Resurgence and Repurposing of Antifungal Azoles by Transition Metal Coordination for Drug Discovery
Abstract
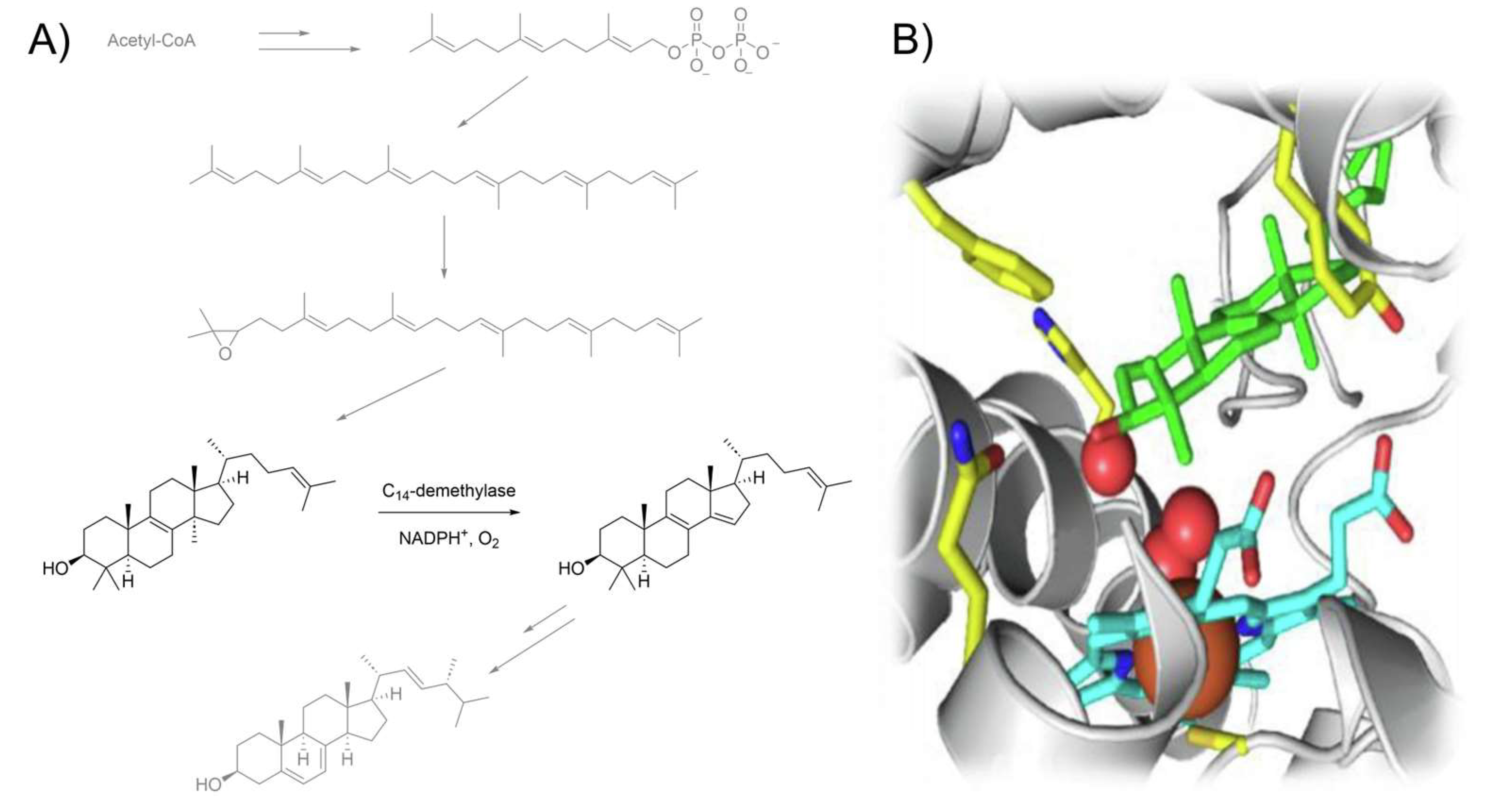
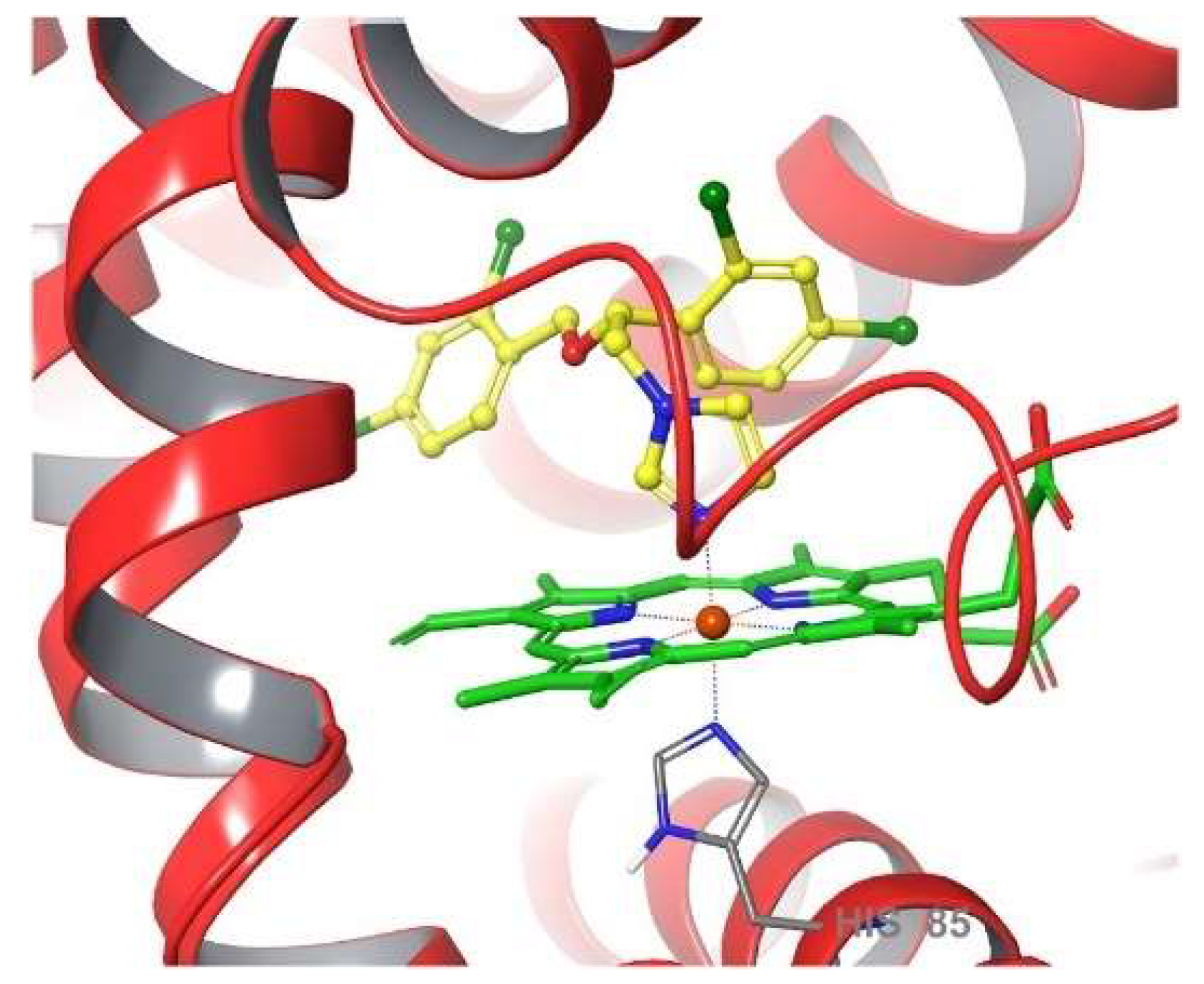
1. Introduction
2. Antimicrobial and Anticancer Activity of Transition Metal Coordination Compounds Bearing AAs
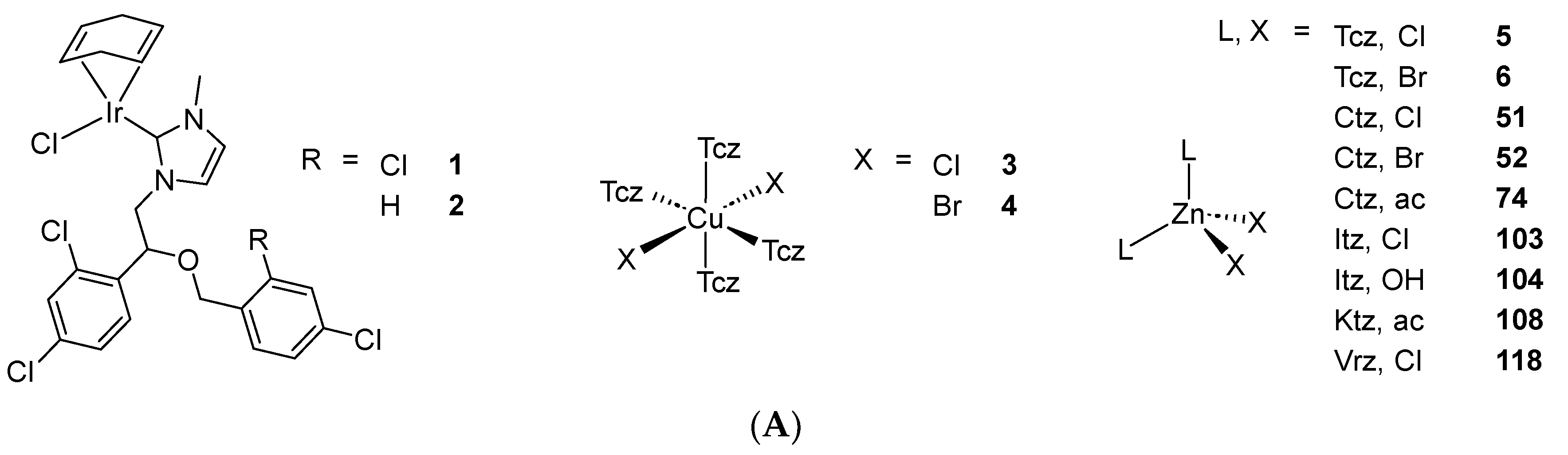
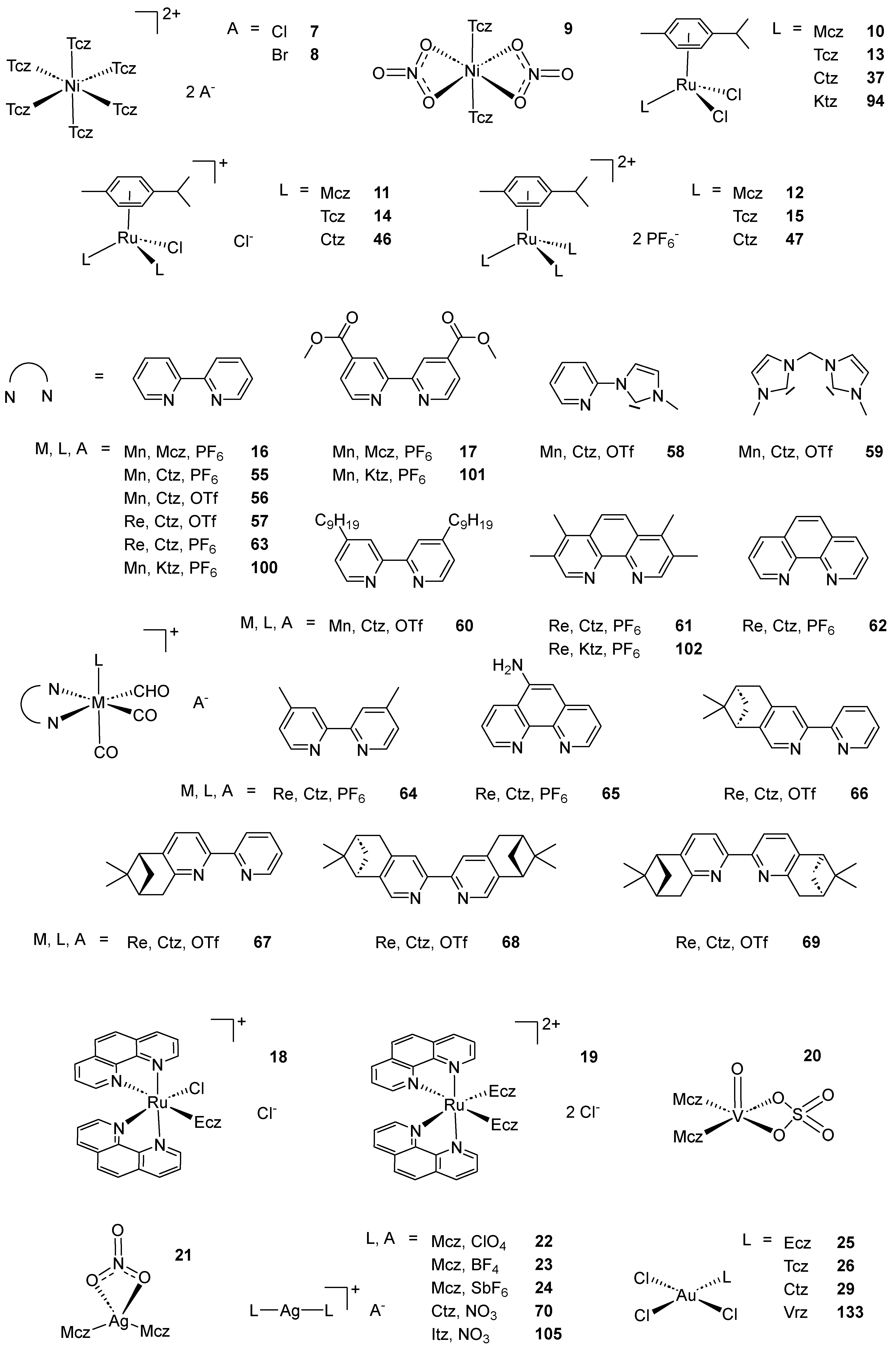
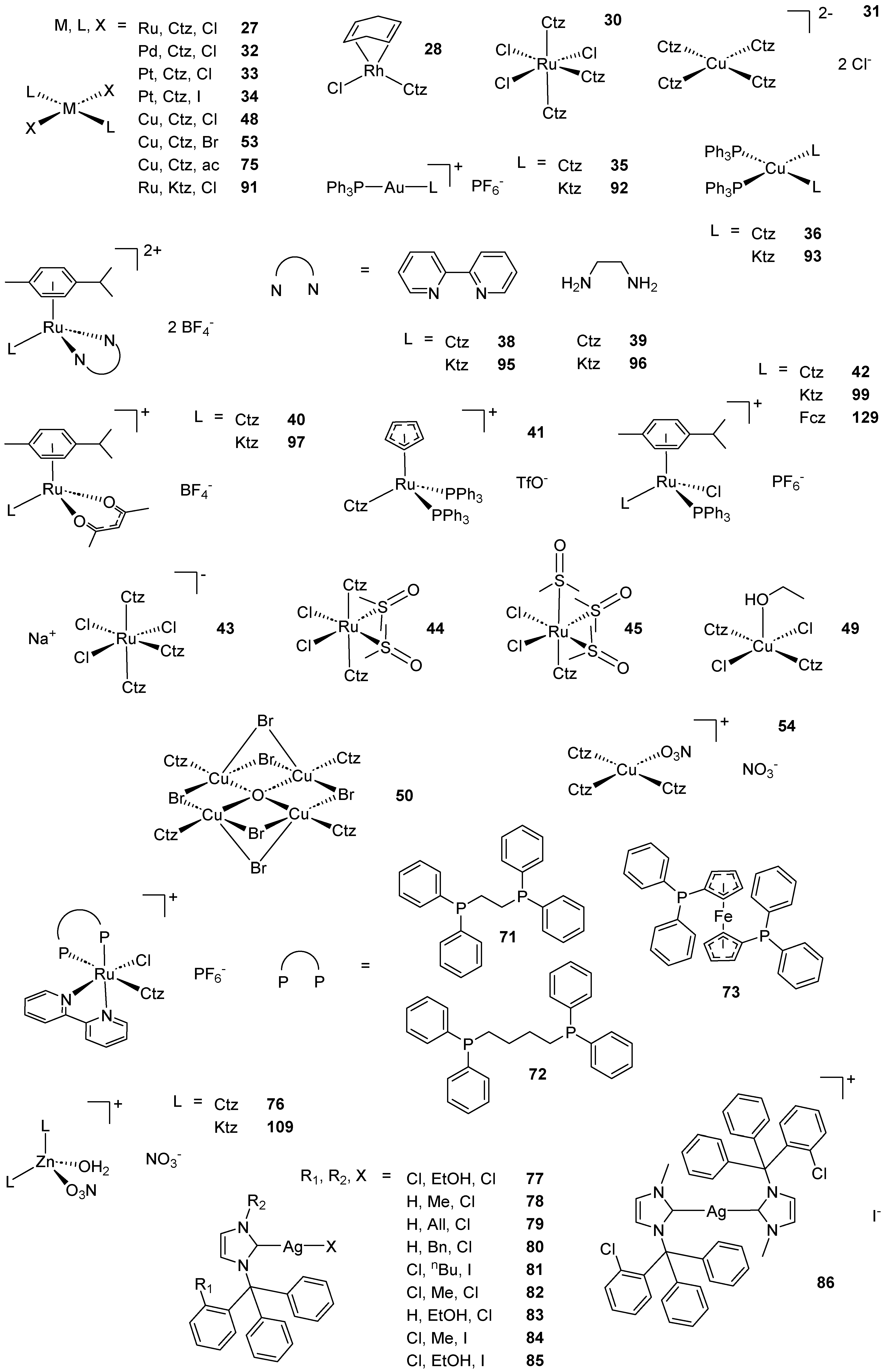
2.1. Coordination Compounds of the Mcz Family of AAs
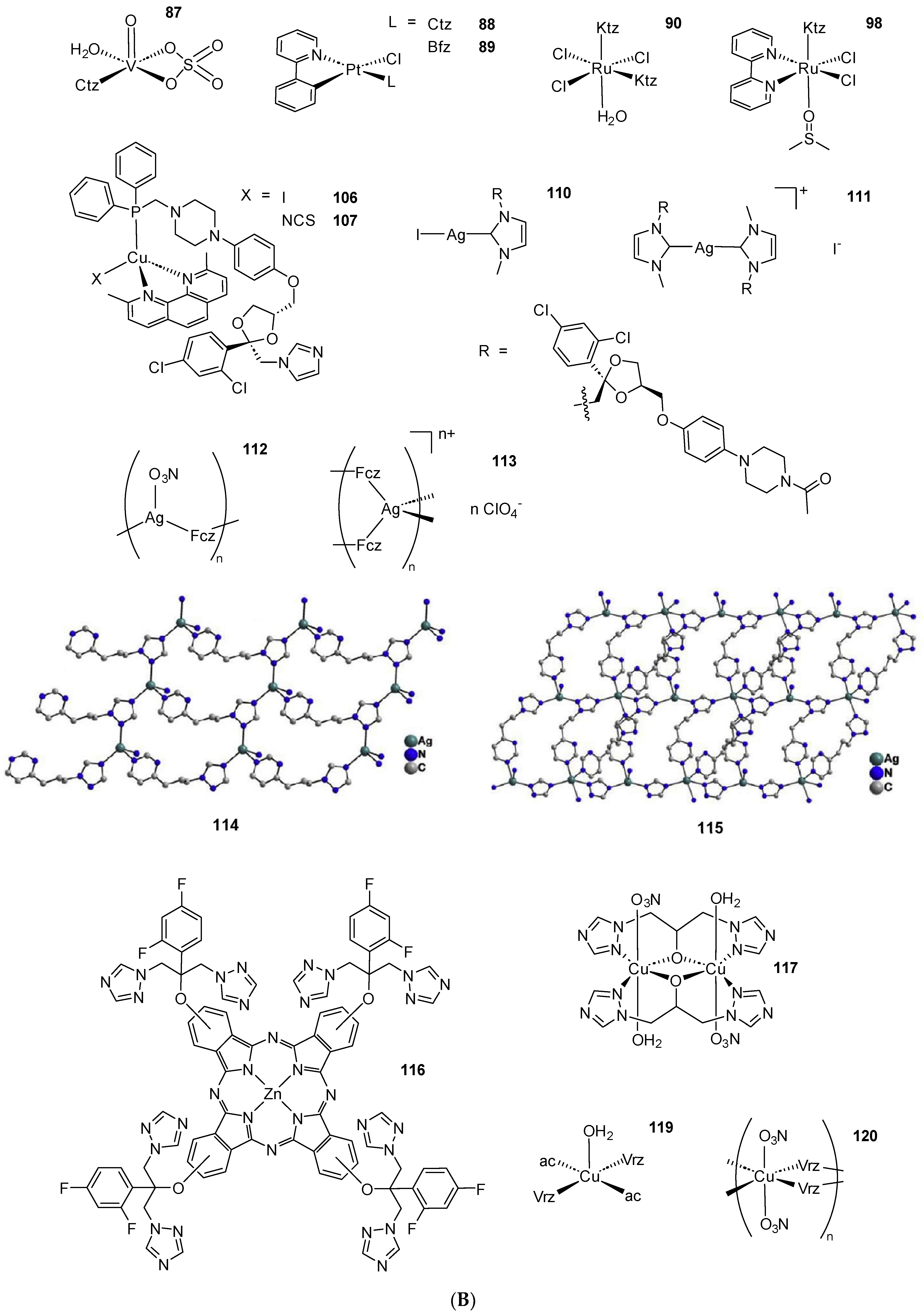
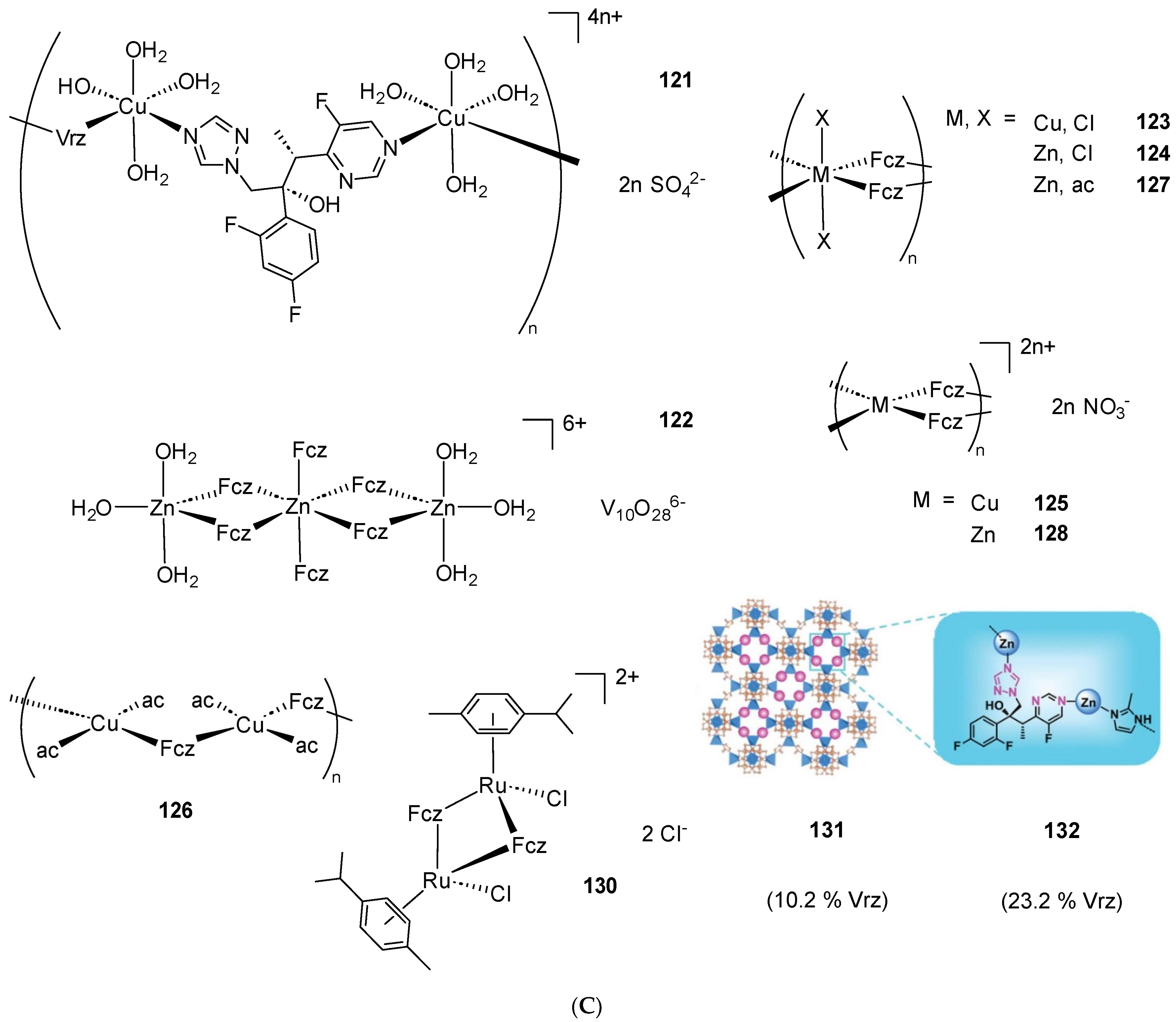
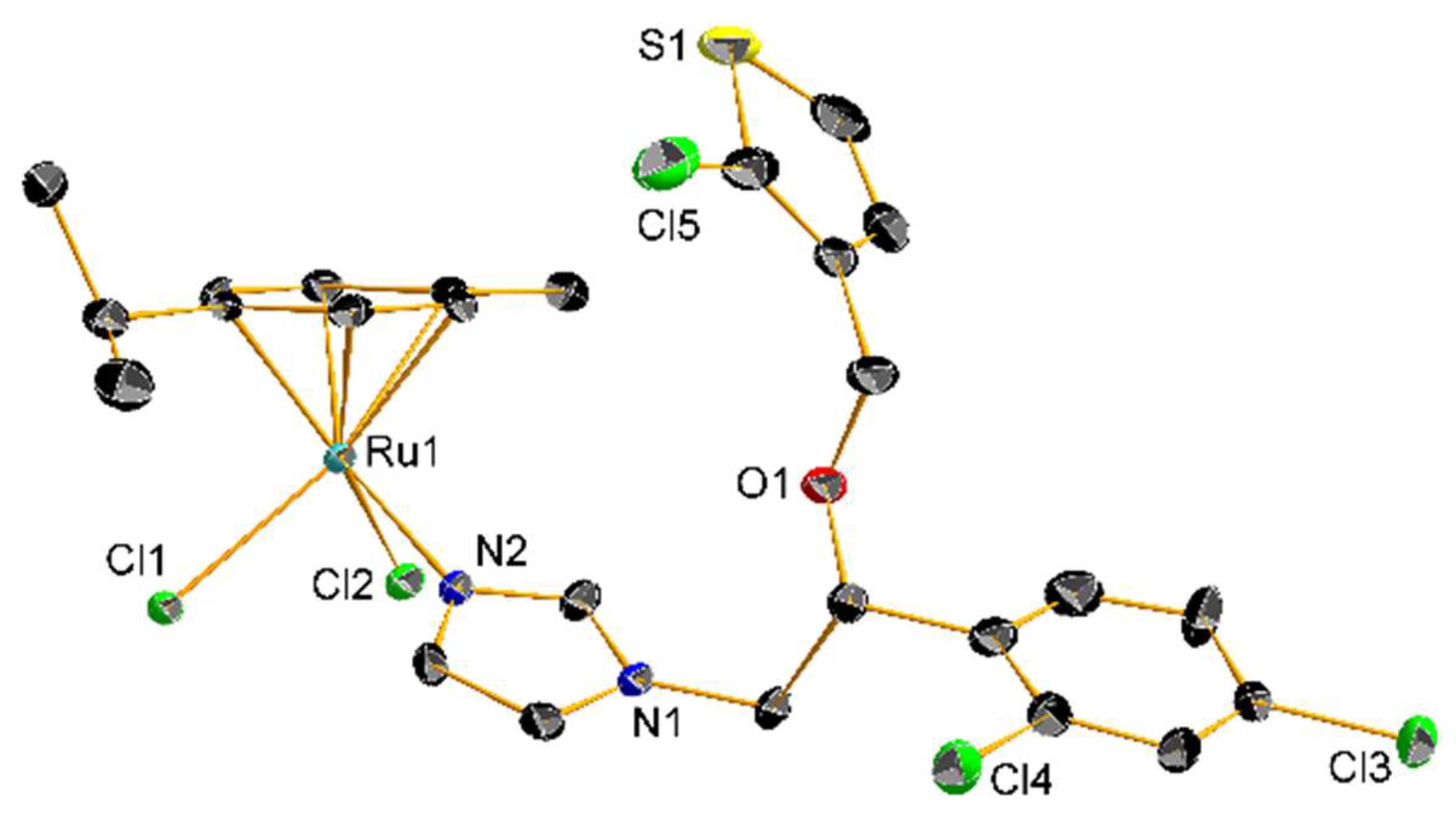
2.2. Coordination Compounds of the Ctz Family of AAs
2.3. Coordination Compounds of the Ktz Family of AAs
2.4. Coordination Compounds of the Fcz Family of AAs
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for all; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016; p. 80. [Google Scholar]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50–S52. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Yuzo, Y.; Yuri, A. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochem. Pharmacol. 1987, 36, 229–235. [Google Scholar] [CrossRef]
- Yoshida, Y. Cytochrome P450 of Fungi: Primary Target for Azole Antifungal Agents. In Current Topics in Medical Mycology; McGinnis, M.R., Ed.; Springer: New York, NY, USA, 1988; pp. 388–418. [Google Scholar]
- Aoyama, Y.; Yoshida, Y.; Sonoda, Y.; Sato, Y. Deformylation of 32-oxo-24,25-dihydrolanosterol by the purified cytochrome P-45014DM (lanosterol 14 α-demethylase) from yeast evidence confirming the intermediate step of lanosterol 14 α-demethylation. J. Biol. Chem. 1989, 264, 18502–18505. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Horne, T.; Hollomon, D.W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol. Lett. 1997, 149, 141–149. [Google Scholar] [CrossRef]
