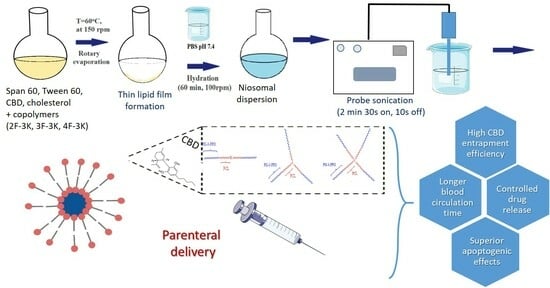
Development, Characterization and Pharmacological Evaluation of Cannabidiol-Loaded Long Circulating Niosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
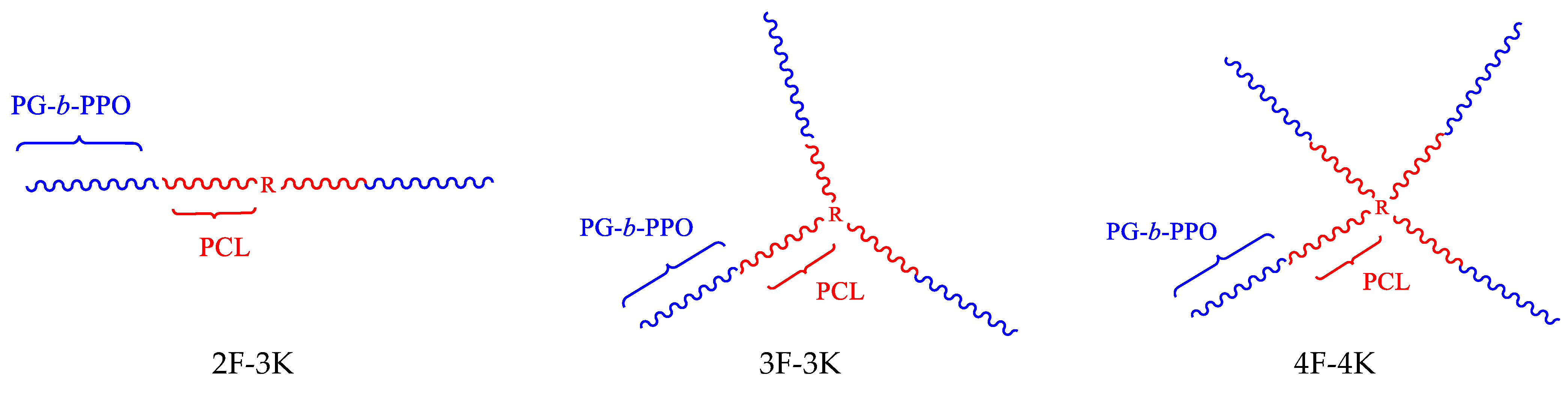
2.2.1. Synthesis of the Star-like 4-Armed Copolymer
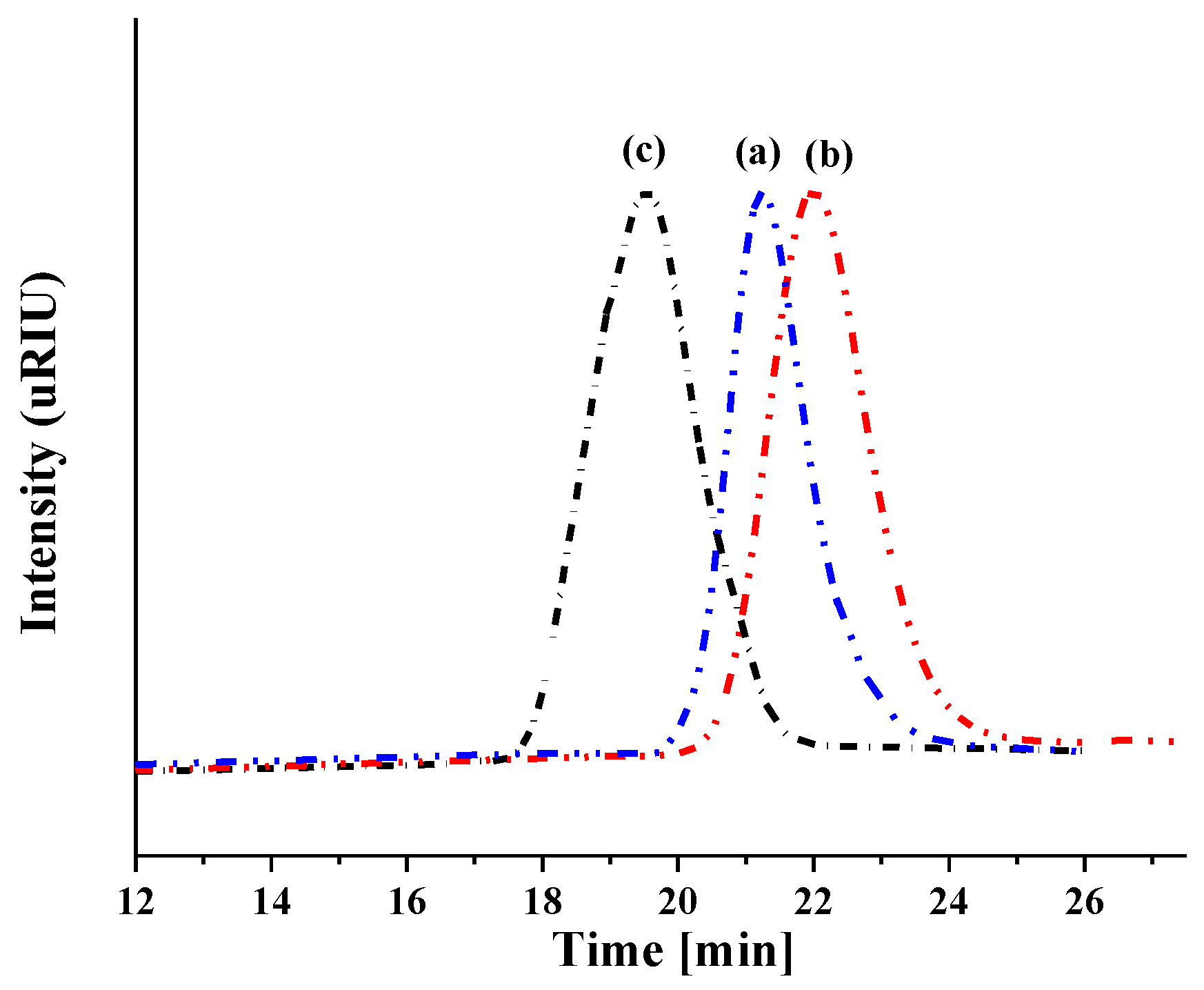
2.2.2. Size Exclusion Chromatography (SEC)
2.2.3. Proton Nuclear Magnetic Resonance (1H-NMR)
2.2.4. Preparation of Cannabidiol-Loaded Conventional and Sterically Stabilized Niosomes
2.2.5. Entrapment Efficiency
2.2.6. Size, Size Distribution, and Zeta Potential Evaluation
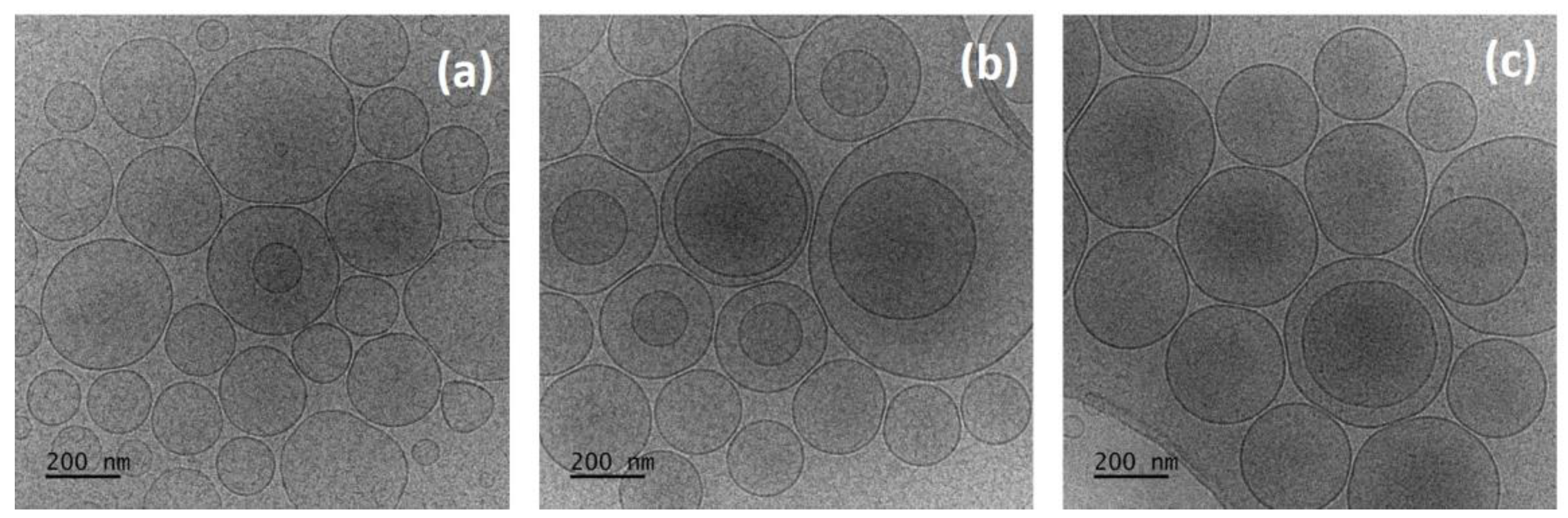
2.2.7. Cryogenic Transmission Electron Microscopy (cryo-TEM)
2.2.8. Carboxyfluorescein (CF) Release Studies
2.2.9. In Vitro Cannabidiol Release from Niosomes
2.2.10. Physical Stability Studies
2.2.11. Evaluation of Cytotoxicity of Cannabidiol and CBD-Loaded Conventional and Copolymer-Modified Niosomes
Cell Lines and Culture Conditions
MTT Colorimetric Assay
2.2.12. Proteome Profiler Analysis of Apoptosis and Inflammation Related Signal Transduction Key Proteins
3. Results and Discussion
3.1. Synthesis of Copolymers
3.2. Characterization of Conventional and Copolymer-Modified Niosomes
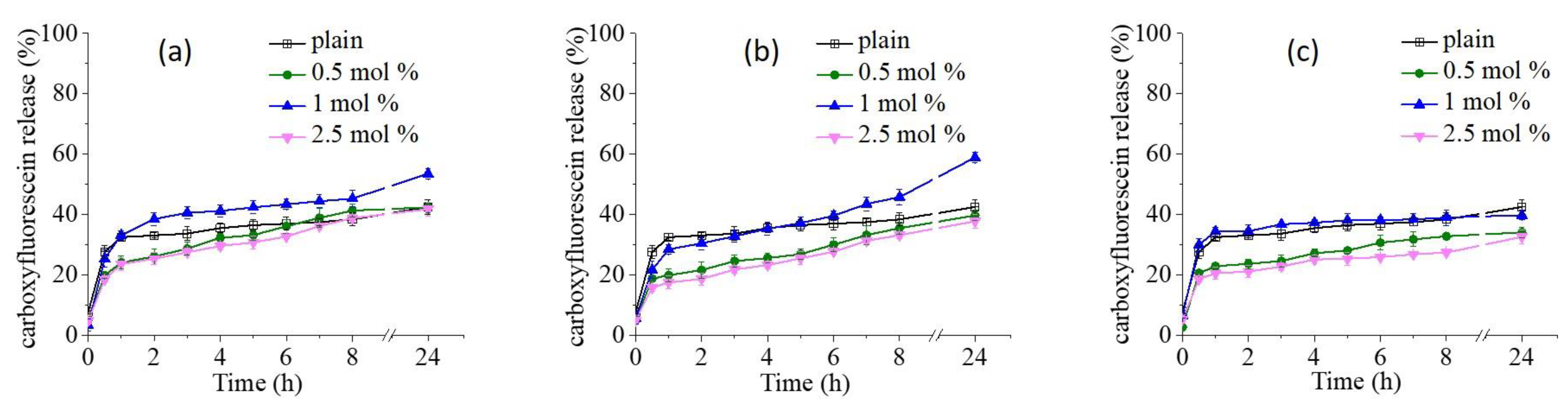
3.3. Carboxyfluorescein Release from Conventional and Copolymer-Modified Niosomes
3.4. In Vitro Release of Cannabidiol from Optimal Plain and Copolymer-Modified Niosomes
3.5. Physical Stability Evaluation
3.6. Cytotoxicity Studies
3.6.1. MTT—Assay
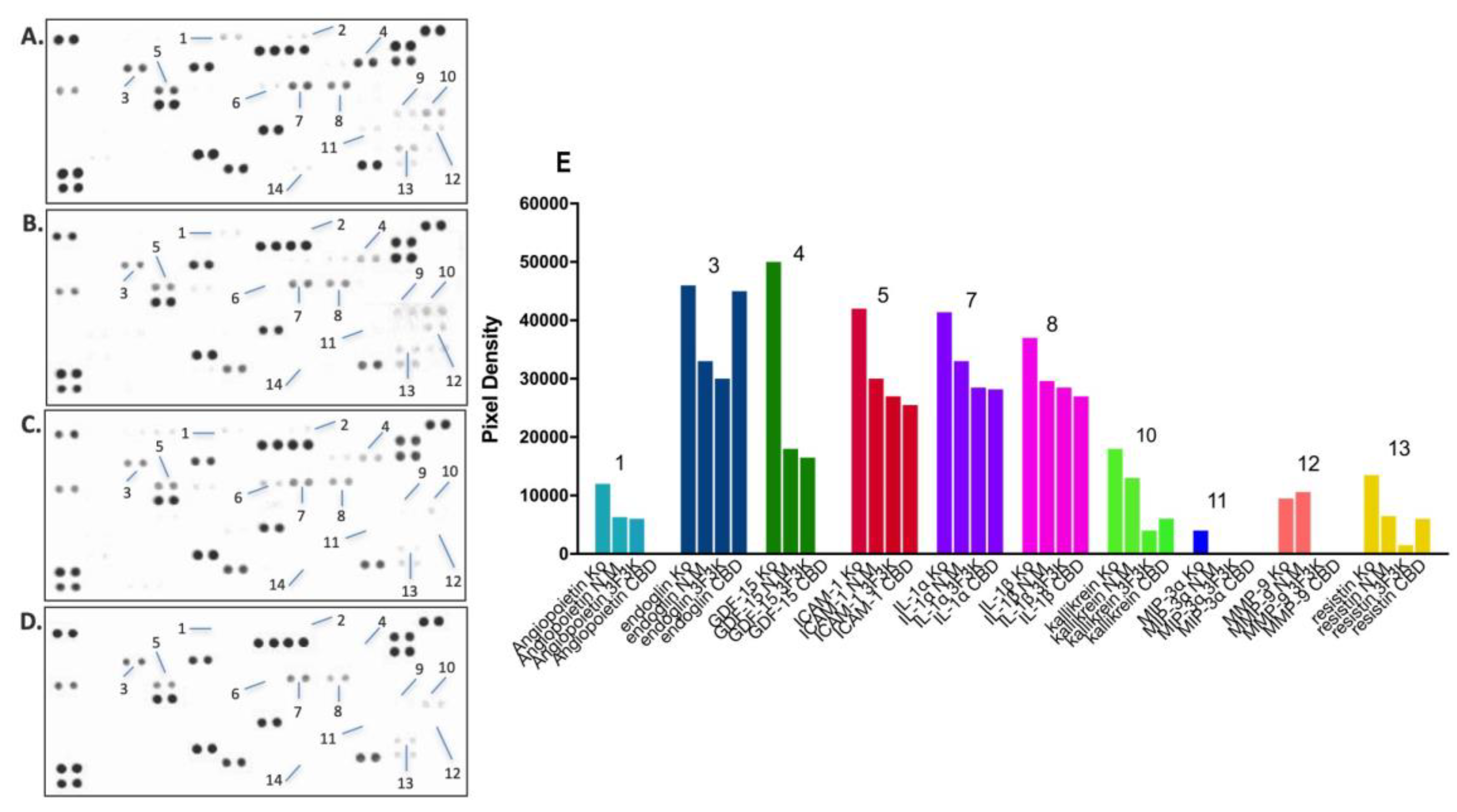
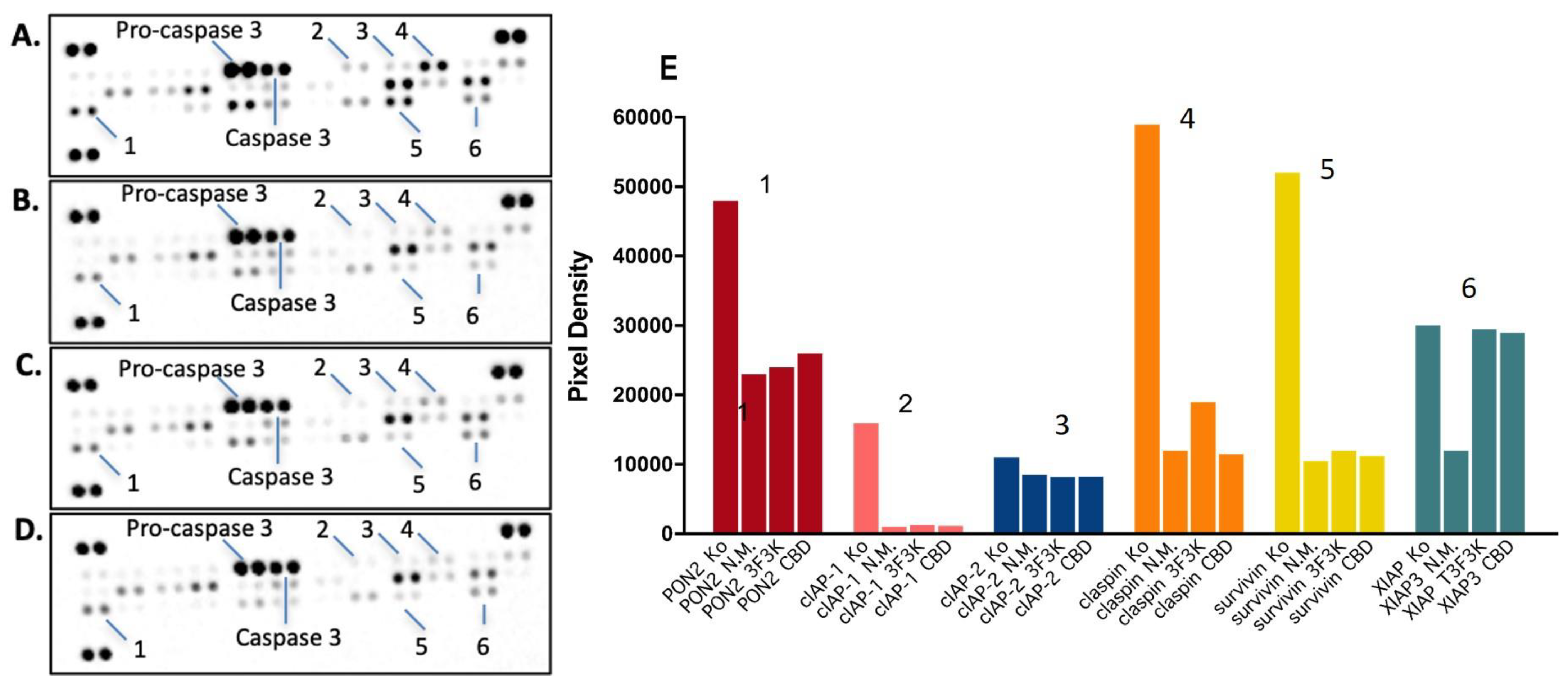
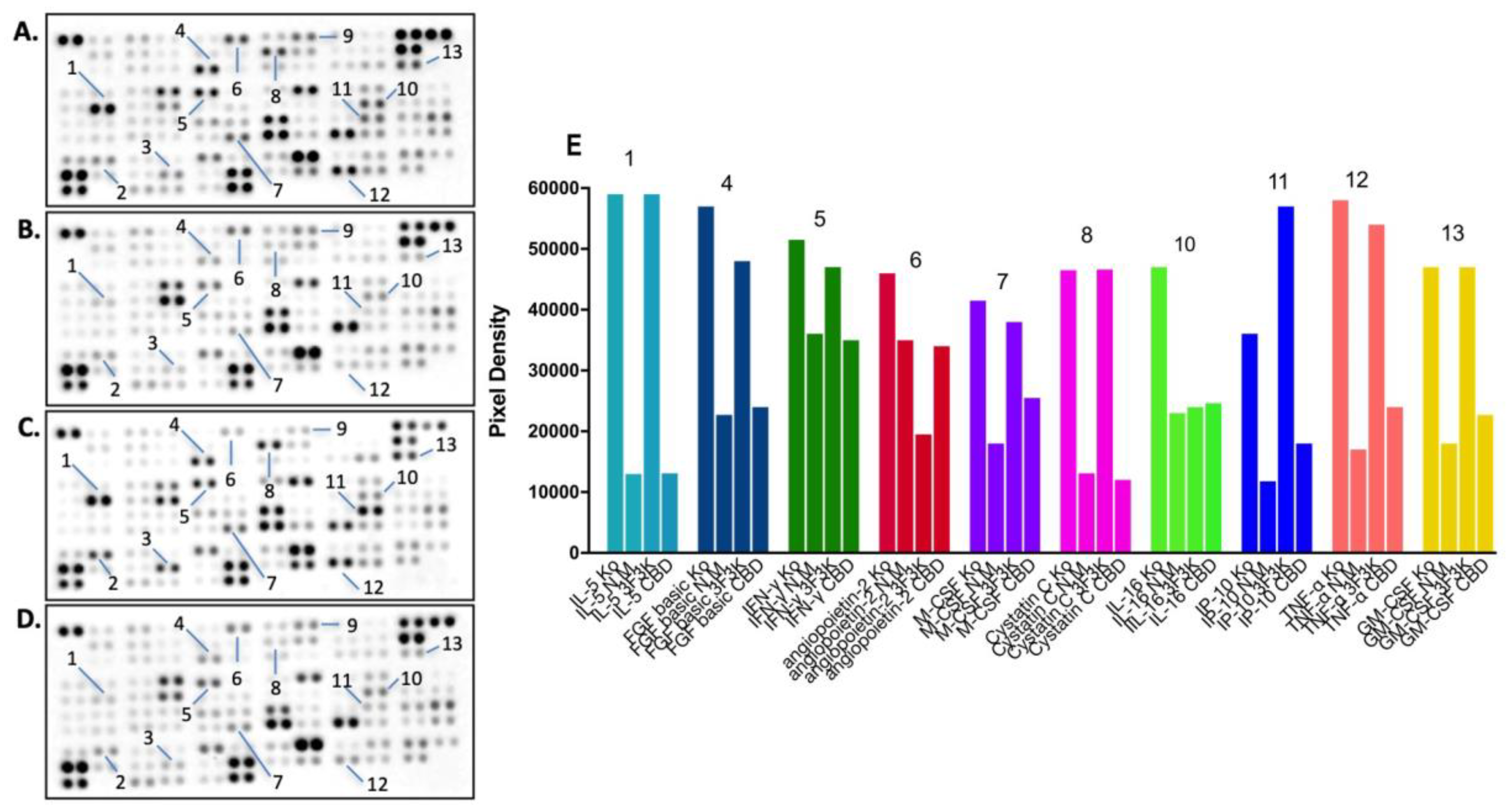
3.6.2. Proteome Profiler Analysis after Treatment with Free or Niosomal CBD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Panda, P.K.; Jain, S.K. Niosome as a promising vesicular tool for therapy and diagnosis. In Advanced Nanoformulations Theranostic Nanosystems, 1st ed.; Hasnain, M.S., Nayak, A.K., Aminabhavi, T.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 3, pp. 233–254. