Abstract
Cancer is one of the most well-studied diseases and there have been significant advancements over the last few decades in understanding its molecular and cellular mechanisms. Although the current treatments (e.g., chemotherapy, radiotherapy, gene therapy and immunotherapy) have provided complete cancer remission for many patients, cancer still remains one of the most common causes of death in the world. The main reasons for the poor response rates for different cancers include the lack of drug specificity, drug resistance and toxic side effects (i.e., in healthy tissues). For addressing the limitations of conventional cancer treatments, nanotechnology has shown to be an important field for constructing different nanoparticles for destroying cancer cells. Due to their size (i.e., less than 1 μm), nanoparticles can deliver significant amounts of cancer drugs to tumors and are able to carry moieties (e.g., folate, peptides) for targeting specific types of cancer cells (i.e., through receptor-mediated endocytosis). Liposomes, composed of phospholipids and an interior aqueous core, can be used as specialized delivery vehicles as they can load different types of cancer therapy agents (e.g., drugs, photosensitizers, genetic material). In addition, the ability to load imaging agents (e.g., fluorophores, radioisotopes, MRI contrast media) enable these nanoparticles to be used for monitoring the progress of treatment. This review examines a wide variety of different liposomes for cancer theranostics, with the different available treatments (e.g., photothermal, photodynamic) and imaging modalities discussed for different cancers.
1. Introduction
Nanomaterials have shown to be effective systems for a wide variety of biomedical applications, and have been used for treatment [1], delivery of therapeutics [2] and bio-imaging [3]. Significant developments have arisen in nanotechnology, focusing on nanoparticles for both imaging and therapy, referred to as theranostics [4,5,6,7]. Nanoparticles are advantageous due to their physical and chemical properties, enabling them to be used for tumor monitoring and treatment. Materials such as gold nanoparticles [8,9], quantum dots [10,11], magnetic nanoparticles [12,13], upconversion nanoparticles [14,15], nanoemulsions [16,17,18], nanogels [19,20], micro/nano-bubbles [21,22,23], carbon nanoparticles [24,25], polymeric nanoparticles [26,27] and micelles [28,29] have all successfully been used for the diagnosis and treatment of cancer. Liposomes are unique nanoparticles that have been used for cancer theranostics, due to their ability to load both hydrophobic (i.e., in their lipophilic shell) and hydrophilic therapeutic and imaging agents (i.e., in the aqueous core). The advantages of liposomes include the high loading efficiency of agents, high stability in biological conditions and controllable release kinetics due to stimuli responsiveness and biocompatibility, providing better pharmacokinetics and biodistribution of theranostic agents than many other carriers [30].
This review article focuses on the different types of therapies and imaging techniques that are available and that have been reported when using liposomes for cancer theranostics. The different types of liposomes are presented, with a focus on the results from the treatment and imaging of different kinds of cancers. The goal is to highlight the benefits of using liposomes and the variety of treatment and imaging options available for cancer theranostics.
2. Common Cancer Therapies
A wide variety of therapies are available when using nanoparticles (e.g., liposomes) for cancer treatment, depending on the stimuli (i.e., ionizing or non-ionizing) used for excitation and the mechanism for cancer cell death (e.g., apoptosis, ablation). Each treatment option has unique advantages that can be attractive when using specific types of nanoparticles, as well as disadvantages limiting their widespread use (Table 1). Common therapies that can use nanoparticles for improving treatment outcomes include chemotherapy, gene therapy, immunotherapy, photothermal therapy, photodynamic therapy, magneto-thermal therapy, ultrasound responsive therapy and radiotherapy.

Table 1.
Advantages and disadvantages of different cancer therapies.
2.1. Chemotherapy
Biocompatible and biodegradable nanoparticles such as liposomes can be used for the delivery of chemotherapeutic agents (e.g., paclitaxel, docetaxel, doxorubicin, celecoxib) [31]. Drug-loaded nanoparticles reduce systemic toxicity due to slow drug release rates, increasing the amount of therapeutic agents that can accumulate in tumor tissue (i.e., through the enhanced permeability and retention effect, EPR) [32,33,34,35]. Resistance to chemotherapy remains a significant issue in oncology, reducing its efficacy in treating metastatic tumors. The drug resistance mechanism is highly intricate and various factors reduce the effectiveness of therapeutic agents in inhibiting tumor growth. Drug transporters are responsible for drug efflux, resulting in low therapeutic doses [36,37], while gene mutations, genomic instability, epigenetic alterations (e.g., DNA methylation, protein acetylation), suppression of apoptotic signaling, and overexpression of anti-apoptotic molecules contribute towards multi-drug resistance (MDR) [38]. To reverse MDR (i.e., resistance to anticancer drugs), conventional strategies include the development of inhibitors for ABC transporters (e.g., monoclonal antibodies, compounds), using high doses of chemotherapy, targeting specific messenger RNAs (mRNAs) for rendering MDR genes ineffective and the development of chemotherapeutics that are not substrates of P-glycoprotein or P-gp (i.e., a MDR drug efflux protein) [39]. To better combat multi-drug-resistant cancer, various nanoparticles have been developed, including those containing inhibitors of drug efflux transporters (e.g., tyrosine kinase inhibitors, small interfering RNA) that block function or silence the MDR mRNA [40].
2.2. Gene Therapy
Nanoparticles such as liposomes can be used for the delivery of genetic material such as plasmid DNA, mRNA, microRNA (miRNA), small interfering RNA (siRNA), short hairpin RNA (shRNA), and antisense oligonucleotides [41,42]. There are several mechanisms by which gene therapies work, for example by replacing a cancer-causing gene with a healthy copy of the gene and/or inactivating a non-functional cancer-causing gene. Replacement of genes can be accomplished by gene transduction, stability maintenance and complete gene expression, or by the correction of gene mutations into its wild-type form [43]. Targets for gene replacement therapy include tumor suppressor genes (e.g., TP53, P21, PTEN). Gene silencing on the other hand involves introducing siRNA or shRNA in tumor cells for targeting a specific complementary sequence to mRNA for its degradation or by blocking the synthesis of proteins [44]. Targets for gene silencing therapy include drug resistance oncogenes (e.g., cMYC, KRAS). Antisense therapy uses antisense oligonucleotides to target mRNA for the downregulation of gene expression (e.g., that are associated with regulating apoptosis, cell growth, metastasis, and angiogenesis) [45]. The miRNA-targeted therapy involves restoring levels of miRNAs that have been altered, using miRNA-duplexes to replace levels of under-expressed miRNAs, or siRNA complementary to the seed sequence of the miRNA of interest [46,47]. Genes can also be introduced, for example in suicide gene therapy, encoding a cytotoxic protein for cancer cell death [48]. In addition, genome editing therapy has recently gained much attention for being able to modify intracellular DNA in a sequence-specific manner (i.e., by insertion, deletion, integration or sequence substitution), using nucleases such as zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN), meganucleases, and CRISPR/Cas9 systems [49,50].
2.3. Immunotherapy
Immunotherapy and the idea of boosting antitumor activity using the immune system (i.e., via tumor-specific or non-specific immune activation) has established itself as an effective therapy option [51]. For example, a promising and emerging strategy to treat both solid and hematological malignancies is programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) blockade immunotherapy [52]. This therapy is used to wake the immune system from the suppression of activities, leading to the death of cancer cells by T cells. This is an attractive alternative to treatment using chemotherapeutic agents, which can lead to multi-drug resistance by cancer cells through genetic mutations. Nanoparticles such as liposomes can be used as platforms for the delivery of immune modulators, targeting tumor-associated cells (e.g., dendritic cells, T cells, tumor cells, natural killer cells, and macrophages) for enhancing immunological responses and immunotherapy efficacy [53,54]. The group of proteins and drugs (e.g., cytokines, thalidomide, imiquimod) that are used for immunotherapy mainly target pathways that decrease or increase the amount of certain proteins for enabling the immune system to work optimally for the treatment of cancer. Different types of nanocarriers (i.e., <1 μm) can be developed for immunomodulation by carrying specific immunotherapeutic agents, based on inorganic and organic moieties [55].
2.4. Photothermal Therapy
Photothermal therapy (PTT) is a therapeutic strategy wherein near-infrared (NIR) light is used to resonate electrons in nanoparticles for producing heat and ablating malignant tumors [56]. When the local temperature from the heat generated by nanoparticles is in the range 43–50 °C, apoptosis in cancer cells can be induced [57]. If cancer cells are exposed for more than 15 min in this temperature range, irreversible tissue damage can occur, with rapid protein denaturation at temperatures above 60 °C (e.g., from thermal tumor ablation) [58]. To improve the optical properties through surface plasmon resonance (i.e., an electromagnetic response that occurs when plasmons are oscillating with the same frequency on the surface of a material), various elements are used for constructing particles, such as gold, silver and carbon [59,60]. Recently, polymer-based nanoparticles have gained attention because of their biocompatibility and biodegradability and use in different types of treatments with high photothermal conversion efficiencies [61,62].
2.5. Photodynamic Therapy
Photodynamic therapy (PDT) is a minimally invasive treatment for cancer therapy [63]. Light (i.e., usually NIR) is absorbed by photosensitizer molecules (i.e., loaded in nanoparticles for reducing systemic toxicity), after accumulating in tumor tissues. Photosensitizers are important due to their ability to induce chemical and physical alterations of other molecules, upon absorption of light. The excited photosensitizer can undergo electron and/or energy transfer reactions to produce reactive oxygen species (ROS). These generated ROS can interfere with important molecules (e.g., DNA) and proteins, inhibiting their function and activating certain mechanisms such as apoptosis due to generated oxidative stress [64]. Like PTT, PDT treatment only targets the areas under illumination, minimizing toxicity in healthy cells [65]. Since photosensitizers can also provide fluorescence under laser irradiation, they are loaded in many types of nanoparticles including liposomes for cancer theranostics [66,67,68].
2.6. Magneto-Thermal Therapy
Magnetic nanoparticles can be used to generate heat using an alternating current (AC) magnetic field for cancer therapy. Magnetic heating (i.e., hyperthermia from magnetically mediated heating of low-frequency electromagnetic waves) and a temperature-responsive or thermally-rupturable layer (i.e., thermosensitive and disrupted above a certain temperature) are two fundamental features required for magneto-thermal delivery and cancer theranostics [13,69,70,71]. Such properties enable therapeutic agents to be released at high quantities at the tumor site after tumor accumulation and heating using magnetic fields, reducing off-target effects (i.e., in other non-cancerous tissues). A common delivery mechanism for magnetically triggered therapies involves the integration of iron oxide nanoparticles (IONPs) in liposomes. Recent developments have involved magneto-thermal therapies (i.e., magneto-photothermal therapy) using magnetic particles with light absorbing molecules/particles and combining NIR lasers and alternating magnetic field induction for improving cancer treatment [72,73,74].
2.7. Ultrasound Responsive Therapy
Ultrasound can be used for temperature-sensitive nanosystems such as liposomes, where the encapsulated payload can be released locally through mechanical effects (e.g., cavitation, strain) [75,76,77,78,79]. Ultrasound responsive therapy provides an attractive mechanism for the delivery of therapeutic agents, controlling the release with no degradation of molecules [80]. Other types of nanoparticles/bubbles that require ultrasound for cancer therapy include polypyrrole hollow microspheres [81], microbubbles [82,83,84,85,86], biodegradable poly(methacrylic acid)-based nanocapsules [87], superparamagnetic iron oxide acoustic droplets [88], crown-ether-coated core/shell nanoparticles [89], polymer-grafted mesoporous silica nanoparticles [90], and echogenic glycol chitosan nanoparticles (i.e., that are able to generate ultrasound signals from acoustic cavitation of the resulting bubbles formed) [91].
2.8. Radiotherapy
Radiotherapy (RT) using nanoparticles and ionizing radiation (i.e., high energy X-rays or other particles) can cause significant damage in tumor tissue. Nanoparticles such as liposomes serve as radiosensitizers (RSs), containing high atomic number and electron-dense elements for enhancing therapeutic sensitivity [92,93]. High atomic number and electron-dense elements are required for improving the relative dose accumulation of tumors (i.e., for increasing the amount of free radicals and enhancing DNA damage) by enhancing the absorption cross-section of X-rays. A main disadvantage of RT is the dose delivery efficiency in destroying cancer cells, which depends on the tolerance level of normal tissues near tumor tissues.
3. Imaging Modalities Available for Cancer Theranostics
Nanoparticles such as liposomes can load imaging molecules (e.g., radioisotopes, fluorophores, superparamagnetic particles, acoustic scatterers) for enhancing contrast in cancer imaging. Due to the hydrophobic and hydrophilic nature of liposomes, many molecules with varying solubility can be loaded. This makes it possible for liposomes to be used with different imaging modalities such as positron emission tomography, single-photon emission computed tomography, computed tomography, magnetic resonance imaging, optical imaging, ultrasound imaging and photoacoustic imaging, improving the overall benefits and avoiding the limitations of certain imaging techniques (Table 2).

Table 2.
Overview of different imaging techniques used in cancer theranostics.
3.1. Positron Emission Tomography
Positron emission tomography (PET) is routinely used clinically for whole-body imaging, based on visualizing and quantifying positron-emitting radionuclides. These radionuclides are used for emitting pairs of γ-rays for generating imaging contrast and include atoms such as 11C, 13N, 15O, 18F, 44Sc, 62Cu, 64Cu, 68Ga, 72As, 74As, 76Br, 82Rb, 86Y, 89Zr, and 124I [94,95,96,97,98,99,100,101,102]. Because radionuclides are small and are limited by their quick clearance, PET probes can be conjugated to or encapsulated within nanoparticles for improving biodistribution and accumulation at the target site. PET imaging provides high sensitivity (i.e., radionuclide concentrations as low as (sub-)picomolar range) and high signal-to-noise ratios, providing unlimited penetration depth. Disadvantages associated with PET imaging include the lack of anatomical information, the relatively low spatial resolution and the necessity for using radioactive probes, which can be toxic to healthy cells. Despite this, PET imaging can be combined with other imaging modalities such as computed tomography (i.e., PET-CT) and magnetic resonance imaging (i.e., PET-MRI) for overcoming the disadvantages of CT or MRI. Various studies have reported on the use of liposomes with PET imaging (i.e., with radionuclides incorporated) [103] for enhancing tumor accumulation and improving pharmacokinetic properties for cancer theranostics [104].