- White Theodore, C.; Marr Kieren, A.; Bowden Raleigh, A. Clinical, Cellular, and Molecular Factors That Contribute to Antifungal Drug Resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Mast, N.; Zheng, W.; Stout, C.D.; Pikuleva, I.A. Antifungal Azoles: Structural Insights into Undesired Tight Binding to Cholesterol-Metabolizing CYP46A1. Mol. Pharmacol. 2013, 84, 86. [Google Scholar] [CrossRef]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 2020, 104, 104240. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Kowalczyk, W. Azole Antimycotics—A Highway to New Drugs or a Dead End? Curr. Med. Chem. 2012, 19, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Podust, L.M.; Roush, W.R. Drug Strategies Targeting CYP51 in Neglected Tropical Diseases. Chem. Rev. 2014, 114, 11242–11271. [Google Scholar] [CrossRef]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef]
- Alsterholm, M.; Karami, N.; Faergemann, J. Antimicrobial activity of topical skin pharmaceuticals—An in vitro study. Acta Derm. Venereol. 2010, 90, 239–245. [Google Scholar] [CrossRef]
- Helmick Ryan, A.; Fletcher Arin, E.; Gardner Anne, M.; Gessner Christopher, R.; Hvitved Angela, N.; Gustin Michael, C.; Gardner Paul, R. Imidazole Antibiotics Inhibit the Nitric Oxide Dioxygenase Function of Microbial Flavohemoglobin. Antimicrob. Agents Chemother. 2005, 49, 1837–1843. [Google Scholar] [CrossRef]
- Sari, S.; Avci, A.; Koçak, E.; Kart, D.; Sabuncuoğlu, S.; Doğan, İ.S.; Özdemir, Z.; Bozbey, İ.; Karakurt, A.; Saraç, S.; et al. Antibacterial azole derivatives: Antibacterial activity, cytotoxicity, and in silico mechanistic studies. Drug Dev. Res. 2020, 81, 1026–1036. [Google Scholar] [CrossRef]
- Amery, W.K.; De Coster, R.; Caers, I. Ketoconazole: From an antimycotic to a drug for prostate cancer. Drug Dev. Res. 1986, 8, 299–307. [Google Scholar] [CrossRef]
- Weng, N.; Zhang, Z.; Tan, Y.; Zhang, X.; Wei, X.; Zhu, Q. Repurposing antifungal drugs for cancer therapy. J. Adv. Res. 2022, 48, 259–273. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Ueda, T.; Inoue, K.; Sakata, K.; Shibahara, H.; Sonoda, T. Repurposing itraconazole as an anticancer agent (Review). Oncol. Lett. 2017, 14, 1240–1246. [Google Scholar] [CrossRef]