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.G.; Sharma, N. Nanobiomaterials in cosmetics: Current status and future prospects. In Nanobiomaterials in Galenic Formulations and Cosmetics Applications of Nanobiomaterials, 1st ed.; Grumezescu, A.M., Ed.; William Andrew Applied Science Publishers: Norwich, NY, USA, 2016; Volume 10, pp. 149–174. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid. Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Ag Seleci, D.; Maurer, V.; Stahl, F.; Scheper, T.; Garnweitner, G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. Int. J. Mol. Sci. 2019, 20, 4696. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Moammeri, A.; Abbaspour, K.; Zafarian, A.; Jamshidifar, E.; Motasadizadeh, H.; Dabbagh Moghaddam, F.; Salehi, Z.; Makvandi, P.; Dinarvand, R. pH-Responsive, Adorned Nanoniosomes for Codelivery of Cisplatin and Epirubicin: Synergistic Treatment of Breast Cancer. ACS Appl. Bio. Mater. 2022, 5, 675–690. [Google Scholar] [CrossRef]
- Shi, B.; Fang, C.; Pei, Y. Stealth PEG-PHDCA niosomes: Effects of Chain Length of PEG and Particle Size on Niosomes Surface Properties, In Vitro Drug Release, Phagocytic Uptake, In Vivo Pharmacokinetics and Antitumor Activity. J. Pharm. Sci. 2006, 95, 1873–1887. [Google Scholar] [CrossRef]
- Shehata, T.; Kimura, T.; Higaki, K.; Ogawara, K. In-vivo disposition characteristics of PEG niosome and its interaction with serum proteins. Int. J. Pharm. 2016, 512, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Elsewedy, H.S.; Shehata, T.M.; Tratrat, C.; Al Dhubiab, B.E.; Venugopala, K.N.; Almostafa, M.M.; Kochkar, H.; Elnahas, H.M. Significant of injectable brucine PEGylated niosomes in treatment of MDA cancer cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103322. [Google Scholar] [CrossRef]
- He, R.-X.; Ye, X.; Li, R.; Chen, W.; Ge, T.; Huang, T.-Q.; Nie, X.-J.; Chen, H.-J.; Peng, D.-Y.; Chen, W.-D. PEGylated niosomes-mediated drug delivery systems for Paeonol: Preparation, pharmacokinetics studies and synergistic anti-tumor effects with 5-FU. J. Liposome Res. 2016, 27, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Storozhuk, M.V. Cannabidiol: Potential in treatment of neurological diseases, flax as a possible natural source of cannabidiol. Front. Cell Neurosci. 2023, 17, 1131653. [Google Scholar] [CrossRef]