3.2. Single-Photon Emission Computed Tomography
Single-photon emission computed tomography (SPECT) imaging uses the non-coincident γ-rays generated by radionuclides such as 99mTc, 111In, 123I, 125I, and 201Tl. Because SPECT is analogous to PET, it shares advantages and disadvantages (i.e., previously mentioned for PET). The sensitivity of SPECT is about an order of magnitude lower than that of PET and quantification is somewhat more difficult. In addition, compared to PET where all the emitted photons have the same energy, the energies from radionuclides in SPECT are often different, enabling the assessment of different radiotracers/radiolabeled nanoprobes at the same time. SPECT can be combined with other imaging modalities such as CT (i.e., SPECT/CT) and MRI (i.e., SPECT/MRI) for monitoring tumors and assessing biodistribution using liposomes [105,106,107].
3.3. Computed Tomography
X-ray computed tomography (CT) imaging is a modality that uses computer processed X-ray scans to produce tomographic images and 3D visualization of tumors. Contrast images are a result of distinctions in X-ray absorption and attenuation by different components of the body, which can be enhanced by the use of nanoparticles. CT contrast agents have large atomic weight (i.e., high Z value) elements such as iodine (non-radioactive), gold, platinum, bismuth, tantalum, and ytterbium. High atomic number element containing liposome contrast agents for CT, especially CT-based multimodal imaging liposomes, have been used for increasing circulation lifetime and enhancing the accumulation at the tumor site [108,109,110]. CT imaging can provide high spatial resolution with unlimited tissue penetration. However, a relatively high dose of ionizing radiation is required for CT imaging to be effectively used for cancer theranostics.
3.4. Magnetic Resonance Imaging
MRI is a clinically applied imaging modality, which depends on the spin–lattice relaxation and the spin–spin relaxation time of protons contained in different tissues or organs, with the ability to enhance image contrast using nanoparticles (e.g., liposomes) [111,112,113,114,115]. MRI does not use any radiation and can provide excellent anatomic detail and high spatial resolution. Paramagnetic ions, such as manganese (Mn2+), iron (Fe3+), and gadolinium (Gd3+), are usually used to provide MRI contrast. The nanoparticles can alter the spin–lattice and/or spin–spin relaxation time of protons for providing T1 and/or T2 contrast [116]. PET, SPECT and optical imaging can be used with MRI to improve sensitivity and image resolution, while the addition of CT improves temporal resolution [117]. Although MRI can be used for (pre-) clinical diagnosis and therapy monitoring, it has several disadvantages such as relatively low contrast agent sensitivity, difficult quantification procedures, and significant time and cost involved.
3.5. Optical Imaging
Optical imaging (OI) is a non-ionizing, non-invasive imaging modality based on the optical characteristics of tissue components (i.e., from light emission/absorption), which can be enhanced through the introduction of nanoparticles (e.g., liposomes with fluorescent probes and/or upconverting nanoparticles, UCNPs) [118,119]. Biomedical optical imaging can be used for quantitative measurements in real time, providing a wide range of image resolutions for cancer diagnosis and monitoring of treatment. Advantages of OI include the simplicity of use, simultaneous detection of multiple markers, and wide spatial scales ranging from subcellular structures to tissues. However, a main disadvantage of OI is that it cannot be used for deeply penetrating tumors due to limited penetration depth. Compared to conventional fluorophores, UCNPs emit higher-energy visible light when excited by NIR light and can vary in composition for use in multiple imaging modalities. Even though an absolute quantification of accumulation of nanoparticles in tumors can be made using 3D fluorescence molecular tomography (FMT), diffusive scattering of fluorescence emission in the body and strong light absorption by organs and tissues limits the assessment of the biodistribution.
3.6. Ultrasound and Photoacoustic Imaging
Ultrasound imaging (USI) is based on the principle that back-scattered signals from acoustic waves vary depending on the reflection by different tissues, as well as by US contrast agents. USI is a versatile technique, providing a clear depiction of the area of interest, with high temporal and spatial resolution. Particles such as nanoscale liquid–liquid nanoparticles, gas–liquid nanoparticles, and solid nanoparticles have also been reported to contribute towards enhanced US contrast and therapy [120,121,122]. Despite this, USI is limited by relatively low resolution and sensitivity, with a penetration depth that depends on the US frequency used. An imaging modality that is commonly used alongside USI imaging is photoacoustic imaging (PAI). PAI is commonly used for the illumination of light-absorbing molecules and nanoprobes [123,124] in tissues using pulsed laser light for providing signals based on energy absorption, heat generation, and thermoelastic expansion from particles and tissue [125,126,127]. The expansion can be detected using ultrasound detectors, with signals detected at deeper depths and with higher sensitivity when USI is combined.
4. Biomedical Applications of Cancer Theranostic Liposomes
A variety of liposomes have been developed for therapy and imaging of different types of cancers. Liposomes can be produced that either passively (i.e., through EPR effect and accumulation) or actively (i.e., through ligand–receptor interactions) target cancer cells. Both kinds of liposomes have been shown to be effective, enhancing pharmacokinetics (e.g., half-life) for cancer treatment. In addition to promising in vitro and in vivo results, many liposomes are undergoing or have undergone different clinical phase trials (Table 3).
4.1. Breast Cancer
Theranostic dual-layered gold (Au)-containing liposomes can be developed for effective tumor targeting and photothermal therapy [128]. A liposomal layer is added to Au-coated liposomes (i.e., ALs) for producing dual layered NPs (i.e., liposomal ALs called LALs). The NPs are functionalized with polyethylene glycol (PEG) for improving the in vivo stability with radioisotopes (i.e., 64Cu-LAL) used for enabling in vivo PET imaging. The addition of Au NPs contributes to PTT due to the excellent photothermal conversion efficiency (i.e., due to surface plasmon resonance phenomenon) and tunability of the absorption band [129,130,131]. Transmission electron microscopy (TEM) was used to visualize the NPs, confirming the decoration of Au NPs on liposomes for ALs and the outer liposomal layer over ALs (i.e., in samples containing LALs). The sizes determined using TEM for ALs and LALs were 61.02 ± 29.22 nm and 72.84 ± 22.49 nm, respectively. The zeta potential decreased from −12.0 mV to −23.7 mV after Au coating on liposomes. The zeta potential increased to −17.7 mV after AL was covered with a liposomal layer, due to the PEG moiety. The LALs were stable in physiological solutions (i.e., deionized water, phosphate-buffered saline or PBS, cell media with 10% fetal bovine serum), with the sizes maintained (i.e., ranging from 60 to 80 nm) over a period of 14 days. Both ALs and LALs have a broad absorbance band with a peak at 800 nm. Under 808 nm laser irradiation with 1 W intensity, the temperatures from solutions containing ALs and LALs increased from ~25 °C (i.e., before laser irradiation) to ~42 °C and ~44 °C, respectively, after 40 min of irradiation (i.e., NPs containing the same Au concentration, ~24 μg/mL). Furthermore, both ALs and LALs were stable in terms of temperature elevation during four repeats of laser irradiation (i.e., on/off cycles), suggesting that the NPs can be irradiated multiple times for PTT with a single injection of NPs. The calculated photothermal efficiency values of ALs and LALs were 34.13% and 37.46%, respectively, which are higher than the values from other photothermal nanomaterials [132,133,134,135,136,137]. In vitro experiments in 4T1 breast cancer cells were carried out to determine the biocompatibility of NPs (i.e., without laser irradiation) and cancer cell viability from PTT using ALs and LALs. The cytotoxicity (i.e., from NPs only) was not significant, showing over 80% survival of cells (i.e., up to Au concentrations of ~12 μg/mL). However, when 4T1 cancer cells are treated with AL and LAL (i.e., both with ~12 μg/mL Au concentration) and laser irradiation (i.e., 2 W/cm2, 5 min), the cell viability is as low as ~30% for both types of NPs (i.e., ALs, LALs). The excellent PTT effect of NPs is due to the high rate of cellular uptake of NPs, DNA damage and temperature change from laser irradiation. To demonstrate the ability to be used for in vivo PET imaging, 64Cu-LAL (i.e., 4.74 μg of Au) was used. The tumor uptake of NPs increased gradually with time, with the percent injected dose per gram of tumor tissue (ID/g) up to ~16.4%, after 24 h from injection. This is much higher than other nanoparticles used for PTT, such as bare AuNPs (i.e., ~1.6% ID/g) and modified AuNPs (i.e., 5–10% ID/g) after 12–48 h from intravenous injection [138,139,140,141,142,143,144]. Using 808 nm laser irradiation on each tumor site with 2.5 W/cm2 (i.e., two times treatment, 24 and 48 h after intravenous injection), the tumor growth inhibition rate of each group was as follows: normal saline + laser was 12.2%, ALs was 14.2%, ALs + laser was 20.4%, LALs was 7.52%, and LALs + laser was 79.4%. This shows that the NPs (i.e., especially LALs) can effectively treat tumors through PTT, while being able to be used for cancer imaging.
Iron oxide nanoparticles (i.e., synthesized using a modified co-precipitation method) and chemotherapeutic drug doxorubicin (DOX) can be passively encapsulated into PEGylated liposomes (i.e., forming magnetoliposomes) [145]. Superparamagnetic iron oxide NPs (i.e., magnetite (Fe3O4), maghemite (γ-Fe2O3)) can be used for different biomedical applications [146] such as drug delivery [147], MRI [148] and hyperthermia [149]. Dextrose (Dex) coating around magnetic nanoparticles (MNPs) was confirmed using Fourier transform infrared (FTIR) spectroscopy, with a peak at 523 cm−1, shifted from 582 cm−1 (i.e., from vibration of Fe–O from MNPs) due to the interaction between Fe–O and OH of Dex molecules [150,151]. A band at 1071 cm−1 also shifted to 1026 cm−1 for Dex, possibly due to the occurrence of vibrational interactions between MNP and Dex molecules [150,151,152]. The Dex coating of MNPs reduced the aggregation and agglomeration of MNPs (i.e., improved dispersity), resulting in an increase in the zeta potential of nanoparticles. This was further confirmed by TEM and visually, with a greater sedimentation rate of MNPs without the Dex coating. Crystallite sizes from X-ray diffraction for MNP, MNP-Dex5% and MNP-Dex10% were determined using the Debye–Scherrer equation, assuming spherical shape (shape constant of 0.9) [153], and calculated to be 14.7 nm, 13.5 nm, and 11.5 nm, respectively. The saturation magnetization for MNP-Dex10% and MNP from the vibrating sample magnetometer (VSM) hysteresis curves was about 58 emu/g, showing the potential of NPs to be used for MRI, with similar or higher saturation magnetization when compared to other types of MNPs [154,155]. The size of the spherical DOX-loaded magnetoliposomes (DMLs) was determined to be ~100 nm (i.e., 2 times larger than plain liposomes), with a zeta potential of −3.6 mV. Compared to DOX-loaded liposomes (DLs), DMLs are more slow-releasing in terms of drug release, which could be due to the interactions between DOX and Dex-coated MNPs. Drug release studies showed that about 50% of the total DOX loaded on DMLs was released in 150 h in a sustained manner (i.e., at 37 °C) due to the stability of liposomes. The cytotoxicity in MCF-7 breast cancer cells was ~40% with treatment with DMLs and was very similar to cytotoxicity values when cells were treated with DOX only (i.e., using same concentration of 25 μM).
Liposomes can also be constructed for active targeting, combining methylene blue (MB) attached upconversion nanoparticles (i.e., NaYF4:Yb, Er UCNPs) for NIR-activated bioimaging and PDT against HER-2 positive breast cancer [156]. The UCNPs can act as an upconverting energy source for the photosensitizer dye, methylene blue (Figure 1a) [157,158,159,160,161,162]. Upon NIR laser irradiation of MB@UCNPs, ROS are produced, which can be used for damaging important biological molecules (e.g., DNA, RNA, proteins) and causing cancer cell death (Figure 1b) [163]. The NPs can specifically target HER-2 positive breast cancer cells by loading anti-HER2 peptides for selective tracking and high cell penetration capabilities [164]. TEM images revealed that the encapsulation of UCNPs was within the hydrophilic core of liposomes. The size of MB@UCNPs was determined to be 15 nm using dynamic light scattering (DLS), with the size of liposomes containing MB@UCNPs being ~77 nm (i.e., LPs). Anti-HER2 peptide attachment on PEGylated LPs (i.e., LPs + DSPE-PEG(2000) maleimide + anti-HER2 peptide) further increased the size of particles to ~91 nm (i.e., with a zeta potential of −18 mV). The amount of MB released from LPs at highly acidic conditions (i.e., 3.5, 4.5) was higher compared to that at physiological pH (i.e., 7.5), showing that the LPs can function as controlled release delivery vehicles, releasing cargo upon late endosomal acidification (i.e., after endocytosis of LPs in cancer cells). The amount of fluorescence in cells from LPs with peptides and UCNPs was significantly greater than the signals from LPs with UCNPs only. To determine the type of nanoparticle that would produce the most ROS for cancer therapy, the energy transfer efficacy (η) was evaluated. The efficacy (η) was found to be 38% for LPs containing peptides, UCNPs and free MB (i.e., Group 3 LPs) and 57% for LPs containing peptides and MB@UCNPs (i.e., Group 4 LPs). This reveals that the energy transfer effectiveness for photoactivating the MB for ROS generation in Group 4 LPs containing peptides and MB@UCNPs is higher than that of Group 3 LPs containing peptides, UCNPs and free MB. This led to the 1O2 generation capability being lower in the Group 3 LPs, compared to Group 4 LPs. Fluorescence experiments also revealed that the fluorescence intensity of ROS fluorogenic markers (i.e., using 2′,7′-dichlorodihydrofluorescein diacetate, DCF-DA) in the Group 4 LPs was higher than the Group 3 LPs, which correlates to the enhanced ROS generated (Figure 1c). The cell viability in SKBR-3 breast cancer cells after treatment with 30 μM Group 4 liposomes and 975 nm NIR laser excitation (i.e., for 5 min) was ~15%, which was about 2 times lower than the cell viability after treatment with 30 μM Group 3 liposomes at the same experimental conditions (Figure 1d).
