Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier
Abstract
1. Introduction
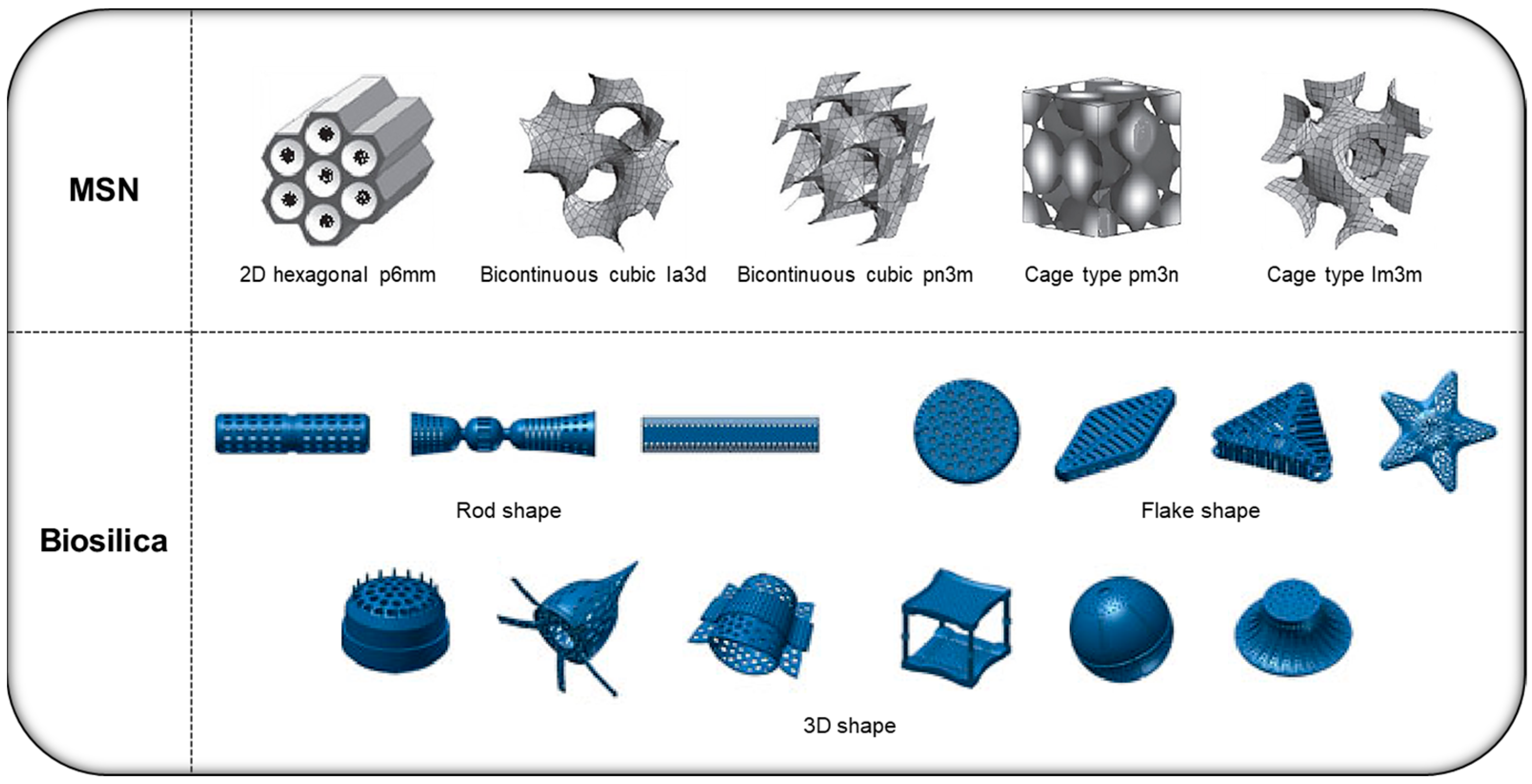
2. Various Types of Silica-Based DDS Platforms
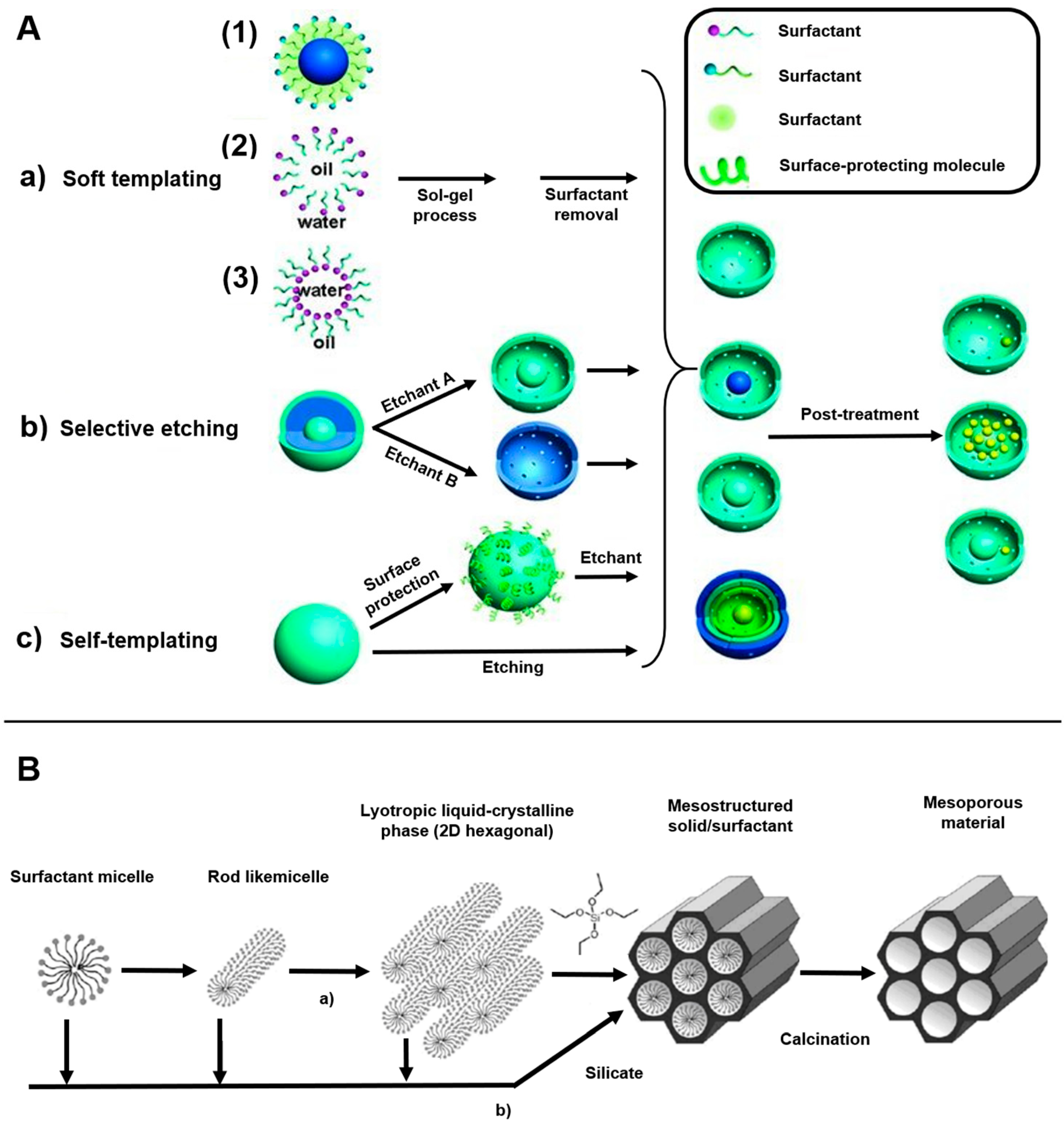
2.1. MSN
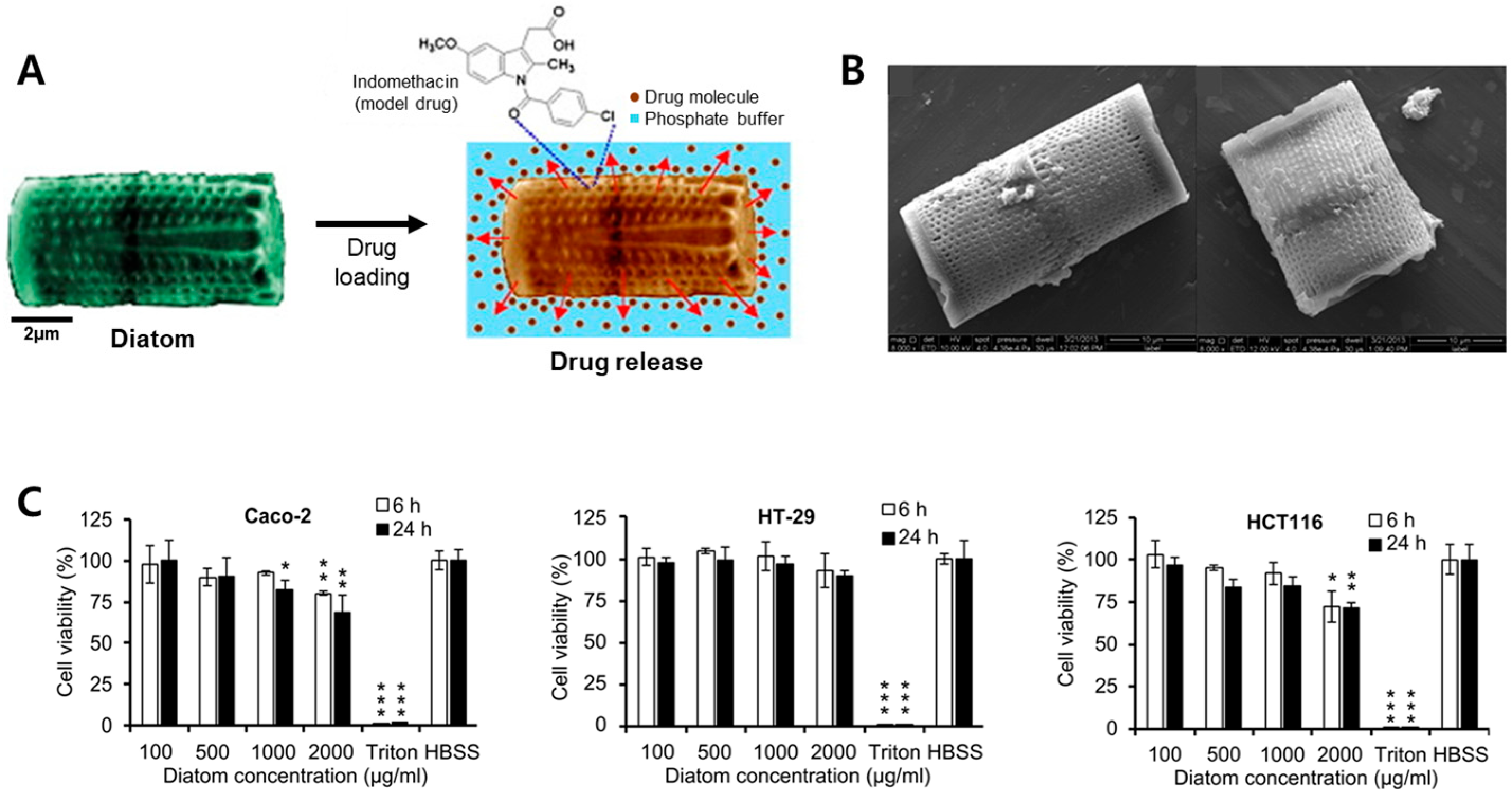
2.2. Diatom Biosilica
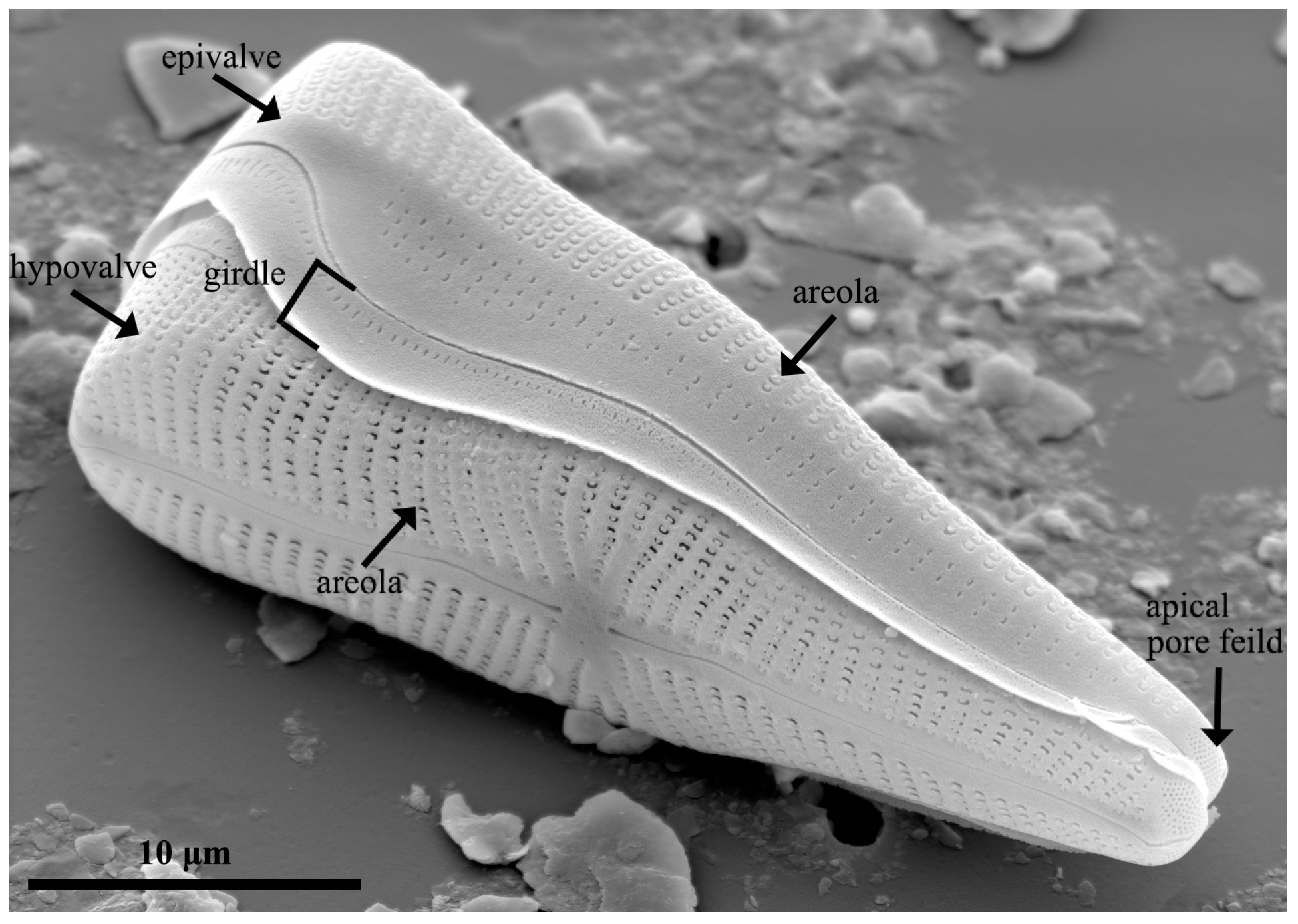
2.3. Frustule of Diatoms
3. Application of MS-DDS Platforms According to Various Administration Routes
3.1. Oral
3.2. Rectal
3.3. Skin
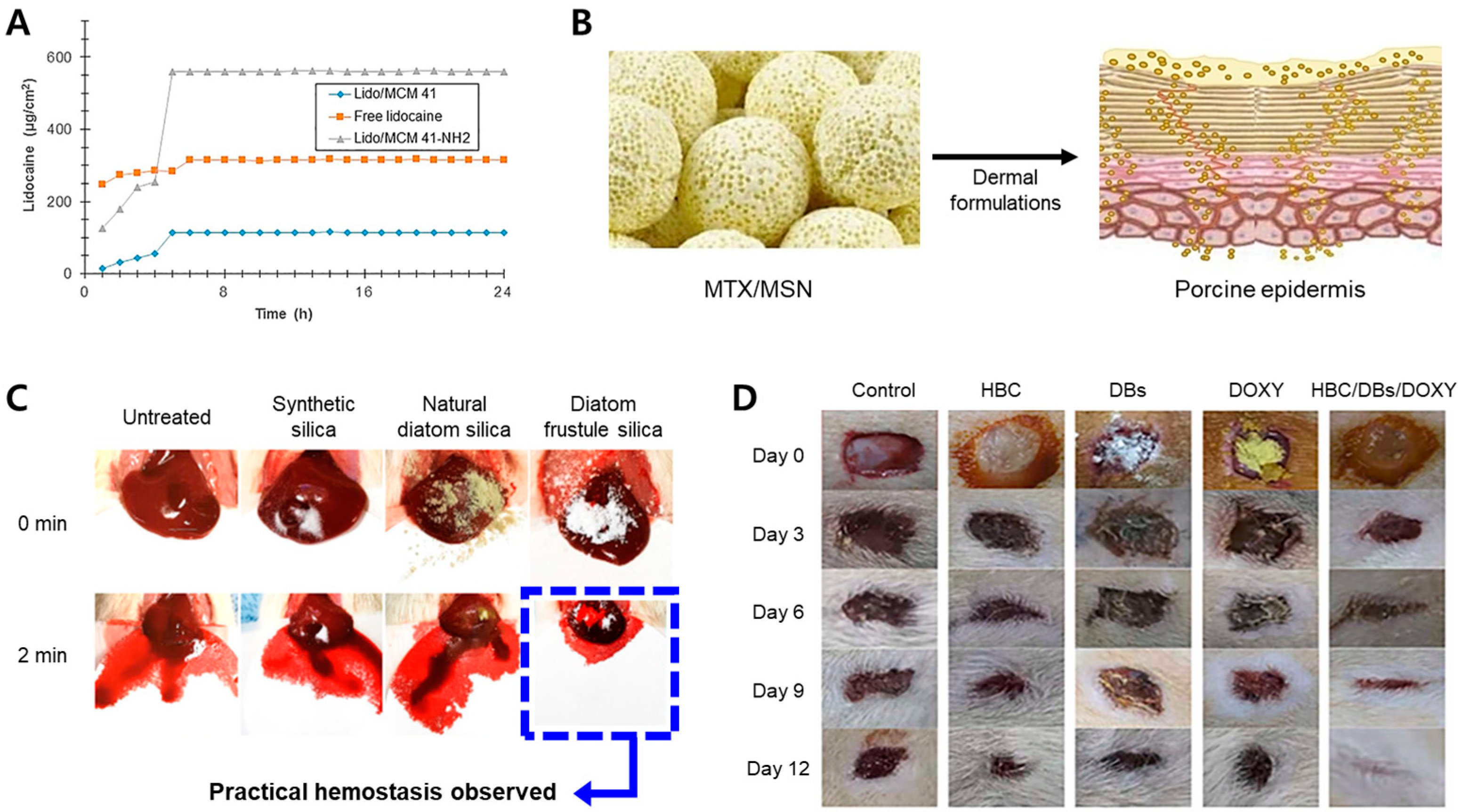
3.4. Injections
4. Value of Diatom-Based DDS Platforms
4.1. Limitations of Diatom-Based DDS Platforms by Injection
4.2. Benefits of Biosilica in Oral Administration
4.3. Surface Modification of Biosilica
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khattak, R.Z.; Nawaz, A.; Alnuwaiser, M.A.; Latif, M.S.; Rashid, S.A.; Khan, A.A.; Alamoudi, S.A. Formulation, In Vitro Characterization and Antibacterial Activity of Chitosan-Decorated Cream Containing Bacitracin for