- Penso, J.; Beitner, R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur. J. Pharmacol. 1998, 342, 113–117. [Google Scholar] [CrossRef]
- Sebastian, J.; Rathinasamy, K. Cytotoxic mechanism of tioconazole involves cell cycle arrest at mitosis through inhibition of microtubule assembly. Cytotechnology 2022, 74, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Unadkat, J.D.; Mao, Q. Interactions of azole antifungal agents with the human breast cancer resistance protein (BCRP). J. Pharm. Sci. 2007, 96, 3226–3235. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Rex, J.H.; Sobel, J.D. Prophylactic Antifungal Therapy in the Intensive Care Unit. Clin. Infect. Dis. 2001, 32, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Teixeira-Santos, R.; Silva, A.P.; Cruz, L.; Ricardo, E.; Pina-Vaz, C.; Rodrigues, A.G. The effect of antibacterial and non-antibacterial compounds alone or associated with antifugals upon fungi. Front. Microbiol. 2015, 6, 669. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Mamak, T.; Hadiseh, H.; Shirin, F.; Masoud, P.; Mohammadreza, S.; Mahsa, A. Antibiotic Treatment in End Stage Cancer Patients; Advantages and Disadvantages. Cancer Inform. 2023, 22, 11769351231161476. [Google Scholar] [CrossRef] [PubMed]
- Chellan, P.; Sadler, P.J. Enhancing the Activity of Drugs by Conjugation to Organometallic Fragments. Chem. Eur. J. 2020, 26, 8676–8688. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Ferraro, G.; Merlino, A. Metallodrugs: Mechanisms of Action, Molecular Targets and Biological Activity. Int. J. Mol. Sci. 2022, 23, 3504. [Google Scholar] [CrossRef]
- Farrell, N. Transition Metal Complexes as Drugs and Chemotherapeutic Agents; Springer: Berlin/Heidelberg, Germany, 1989; Volume 11. [Google Scholar]