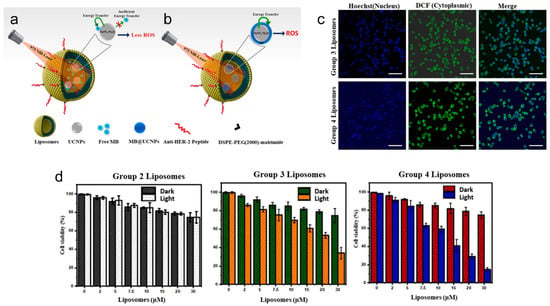
Figure 1.
Ligand-targeted theranostic liposomes combining methylene blue attached upconversion nanoparticles for NIR-activated bioimaging and photodynamic therapy against HER-2 positive breast cancer. Illustration shows anti-HER2 peptide conjugated liposomes for selective bioimaging and PDT (i.e., using 975 nm NIR-laser) using two different combinations with LPs with free MB and UCNPs (a) and LPs with MB@UCNPs (b). Confocal images show that ROS can be generated in Group 3 LPs and Group 4 LPs using DCF-DA as a green fluorescent indicator after 5 min of laser excitation (i.e., at 975 nm) (c). Nuclei were counterstained with Hoechst shown in blue color. Scale represents: 50 μm. In vitro cell viability was determined using XTT assay to assess the SKBR-3 cell viability with different concentrations of LPs Groups (0–30 μM) and 975 nm NIR laser excitation for 5 min (d). Group 2 LPs represent LPs with UCNPs, Group 3 LPs represent LPs with free MB and UCNPs, and Group 4 LPs represent LPs with MB@UCNPs. Data are presented as mean ± SD (n = 3). Reprinted from Journal of Luminescence from [156], Copyright (2021), with permission from Elsevier.
Other types of NPs that can be used for fluorescence imaging include graphene-oxide-supported liposomes (i.e., for phototriggered tissue visualization and tumor regression) [165]. The graphene oxide flake decorated liposomal (GOF-Lipo) nanohybrid can carry the anticancer drug doxorubicin hydrochloride (DOX·HCL) [166,167] and be functionalized with folic acid [166] for tumor bonding ability, through the use of the film hydration and extrusion method. DOX is used in NPs to examine the multi-stimuli (i.e., NIR light and pH)-triggered response of folic-acid-attached GOF-Lipo (GOF-Lipo-FA) under NIR light irradiation. The spherical red emissive GOF-Lipo has a size of 200 nm, with stability for at least a month and no premature drug release in physiological conditions. The elemental composition of the GOF-Lipo nanohybrid (i.e., containing P, O, C, and N elements) can be determined using scanning transmission electron microscope (STEM) elemental mapping. The temperature was found to increase from ~37 °C to ~54 °C for GOF-Lipo (i.e., at concentration of 2.5 mg/mL) after 5 min of NIR exposure (i.e., at 808 nm, 1 W). This reveals the promising photothermal response of the designed nanohybrid due to the uniform support of GOF with liposomes. Furthermore, the DOX-GOF-Lipo showed a ~3% release in hyperthermia conditions (i.e., 43 °C), with drug release more than 90% at the photothermal ablation temperature (48 °C), due to disintegration of nanohybrids. Due to the protonation of a functional group that causes the disintegration of GOF-Lipo, the designed DOX-GOF-Lipo nanohybrid showed about 100% drug release in late endosomal conditions (i.e., pH 2 and 4). The combined effect of the drug and heat generated from DOX-GOF-Lipo-FA during NIR exposure led to high MDA-MB-231 breast cancer cell death (i.e., more than 95%) and 4T1 breast cancer cell death (i.e., ~90%). Very high emissive intensity at the tumor site after 24 h post-injection of GOF-Lipo-FA was seen due to the high accumulation through specific receptor-ligand binding. The tumor volume stayed relatively constant over a period of 21 days from combined chemo-PTT treatment using the nanohybrid (i.e., three sets of treatment repeated after 2-day time intervals using 808 nm light for 5 min), with tumor weight (i.e., ~0.15 g) after treatment lower than that compared to chemotherapy treatment (i.e., ~0.2 g) and control (i.e., ~0.37 g).
4.2. Cervical Cancer
Nanoliposome (NL) co-encapsulating X-ray CT imaging contrast agents (i.e., iodixanol) and photosensitizers (i.e., meso-tetrakis(4-sulphonatophenyl)porphine, TPPS4) can be produced for enhanced-imaging-guided photodynamic therapy of cancer [109]. Iodinated iodixanol (Visipaque®) is clinically approved and used for CT imaging, while TPPS4 is used in concurrent CT and fluorescence (FL) imaging-guided PDT, through the use of NPs (i.e., referred to as NL co-encapsulation of iodixanol and TPPS4 or LIT). For enhancing singlet oxygen generation and PDT efficacy through the intraparticle heavy atom (iodine) effect on the photosensitizer (PS), the PS TPPS4 and iodinated iodixanol can be co-encapsulated within LIT (Figure 2a). The size of the LIT was determined to be ~117 nm, suitable for uptake by the EPR effect [168], with a zeta potential of 13.5 mV. The TPPS4 and iodixanol loading efficiencies in LIT were determined to be ~67% and ~64%, respectively. Compared to nanoliposomes without iodine and with only TPPS4 (i.e., LT), LIT formulations produced significantly higher phosphorescence signals, which corresponds to the higher amount of singlet oxygen sensitized by TPPS4 within LIT. This enhancement is due to increased intersystem crossing (ISC) rate in the PS molecule produced by the spin–orbit coupling enhanced by external iodine atoms (i.e., heavy atom effect) [169,170,171]. Strong fluorescence signals from HeLa cervical cancer cells were seen due to internalization of LIT (Figure 2b). The cell survival percentage of the LIT group under laser irradiation at 552 nm (i.e., using 15 J/cm2) and 640 nm (i.e., using 15 J/cm2) was measured to be ~23% and ~37%, respectively, due to the generation of ROS (Figure 2c). Fluorescence and X-ray CT signals from tumors with LIT were still strong after 96 h post-injection, with LIT mainly accumulating in the tumor (i.e., much greater signals in tumors compared to other organs). Tumor signals from LIT from CT imaging were still strong after 96 h post-injection of particles, and stronger than those from iodixanol (Figure 2d). A significant suppression of tumor growth was exhibited for the group of mice injected with LIT followed by irradiation, with the relative tumor volume being more than 6 times lower compared to other groups (i.e., LIT (no treatment), T or TPPS4 (irradiation), LT (irradiation)).
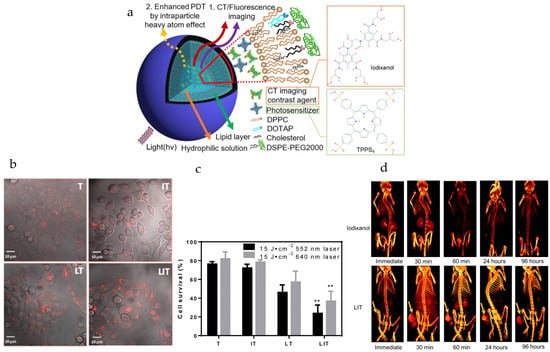
Figure 2.
Nanoliposomes co-encapsulating CT imaging contrast agent and photosensitizer for enhanced imaging-guided photodynamic therapy of cancer. (a) Schematic diagram illustrates nanoliposomes co-encapsulating CT imaging contrast agent (CTIA) and photosensitizer (PS). (b) Optical imaging was used for visualizing cellular internalization of TPPS4 after 18 h incubation with free TPPS4 (T), free iodixanol and TPPS4 (IT), nanoliposomes encapsulating TPPS4 (LT) or nanoliposomes co-encapsulating iodixanol and TPPS4 (LIT). Images merging the transmission and fluorescence confocal channels are shown. Fluorescence of TPPS4 (red pseudocolor) was excited by a 543 nm laser. (c) Survival of HeLa cells was determined one day post-PDT. The dark bars indicate 552 nm laser irradiation and the light ones indicate 640 nm laser irradiation at the same irradiation dose (15 J cm−2). (** p < 0.01 compared with T, IT and LT at the same laser irradiation dose). (d) Three-dimensional volume-rendered images were acquired from the nude mice bearing HeLa tumor xenografts after injection of free iodixanol or NLs with co-encapsulated iodixanol and TPPS4 (LIT). Arrows indicate the location of the tumor. Reprinted with permission from Theranostics from [109]. Copyright (2019) Ivyspring International Publisher (distributed under Creative Commons Attribution (CC BY-NC) License at https://creativecommons.org/licenses/by-nc/4.0/ with no changes).
Multifunctional thermosensitive liposomes based on natural phase-change material can be synthesized for NIR light-triggered drug release and multimodal imaging-guided cancer combination therapy [172]. The indocyanine green (ICG)/DOX loaded and gadolinium (Gd) chelate conjugated temperature sensitive liposome nanoplatforms (ID@TSL-Gd NPs) can exhibit NIR-triggered drug release and prominent chemo-, photothermal, and photodynamic therapy properties. Due to the fluorescence, photoacoustic, photothermal and photodynamic properties of ICG [173,174,175] and magnetic properties of Gd, the NPs can be used for fluorescence/photoacoustic/magnetic resonance imaging triple-modal imaging-guided combination tumor therapy (i.e., chemotherapy, photothermotherapy, and photodynamic therapy). With the modification of folic acid (FA)-phospholipids, the accumulation of NPs in tumors can be enhanced for theranostics. In the cases of 5, 10, 20, and 40 μg/mL of ID@TSL-Gd, after 5 min of laser irradiation, the temperatures reached 45.7, 53.5, 58.5, and 61.2 °C, respectively. These temperature values are all higher than that required for irreversible apoptosis of tumor cells (i.e., 43 °C) [176]. A final DOX release of 55% could be achieved by irradiating the ID@TSL-Gd NPs in an on–off formation within 100 min (i.e., at 808 nm, 0.5 W/cm2), far higher than that without NIR irradiation. This stimuli response can be used to deliver greater amounts of drugs to cancer cells once the NPs have accumulated at the tumor site, while also being able to generate ROS from laser irradiation. In vitro fluorescence imaging revealed that folic acid on NPs plays a vital role in enhancing cellular uptake of imaging and therapeutic agents [176,177]. Increased cytotoxicity was seen in HeLa cervical cancer cells, induced by NIR-triggered drug release from ID@TSL-Gd (i.e., using 808 nm radiation, 0.5 W/cm2 for 5 min). The cell viability was as low as ~5% from treatment with the ID@TSL-Gd + laser, with a significant amount of apoptotic or damaged cells (i.e., visualized from calcein/propidium iodide staining). Furthermore, the in vivo fluorescence intensity from ID@TSL-Gd from tumors was twice the value from ICG only (i.e., after 48 h post-injection). The photoacoustic intensity and MRI signals from T1-weighted MR images of the tumor from the accumulation of ID@TSL-Gd peaked at 12 h post-injection. The ID@TSL-Gd + laser group showed the greatest tumor growth inhibition, compared to other groups (i.e., ICG@TSL-Gd + laser, ID@TSL-Gd, PBS + laser, PBS).
4.3. Brain Cancer
Theranostic verteporfin-loaded lipid-polymer liposomes for photodynamic applications can be designed by a simple and fast thin-film hydration method [178]. Verteporfin (VP) is a PS that can be used for PDT and can function as a drug for the destruction of cancerous cells (i.e., with high metastatic potential) [179]. Furthermore, liposomes with the copolymer F127, modified with fluorescent probe 5(6)-carboxyfluorescein (CF), enable imaging of cancer cells (Figure 3a) [180,181]. It was found that sonication for 480 s of a solution containing 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine or DPPC, F127 and F127-CF led to liposomes with the smallest size (i.e., ~88 nm) and low polydispersity index (i.e., 0.15). These liposomes were highly stable in solution (i.e., PBS) for more than a week. TEM images further confirmed the monodisperse size distribution of the liposomes, with an inner, dark and dense region representing phospholipids organized in the lipid bilayer and a lighter region representing the steric coating layer for the vesicle (i.e., from EO groups of the modified copolymer) [182]. Loading of VP in liposomes (i.e., F127-CF/DPPC/VP) increased the size from ~88 nm to ~112 nm and decreased the zeta potential from ~2.9 mV to ~1 mV, with a loading efficiency of ~93%. The absorption and emission spectra of liposomes containing F127-CF and DPPC revealed that VP is well entrapped within vesicles mostly in its monomeric state (i.e., from typical Q-band, Soret band, emission λMAX = 688 nm) (Figure 3b,c). The fluorescence quantum yield of CF in F127-CF/DPPC/VP liposomes (i.e., ϕF = 0.57) still ensures its use as a strong diagnostic tool for cancer imaging. For PDT, T98G brain cancer cells containing DPPC/F127-CF/VP liposomes were irradiated for 20 min by a blue light emitting diode (LED) at 5.52 mW/cm2. At a concentration of 1 and 3 μM for VP in liposomes, the T98G cancer cell viability was less than 5%, with the cell viability being greater than 75% with no light treatment (i.e., with DPPC/F127-CF/VP liposomes only) (Figure 3d).