Topical Delivery. Antibiotics 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.D.; Nimbalwar, M.G.; Singhal, N.S.; Panchale, W.A.; Manwar, J.V.; Bakal, R.L. Comprehensive Review on Characterizations and Application of Gastro-Retentive Floating Drug Delivery System. GSC Adv. Res. Rev. 2021, 7, 35–44. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, Q.; Wang, J.; Wu, S.; Luo, Z.; Lu, W. Drug Nanocrystals for Active Tumor-Targeted Drug Delivery. Pharmaceutics 2022, 14, 797. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Xu, H.; Schmidt, O.G. Micro-and Nano-Motors: The New Generation of Drug Carriers. Ther. Deliv. 2018, 9, 303–316. [Google Scholar] [CrossRef]
- Sadab, S.; Sahu, S.; Patel, S.; Khan, R.; Khare, B.; Thakur, B.S.; Jain, A.; Jain, P.K. A Comprehensive Review: Transdermal Drug Delivery System: A Tool for Novel Drug Delivery System. Asian J. Dent. Health Sci. 2022, 2, 40–47. [Google Scholar] [CrossRef]
- Allen, T.M.; Cheng, K.; Wilson, W.; Hare, J.I.; Laginha, K.M. Pharmacokinetics and Pharmacodynamics of Lipidic Nano-Particles in Cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2006, 6, 513–523. [Google Scholar] [CrossRef]
- Cegnar, M.; Kristl, J.; Kos, J. Nanoscale Polymer Carriers to Deliver Chemotherapeutic Agents to Tumours. Expert Opin. Biol. Ther. 2005, 5, 1557–1569. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic Nano-Carriers Based Smart Drug Delivery Systems for Tumor Therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 598722. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, N.; Philip, M.; Krishna, S.S.; Viswanad, V.; Sahu, R.K.; Shiroorkar, P.N.; Aasif, A.H.; Fattepur, S.; Asdaq, S.M.B.; Nair, A.B. Multifunctional Mesoporous Silica Nanoparticles for Oral Drug Delivery. Coatings 2022, 12, 358. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous Silica Microshells from Diatoms as Biocarrier for Drug Delivery Applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. ISBN 0521363187. [Google Scholar]
- Mann, D.G. The Species Concept in Diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Scala, S.; Bowler, C. Molecular Insights into the Novel Aspects of Diatom Biology. Cell. Mol. Life Sci. C 2001, 58, 1666–1673. [Google Scholar] [CrossRef]
- Lopez, P.J.; Descles, J.; Allen, A.E.; Bowler, C. Prospects in Diatom Research. Curr. Opin. Biotechnol. 2005, 16, 180–186. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Kromkamp, J. Primary Production by Phytoplankton and Microphytobenthos in Estuaries. Adv. Ecol. Res. 1999, 29, 93–153. [Google Scholar]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Iudicone, D. Influence of Diatom Diversity on the Ocean Biological Carbon Pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; National University of Ireland: Galway, Ireland, 2008. [Google Scholar]
- NIBR. National List of Species of Korea (Algae); National Institute of Biological Resources: Incheon, Republic of Korea, 2023.
