fructus. Pharmaceutics 2023, 15, 2454. https://doi.org/10.3390/pharmaceutics15102454
Kim SC, Gu DR, Yang H, Lee S-J, Ryuk JA, Ha H. Isolation and Characterization of an Anti-Osteoporotic Compound from Melia toosendan Fructus. Pharmaceutics. 2023; 15(10):2454. https://doi.org/10.3390/pharmaceutics15102454
Chicago/Turabian StyleKim, Seong Cheol, Dong Ryun Gu, Hyun Yang, Sung-Ju Lee, Jin Ah Ryuk, and Hyunil Ha. 2023. "Isolation and Characterization of an Anti-Osteoporotic Compound from Melia toosendan Fructus" Pharmaceutics 15, no. 10: 2454. https://doi.org/10.3390/pharmaceutics15102454
APA StyleKim, S. C., Gu, D. R., Yang, H., Lee, S.-J., Ryuk, J. A., & Ha, H. (2023). Isolation and Characterization of an Anti-Osteoporotic Compound from Melia toosendan Fructus. Pharmaceutics, 15(10), 2454. https://doi.org/10.3390/pharmaceutics15102454