- O’Brien, K. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol aqueous solubility enhancement: Comparison of three amorphous formulations strategies using different type of polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–17804. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Muresan, P.; Woodhams, S.; Smith, F.; Taresco, V.; Shah, J.; Wong, M.; Chapman, V.; Smith, S.; Hathway, G.; Rahman, R.; et al. Evaluation of cannabidiol nanoparticles and nanoemulsion biodistribution in the central nervous system after intrathecal administration for the treatment of pain. Nanomedicine 2023, 49, 102664. [Google Scholar] [CrossRef] [PubMed]
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703. [Google Scholar] [CrossRef] [PubMed]
- Morakul, B.; Junyaprasert, V.B.; Sakchaisri, K.; Teeranachaideekul, V. Cannabidiol-Loaded Nanostructured Lipid Carriers (NLCs) for Dermal Delivery: Enhancement of Photostability, Cell Viability, and Anti-Inflammatory Activity. Pharmaceutics 2023, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Momekova, D.; Ivanov, E.; Konstantinov, S.; Ublekov, F.; Petrov, P.D. Nanocomposite cryogel carriers from 2-hydroxyethyl cellulose network and cannabidiol-loaded polymeric micelles for sustained topical delivery. Polymers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Dimitrov, E.; Grancharov, G.; Momekova, D.; Petrov, P.; Rangelov, S. Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol. Pharmaceutics 2023, 15, 2128. [Google Scholar] [CrossRef]
- Shilo-Benjamini, Y.; Cern, A.; Zilbersheid, D.; Hod, A.; Lavy, E.; Barasch, D.; Barenholz, Y. A Case Report of Subcutaneously Injected Liposomal Cannabidiol Formulation Used as a Compassion Therapy for Pain Management in a Dog. Front. Vet. Sci. 2022, 9, 892306. [Google Scholar] [CrossRef]