- Sarachek, A.; Henderson, L.A. Modification of responses of Candida albicans to cisplatin by membrane damaging antimycotic agents. Mycoses 1991, 34, 177–182. [Google Scholar] [CrossRef]
- Minn, Y.; Brummer, E.; Stevens, D.A. Effect of iron on fluconazole activity against Candida albicans in presence of human serum or monocyte-derived macrophages. Mycopathologia 1997, 138, 29–35. [Google Scholar] [CrossRef]
- Uthman, A.; Rezaie, S.; Dockal, M.; Ban, J.; Söltz-Szöts, J.; Tschachler, E. Fluconazole downregulates metallothionein expression and increases copper cytotoxicity in Microsporum canis. Biochem. Biophys. Res. Commun. 2002, 299, 688–692. [Google Scholar] [CrossRef]
- Eshwika, A.; Coyle, B.; Devereux, M.; McCann, M.; Kavanagh, K. Metal complexes of 1,10-phenanthroline-5,6-dione alter the susceptibility of the yeast Candida albicans to Amphotericin B and Miconazole. BioMetals 2004, 17, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.M.; Oyama, T.B.; Oyama, K.; Sakanashi, Y.; Morimoto, M.; Matsui, H.; Oyama, Y. Clotrimazole, an antifungal drug possessing diverse actions, increases membrane permeation of cadmium in rat thymocytes. Toxicol. Vitr. 2007, 21, 1505–1512. [Google Scholar] [CrossRef]
- Oyama, Y.; Matsui, H.; Morimoto, M.; Sakanashi, Y.; Nishimura, Y.; Ishida, S.; Okano, Y. Synergic cytotoxic action induced by simultaneous application of zinc and clotrimazole in rat thymocytes. Toxicol. Lett. 2007, 171, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Sakanashi, Y.; Oyama, T.M.; Oyama, Y.; Yokota, S.-i.; Ishida, S.; Okano, Y.; Oyama, T.B.; Nishimura, Y. Imidazole antifungals, but not triazole antifungals, increase membrane Zn2+ permeability in rat thymocytes: Possible contribution to their cytotoxicity. Toxicology 2008, 248, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kinazaki, A.; Sakanashi, Y.; Oyama, T.M.; Shibagaki, H.; Yamashita, K.; Hashimoto, E.; Nishimura, Y.; Ishida, S.; Okano, Y.; Oyama, Y. Micromolar Zn2+ potentiates the cytotoxic action of submicromolar econazole in rat thymocytes: Possible disturbance of intracellular Ca2+ and Zn2+ homeostasis. Toxicol. Vitr. 2009, 23, 610–616. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Franz, K.J. Copper potentiates azole antifungal activity in a way that does not involve complex formation. Dalton Trans. 2019, 48, 9654–9662. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Franz, K.J. Candida albicans reprioritizes metal handling during fluconazole stress. Metallomics 2019, 11, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- da Silva Hellwig, A.H.; Pagani, D.M.; Rios, I.d.S.; Ribeiro, A.C.; Zanette, R.A.; Scroferneker, M.L. Influence of iron on growth and on susceptibility to itraconazole in Sporothrix spp. Med. Mycol. 2021, 59, 400–403. [Google Scholar] [CrossRef]
- Gaspar-Cordeiro, A.; Amaral, C.; Pobre, V.; Antunes, W.; Petronilho, A.; Paixão, P.; Matos, A.P.; Pimentel, C. Copper Acts Synergistically with Fluconazole in Candida glabrata by Compromising Drug Efflux, Sterol Metabolism, and Zinc Homeostasis. bioRxiv 2021, 13, 920574. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Yu, C.-H.A.; Franz, K.J. Copper Availability Influences the Transcriptomic Response of Candida albicans to Fluconazole Stress. G3 Genes Genomes Genet. 2021, 11, jkab065. [Google Scholar] [CrossRef]
- Alapi, E.M.; Fischer, J. Table of Selected Analogue Classes. In Analogue-Based Drug Discovery; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 441–552. [Google Scholar]
- Davis, J.H.; Lake, C.M.; Bernard, M.A. Azolidene Carbenes Derived from Biologically Relevant Molecules. 1 Synthesis and Characterization of Iridium Complexes of Imidazolidene Ligands Based upon the Antifungal Drugs Econazole and Miconazole. Inorg. Chem. 1998, 37, 5412–5413. [Google Scholar] [CrossRef]
- Ou, Y.-H.; Du, R.-K.; Zhang, S.-P.; Ling, Y.; Li, S.; Zhao, C.-J.; Zhang, W.-Z.; Zhang, L. Synthesis, crystal structure and in vitro antifungal activity of two-dimensional silver(I)-voriconazole coordination complexes. J. Mol. Struct. 2020, 1215, 128229. [Google Scholar] [CrossRef]