- Phogat, S.; Saxena, A.; Kapoor, N.; Aggarwal, C.; Tiwari, A. Diatom Mediated Smart Drug Delivery System. J. Drug Deliv. Sci. Technol. 2021, 63, 102433. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.-A.; Mäkilä, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom Silica Microparticles for Sustained Release and Permeation Enhancement Following Oral Delivery of Prednisone and Mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-Functionalized Diatom Microcapsules for Drug Delivery of Water-Insoluble Drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bai, S.; Wu, X.; Tan, S.; He, Y. Biodegradability of Mesoporous Silica Nanoparticles. Ceram. Int. 2021, 47, 31031–31041. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Wang, Y.; Kaur, G.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Evdokiou, A.; Losic, D. From the Mine to Cancer Therapy: Natural and Biodegradable Theranostic Silicon Nanocarriers from Diatoms for Sustained Delivery of Chemotherapeutics. Adv. Healthc. Mater. 2016, 5, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Mudakavi, R.J.; Raichur, A.M.; Chakravortty, D. Lipid Coated Mesoporous Silica Nanoparticles as an Oral Delivery System for Targeting and Treatment of Intravacuolar Salmonella Infections. RSC Adv. 2014, 4, 61160–61166. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Cai, J.; Pan, J.; Jiang, X.; Jiang, Y. Bio-Manufacturing Technology Based on Diatom Micro-and Nanostructure. Chin. Sci. Bull. 2012, 57, 3836–3849. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yang, B.; Xu, L.; Zheng, N.; Chen, H.; Li, S. Control-Release Microcapsule of Famotidine Loaded Biomimetic Synthesized Mesoporous Silica Nanoparticles: Controlled Release Effect and Enhanced Stomach Adhesion In Vitro. Mater. Sci. Eng. C 2016, 58, 273–277. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous Silica Nanoparticles in Medicine—Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Yang, B.; Li, J.; Xu, L.; Liu, H.; Li, S.; Xu, J.; Yang, M.; Wei, M. Evaluation of Biomimetically Synthesized Mesoporous Silica Nanoparticles as Drug Carriers: Structure, Wettability, Degradation, Biocompatibility and Brain Distribution. Mater. Sci. Eng. C 2019, 94, 453–464. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Vazquez, N.I.; Gonzalez, Z.; Ferrari, B.; Castro, Y. Synthesis of Mesoporous Silica Nanoparticles by Sol–Gel as Nanocontainer for Future Drug Delivery Applications. Boletín Soc. Española Cerám. Vidr. 2017, 56, 139–145. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.; Na, J.; Lee, C.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous Materials for Clean Energy Technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Dudhipala, N. A Systemic Review on Development of Mesoporous Nanoparticles as a Vehicle for Transdermal Drug Delivery. Nanotheranostics 2023, 7, 70. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification—Moving Forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Pamirsky, I.E.; Golokhvast, K.S. Silaffins of Diatoms: From Applied Biotechnology to Biomedicine. Mar. Drugs 2013, 11, 3155–3167. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- Chao, J.T.; Biggs, M.J.P.; Pandit, A.S. Diatoms: A Biotemplating Approach to Fabricating Drug Delivery Reservoirs. Expert Opin. Drug Deliv. 2014, 11, 1687–1695. [Google Scholar] [CrossRef]
- Saxena, A.; Dutta, A.; Kapoor, N.; Kumar, A.; Tiwari, A. Envisaging Marine Diatom Thalassiosira Weissflogii as a “SMART” Drug Delivery System for Insoluble Drugs. J. Drug Deliv. Sci. Technol. 2022, 68, 102983. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Kim, S.-K. Application of Diatom Biosilica in Drug Delivery. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 245–254. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms Green Nanotechnology for Biosilica-Based Drug Delivery Systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef]
- Zobi, F. Diatom Biosilica in Targeted Drug Delivery and Biosensing Applications: Recent Studies. Micro 2022, 2, 342–360. [Google Scholar] [CrossRef]
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Hussein, H.A.; Nazir, M.S.; Azra, N.; Qamar, Z.; Seeni, A.; Tengku Din, T.A.D.A.-A.; Abdullah, M.A. Novel Drug and Gene Delivery System and Imaging Agent Based on Marine Diatom Biosilica Nanoparticles. Mar. Drugs 2022, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Brühwiler, D. Postsynthetic Functionalization of Mesoporous Silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Townley, H.E.; Parker, A.R.; White-Cooper, H. Exploitation of Diatom Frustules for Nanotechnology: Tethering Active Biomolecules. Adv. Funct. Mater. 2008, 18, 369–374. [Google Scholar] [CrossRef]