- Volmajer Valh, J.; Peršin, Z.; Vončina, B.; Vrezner, K.; Tušek, L.; Fras Zemljič, L. Microencapsulation of Cannabidiol in Liposomes as Coating for Cellulose for Potential Advanced Sanitary Material. Coatings 2021, 11, 3. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol—Transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Lazzarotto Rebelatto, E.R.; Rauber, G.S.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef]
- Taton, D.; Le Borgne, A.; Sepulchre, M.; Spassky, N. Synthesis of chiral and racemic functional polymers from glycidol and thioglycidol. Macromol. Chem. Phys. 1994, 195, 139–148. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Bakardzhiev, P.; Rangelov, S.; Trzebicka, B.; Forys, A.; Petrov, P.D. Linear Amphiphilic Polyglycidol/Poly(ε-caprolactone) Block Copolymers Prepared via “Click” Chemistry-Based Concept. Macromolecules 2019, 52, 3435–3447. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Varma, R.S. Solvent-free tetrahydropyranylation (THP) of alcohols and phenols and their regeneration by catalytic aluminum chloride hexahydrate. Tetrahedron Lett. 2002, 43, 1143–1146. [Google Scholar] [CrossRef]
- Dimitrov, P.; Rangelov, S.; Dworak, A.; Haraguchi, N.; Hirao, A.; Tsvetanov, C.B. Triblock and Radial Star-Block Copolymers Comprised of Poly(ethoxyethyl glycidyl ether), Polyglycidol, Poly(propylene oxide) and Polystyrene Obtained by Anionic Polymerization Initiated by Cs Initiators. Macromol. Symp. 2004, 215, 127–140. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. The Effects of Hydration Parameters and Co-Surfactants on Methylene Blue-Loaded Niosomes Prepared by the Thin Film Hydration Method. Pharmaceuticals 2019, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Shilakari Asthana, G.; Sharma, P.K.; Asthana, A. In Vitro and In Vivo Evaluation of Niosomal Formulation for Controlled Delivery of Clarithromycin. Scientifica 2016, 2016, 6492953. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Sakellariou, G.; Chatzichristidi, M. Polymers with Star-Related Structures: Synthesis, Properties, and Applications. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 29–111. [Google Scholar] [CrossRef]