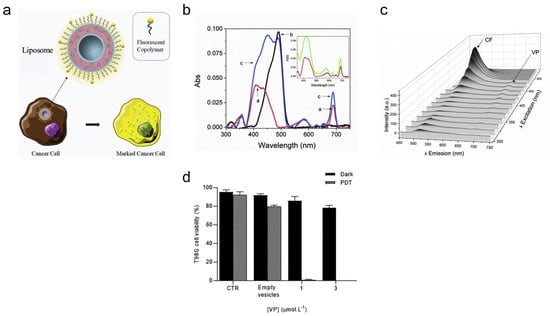
Figure 3.
Verteporfin-loaded lipid-polymer liposomes for cancer theranostics. (a) Copolymer F127 modified with 5(6)-carboxyfluorescein and verteporfin can be loaded in liposomal system for cancer. (b) Absorption spectra of the vesicles are shown for DPPC/VP (red), F127-CF/DPPC (black) and F127-CF/DPPC/VP (blue). The insert shows the spectral overlap between the VP in methanol and in lipid-copolymer fluorescent liposomes. (c) The emission is shown as a function of the excitation wavelength. [DPPC] = 1.5 mmolL−1; [F127] = 0.015% (w/V); [F127-CF] = 0.005% (w/V); [VP] = 1.0 μmolL−1. (d) Cell viability of T98G cells was determined before and after the treatment with DPPC/F127-CF/VP. ([DPPC] = 1.5 mmolL−1; [F127] = 0.015% w/V; [F127-CF] = 0.005% w/V; [VP] = 1.0 μmolL−1). In PDT, the cell was irradiated for 20 min by a blue LED (5.52 mWcm−2). Incubation time = 2 h. Reprinted from Journal of Photochemistry and Photobiology B: Biology from [178], Copyright (2020), with permission from Elsevier.
Transferrin(Tf)-receptor-targeted gold-based theranostic liposomes can be produced, containing both docetaxel (DCX) and glutathione-reduced gold nanoparticles (AuGSH), for brain-targeted drug delivery and imaging [183]. Transferrin is a glycoprotein used for receptor-mediated endocytosis of nanoparticles for enhancing the uptake of cancer therapy drugs such as docetaxel [184,185,186,187]. Gold nanoparticles can be used in various imaging modalities such as photoacoustic and CT imaging and due to their attractive properties, such as strong surface plasmon absorption, biosafety, stability, and ease of modification, they are ideal for both imaging and therapy applications [188,189,190]. DCX and AuGSH can be co-loaded into liposomes using a solvent injection technique and Tf post-conjugated on the surface of the liposomes using a linker (i.e., carboxylated Vit-E TPGS (TPGS-COOH)). The size, polydispersity index (PDI), zeta potential and encapsulation efficiency (i.e., of DCX) for DCX-AuGSH-TPGS-Tf (targeted) liposomes were determined to be ~270 nm, ~0.6, ~−6 mV and ~70%, respectively. A sustained mode of release was observed for about 48 h from targeted gold liposomes (DCX-AuGSH-TPGS-Tf) without any signs of burst release, which is important for reducing systemic toxicity in vivo, with surface conjugation of Tf on liposomes slowing down drug release [191]. The half maximal inhibitory concentration (IC50) from the treatment of C6 glioma cells with DCX-AuGSH-TPGS-Tf (targeted) liposomes was 26.82 μg/mL, much lower than IC50 (i.e., 42.69 μg/mL) from the treatment with DCX-AuGSH-TPGS (non-targeted) and Docel™ drug treatment alone (i.e., 79.24 μg/mL). The cell viability results that showed higher potency from the use of DCX-AuGSH-TPGS-Tf (targeted) liposomes were confirmed by fluorescence imaging, showing the greater uptake of targeted theranostic liposomes, compared to non-targeted liposomes due to receptor-mediated endocytosis (i.e., transferrin-mediated). Furthermore, the in vivo results demonstrated that targeted gold liposomes were able to deliver DCX into the brain by 3.70-, 2.74- and 4.08-folds higher than Docel™ after 30, 120 and 240 min of the treatment, respectively. Overall, the results show that the Tf-decorated AuGSH and DCX co-loaded liposomes are a promising platform for targeted nano-theranostics.
4.4. Lung Cancer
DNA-biodot-based targeted theranostic nanoparticles can be used for the imaging and treatment of non-small cell lung cancer (NSCLC) [192]. Cetuximab (CTX) is an epidermal growth factor receptor (EGFR) inhibitor [65,193], and etoposide (ETP) is a topoisomerase-II blocker, which are loaded with DNA biodots in liposomes for targeted imaging and treatment of advanced-stage NSCLC (Figure 4a). DNA, under high pressure and temperature, condenses to form luminescent biodots (BD) [194] and can be loaded in liposomes through the solvent injection method. The particle size, PDI, zeta potential, encapsulation efficiency of ETP, encapsulation efficiency of DNA BDs and IC50 value (i.e., from EGFR-positive A549 lung cancer cells) of targeted CTX-BD-ETP-liposomes were determined to be ~182 nm, ~0.2, −29 mV, 70%, 42% and 0.45 μg/mL, respectively. While the particle size, PDI, zeta potential, encapsulation efficiency of ETP, and encapsulation efficiency of DNA of non-targeted particles (i.e., BD-ETP-liposomes) were very similar to those of targeted CTX-BD-ETP-liposomes, the IC50 value was much greater (i.e., 9.7 μg/mL) for non-targeted liposomes, due to the lack of receptor targeting towards A549 lung cancer cells. The amount and rate of ETP released from both non-targeted BD-ETP-liposomes and targeted CTX-BD-ETP-liposomes were significantly higher at acidic pH (pH 5.5) than at pH 7.4 and pH 6.4. The greater amount of ETP release at acidic pH is attributed to the increased fluidity of the bilayer membrane at lower pH due to the hexagonal phase transition of 1,2-distearoyl-sn-glycerol-3-phosphoethanolamine (DSPE) component in the liposomes. This can be used to improve antitumor efficacy and lower systemic toxicity as the pH at the tumor site is acidic. Images from fluorescence microscopy showed that cetuximab targeted liposomes have the highest uptake, greater than the cellular uptake of non-targeted and unencapsulated BD (Figure 4b). The cetuximab pre-treated group had less uptake than that of targeted liposomes group due to the blocking of the EGFR receptor, indicating the important role of the EGFR receptor in the cellular uptake of targeted liposomes. To determine the biocompatibility of nanoparticles, the levels of biochemical components (i.e., lactate dehydrogenase, alkaline phosphatase, total protein count) were measured (Figure 4c). The levels of all the biochemical components started to reduce with time after a period of 14 days.
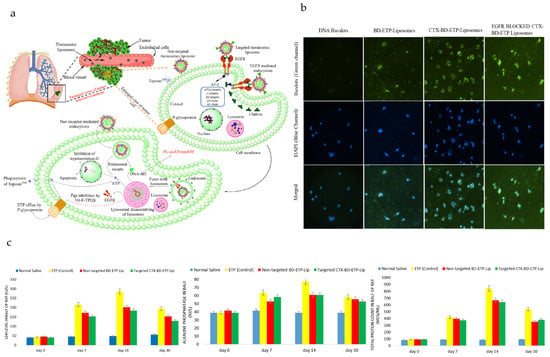
Figure 4.
Targeted theranostic DNA-biodot-based agents for imaging and treatment of non-small cell lung cancer. (a) The mechanism of targeted delivery of etoposide (ETP) and DNA biodots (DNA-BD) loaded, non-targeted and targeted (cetuximab-conjugated) theranostic liposomes in lung cancer imaging and therapy is shown. (b) Fluorescence microscopy images were taken using A-549 adenocarcinoma cells after 48 h incubation with free DNA-BD, BD-ETP-Liposomes, CTX-BD-ETP-Liposomes and cetuximab pretreated CTX-BD-ETP-Liposomes. (c) Levels of alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and Total Protein Count from female rats at day 0, day 7, day 14, day 30, after 7 day administration of normal saline, ETP (control), BD-ETP-Liposomes and CTX-BD-ETP-Liposomes were determined, (dose = 10 mg/kg), (n = 4). Reprinted from International Journal of Biological Macromolecules from [192], Copyright (2020), with permission from Elsevier.
Folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer can be constructed [195]. Paclitaxel (PCX) and vinorelbine (VNB) are both chemotherapeutic agents that have been approved for the treatment of NSCLC [196,197]. Folate in nanoparticles can be used for targeting folate receptors on the membranes of cancer cells [198], while the Tc-99m radioisotope in particles can be used for SPECT/CT imaging. Tc-99m has many advantages for imaging, including its pure gamma energy and optimal half-life [199]. The size determined for liposomes with folate, PCX and VNB (Fol-Lipo/PCX/VNB) was 192 nm, with an encapsulation efficiency of 18% and 23% for PCX and VNB, respectively, and radiolabeling efficiency of 85%. Both Lipo and Fol-Lipo formulations were highly internalized by LLC1 lung cancer cells, with greater fluorescence intensity and signals from radiolabeled liposomes (targeted nanoparticles). The IC50 values for PCX and VNB for Fol-Lipo/PCX/VNB were determined to be 4.25 nM and 11.25 nM, respectively, and much lower (i.e., greater potency) than IC50 values for free PCX (i.e., 54.05 nM) and VNB (i.e., 48.91 nM). The IC50 values for each drug in Fol-Lipo/PCX/VNB were ~6 times lower than IC50 values for liposomes, with each drug only (i.e., Fol-Lipo/PCX, Fol-Lipo/VNB). The tumor uptake of 99mTc-Fol-Lipo/PCX/VNB was significantly enhanced with time, and was found to be higher than the tumor uptake of 99mTc-Lipo/PCX/VNB at 24 h post-administration. The Fol-Lipo/PCX/VNB formulation significantly inhibited tumor growth with a much greater weight change in tumor bearing mice, compared with the control (Fol-Lipo) and free drug combination groups (i.e., containing the same amount of PCX and VNB as in liposome formulations). These results were further confirmed by micro-CT images obtained from mice before and after the treatment, with therapeutic evaluation from measurements of tumor size.
4.5. Prostate Cancer
Liposomes can be developed for paclitaxel-potentiated photodynamic theranostics, for synergistic tumor ablation and precise anticancer efficacy monitoring [200]. The dual-functional theranostic photosensitizer (PS), TPCI, possesses a very high 1O2 quantum yield of ~99% in water and can simultaneously self-report the PDT therapeutic response from the beginning of the treatment [201]. TPCI along with a first-line chemotherapy agent (i.e., paclitaxel, PTX) [202,203,204] can be co-encapsulated in liposomes for a synergistic anticancer effect against a series of tumor cell lines, including those of prostate cancer. TPCI/PTX@Lipo (i.e., liposomes with TPCI and PTX) are spherical with an average size of 100–120 nm and encapsulation efficiency of 89% for PTX and 87% for TPCI. In addition, TPCI/PTX@Lipo were stable for at least 2 weeks, in terms of size, with no obvious aggregation or precipitation. The cumulative release values of PTX from PTX@Lipo and TPCI/PTX@Lipo were 80% and 92% after 48 h, respectively, with similar drug release kinetics for PTX and TPCI from TPCI/PTX@Lipo in release media with different pH values (7.4 and 6.5). This suggests that the release of agents from TPCI/PTX@Lipo is independent of the microenvironment. Since the red fluorescence of BODIPY C11 (i.e., a detector of ROS) decreased drastically with the irradiation time (i.e., at 460 nm excitation, 4 mW/cm2) in the presence of TPCI@Lipo or TPCI/PTX@Lipo, the liposomes were very efficient in generating ROS. Upon irradiation at 460 nm and 1 mW/cm2 for 10 min, the red fluorescence from BODIPY C11 in PC3 cells pretreated with TPCI/PTX@Lipo was dramatically weakened, suggesting excellent ROS generation efficiency. The IC50 values of PTX in TPCI/PTX@Lipo in treating various cancer cells (i.e., at 460 nm, 1 mW/cm2, 10 min) were more than 9 times lower than the IC50 values of PTX in PTX@Lipo, and more than 4 times lower than IC50 values of TPCI in TPCI@Lipo, due to the effect from PDT and PTX, respectively. The combination index (CI) values of TPCI/PTX@Lipo were below 0.5 for all examined cell lines (i.e., PC3, EJ, J82, UMUC3, MCF-7), suggesting significant synergism [205,206], from the combined PDT and chemotherapy. For the PC3 prostate cancer cells treated with PTX@Lipo, the live cells were over 80% regardless of irradiation. However, after the cells were treated by TPCI/PTX@Lipo and irradiation, only 26.9% of the total cells remained alive, suggesting an extremely strong synergistic effect in killing cancer cells. After being incubated for 12 h, the fluorescence of TPCI from TPCI/PTX@Lipo in PC3 prostate cancer cells overlapped with that of Lysotracker (i.e., labelling lysosomes), suggesting effective endocytosis of the liposomes. The fluorescence of lysosomes in PC3 cells attenuated as the scanning time increased due to ROS-mediated lysosomal rupture, with the released TPCI translocated from the cytoplasm to the nucleus during the cell death process. Due to treatment with TPCI/PTX@Lipo and irradiation, the expression of the protein that pumps PTX out from the cytoplasm (i.e., P-gp) was downregulated and cleaved PARP (i.e., biomarker of cell apoptosis) [207] expression was upregulated. The PC3 cells treated with TPCI/PTX@Lipo and blue light irradiation showed the highest expression of anti-apoptotic proteins (i.e., Bcl-XL and Bcl-2) and the lowest expression of pro-apoptotic proteins (i.e., Bax and Bad), suggesting that irradiated TPCI/PTX@Lipo induces PC3 cell apoptosis through the mitochondrial pathway [208]. With the proceeding of the treatment, TPCI can be released from the liposomes and enter the nuclei during the cell death process. After binding with chromatin within the nuclei, strong fluorescence is emitted from TPCI [209,210]. The fluorescence of TPCI in PC3 cells treated with TPCI/PTX@Lipo increased 2.6-fold after irradiation, occurring concurrently with cell death (i.e., during therapy). TPCI in TPCI/PTX@Lipo can also report the cell death triggered by PTX as the TPCI fluorescence in cells pretreated with TPCI/PTX@Lipo was 2.8-fold higher than that in cells pretreated with TPCI@Lipo. Enhanced fluorescence at the injection sites of mice that received irradiation was seen, implying that tumor cells could be damaged severely, with the un-irradiated group exhibiting a negligible change in fluorescence. The results show that TPCI is effective in reporting the cell death triggered by chemotherapy, with tumor weights after treatment (i.e., from TPCI/PTX@Lipo + laser) being more than 2 times smaller, compared to other groups (i.e., saline, PTX@Lipo, TPCI/PTX@Lipo, TPCI@Lipo + laser).