- Yu, Y.; Addai-Mensah, J.; Losic, D. Functionalized Diatom Silica Microparticles for Removal of Mercury Ions. Sci. Technol. Adv. Mater. 2012, 13, 015008. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-delivery and Antioxidant Properties. ChemPlusChem 2015, 80, 1104–1112. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous Silica Nanoparticles: Synthesis, Pharmaceutical Applications, Biodistribution, and Biosafety Assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Fu, W.; Wichuk, K.; Brynjólfsson, S. Developing Diatoms for Value-Added Products: Challenges and Opportunities. New Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-K.; Seibert, M. Prospects for Commercial Production of Diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Saoud, H.A.A.L.; Sprynskyy, M.; Pashaei, R.; Kawalec, M.; Pomastowski, P.; Buszewski, B. Diatom Biosilica: Source, Physical-chemical Characterization, Modification, and Application. J. Sep. Sci. 2022, 45, 3362–3376. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom Silica for Biomedical Applications: Recent Progress and Advances. Adv. Healthc. Mater. 2018, 7, 1800552. [Google Scholar] [CrossRef]
- Nafisi, S.; Samadi, N.; Houshiar, M.; Maibach, H.I. Mesoporous Silica Nanoparticles for Enhanced Lidocaine Skin Delivery. Int. J. Pharm. 2018, 550, 325–332. [Google Scholar] [CrossRef]
- Salonen, J.; Kaukonen, A.M.; Hirvonen, J.; Lehto, V.-P. Mesoporous Silicon in Drug Delivery Applications. J. Pharm. Sci. 2008, 97, 632–653. [Google Scholar] [CrossRef]
- Rea, I.; De Stefano, L. Special Issue on New Frontiers in Diatom Nanotechnology. Appl. Sci. 2022, 12, 10332. [Google Scholar] [CrossRef]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous Silica Nanoparticles as a Promising Skin Delivery System for Methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.P.; Sprynskyy, M.; Brzozowska, W.; Lisowska-Oleksiak, A. Electrochemical Behavior of a Composite Material Containing 3D-Structured Diatom Biosilica. Algal Res. 2019, 41, 101538. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine Diatom Thalassiosira Weissflogii Based Biorefinery for Co-Production of Eicosapentaenoic Acid and Fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface Functionalisation of Diatoms with Dopamine Modified Iron-Oxide Nanoparticles: Toward Magnetically Guided Drug Microcarriers with Biologically Derived Morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tatè, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite Silica Nanoparticles for Drug Delivery. Nanoscale Res. Lett. 2014, 9, 329. [Google Scholar] [CrossRef]
- Suib, S.L. A Review of Recent Developments of Mesoporous Materials. Chem. Rec. 2017, 17, 1169–1183. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; De Stefano, L. Synthetic vs. Natural: Diatoms Bioderived Porous Materials for the next Generation of Healthcare Nanodevices. Adv. Healthc. Mater. 2017, 6, 1601125. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled Synthesis of Monodispersed Mesoporous Silica Nanoparticles: Particle Size Tuning and Formation Mechanism Investigation. Microporous Mesoporous Mater. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Kurdyukov, D.A.; Eurov, D.A.; Kirilenko, D.A.; Kukushkina, J.A.; Sokolov, V.V.; Yagovkina, M.A.; Golubev, V.G. High-Surface Area Spherical Micro-Mesoporous Silica Particles. Microporous Mesoporous Mater. 2016, 223, 225–229. [Google Scholar] [CrossRef]
- Fischer, C.; Adam, M.; Mueller, A.C.; Sperling, E.; Wustmann, M.; van Pée, K.-H.; Kaskel, S.; Brunner, E. Gold Nanoparticle-Decorated Diatom Biosilica: A Favorable Catalyst for the Oxidation of D-Glucose. ACS Omega 2016, 1, 1253–1261. [Google Scholar] [CrossRef]
- Mu, Y.; Fu, Y.; Li, J.; Shao, K.; Pang, J.; Su, C.; Cai, Y.; Sun, X.; Cong, X.; Chen, X. Thrombin Immobilized Polydopamine–Diatom Biosilica for Effective Hemorrhage Control. Biomater. Sci. 2021, 9, 4952–4967. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Alsawat, M.; Kumeria, T.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Losic, D. Luminescent Silicon Diatom Replicas: Self-Reporting and Degradable Drug Carriers with Biologically Derived Shape for Sustained Delivery of Therapeutics. Adv. Funct. Mater. 2015, 25, 5107–5116. [Google Scholar] [CrossRef]
- Shen, L.; Guo, X.; Fang, X.; Wang, Z.; Chen, L. Magnesiothermically Reduced Diatomaceous Earth as a Porous Silicon Anode Material for Lithium Ion Batteries. J. Power Sources 2012, 213, 229–232. [Google Scholar] [CrossRef]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured Biosilica of Diatoms: From Water World to Biomedical Applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Medlin, L.K.; Kooistra, W.H.; Gersonde, R.; Wellbrock, U. Evolution of the Diatoms (Bacillariophyta). II. Nuclear-Encoded Small-Subunit RRNA Sequence Comparisons Confirm a Paraphyletic Origin for the Centric Diatoms. Mol. Biol. Evol. 1996, 13, 67–75. [Google Scholar] [CrossRef]
- Fu, W.; Shu, Y.; Yi, Z.; Su, Y.; Pan, Y.; Zhang, F.; Brynjolfsson, S. Diatom Morphology and Adaptation: Current Progress and Potentials for Sustainable Development. Sustain. Horiz. 2022, 2, 100015. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Kotrc, B. Silica Use through Time: Macroevolutionary Change in the Morphology of the Diatom Fustule. Geomicrobiol. J. 2010, 27, 596–608. [Google Scholar] [CrossRef]