- Su, L.; Li, Y.; Liu, Y.; Ma, R.; Liu, Y.; Huang, F.; An, Y.; Ren, Y.; van der Mei, H.C.; Busscher, H.J.; et al. Antifungal-Inbuilt Metal–Organic-Frameworks Eradicate Candida albicans Biofilms. Adv. Funct. Mater. 2020, 30, 2000537. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Nour El-Dien, F.A.; Mohamed, G.G.; Mohamed, N.A. Synthesis, spectroscopic, thermal characterization, and antimicrobial activity of miconazole drug and its metal complexes. J. Therm. Anal. Calorim. 2012, 109, 883–892. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Nour El-Dien, F.A.; Mohamed, G.G.; Mohamed, N.A. Chelating behavior, thermal studies and biocidal efficiency of tioconazole and its complexes with some transition metal ions. J. Therm. Anal. Calorim. 2013, 111, 173–181. [Google Scholar] [CrossRef]
- Crisóstomo-Lucas, C.; García-Holley, P.; Hernández-Ortega, S.; Sánchez-Bartéz, F.; Gracia-Mora, I.; Barba-Behrens, N. Structural characterization and cytotoxic activity of tioconazole coordination compounds with cobalt(II), copper(II), zinc(II) and cadmium(II). Inorg. Chim. Acta 2015, 438, 245–254. [Google Scholar] [CrossRef]
- Crisostomo-Lucas, C.; Navarro-Peñaloza, R.; Ortiz-Pastrana, N.; Sanchez-Bartez, F.; Gracia-Mora, I.; Barba-Behrens, N. Synthesis, Characterization and Cytotoxic Activity of Tioconazole Coordination Compounds with Nickel(II), Palladium(II) and Platinum(II). J. Mex. Chem. Soc. 2018, 62, 225–237. [Google Scholar]
- Karaoun, N.; Renfrew, A.K. A luminescent ruthenium(ii) complex for light-triggered drug release and live cell imaging. Chem. Comm. 2015, 51, 14038–14041. [Google Scholar] [CrossRef]
- Basaleh, A.S.; Alomari, F.Y.; Sharfalddin, A.A.; Al-Radadi, N.S.; Domyati, D.; Hussien, M.A. Theoretical Investigation by DFT and Molecular Docking of Synthesized Oxidovanadium(IV)-Based Imidazole Drug Complexes as Promising Anticancer Agents. Molecules 2022, 27, 2796. [Google Scholar] [CrossRef] [PubMed]