Degradable multifunctional gold liposomes (i.e., Lipogold) as an all-in-one theranostic platform for image-guided radiotherapy (IGRT) can be synthesized for prostate cancer [211]. Both doxorubicin and iohexol can be loaded in the core of liposomes, as chemotherapeutic and computed tomography (X-ray) contrast agents, respectively (Figure 5a). The ratio of gold to ascorbic acid was varied, producing a variety of blue-green-colored nanoshells. Including more gold resulted in more intense and red-shifted plasmons, which enables longer wavelengths of light to be used for penetrating the tissue deeper for theranostics. The size of gold-containing liposomes (Lipogold) was determined to be around 100 nm, with a PDI of ~0.3. Lipogold alone is largely non-toxic, although oxidative stress following uptake may occur and impact the radiation response. After 5 min of irradiation (i.e., at 808 nm, 1500 mW/cm2), photothermal therapy alone was sufficient to kill ~62% of PC-3 prostate cancer cells as determined via the clonogenic assay (Figure 5b). The irradiation time of 5 min was sufficient to fully release encapsulated DOX from the Lipogold due to degraded/burst nanoshells, with an increase in the temperature from ~25 °C to ~65 °C. During CT scanning, encapsulation was found to significantly improve the contrast effect of the Lipogold through the addition of both gold and iodine. A two-phase pattern was seen in the release profiles of Lipogold, with a fast release of the entrapped iohexol (i.e., ~20–30% within the first 6 h) before a slower leakage of the remaining amount. Similar release profiles have been previously seen for other liposomes with both iohexol and iodixanol [212,213,214,215]. Lipogold was found to be a highly effective radiosensitizer under low doses of radiation, with the largest decrease in survival of PC-3 prostate cancer cells (i.e., ~2.65-fold difference compared to control) observed at a 1.5 Gy radiation dose (i.e., of 6 MV X-ray irradiation), with a surviving fraction of ~0.25 (Figure 5c).
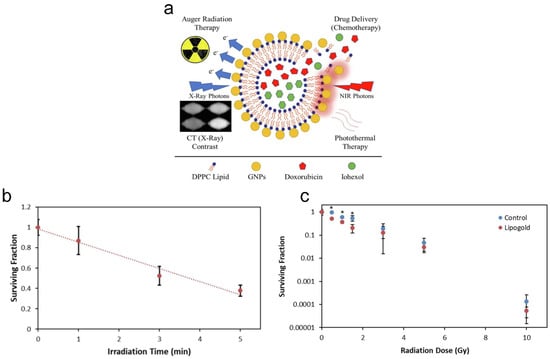
Figure 5.
Multifunctional gold liposomes as a theranostic platform for image-guided radiotherapy. (a) Gold nanoparticle-coated liposomes (i.e., Lipogold) as an all-in-one platform for cancer therapies. (b) Clonogenic survival of PC-3 cells was determined following photothermal therapy (PTT). (c) Dose survival curve was determined under different doses of 6 MV X-ray irradiation. N ≥ 5 with errors bars representing the standard deviation. * p < 0.05. Reprinted from International Journal of Pharmaceutics from [211], Copyright (2022), with permission from Elsevier.
4.6. Skin Cancer
Theranostic liposomes (i.e., ROS-responsive liposomes, Lipo@BODIPY11) with stimulus sensing and controlled drug release properties can be fabricated for skin cancer [216]. Upon the addition of hydrogen peroxide (i.e., reactive oxygen species, H2O2), a significant increase in FL515 (i.e., fluorescence at 515 nm) of Lipo@BODIPY11 along with a fluorescence decline at 595 nm was observed, due to the ROS-induced oxidation of the diene between the BODIPY chromophore and the phenyl group. With the increase in concentration of H2O2, the FL595 intensity increased slightly at first and then significantly dropped off for concentrations between 1 and 10 mM for H2O2. However, FL515 intensity continuously grows with H2O2 concentration. The trends seen in fluorescence intensity at low concentrations of H2O2 might possibly be due to the oxidation-induced fluorescence dequenching of C11-BODIPY (581/591) on liposome bilayers. Liposomes with a higher degree of functionalization showed a stronger tendency to undergo ROS-induced detachment of C11-BODIPY (581/591). However, over-modification (i.e., >10%) leads to the formation of undesired C11-BODIPY (581/591) aggregates, which can influence the ROS-responsive behavior of Lipo@ BODIPY11. The shifts of green FL and red FL from cytometry experiments referred to the ROS sensing and the corresponding drug release behavior (i.e., of mitoxantrone, MXT) of liposomes (i.e., Lipo@BODIPY11&MXT), respectively. The time-dependent elevation of fluorescence intensity on both channels (i.e., FL530 and FL610) was observed, due to the increased internalization level (i.e., in KB cells) or the ROS-triggered colorimetric response. Quantitative analysis revealed about a three-fold (i.e., 0.91–3.01) increase in FL530/FL610 from Lipo-FA@BODIPY11 (i.e., folate containing) after H2O2 treatment. On the other hand, the ROS scavenger, N-acetyl-L-cysteine (NAC), dramatically suppressed the H2O2-induced change in FL530, FL610 and FL530/FL610. Furthermore, there was a concentration-dependent cytotoxicity to KB cells from MXT-loaded Lipo-FA@BODIPY11, with IC50 lower than 10 μM MXT. The cytotoxicity of Lipo-FA@BODIPY11&MXT was enhanced upon H2O2 treatment. However, Lipo-FA@MXT without C11-BODIPY (581/591) modification displayed relatively weak cytotoxicity and showed no observable H2O2-dependent variation. Overall, the results show that the Lipo-FA@BODIPY11&MXT can be used for enhancing cytotoxicity and fluorescence imaging.
Chemiluminescent (CL) liposomes as theranostic carriers can be used for the detection of tumor cells under oxidative stress [217]. The chemiluminescent liposomes are composed of peroxyoxalate (PO) [218,219] and fluorophore curcumin [220,221,222] for chemiluminescence and treatment of cancer cells, respectively (Figure 6a). The CL liposomes containing these agents can detect hydrogen peroxide using chemiluminescence intensity. Curcumin plays the dual role both as an activator in PO-CL reactions as well as an effective photosensitizer for singlet oxygen generation for PDT. The size of spherical curcumin-encapsulated peroxalate liposomes was determined to be ~170 nm, with a zeta potential of ~−9 mV. The association of curcumin with liposomes led to the peak broadening of absorption spectra of curcumin in liposomes, with a relatively small shoulder at 397 nm. The maxima shifted in fluorescence spectra from 525 nm in methanol to 515 nm after loading into liposomes, indicating that curcumin is partitioning into the vesicle. Also, the sensitized PO-CL emission was similar to the fluorescence spectrum of curcumin, under comparable experimental conditions (i.e., intensity maximum ~515 nm). These changes in the spectra confirm the singlet excited state of the fluorescent activator that formed in the CL reaction and the emitting species [218,219]. Even though experiments have revealed that the chemiluminescence intensity of free curcumin was higher (i.e., about 4-fold) than that of the encapsulated curcumin under the same experimental condition, the stability and bioavailability of lipophilic curcumin was increased by encapsulation in liposomes. The chemiluminescence intensity increased with the increasing concentration of imidazole and hydrogen peroxide, with a linear relationship between chemiluminescence intensity and concentration of H2O2 (i.e., at physiological concentrations). The viability of cells after treatment with curcumin-encapsulated peroxyoxalate liposomes at 0.4 mg/mL encapsulated curcumin was ~12%, while the cell viability was ~40% with 1 mM of H2O2 (Figure 6b). The level of ROS generation in cells with liposomes was ~28% at a concentration of 200 μM H2O2. Strong fluorescence signals were observed in melanoma cancer cells, incubated with chemiluminescent liposomes (i.e., from auto-fluorescence of curcumin). Signals from liposomes were found in the cytoplasm. Fluorescence imaging also showed that curcumin liposomes gained more efficient entry into cancer cells than free curcumin. Furthermore, the addition of chemiluminescent liposomes and imidazole to the cells under oxidative stress conditions (i.e., about 4.5 million) resulted in a detectable emission of light, while the intensity of light depended on cell density (Figure 6c).


Table 3.
Liposomes for cancer therapy and imaging in different clinical phase trials.
Table 3.
Liposomes for cancer therapy and imaging in different clinical phase trials.
| Type | Therapeutic/Imaging Agent(s) | Cancer(s) | Phase(s) | Remarks | ClinicalTrials.gov Identifier | Reference(s) |
|---|---|---|---|---|---|---|
| liposomal doxorubicin | doxorubicin | metastatic breast cancer (MBC) | phase II | Results from clinical trials revealed liposomal doxorubicin is well tolerated and has activity similar to weekly docetaxel. | NCT00193037 | [223] |
| pegylated liposomal doxorubicin (PLD) | bevacizumab doxorubicin | metastatic breast cancer (MBC) | phase II | Combination of bimonthly PLD and antibody bevacizumab led to modest activity for the treatment of MBC. | NCT00445406 | [224] |
| nanoliposomal irinotecan (nal-IRI, MM-398) | irinotecan 5-fluorouracil (5-FU) leucovorin (LV) oxaliplatin | metastatic pancreatic cancer | phase I/II | The nal-IRI with oxaliplatin, 5-FU and LV (NALIRIFOX) was tolerable and generally manageable for patients with locally advanced/metastatic pancreatic ductal adenocarcinoma (mPDAC). | NCT02551991 | [225,226] |
| liposome entrapped paclitaxel easy to use (LEP-ETU) | paclitaxel | many different advanced cancers | phase I | LEP-ETU is well tolerated and safe at doses below 225 mg/m2 and showed bioequivalence with paclitaxel formulated with polyethoxylated castor oil. | NCT00080418 | [227,228] |
| DsiRNA lipid nanoparticle for MYC oncogene silencing (DCR-MYC) | double-stranded RNA | liver cancer | phase Ib/II | The lipid particles can inhibit cancer cell growth by targeting oncogene MYC. | NCT02314052 | [229] |
| cationic liposome-DNA complexes (JVRS-100) | plasmid DNA complex | leukemia | phase I | JVRS-100 can be used for immunotherapy for eliciting cytokines important in mediating host defense against cancer. | NCT00860522 | [230] |
| thermosensitive liposomal doxorubicin (ThermoDox®) | doxorubicin | liver cancer | phase III | There is a therapeutic benefit from combining thermosensitive liposomal doxorubicin with radiofrequency ablation (RFA). | NCT00617981 | [231] |
| 99mTc-labeled, pegylated liposomal doxorubicin (Caelyx®, PLD) | technetium-99m (99mTc) doxorubicin cyclophosphamide trastuzumab | many different advanced cancers | phase II | PLD and cyclophosphamide ± trastuzumab (antibody) followed by docetaxel is highly active in patients with planar gamma scintigraphy images showing accumulation of particles. | NCT01206881 | [232,233] |
| PEGylated liposomal doxorubicin targeted against HER2 (MM-302) | copper-64 (64Cu) doxorubicin | advanced breast cancer | phase I | High 64Cu-MM-302 deposition was associated with more favorable treatment outcomes using radiotherapy and PET imaging. | NCT01304797 | [234] |
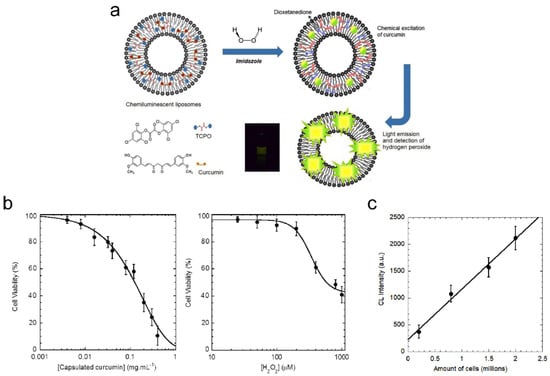
Figure 6.
Chemiluminescent liposomes for cancer theranostics under oxidative stress. (a) A schematic diagram of chemiluminescent liposomes composed of peroxyoxalate and curcumin. The liposomes generate light emission instantaneously in response to hydrogen peroxide. (b) Relative viability values of control cells and target cells were determined after treatment with curcumin-encapsulated peroxyoxalate liposomes at different concentrations for 24 h with cytotoxicity evaluations of hydrogen peroxide on cells after 1 day. Reported values are means ± SD for three independent determinations. (c) The dependence of the integral light emitted during PO-CL reaction in the cells is based on their amount in the sample (y = 935.47x + 231.02, R = 0.995). Reported values are means ± SD for three independent determinations. Reprinted from Analytica Chimica Acta from [217], Copyright (2019), with permission from Elsevier.