- Cox, E.J. Morphology, Cell Wall, Cytology, Ultrastructure and Morphogenetic Studies: Overview and Specific Observations. In The Diatom World; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–45. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I. Diatomite Biosilica Nanocarriers for SiRNA Transport inside Cancer Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- López-Cebral, R.; Peng, G.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Chen, J.; Silva, T.H.; Reis, R.L. Dual Delivery of Hydrophilic and Hydrophobic Drugs from Chitosan/Diatomaceous Earth Composite Membranes. J. Mater. Sci. Mater. Med. 2018, 29, 21. [Google Scholar] [CrossRef]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning Drug Loading and Release Properties of Diatom Silica Microparticles by Surface Modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, J.; Landfester, K.; Mailänder, V. The Challenges of Oral Drug Delivery via Nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Thelen, K.; Dressman, J.B. Cytochrome P450-Mediated Metabolism in the Human Gut Wall. J. Pharm. Pharmacol. 2009, 61, 541–558. [Google Scholar] [CrossRef]
- Liu, J.; Leng, P.; Liu, Y. Oral Drug Delivery with Nanoparticles into the Gastrointestinal Mucosa. Fundam. Clin. Pharmacol. 2021, 35, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in Rectal Drug Delivery Systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal Route in the 21st Century to Treat Children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef]
- Rathi, R.; Sanshita; Kumar, A.; Vishvakarma, V.; Huanbutta, K.; Singh, I.; Sangnim, T. Advancements in Rectal Drug Delivery Systems: Clinical Trials, and Patents Perspective. Pharmaceutics 2022, 14, 2210. [Google Scholar] [CrossRef]
- Özgüney, I.; Kardhiqi, A.; Yıldız, G.; Ertan, G. In vitro–in vivo evaluation of in situ gelling and thermosensitive ketoprofen liquid suppositories. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 283–291. [Google Scholar] [CrossRef]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-Targeted Release of a Ruthenium Anticancer Agent from Vitamin B 12 Functionalized Marine Diatom Microalgae. Dalt. Trans. 2018, 47, 17221–17232. [Google Scholar] [CrossRef]
- Prasanna, J.L.; Deepthi, B.; Rao, N.R. Rectal Drug Delivery: A Promising Route for Enhancing Drug Absorption. Asian J. Res. Pharm. Sci. 2012, 2, 143–149. [Google Scholar]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal Patches: History, Development and Pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating a Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and Biophysical Characteristics of Human Skin in Maintaining Proper Epidermal Barrier Function. Adv. Dermatol. Allergol. 2016, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P. Nanoparticles and Microparticles for Skin Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.-H. Penetration and Release Studies of Positively and Negatively Charged Nanoemulsions—Is There a Benefit of the Positive Charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Elango, T.; Thirupathi, A.; Subramanian, S.; Dayalan, H.; Gnanaraj, P. Methotrexate Normalized Keratinocyte Activation Cycle by Overturning Abnormal Keratins as Well as Deregulated Inflammatory Mediators in Psoriatic Patients. Clin. Chim. Acta 2015, 451, 329–337. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.A.; Shin, M.; Juang, L.J.; Kastrup, C.J.; Go, G.M.; Lee, H. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. ACS Nano 2020, 14, 4755–4766. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-Coated Diatom Silica as Hemostatic Agent for Hemorrhage Control. ACS Appl. Mater. Interfaces 2016, 8, 34234–34243. [Google Scholar] [CrossRef]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The Complex Hydrogel Based on Diatom Biosilica and Hydroxybutyl Chitosan for Wound Healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, a Complex Rate-Controlling Membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Usach, I.; Martinez, R.; Festini, T.; Peris, J.-E. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv. Ther. 2019, 36, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted Drug Delivery Using Genetically Engineered Diatom Biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hao, N.; Zhang, H.; Guo, Z.; Liu, R.; He, B.; Li, S. Mesoporous Iron-Carboxylate Metal–Organic Frameworks Synthesized by the Double-Template Method as a Nanocarrier Platform for Intratumoral Drug Delivery. Biomater. Sci. 2017, 5, 1032–1040. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zou, Q.; Sun, S.-K.; Yu, C.; Zhang, X.; Li, R.-J.; Fu, Y.-Y. Theranostic Metal–Organic Framework Core–Shell Composites for Magnetic Resonance Imaging and Drug Delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Todd, T.; Zhen, Z.; Tang, W.; Chen, H.; Wang, G.; Chuang, Y.-J.; Deaton, K.; Pan, Z.; Xie, J. Iron Oxide Nanoparticle Encapsulated Diatoms for Magnetic Delivery of Small Molecules to Tumors. Nanoscale 2014, 6, 2073–2076. [Google Scholar] [CrossRef]
- Borak, B.; Biernat, P.; Prescha, A.; Baszczuk, A.; Pluta, J. In Vivo Study on the Biodistribution of Silica Particles in the Bodies of Rats. Adv. Clin. Exp. Med. 2012, 21, 13–18. [Google Scholar]
- Barbe, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Zhong, D.; Du, Z.; Zhou, M. Algae: A Natural Active Material for Biomedical Applications. View 2021, 2, 20200189. [Google Scholar] [CrossRef]