- Stryjska, K.; Radko, L.; Chęcińska, L.; Kusz, J.; Posyniak, A.; Ochocki, J. Synthesis, Spectroscopy, Light Stability, Single-Crystal Analysis, and In Vitro Cytotoxic Activity on HepG2 Liver Cancer of Two Novel Silver(I) Complexes of Miconazole. Int. J. Mol. Sci. 2020, 21, 3629. [Google Scholar] [CrossRef] [PubMed]
- Stryjska, K.; Korona-Glowniak, I.; Chęcińska, L.; Kusz, J.; Ochocki, J. Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug. Int. J. Mol. Sci. 2021, 22, 1510. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Martínez, A.; Varela-Ramirez, A.; Sánchez-Delgado, R.A.; Aguilera, R.J. Analysis of the cytotoxic effects of ruthenium–ketoconazole and ruthenium–clotrimazole complexes on cancer cells. Cell Biol. Toxicol. 2013, 29, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Arce, E.; Sarniguet, C.; Moraes, T.S.; Vieites, M.; Tomaz, A.I.; Medeiros, A.; Comini, M.A.; Varela, J.; Cerecetto, H.; González, M.; et al. A new ruthenium cyclopentadienyl azole compound with activity on tumor cell lines and trypanosomatid parasites. J. Coord. Chem. 2015, 68, 2923–2937. [Google Scholar] [CrossRef]
- Colina-Vegas, L.; Oliveira, K.M.; Cunha, B.N.; Cominetti, M.R.; Navarro, M.; Azevedo Batista, A. Anti-Proliferative and Anti-Migration Activity of Arene–Ruthenium(II) Complexes with Azole Therapeutic Agents. Inorganics 2018, 6, 132. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Gómez-Ruiz, C.; Barrón-Sosa, L.R.; Gracia-Mora, I.; Flores-Álamo, M.; Barba-Behrens, N. Cytotoxic copper(II), cobalt(II), zinc(II), and nickel(II) coordination compounds of clotrimazole. J. Inorg. Biochem. 2012, 114, 82–93. [Google Scholar] [CrossRef]