5. Future Directions
In order to achieve significant progress/advancement in the development of liposomes for cancer theranostics, some key improvements are required. The intrinsic characteristics of agents (e.g., for PTT and PDT) loaded in liposomes need to be optimized for higher efficacy, greater tissue penetration and effectiveness against tumor microenvironments. As research progresses in nanoparticle development, greater emphasis will be placed on nanoparticles that respond specifically toward the tumor environment (e.g., specific pH or proteins/enzymes expressed at high levels in tumor regions). To reduce the limitations present for imaging modalities and treatment techniques, the identification of more imaging and therapeutic agents that can be loaded in liposomes is required for combined therapy and imaging. This will enable image-guided therapy and theranostics of a wide variety of complex tumors. In addition, targeted theranostics and nanomedicine tailored according to an individual’s genetic profile will be important for clinical translation, as tumor properties governing recurrence vary based on factors such as age, race and ethnicity.
6. Conclusions
Nanoparticles are promising vehicles for theranostics, having the ability to deliver both therapeutics and imaging agents in cancer cells. Encapsulation in liposomes enables greater accumulation of theranostic agents in tumors, while reducing the risk of significant damage in healthy tissues (i.e., high when using therapeutic molecules alone). Liposomes are biocompatible and biodegradable and can be modified to carry a variety of molecules for different treatments and imaging modalities (i.e., through image-guided therapy). This provides the ability of liposomes to treat different types of cancers at different levels of severity, preventing the recurrence of tumors by destroying a significant amount of cancer cells. Many of the different liposomes developed for cancer theranostics show promise, potentially leading to breakthroughs in treating more complex forms of cancer that otherwise could not be treated successfully through conventional treatments (e.g., using chemotherapy and radiotherapy).
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Anderson, R.C.; Lan, X.; Conti, P.S.; Chen, K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med. Res. Rev. 2020, 40, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Fernandes, D.A. Review on the applications of nanoemulsions in cancer theranostics. J. Mater. Res. 2022, 37, 1953–1977. [Google Scholar] [CrossRef]
- Fernandes, D.A. Theranostic Nanoparticles for Therapy and Imaging in Cancer Detection. In Nanomaterials for Cancer Detection Using Imaging Techniques and Their Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–177. [Google Scholar]
- Guo, J.; Rahme, K.; He, Y.; Li, L.-L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold nanoparticles in cancer theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Dhas, N.; Pastagia, M.; Sharma, A.; Khera, A.; Kudarha, R.; Kulkarni, S.; Soman, S.; Mutalik, S.; Barnwal, R.P.; Singh, G. Organic quantum dots: An ultrasmall nanoplatform for cancer theranostics. J. Control. Release 2022, 348, 798–824. [Google Scholar] [CrossRef]
- Tripathi, S.; Kaur, G.; Khurana, R.K.; Kapoor, S.; Singh, B. Quantum dots and their potential role in cancer theranostics. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 461–502. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Li, K.; Hong, E.; Wang, B.; Wang, Z.; Zhang, L.; Hu, R.; Wang, B. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Fernandes, D.D.; Malik, A.; Gomes, G.-N.W.; Appak-Baskoy, S.; Berndl, E.; Gradinaru, C.C.; Kolios, M.C. Multifunctional nanoparticles as theranostic agents for therapy and imaging of breast cancer. J. Photochem. Photobiol. B Biol. 2021, 218, 112110. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Appak-Baskoy, S.; Berndl, E.; Kolios, M.C. Laser activatable perfluorocarbon bubbles for imaging and therapy through enhanced absorption from coupled silica coated gold nanoparticles. RSC Adv. 2021, 11, 4906–4920. [Google Scholar] [CrossRef]
- Wang, H.; Picchio, M.L.; Calderon, M. One stone, many birds: Recent advances in functional nanogels for cancer nanotheranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1791. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Jangjou, A.; Meisami, A.H.; Jamali, K.; Niakan, M.H.; Abbasi, M.; Shafiee, M.; Salehi, M.; Hosseinzadeh, A.; Amani, A.M.; Vaez, A. The promising shadow of microbubble over medical sciences: From fighting wide scope of prevalence disease to cancer eradication. J. Biomed. Sci. 2021, 28, 49. [Google Scholar] [CrossRef]
- Zahiri, M.; Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Theranostic nanobubbles towards smart nanomedicines. J. Control. Release 2021, 339, 164–194. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.H.; Dayton, P.A. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Pan, T.; Yin, Y.; Mei, Y.; Xiao, Q.; Wang, R.; Yan, Z.; Wang, W. Versatile carbon nanoplatforms for cancer treatment and diagnosis: Strategies, applications and future perspectives. Theranostics 2022, 12, 2290. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef] [PubMed]
- Indoria, S.; Singh, V.; Hsieh, M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid-and polymer-based nanostructures for cancer theranostics. Theranostics 2012, 2, 1117. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Gerlowski, L.E.; Jain, R.K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.A.; Malla, S.; Pasternak, M.A.; Terrero, D.; Brown, N.G.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.-S.; Tiwari, A.K. The role of endolysosomal trafficking in anticancer drug resistance. Drug Resist. Updat. 2021, 57, 100769. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A. Emerging nanotherapeutic approaches to overcome drug resistance in cancers with update on clinical trials. Pharmaceutics 2022, 14, 866. [Google Scholar] [CrossRef]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life 2020, 72, 855–871. [Google Scholar] [CrossRef]
- Yadav, P.; Ambudkar, S.V.; Rajendra Prasad, N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 423. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; Tofani, L.B.; de Araújo, J.T.C.; Di Filippo, L.D.; Marchetti, J.M.; Chorilli, M. Targeted liposomes: A nonviral gene delivery system for cancer therapy. Pharmaceutics 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gerbi, S.A. Making ends meet: Targeted integration of DNA fragments by genome editing. Chromosoma 2018, 127, 405–420. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Stahel, R.A.; Zangemeister-Wittke, U. Antisense oligonucleotides for cancer therapy—An overview. Lung Cancer 2003, 41, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide gene therapy for cancer–current strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar]
- Maeder, M.L.; Gersbach, C.A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 2016, 24, 430–446. [Google Scholar] [CrossRef]
- Yeh, C.D.; Richardson, C.D.; Corn, J.E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 2019, 21, 1468–1478. [Google Scholar] [CrossRef]
- Sheng, W.-Y.; Huang, L. Cancer immunotherapy and nanomedicine. Pharm. Res. 2011, 28, 200–214. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Q.; Zhang, R. PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy. Cancer Biol. Med. 2018, 15, 116. [Google Scholar]
- Gao, A.; Hu, X.-l.; Saeed, M.; Chen, B.-f.; Li, Y.-p.; Yu, H.-j. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019, 40, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Alhamhoom, Y.; Kakinani, G.; Rahamathulla, M.; Osmani, R.A.M.; Hani, U.; Thajudeen, K.Y.; Gowda, D.V. Recent Advances in the Liposomal Nanovesicles Based Immunotherapy in the Treatment of Cancer: A Review. Saudi Pharm. J. 2022, 31, 279–294. [Google Scholar] [CrossRef]
- Debele, T.A.; Yeh, C.-F.; Su, W.-P. Cancer immunotherapy and application of nanoparticles in cancers immunotherapy as the delivery of immunotherapeutic agents and as the immunomodulators. Cancers 2020, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; He, S.; Seare, W.J.; Almutairi, A. Review of the progress toward achieving heat confinement—The holy grail of photothermal therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar] [CrossRef]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Qi, T.; Wu, Y.; Xie, X.; Chen, F.; Shao, D.; Liao, J. Biomedical applications and prospects of temperature-orchestrated photothermal therapy. MedComm Biomater. Appl. 2022, 1, e25. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, H.; Chen, Y.; Zhu, R.; Yin, L. Photoresponsive drug/gene delivery systems. Biomacromolecules 2018, 19, 1840–1857. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Derycke, A.S.; De Witte, P.A. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Feron, O.; Amorim, C.A. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. J. Control. Release 2021, 339, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Chuang, C.-C.; Chen, J.-P. Liposomal IR-780 as a highly stable nanotheranostic agent for improved photothermal/photodynamic therapy of brain tumors by convection-enhanced delivery. Cancers 2021, 13, 3690. [Google Scholar] [CrossRef]
- Brazel, C.S. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009, 26, 644–656. [Google Scholar] [CrossRef]
- Shevtsov, M.; Kaesler, S.; Posch, C.; Multhoff, G.; Biedermann, T. Magnetic nanoparticles in theranostics of malignant melanoma. EJNMMI Res. 2021, 11, 127. [Google Scholar] [CrossRef]
- Heidarli, E.; Dadashzadeh, S.; Haeri, A. State of the art of stimuli-responsive liposomes for cancer therapy. Iran. J. Pharm. Res. IJPR 2017, 16, 1273. [Google Scholar]
- TS, A.; Shalumon, K.; Chen, J.-P. Applications of magnetic liposomes in cancer therapies. Curr. Pharm. Des. 2019, 25, 1490–1504. [Google Scholar]
- Shivanna, A.T.; Dash, B.S.; Chen, J.-P. Functionalized magnetic nanoparticles for alternating magnetic field-or near infrared light-induced cancer therapies. Micromachines 2022, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- TS, A.; Lu, Y.-J.; Chen, J.-P. Optimization of the preparation of magnetic liposomes for the combined use of magnetic hyperthermia and photothermia in dual magneto-photothermal cancer therapy. Int. J. Mol. Sci. 2020, 21, 5187. [Google Scholar]
- Yudina, A.; De Smet, M.; Lepetit-Coiffe, M.; Langereis, S.; Van Ruijssevelt, L.; Smirnov, P.; Bouchaud, V.; Voisin, P.; Grüll, H.; Moonen, C. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J. Control. Release 2011, 155, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Staruch, R.; Chopra, R.; Hynynen, K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int. J. Hyperth. 2011, 27, 156–171. [Google Scholar] [CrossRef]
- Ibsen, S.; Benchimol, M.; Simberg, D.; Schutt, C.; Steiner, J.; Esener, S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J. Control. Release 2011, 155, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Boffa, C.; Castelli, D.D.; Cutrin, J.C.; Aime, S.; Terreno, E. In vivo MRI visualization of release from liposomes triggered by local application of pulsed low-intensity non-focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e901–e904. [Google Scholar] [CrossRef]
- Ranjan, A.; Jacobs, G.C.; Woods, D.L.; Negussie, A.H.; Partanen, A.; Yarmolenko, P.S.; Gacchina, C.E.; Sharma, K.V.; Frenkel, V.; Wood, B.J. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release 2012, 158, 487–494. [Google Scholar] [CrossRef]
- Deckers, R.; Moonen, C.T. Ultrasound triggered, image guided, local drug delivery. J. Control. Release 2010, 148, 25–33. [Google Scholar] [CrossRef]
- Zha, Z.; Wang, J.; Qu, E.; Zhang, S.; Jin, Y.; Wang, S.; Dai, Z. Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation. Sci. Rep. 2013, 3, 2360. [Google Scholar] [CrossRef]
- Gao, Y.; Chan, C.U.; Gu, Q.; Lin, X.; Zhang, W.; Yeo, D.C.L.; Alsema, A.M.; Arora, M.; Chong, M.S.K.; Shi, P. Controlled nanoparticle release from stable magnetic microbubble oscillations. NPG Asia Mater. 2016, 8, e260. [Google Scholar] [CrossRef]
- Kang, S.-T.; Lin, J.-L.; Wang, C.-H.; Chang, Y.-C.; Yeh, C.-K. Internal polymer scaffolding in lipid-coated microbubbles for control of inertial cavitation in ultrasound theranostics. J. Mater. Chem. B 2015, 3, 5938–5941. [Google Scholar] [CrossRef] [PubMed]
- Geers, B.; De Wever, O.; Demeester, J.; Bracke, M.; De Smedt, S.C.; Lentacker, I. Targeted liposome-loaded microbubbles for cell-specific ultrasound-triggered drug delivery. Small 2013, 9, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef]
- Dwivedi, P.; Kiran, S.; Han, S.; Dwivedi, M.; Khatik, R.; Fan, R.; Mangrio, F.A.; Du, K.; Zhu, Z.; Yang, C. Magnetic targeting and ultrasound activation of liposome–microbubble conjugate for enhanced delivery of anticancer therapies. ACS Appl. Mater. Interfaces 2020, 12, 23737–23751. [Google Scholar] [CrossRef]
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly (methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Kang, S.-T.; Yeh, C.-K. Superparamagnetic iron oxide and drug complex-embedded acoustic droplets for ultrasound targeted theranosis. Biomaterials 2013, 34, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-F.; Zhu, X.-M.; Wang, Y.-X.J.; Xuan, S.-H.; You, Q.; Chan, W.-H.; Wong, C.-H.; Wang, F.; Yu, J.C.; Cheng, C.H. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef]