- Ahire, E.; Thakkar, S.; Darshanwad, M.; Misra, M. Parenteral nanosuspensions: A brief review from solubility enhancement to more novel and specific applications. Acta Pharm. Sin. B. 2018, 8, 733–755. [Google Scholar] [CrossRef]
- Yoshioka, T.; Sternberg, B.; Florence, A. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int. J. Pharm. 1994, 105, 1–6. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Akbarzadeh, I.; Tavakkoli Yaraki, M.; Lajevardi, A.; Fatemizadeh, M.; Heidarpoor Saremi, L. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Hisham, S.F.; Parkir, N. Effects of cholesterol and charging additives on stability of curcumin niosomes. J. Eng. Appl. Sci. 2017, 12, 8537–8541. [Google Scholar] [CrossRef]
- New, R.R.C. Liposomes: A Practical Approach, 1st ed.; IRL Press at Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Kashchiev, D.; Exerowa, D. Bilayer lipid membrane permeation and rupture due to hole formation. Biochim. Biophys. Acta. 1983, 732, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Volkov, A.G.; Van Hoek, A.N.; Haines, T.H.; Deamer, D.W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Silvander, M.; Johnsson, M.; Edwards, K. Effects of PEG-lipids on permeability of phosphatidylcholine/cholesterol liposomes in buffer and in human serum. Chem. Phys. Lipids 1998, 97, 15–26. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.E.; Spoth, K.A.; Kourkoutis, L.F.; Rizvi, S.S.H. Stability of niosomes with encapsulated vitamin D3 and ferrous sulfate generated using a novel supercritical carbon dioxide method. J. Liposome Res. 2015, 26, 261–268. [Google Scholar] [CrossRef]
- García-Díaz, N.; Piris, M.Á.; Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis Fungoides and Sézary Syndrome: An Integrative Review of the Pathophysiology, Molecular Drivers, and Targeted Therapy. Cancers 2021, 13, 1931. [Google Scholar] [CrossRef]
- Seidl, C. Targets for Therapy of Bladder Cancer. Semin. Nucl. Med. 2020, 50, 162–170. [Google Scholar] [CrossRef]
- Chu, C.; Pietzak, E. Immune mechanisms and molecular therapeutic strategies to enhance immunotherapy in non-muscle invasive bladder cancer: Invited review for special issue “Seminar: Treatment Advances and Molecular Biology Insights in Urothelial Carcinoma”. Urol. Oncol. Semin. Orig. 2022. [Google Scholar] [CrossRef]