- Zhai, W.; He, C.; Wu, L.; Zhou, Y.; Chen, H.; Chang, J.; Zhang, H. Degradation of Hollow Mesoporous Silica Nanoparticles in Human Umbilical Vein Endothelial Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, M.; De Stefano, L.; Tortiglione, C.; Tino, A.; Rea, I. In Vivo Toxicity Assessment of Hybrid Diatomite Nanovectors Using Hydra Vulgaris as a Model System. Adv. Biosyst. 2019, 3, 1800247. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Lee, S.-Y.; Lee, E.J.; Park, S.H.; Seong, N.; Seo, H.-S.; Shin, S.-S.; Kim, S.-J.; Meang, E.-H.; Park, M.-K. Toxicity of Colloidal Silica Nanoparticles Administered Orally for 90 Days in Rats. Int. J. Nanomed. 2014, 9, 67–78. [Google Scholar] [CrossRef][Green Version]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-Based Systems for Oral Delivery of Drugs, Macromolecules and Cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Keckeis, V. Advances in Drug Delivery Systems: Work in Progress Still Needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Czuryszkiewicz, T.; Areva, S.; Honkanen, M.; Lindén, M. Synthesis of Sol–Gel Silica Materials Providing a Slow Release of Biphosphonate. Colloids Surf. A Physicochem. Eng. Asp. 2005, 254, 69–74. [Google Scholar] [CrossRef]
- Ki, M.-R.; Nguyen, T.K.M.; Jun, H.S.; Pack, S.P. Biosilica-Enveloped Ferritin Cage for More Efficient Drug Deliveries. Process Biochem. 2018, 68, 182–189. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Z.; Wu, H.; Feng, Y.; Li, J. Protamine-Induced Biosilica as Efficient Enzyme Immobilization Carrier with High Loading and Improved Stability. Mater. Sci. Eng. C 2009, 29, 2029–2035. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Schneider, F.; Jedamzik, P.; Sager, M.; Kühn, J.-P.; Siegmund, W.; Weitschies, W. Navigating the Human Gastrointestinal Tract for Oral Drug Delivery: Uncharted Waters and New Frontiers. Adv. Drug Deliv. Rev. 2016, 101, 75–88. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Wang, J.; Liu, C.; Kong, N.; Farokhzad, O.C.; Tao, W. Oral Insulin Delivery Platforms: Strategies to Address the Biological Barriers. Angew. Chem. Int. Ed. 2020, 59, 19787–19795. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, P.; Schätzlein, A.G. Biological Barriers: Transdermal, Oral, Mucosal, Blood Brain Barrier, and the Blood Eye Barrier. In Fundamentals of Pharmaceutical Nanoscience; Springer: Berlin/Heidelberg, Germany, 2013; pp. 301–336. [Google Scholar] [CrossRef]
- Managò, S.; Tramontano, C.; Delle Cave, D.; Chianese, G.; Zito, G.; De Stefano, L.; Terracciano, M.; Lonardo, E.; De Luca, A.C.; Rea, I. SERS Quantification of Galunisertib Delivery in Colorectal Cancer Cells by Plasmonic-assisted Diatomite Nanoparticles. Small 2021, 17, 2101711. [Google Scholar] [CrossRef] [PubMed]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of Stimulus-Responsive Diatom Biosilica Microcapsules for Antibiotic Drug Delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, M.; Shahbazi, M.A.; Correia, A.; Rea, I.; Lamberti, A.; De Stefano, L.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074. [Google Scholar] [CrossRef]
| Diatom Biosilica | Mesoporous Silica Nanoparticle | Reference | |
|---|---|---|---|
| Surface functionalization | Silanization | Co-condensation (direct or one-pot synthesis)/postsynthetic treatment (often referred to as grafting) | [51,52,53,54,55] |
| Production | Diatom culture, H2O2-based purification | Sol-gel, microwave synthesis, hydrothermal synthesis, template synthesis, modified aerogel methods, soft and hard templating methods, fast self-assembly, etc. | [13,56,57,58,59,60] |
| Expense | Cheap (≈$200 per tonne (DE)) | Expensive | [22,44,48,49,51,61] |
| Environment | Nature (environmentally friendly, “green” material) | Toxicity (CTAB/MeOH), highly toxic and highly polluted waste is generated. | [13,44,49,60,62,63,64] |
| Bulk up | Easy | Difficult | [48,49,59,65,66,67] |
| Pore size | Alignment (diameter 30 nm to 500 nm) | Alignment (2–130 nm, typically ca. 2–6.5 nm) | [13,62,63,68,69,70] |
| Particle size | Varied (less than 350 nm on average (100 nm to 2 mm)) | Varied (20 nm to 1 μm when adjusting parameters) | [36,61,69,71,72] |
| Specific surface area Brunauer–Emmett–Teller method (BET SSA) | Large (6–200 m2/g) | Large (~1000 m2/g) | [35,54,61,70,71,73,74,75,76,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics 2023, 15, 2434. https://doi.org/10.3390/pharmaceutics15102434
Lim H, Seo Y, Kwon D, Kang S, Yu J, Park H, Lee SD, Lee T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics. 2023; 15(10):2434. https://doi.org/10.3390/pharmaceutics15102434
Chicago/Turabian StyleLim, Hayeon, Yoseph Seo, Daeryul Kwon, Sunggu Kang, Jiyun Yu, Hyunjun Park, Sang Deuk Lee, and Taek Lee. 2023. "Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier" Pharmaceutics 15, no. 10: 2434. https://doi.org/10.3390/pharmaceutics15102434
APA StyleLim, H., Seo, Y., Kwon, D., Kang, S., Yu, J., Park, H., Lee, S. D., & Lee, T. (2023). Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics, 15(10), 2434. https://doi.org/10.3390/pharmaceutics15102434