- Min, H.S.; You, D.G.; Son, S.; Jeon, S.; Park, J.H.; Lee, S.; Kwon, I.C.; Kim, K. Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics 2015, 5, 1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-E.; Yu, H.-M.; Lu, Y.-C.; Heish, N.-N.; Tseng, Y.-L.; Huang, K.-L.; Chuang, K.-T.; Chen, C.-H.; Hwang, J.-J.; Lin, W.-J. Internal radiotherapy and dosimetric study for 111In/177Lu-pegylated liposomes conjugates in tumor-bearing mice. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 569, 533–537. [Google Scholar] [CrossRef]
- Stolarz, A.J.; Chhetri, B.P.; Borrelli, M.J.; Jenkins, S.V.; Jamshidi-Parsian, A.; Phillips, J.H.; Fologea, D.; Gandy, J.; Griffin, R.J. Liposome formulation for tumor-targeted drug delivery using radiation therapy. Int. J. Mol. Sci. 2022, 23, 11662. [Google Scholar] [CrossRef]
- Bar-Shalom, R.; Valdivia, A.Y.; Blaufox, M.D. PET imaging in oncology. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2000; pp. 150–185. [Google Scholar]
- Adam, M.J.; Wilbur, D.S. Radiohalogens for imaging and therapy. Chem. Soc. Rev. 2005, 34, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A. [74As]-Labeled monoclonal antibody against anionic phospholipids. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Pressly, E.D.; Rossin, R.; Hagooly, A.; Fukukawa, K.-i.; Messmore, B.W.; Welch, M.J.; Wooley, K.L.; Lamm, M.S.; Hule, R.A.; Pochan, D.J. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined 64Cu-labeled nanoparticles comprised of amphiphilic block graft copolymers. Biomacromolecules 2007, 8, 3126–3134. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Keliher, E.J.; Thurber, G.M.; Nahrendorf, M.; Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjugate Chem. 2009, 20, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Herth, M.M.; Barz, M.; Jahn, M.; Zentel, R.; Rösch, F. 72/74As-labeling of HPMA based polymers for long-term in vivo PET imaging. Bioorganic Med. Chem. Lett. 2010, 20, 5454–5458. [Google Scholar] [CrossRef]
- Roesch, F. Scandium-44: Benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr. Radiopharm. 2012, 5, 187–201. [Google Scholar] [CrossRef]
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; van der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the decay half-life with the biological half-life: ImmunoPET imaging with 44Sc-labeled cetuximab Fab fragment. Bioconjugate Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Gawne, P.J.; de Rosales, R.T. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Chakravarty, R.; Hong, H.; Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.; Langereis, S.; van den Bosch, S.; Bitter, K.; Hijnen, N.M.; Heijman, E.; Grüll, H. SPECT/CT imaging of temperature-sensitive liposomes for MR-image guided drug delivery with high intensity focused ultrasound. J. Control. Release 2013, 169, 82–90. [Google Scholar] [CrossRef]
- Head, H.W.; Dodd, G.D., III; Bao, A.; Soundararajan, A.; Garcia-Rojas, X.; Prihoda, T.J.; McManus, L.M.; Goins, B.A.; Santoyo, C.A.; Phillips, W.T. Combination radiofrequency ablation and intravenous radiolabeled liposomal Doxorubicin: Imaging and quantification of increased drug delivery to tumors. Radiology 2010, 255, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Silindir, M.; Erdoğan, S.; Özer, A.Y.; Doğan, A.L.; Tuncel, M.; Uğur, Ö.; Torchilin, V.P. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J. Liposome Res. 2013, 23, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, X.; Tournier, H.; Khan, H.; Sabitha, S.; Burkhardt, S.; Terrier, F.; Schneider, M. Enhancement of computed tomography liver contrast using iomeprol-containing liposomes and detection of small liver tumors in rats. Acad. Radiol. 1995, 2, 576–583. [Google Scholar] [CrossRef]
- Xu, H.; Ohulchanskyy, T.Y.; Yakovliev, A.; Zinyuk, R.; Song, J.; Liu, L.; Qu, J.; Yuan, Z. Nanoliposomes co-encapsulating CT imaging contrast agent and photosensitizer for enhanced, imaging guided photodynamic therapy of cancer. Theranostics 2019, 9, 1323. [Google Scholar] [CrossRef]
- Zheng, J.; Jaffray, D.; Allen, C. Quantitative CT imaging of the spatial and temporal distribution of liposomes in a rabbit tumor model. Mol. Pharm. 2009, 6, 571–580. [Google Scholar] [CrossRef]
- Kircher, M.F.; Willmann, J.K. Molecular body imaging: MR imaging, CT, and US. part I. principles. Radiology 2012, 263, 633–643. [Google Scholar] [CrossRef]
- Willmann, J.K.; Van Bruggen, N.; Dinkelborg, L.M.; Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 591–607. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, H.; Lv, R.; Zhao, P.; Sun, X.; Wang, S.; Su, W.; Niu, R.; Chang, J. Polymeric liposomes-coated superparamagnetic iron oxide nanoparticles as contrast agent for targeted magnetic resonance imaging of cancer cells. Langmuir 2011, 27, 3100–3105. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-C.; Ren, W.; Zhang, S.; Zhong, T.; Duan, X.-C.; Yin, Y.-F.; Xu, M.-Q.; Hao, Y.-L.; Li, Z.-T.; Li, H. The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kattel, K.; Park, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Paramagnetic nanoparticle T 1 and T 2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Kalber, T.L.; Cooper, M.S.; Sunassee, K.; Chalker, S.L.; Shaw, K.P.; Ordidge, K.L.; Badar, A.; Janes, S.M.; Blower, P.J. Incorporation of paramagnetic, fluorescent and PET/SPECT contrast agents into liposomes for multimodal imaging. Biomaterials 2013, 34, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 2016, 6, 1336. [Google Scholar] [CrossRef]
- Zhou, L.-Q.; Li, P.; Cui, X.-W.; Dietrich, C.F. Ultrasound nanotheranostics in fighting cancer: Advances and prospects. Cancer Lett. 2020, 470, 204–219. [Google Scholar] [CrossRef]
- Lin, X.; Qiu, Y.; Song, L.; Chen, S.; Chen, X.; Huang, G.; Song, J.; Chen, X.; Yang, H. Ultrasound activation of liposomes for enhanced ultrasound imaging and synergistic gas and sonodynamic cancer therapy. Nanoscale Horiz. 2019, 4, 747–756. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics. ACS Nano 2017, 11, 1509–1519. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to drug delivery and responses via photoacoustic imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nano-Micro Lett. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Razansky, D.; Ntziachristos, V. Hybrid photoacoustic fluorescence molecular tomography using finite-element-based inversion. Med. Phys. 2007, 34, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, G.; Lee, W.; Baek, S.; Jung, H.N.; Im, H.-J. Development of theranostic dual-layered Au-liposome for effective tumor targeting and photothermal therapy. J. Nanobiotechnology 2021, 19, 262. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.-J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Goh, D.; Gong, T.; Dinish, U.; Maiti, K.K.; Fu, C.Y.; Yong, K.-T.; Olivo, M. Pluronic triblock copolymer encapsulated gold nanorods as biocompatible localized plasmon resonance-enhanced scattering probes for dark-field imaging of cancer cells. Plasmonics 2012, 7, 595–601. [Google Scholar] [CrossRef]
- Liopo, A.V.; Conjusteau, A.; Chumakova, O.V.; Ermilov, S.A.; Su, R.; Oraevsky, A.A. Highly purified biocompatible gold nanorods for contrasted optoacoustic imaging of small animal models. Nanosci. Nanotechnol. Lett. 2012, 4, 681–686. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Huang, J.; Zhu, J. Synthesis and solar photo-thermal conversion of Au, Ag, and Au-Ag blended plasmonic nanoparticles. Energy Convers. Manag. 2016, 127, 293–300. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall black phosphorus quantum dots: Synthesis and use as photothermal agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Santos, G.M.; Zhao, F.; Zeng, J.; Shih, W.-C. Characterization of nanoporous gold disks for photothermal light harvesting and light-gated molecular release. Nanoscale 2014, 6, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yao, D.; Wang, Y.; Yang, W.; Zhang, B.; Wang, D. Enzyme-triggered self-assembly of gold nanoparticles for enhanced retention effects and photothermal therapy of prostate cancer. Chem. Commun. 2018, 54, 9841–9844. [Google Scholar] [CrossRef]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W. Bacteria-like mesoporous silica-coated gold nanorods for positron emission tomography and photoacoustic imaging-guided chemo-photothermal combined therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, L.; Wang, G.; Yang, K.; Chen, M.; Tian, R.; Ma, Q.; Zhu, L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 2016, 79, 36–45. [Google Scholar] [CrossRef]
- You, J.; Zhang, R.; Xiong, C.; Zhong, M.; Melancon, M.; Gupta, S.; Nick, A.M.; Sood, A.K.; Li, C. Effective Photothermal Chemotherapy Using Doxorubicin-Loaded Gold Nanospheres That Target EphB4 Receptors in TumorsConcerted Chemo-Photothermal Therapy Targeting EphB4. Cancer Res. 2012, 72, 4777–4786. [Google Scholar] [CrossRef]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K.; Liu, J.; Guo, X.; Wei, Y.; Gao, W. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef]
- Askari, A.; Tajvar, S.; Nikkhah, M.; Mohammadi, S.; Hosseinkhani, S. Synthesis, characterization and in vitro toxicity evaluation of doxorubicin-loaded magnetoliposomes on MCF-7 breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 55, 101447. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- German, S.; Navolokin, N.; Kuznetsova, N.; Zuev, V.; Inozemtseva, O.; Anis’Kov, A.; Volkova, E.; Bucharskaya, A.; Maslyakova, G.; Fakhrullin, R. Liposomes loaded with hydrophilic magnetite nanoparticles: Preparation and application as contrast agents for magnetic resonance imaging. Colloids Surf. B Biointerfaces 2015, 135, 109–115. [Google Scholar] [CrossRef]
- Mueller, S. Magnetic fluid hyperthermia therapy for malignant brain tumors—An ethical discussion. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Can, H.K.; Kavlak, S.; ParviziKhosroshahi, S.; Güner, A. Preparation, characterization and dynamical mechanical properties of dextran-coated iron oxide nanoparticles (DIONPs). Artif. Cells Nanomed. Biotechnol. 2018, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Herea, D.; Chiriac, H.; Lupu, N.; Grigoras, M.; Stoian, G.; Stoica, B.; Petreus, T. Study on iron oxide nanoparticles coated with glucose-derived polymers for biomedical applications. Appl. Surf. Sci. 2015, 352, 117–125. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Banu, N.; Vallejo-Cardona, A.A.; Lugo-Fabres, P.; Camacho-Villegas, T.A.; Salas, P.; De la Rosa, E. Ligand-targeted Theranostic Liposomes combining Methylene Blue attached Upconversion nanoparticles for NIR activated Bioimaging and Photodynamic therapy against HER-2 positive breast cancer. J. Lumin. 2021, 237, 118143. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Chang, C.A. Optimising FRET-efficiency of Nd 3+-sensitised upconversion nanocomposites by shortening the emitter–photosensitizer distance. Nanoscale 2020, 12, 8742–8749. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Y.; Jiang, Y.; Wu, Y.; Cui, C.; Wang, Y.; Zhang, L.; Teng, I.-T.; Tan, W. Aptamer-based multifunctional ligand-modified UCNPs for targeted PDT and bioimaging. Nanoscale 2018, 10, 10986–10990. [Google Scholar] [CrossRef]
- Feng, L.; He, F.; Liu, B.; Yang, G.; Gai, S.; Yang, P.; Li, C.; Dai, Y.; Lv, R.; Lin, J. g-C3N4 coated upconversion nanoparticles for 808 nm near-infrared light triggered phototherapy and multiple imaging. Chem. Mater. 2016, 28, 7935–7946. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Panikar, S.S.; Ramírez-García, G.; Vallejo-Cardona, A.A.; Banu, N.; Patrón-Soberano, O.A.; Cialla-May, D.; Camacho-Villegas, T.A.; de la Rosa, E. Novel anti-HER2 peptide-conjugated theranostic nanoliposomes combining NaYF 4: Yb, Er nanoparticles for NIR-activated bioimaging and chemo-photodynamic therapy against breast cancer. Nanoscale 2019, 11, 20598–20613. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Yadav, A.S.; Gorain, M.; Chauhan, D.S.; Kundu, G.C.; Srivastava, R.; Selvaraj, K. Graphene oxide supported liposomes as red emissive theranostics for phototriggered tissue visualization and tumor regression. ACS Appl. Bio Mater. 2019, 2, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Aiyer, S.; Chauhan, D.S.; Srivastava, R.; Selvaraj, K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale 2016, 8, 4537–4546. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-García, B.E.; Medina, L.A.; López-Téllez, G.; Gibbens-Bandala, B.V.; Izquierdo-Sánchez, V. Biodegradable poly (D, L-lactide-co-glycolide)/poly (L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2017, 76, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef]
- Lim, C.-K.; Shin, J.; Kwon, I.C.; Jeong, S.Y.; Kim, S. Iodinated photosensitizing chitosan: Self-assembly into tumor-homing nanoparticles with enhanced singlet oxygen generation. Bioconjug. Chem. 2012, 23, 1022–1028. [Google Scholar] [CrossRef]
- Kim, S.; Ohulchanskyy, T.Y.; Bharali, D.; Chen, Y.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles with intraparticle heavy-atom effect on the encapsulated photosensitizer for enhanced efficacy of photodynamic therapy. J. Phys. Chem. C 2009, 113, 12641–12644. [Google Scholar] [CrossRef][Green Version]
- Lim, C.K.; Shin, J.; Lee, Y.D.; Kim, J.; Park, H.; Kwon, I.C.; Kim, S. Heavy-Atomic Construction of Photosensitizer Nanoparticles for Enhanced Photodynamic Therapy of Cancer. Small 2011, 7, 112–118. [Google Scholar] [CrossRef]
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional thermosensitive liposomes based on natural phase-change material: Near-infrared light-triggered drug release and multimodal imaging-guided cancer combination therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553. [Google Scholar] [CrossRef]