- Patil, K.; Kuttikrishnan, S.; Khan, Q.A.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86, 382–399. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Fitton, A.O.; Hill, J.; Jane, D.E.; Millar, R. Synthesis of simple oxetanes carrying reactive 2-substituents. Synthesis 1987, 1140–1142. [Google Scholar] [CrossRef]
- Storey, R.F.; Sherman, J.W. Kinetics and Mechanism of the Stannous Octoate-Catalyzed Bulk Polymerization of ε-Caprolactone. Macromolecules 2002, 35, 1504–1512. [Google Scholar] [CrossRef]



| Copolymer | Mn a (g/mol) | Mw b (g/mol) | Mw/Mn b | ||
|---|---|---|---|---|---|
| Topology | Composition c,d,e | Abbreviation | |||
| Linear | R[(CL)9(PO)7(EEGE)23]2 | 9600 | 13,200 | 1.20 | |
| Linear | R[(CL)9(PO)7(G)23]2 | 2F-3K | 5900 | - | - |
| Star-like, 3-armed | R[(CL)6(PO)7(EEGE)23]3 | 13,400 | 17,700 | 1.22 | |
| Star-like, 3-armed | R[(CL)6(PO)7(G)23]3 | 3F-3K | 8300 | - | - |
| Star-like, 4-armed | R[(CL)5(PO)7(EEGE)23]4 | 17,200 | 14,100 | 1.17 | |
| Star-like, 4-armed | R[(CL)5(PO)7(G)23]4 | 4F-3K | 10,400 | - | - |
| Sample | SF:Chol (mol:mol) | Copolymer (mol %) | Dh (nm) ± SD | PDI ± SD | ζ-Potential (mV) ± SD | EE (%) CBD |
|---|---|---|---|---|---|---|
| Conventional niosomes | ||||||
| S1 | Sp20:Chol (6:4) | - | 230 ± 4.2 | 0.26 ± 0.05 | −13.3 ± 1.9 | 65.8 ± 1.7 |
| S2 | Sp80:Chol (6:4) | - | 180 ± 3.2 | 0.22 ± 0.05 | −26.6 ± 2.2 | 64.7 ± 1.6 |
| S3 | Sp60:Chol (6:4) | - | 186 ± 2.8 | 0.4 ± 0.02 | −12.9 ± 1.7 | 80.3 ± 0.7 |
| S4 | Tw60:Sp60:Chol (3:3:4) | - | 177 ±2.3 | 0.39 ± 0.03 | −11.9 ± 0.5 | 85.2 ± 2.3 |
| S5 | Tw60:Sp60:Chol (3.5:3.5:3) | - | 150 ± 1.1 | 0.35 ± 0.07 | −10.3 ± 1.6 | 93.2 ± 2.1 |
| S6 | Tw60:Sp60:Chol (3.5:3.5:3) (blank) | - | 133 ± 1.7 | 0.31 ± 0.09 | −11.2 ± 1.5 | - |
| S7 | Tw60:Sp60:Chol3.5:3.5:3(unsonicated) | - | 387 ± 5.3 | 0.32 ± 0.02 | −11.9 ± 2.1 | 93.5 ± 1.8 |
| S8 | Tw60:Sp60:Chol 3.5:3.5:3 (blank) (unsonicated) | - | 489 ± 3.8 | 0.34 ± 0.06 | −12.3 ± 2.3 | - |