- Zheng, X.; Xing, D.; Zhou, F.; Wu, B.; Chen, W.R. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol. Pharm. 2011, 8, 447–456. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, F.; Wu, B.; Chen, W.R.; Xing, D. Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection. Mol. Pharm. 2012, 9, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-J.; Lee, H.-S.; Lim, J.-Y.; Park, J.-H. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 5683–5691. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A.K.; Thomas, T.; Mulé, J.; Baker, J.R. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm. Res. 2002, 19, 1310–1316. [Google Scholar] [CrossRef]
- Reddy, J.; Allagadda, V.; Leamon, C. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr. Pharm. Biotechnol. 2005, 6, 131–150. [Google Scholar] [CrossRef]
- de Oliveira, D.C.S.; de Freitas, C.F.; Calori, I.R.; Goncalves, R.S.; Cardinali, C.A.E.F.; Malacarne, L.C.; Ravanelli, M.I.; de Oliveira, H.P.M.; Tedesco, A.C.; Caetano, W. Theranostic verteporfin-loaded lipid-polymer liposome for photodynamic applications. J. Photochem. Photobiol. B Biol. 2020, 212, 112039. [Google Scholar] [CrossRef] [PubMed]
- Al-Moujahed, A.; Brodowska, K.; Stryjewski, T.P.; Efstathiou, N.E.; Vasilikos, I.; Cichy, J.; Miller, J.W.; Gragoudas, E.; Vavvas, D.G. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci. Rep. 2017, 7, 7602. [Google Scholar] [CrossRef] [PubMed]
- Mordon, S.; Devoisselle, J.M.; Maunoury, V. In vivo pH measurement and imaging of tumor tissue using a pH-sensitive fluorescent probe (5, 6–Carboxyfluorescein): Instrumental and experimental studies. Photochem. Photobiol. 1994, 60, 274–279. [Google Scholar] [CrossRef]
- Magin, R.L.; Weinstein, J.N. The Design and Characterization of Temperature-Sensitive Liposomes. Liposome Technology, 1st ed.; Gregoriadis, G., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 137–155. [Google Scholar]
- de Freitas, C.F.; Calori, I.R.; da Silva, A.C.P.; de Castro, L.V.; Sato, F.; Pellosi, D.S.; Tessaro, A.L.; Caetano, W.; Hioka, N. PEG-coated vesicles from Pluronic/lipid mixtures for the carrying of photoactive erythrosine derivatives. Colloids Surf. B Biointerfaces 2019, 175, 530–544. [Google Scholar] [CrossRef]
- Sonkar, R.; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; Muthu, M.S. Gold liposomes for brain-targeted drug delivery: Formulation and brain distribution kinetics. Mater. Sci. Eng. C 2021, 120, 111652. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kutty, R.V.; Luo, Z.; Xie, J.; Feng, S.-S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef]
- Sonali; Agrawal, P.; Singh, R.P.; Rajesh, C.V.; Singh, S.; Vijayakumar, M.R.; Pandey, B.L.; Muthu, M.S. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: Preparation, characterization and brain distribution in rats. Drug Deliv. 2016, 23, 1788–1798. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Agrawal, P.; Pandey, B.L.; Koch, B.; Muthu, M.S. Transferrin receptor targeted PLA-TPGS micelles improved efficacy and safety in docetaxel delivery. Int. J. Biol. Macromol. 2016, 83, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging strategies for early diagnosis and therapy of brain cancer. Nanotheranostics 2018, 2, 70. [Google Scholar]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics˗ application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Feng, S.-S. Theranostic Liposomes for Cancer Diagnosis and Treatment: CURRENT Development and Pre-Clinical Success; Taylor & Francis: Abingdon, UK, 2013; Volume 10, pp. 151–155. [Google Scholar]
- Muthu, M.S.; Sahu, A.K.; Sonali; Abdulla, A.; Kaklotar, D.; Rajesh, C.V.; Singh, S.; Pandey, B.L. Solubilized delivery of paliperidone palmitate by D-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv. 2016, 23, 230–237. [Google Scholar] [CrossRef]
- Jha, A.; Viswanadh, M.K.; Burande, A.S.; Mehata, A.K.; Poddar, S.; Yadav, K.; Mahto, S.K.; Parmar, A.S.; Muthu, M.S. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 150, 413–425. [Google Scholar] [CrossRef]
- Rani, D.; Somasundaram, V.H.; Nair, S.; Koyakutty, M. Advances in cancer nanomedicine. J. Indian Inst. Sci. 2012, 92, 187–218. [Google Scholar]
- Kuang, L.; Cao, S.-P.; Zhang, L.; Li, Q.-H.; Liu, Z.-C.; Liang, R.-P.; Qiu, J.-D. A novel nanosensor composed of aptamer bio-dots and gold nanoparticles for determination of thrombin with multiple signals. Biosens. Bioelectron. 2016, 85, 798–806. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2021, 156, 105576. [Google Scholar] [CrossRef]
- Jung, M.; Grunberg, S.; Timblin, C.; Buder-Hoffman, S.; Vacek, P.; Taatjes, D.J.; Mossman, B.T. Paclitaxel and vinorelbine cause synergistic increases in apoptosis but not in microtubular disruption in human lung adenocarcinoma cells (A-549). Histochem. Cell Biol. 2004, 121, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Huerter, M.M.; Meza, J.L.; Copur, M.S.; Tolentino, A.; Marr, A.S.; Ketcham, M.; DeSpiegelaere, H.; Kruse, S.; Kos, M.E.; Swenson, K. Weekly vinorelbine and paclitaxel in older patients with advanced non-small cell lung cancer: A phase II Fred and Pamela Buffet Cancer Center Clinical Trials Network study. J. Geriatr. Oncol. 2017, 8, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Ali Mansoori, G.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 18496. [Google Scholar] [CrossRef]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–333. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; He, Z.; Yang, X.; Meng, F.; Liang, H.; Zhang, X.; Luo, L. Paclitaxel-potentiated photodynamic theranostics for synergistic tumor ablation and precise anticancer efficacy monitoring. ACS Appl. Mater. Interfaces 2020, 12, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; He, X.; He, Z.; Yang, X.; Tian, S.; Meng, F.; Ding, D.; Luo, L.; Tang, B.Z. A dual-functional photosensitizer for ultraefficient photodynamic therapy and synchronous anticancer efficacy monitoring. Adv. Funct. Mater. 2019, 29, 1902673. [Google Scholar] [CrossRef]
- Lan, Y.-Q.; Kong, L.-J.; Lin, X.-Y.; Xu, Q.; Gao, X.-Y.; Wu, R.-P.; Wang, X.-L.; Zhong, D.-T. Combination chemotherapy with paclitaxel and oxaliplatin as first-line treatment in patients with advanced gastric cancer. Cancer Chemother. Pharmacol. 2018, 81, 1007–1015. [Google Scholar] [CrossRef]
- Yoshimura, A.; Chihara, Y.; Date, K.; Tamiya, N.; Takemura, Y.; Imabayashi, T.; Kaneko, Y.; Yamada, T.; Ueda, M.; Arimoto, T. A phase II study of S-1 and paclitaxel combination therapy as a first-line treatment in elderly patients with advanced non-small cell lung cancer. Oncologist 2019, 24, 459-e131. [Google Scholar] [CrossRef]
- Sandercock, J.; Parmar, M.; Torri, V.; Qian, W. First-line treatment for advanced ovarian cancer: Paclitaxel, platinum and the evidence. Br. J. Cancer 2002, 87, 815–824. [Google Scholar] [CrossRef]
- Ye, S.; Rao, J.; Qiu, S.; Zhao, J.; He, H.; Yan, Z.; Yang, T.; Deng, Y.; Ke, H.; Yang, H. Rational design of conjugated photosensitizers with controllable photoconversion for dually cooperative phototherapy. Adv. Mater. 2018, 30, 1801216. [Google Scholar] [CrossRef]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. Acs Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- An, W.; Hwang, S.; Trepel, J.; Blagosklonny, M. Protease inhibitor-induced apoptosis: Accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia 2000, 14, 1276–1283. [Google Scholar] [CrossRef][Green Version]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Water-Soluble Tetraphenylethene Derivatives as Fluorescent “Light-Up” Probes for Nucleic Acid Detection and Their Applications in Cell Imaging. Chem. Asian J. 2013, 8, 1806–1812. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zheng, Z.; Ye, R.; Zhang, Y.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. In situ monitoring apoptosis process by a self-reporting photosensitizer. J. Am. Chem. Soc. 2019, 141, 5612–5616. [Google Scholar] [CrossRef]
- Youden, B.; Wang, F.; Zhang, X.; Curry, D.; Majtenyi, N.; Shaaer, A.; Bingham, K.; Nguyen, Q.; Bragg, L.; Liu, J. Degradable multifunctional gold-liposomes as an all-in-one theranostic platform for image-guided radiotherapy. Int. J. Pharm. 2022, 629, 122413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Airan, R.; Han, Z.; Xu, J.; Chan, K.W.; Xu, Y.; Bulte, J.W.; Van Zijl, P.C.; McMahon, M.T. CT and CEST MRI bimodal imaging of the intratumoral distribution of iodinated liposomes. Quant. Imaging Med. Surg. 2019, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Delama, A.; Teixeira, M.I.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Microfluidic encapsulation method to produce stable liposomes containing iohexol. J. Drug Deliv. Sci. Technol. 2019, 54, 101340. [Google Scholar] [CrossRef]
- Mukundan, S., Jr.; Ghaghada, K.B.; Badea, C.T.; Kao, C.-Y.; Hedlund, L.W.; Provenzale, J.M.; Johnson, G.A.; Chen, E.; Bellamkonda, R.V.; Annapragada, A. A liposomal nanoscale contrast agent for preclinical CT in mice. Am. J. Roentgenol. 2006, 186, 300–307. [Google Scholar] [CrossRef]
- Zheng, J.; Perkins, G.; Kirilova, A.; Allen, C.; Jaffray, D.A. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Investig. Radiol. 2006, 41, 339–348. [Google Scholar] [CrossRef]
- Chen, C.; Gao, K.; Lian, H.; Chen, C.; Yan, X. Single-particle characterization of theranostic liposomes with stimulus sensing and controlled drug release properties. Biosens. Bioelectron. 2019, 131, 185–192. [Google Scholar] [CrossRef]
- Mohammadi, S.S.; Vaezi, Z.; Shojaedin-Givi, B.; Naderi-Manesh, H. Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal. Chim. Acta 2019, 1059, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.Y.; Abedirad, S.M.; Vaezi, Z.; Ganjali, M.R. A study of chemiluminescence characteristics of a novel peroxyoxalate system using berberine as the fluorophore. Dye. Pigment. 2012, 95, 751–756. [Google Scholar] [CrossRef]
- Chaichi, M.J.; VAAEZI, Z.; Hosseini, M.; Hosseinkhani, S.; Shamsipur, M. The study of chemiluminescence of acridinium ester in presence of rhodamin B as a fluorescer. Iran. J. Chem. Chem. Eng. 2011, 30, 89–96. [Google Scholar]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Yardley, D.A.; Burris, H.A., III; Spigel, D.R.; Clark, B.L.; Vazquez, E.; Shipley, D.; Barton, J.; Thompson, D.; Montes, I.; Greco, F.A. A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first-line treatment of women with metastatic breast cancer. Clin. Breast Cancer 2009, 9, 247–252. [Google Scholar] [CrossRef]
- Rochlitz, C.; Ruhstaller, T.; Lerch, S.; Spirig, C.; Huober, J.; Suter, T.; Bühlmann, M.; Fehr, M.; Schönenberger, A.; von Moos, R. Combination of bevacizumab and 2-weekly pegylated liposomal doxorubicin as first-line therapy for locally recurrent or metastatic breast cancer. A multicenter, single-arm phase II trial (SAKK 24/06). Ann. Oncol. 2011, 22, 80–85. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Bekaii-Saab, T.; Boland, P.M.; Dayyani, F.; Macarulla, T.; Mody, K.; Belanger, B.; Maxwell, F.; Moore, Y.; Thiagalingam, A. First-line liposomal irinotecan with oxaliplatin, 5-fluorouracil and leucovorin (NALIRIFOX) in pancreatic ductal adenocarcinoma: A phase I/II study. Eur. J. Cancer 2021, 151, 14–24. [Google Scholar] [CrossRef]
- Brendel, K.; Bekaii-Saab, T.; Boland, P.M.; Dayyani, F.; Dean, A.; Macarulla, T.; Maxwell, F.; Mody, K.; Pedret-Dunn, A.; Wainberg, Z.A. Population pharmacokinetics of liposomal irinotecan in patients with cancer and exposure–safety analyses in patients with metastatic pancreatic cancer. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1550–1563. [Google Scholar] [CrossRef]
- Fishman, M.; Elsayed, Y.; Damjanov, N.; Steinberg, J.; Mahany, J.; Nieves, J.; Wanaski, S.; Dul, J.; Sherman, J. Phase I study of liposome entrapped paclitaxel (LEP-ETU) in patients with advanced cancer. J. Clin. Oncol. 2004, 22, 2110. [Google Scholar] [CrossRef]
- Slingerland, M.; Guchelaar, H.-J.; Rosing, H.; Scheulen, M.E.; van Warmerdam, L.J.; Beijnen, J.H.; Gelderblom, H. Bioequivalence of Liposome-Entrapped Paclitaxel Easy-To-Use (LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil: A randomized, two-period crossover study in patients with advanced cancer. Clin. Ther. 2013, 35, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D. Safety and Activity of DCR-MYC, a First-in-Class Dicer-Substrate Small Interfering RNA (DsiRNA) Targeting MYC, in a Phase I Study in Patients with Advanced Solid Tumors; American Society of Clinical Oncology: Alexandria, VA, USA, 2015. [Google Scholar]
- Chang, S.; Herse, Z.; Claxton, D.; Giffon, T.; Lewis, D.; Fairman, J. Immunotherapy of Acute Leukemia with Cationic Lipid DNA Complexes (JVRS-100); Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Celsion. Phase 3 Study of ThermoDox with Radiofrequency Ablation (RFA) in Treatment of Hepatocellular Carcinoma (HCC); National Library of Medicine: Bethesda, MD, USA, 2011. Available online: https://clinicaltrials.gov/ (accessed on 4 August 2023).
- Tuxen, M.K.; Cold, S.; Tange, U.B.; Balslev, E.; Nielsen, D.L. Phase II study of neoadjuvant pegylated liposomal doxorubicin and cyclophosphamide±trastuzumab followed by docetaxel in locally advanced breast cancer. Acta Oncol. 2014, 53, 1440–1445. [Google Scholar] [CrossRef]
- Koukouraki, K.S.; Giatromanolaki, A.; Kakolyris, S.; Georgoulias, V.; Velidaki, A.; Archimandritis, S.; Nikolaos, N. Karkavitsas, M.I. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas: Rationale for combination with radiotherapy. Acta Oncol. 2000, 39, 207–211. [Google Scholar]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).