| Niosomes modified with 2F-3K | ||||||
| S9 | Tw60:Sp60:Chol 3.5:3.5:3 | 0.5 | 224 ± 4.5 | 0.19 ± 0.02 | −12.2 ± 1.8 | - |
| S10 | Tw60:Sp60:Chol 3.5:3.5:3 | 1 | 237 ± 6.9 | 0.26 ± 0.06 | −11.7 ± 0.6 | - |
| S11 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 250 ± 5.3 | 0.31 ± 0.05 | −10.1 ± 1.4 | - |
| S12 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 240 ± 3.3 | 0.38 ±0.03 | −11.3 ± 2.1 | 89.3 ± 2.1 |
| Niosomes modified with 3F-3K | ||||||
| S13 | Tw60:Sp60:Chol 3.5:3.5:3 | 0.5 | 218 ± 8.5 | 0.27 ± 0.02 | −12.4 ± 0.4 | - |
| S14 | Tw60:Sp60:Chol 3.5:3.5:3 | 1 | 234 ± 5.7 | 0.31 ± 0.06 | −11.3 ± 1.5 | - |
| S15 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 240 ± 7.6 | 0.27 ± 0.02 | −10.1 ± 1.7 | - |
| S16 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 235 ± 5.6 | 0.36 ± 0.02 | −11.1 ± 1.7 | 94.1 ± 2.8 |
| Niosomes modified with 4F-3K | ||||||
| S17 | Tw60:Sp60:Chol 3.5:3.5:3 | 0.5 | 234 ± 8.5 | 0.41 ± 0.04 | −10.2 ± 0.2 | - |
| S18 | Tw60:Sp60:Chol 3.5:3.5:3 | 1 | 223 ± 5.7 | 0.31 ± 0.05 | −9.6 ± 0.1 | - |
| S19 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 243 ± 7.6 | 0.36 ± 0.03 | −9.5 ± 0.4 | - |
| S20 | Tw60:Sp60:Chol 3.5:3.5:3 | 2.5 | 238 ± 9.6 | 0.40 ± 0.05 | −8.2 ± 0.3 | 90.1 ± 2.3 |
| Kinetic Model | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Ko (mg/mL)/h | R2 | KF (h−1) | R2 | KH (mg/mL)/h0.5 | R2 | KKP (h−n) | n | t1/2 (h) | |
| Tw60:Sp60:Chol non-modified niosomes (in PBS) | 0.742 | 0.686 | 0.897 | −0.0089 | 0.718 | 7.174 | 0.983 | 12.56 | 0.313 | 82.204 |
| Tw60:Sp60:Chol non-modified niosomes (in PBS+ 20 albumin) | 0.813 | 0.634 | 0.914 | −0.008 | 0.712 | 7.01 | 0.989 | 17.61 | 0.23 | 81.58 |
| Tw60:Sp60:Chol:3F-3K (in PBS) | 0.540 | 0.657 | 0.873 | −0.0066 | 0.594 | 6.001 | 0.985 | 11.24 | 0.290 | 171.6 |
| Tw60:Sp60:Chol:3F-3K (in PBS + 20% albumin) | 0.595 | 0.680 | 0.900 | −0.0072 | 0.596 | 6.542 | 0.982 | 12.25 | 0.290 | 127.7 |
| Sample | Size (nm) | PDI | ζ-Potential (mV) | EE (%) | |
|---|---|---|---|---|---|
| (S5) | Initial | 150 ± 1.1 | 0.35 ± 0.07 | −10.3 ± 1.6 | 92.2 ± 2.1 |
| After 1 month storage | 132 ± 2.8 | 0.41 ± 0.09 | −10.6 ± 2.1 | 90.5 ± 4.1 | |
| (S16) | Initial | 235 ± 5.6 | 0.36 ± 0.02 | −11.1 ± 1.7 | 94.1 ± 2.8 |
| After 1 month storage | 228 ±2.2 | 0.39 ± 0.05 | −11.8 ± 1.2 | 93.8 ± 1.9 | |
| IC50 (μM ± SD) in Terms of CBD Concentration | |||
|---|---|---|---|
| Cell Line Sample | T-24 | MJ | HUT-78 |
| CBD (as solution) | 12.2 ± 2.1 | 58.6 ± 5.9 | 30.1 ± 5.9 |
| Tw60:Sp60:Ch:3F-3K * (S16) | 32.1 ± 6.3 | 74.5 ± 4.4 | 63.9 ± 1.8 |
| Tw60:Sp60:Ch:4F-3K * (S20) | 37.3 ± 5.2 | 74.2 ± 3.8 | 64.6 ± 3.7 |
| Tw60:Sp60:Ch (N.M.) (S5) | 58.9 ± 6.5 | 69.1 ± 6.2 | 86.2 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugleva, V.; Ahchiyska, K.; Georgieva, D.; Mihaylova, R.; Konstantinov, S.; Dimitrov, E.; Toncheva-Moncheva, N.; Rangelov, S.; Forys, A.; Trzebicka, B.; et al. Development, Characterization and Pharmacological Evaluation of Cannabidiol-Loaded Long Circulating Niosomes. Pharmaceutics 2023, 15, 2414. https://doi.org/10.3390/pharmaceutics15102414
Gugleva V, Ahchiyska K, Georgieva D, Mihaylova R, Konstantinov S, Dimitrov E, Toncheva-Moncheva N, Rangelov S, Forys A, Trzebicka B, et al. Development, Characterization and Pharmacological Evaluation of Cannabidiol-Loaded Long Circulating Niosomes. Pharmaceutics. 2023; 15(10):2414. https://doi.org/10.3390/pharmaceutics15102414
Chicago/Turabian StyleGugleva, Viliana, Katerina Ahchiyska, Dilyana Georgieva, Rositsa Mihaylova, Spiro Konstantinov, Erik Dimitrov, Natalia Toncheva-Moncheva, Stanislav Rangelov, Aleksander Forys, Barbara Trzebicka, and et al. 2023. "Development, Characterization and Pharmacological Evaluation of Cannabidiol-Loaded Long Circulating Niosomes" Pharmaceutics 15, no. 10: 2414. https://doi.org/10.3390/pharmaceutics15102414
APA StyleGugleva, V., Ahchiyska, K., Georgieva, D., Mihaylova, R., Konstantinov, S., Dimitrov, E., Toncheva-Moncheva, N., Rangelov, S., Forys, A., Trzebicka, B., & Momekova, D. (2023). Development, Characterization and Pharmacological Evaluation of Cannabidiol-Loaded Long Circulating Niosomes. Pharmaceutics, 15(10), 2414. https://doi.org/10.3390/pharmaceutics15102414