- Colina-Vegas, L.; Dutra, J.L.; Villarreal, W.; de Neto, J.H.A.; Cominetti, M.R.; Pavan, F.; Navarro, M.; Batista, A.A. Ru(II)/clotrimazole/diphenylphosphine/bipyridine complexes: Interaction with DNA, BSA and biological potential against tumor cell lines and Mycobacterium tuberculosis. J. Inorg. Biochem. 2016, 162, 135–145. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Shepherd, S.; William, N.; Blundell, H.A.; Das, M.; Pask, C.M.; Lake, B.R.M.; Phillips, R.M.; Nelson, A.; Willans, C.E. Silver(I) N-Heterocyclic Carbene Complexes Derived from Clotrimazole: Antiproliferative Activity and Interaction with an Artificial Membrane-Based Biosensor. Organometallics 2020, 39, 1318–1331. [Google Scholar] [CrossRef]
- da Silva dos Reis Condé, C.A.; de Andrade Querino, A.L.; Silva, H.; Navarro, M. Silver(I) complexes containing N-heterocyclic carbene azole drugs: Synthesis, characterization, cytotoxic activity, and their BSA interactions. J. Inorg. Biochem. 2023, 246, 112303. [Google Scholar] [CrossRef]
- Fernández-Pampín, N.; Vaquero, M.; Gil, T.; Espino, G.; Fernández, D.; García, B.; Busto, N. Distinct mechanism of action for antitumoral neutral cyclometalated Pt(II)-complexes bearing antifungal imidazolyl-based drugs. J. Inorg. Biochem. 2022, 226, 111663. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Lei, Z.N.; Ali, M.; Kojima, K.; Ahmed, M.; Peng, R.; Yang, D.H.; Haider, S.M.; Ayatollahi, S.A.; Chen, Z.S. Metal (II) Complexes of Fluconazole: Thermal, XRD and Cytotoxicity Studies. Iran J. Pharm. Res. 2020, 19, 171–182. [Google Scholar] [CrossRef]
- BecİT, M.; Aydin, S.; BaŞAran, N. Effects of Pycnogenol and Its Combinations with Cisplatin on Hepatocellular Carcinoma Cell Viability. Fabad J. Pharm. Sci. 2021, 46, 13–22. [Google Scholar]
- Sharfalddin, A.A.; Emwas, A.-H.; Jaremko, M.; Hussien, M.A. Synthesis and theoretical calculations of metal-antibiotic chelation with thiamphenicol: In vitro DNA and HSA binding, molecular docking, and cytotoxicity studies. New J. Chem. 2021, 45, 9598–9613. [Google Scholar] [CrossRef]
- Patra, M.; Joshi, T.; Pierroz, V.; Ingram, K.; Kaiser, M.; Ferrari, S.; Spingler, B.; Keiser, J.; Gasser, G. DMSO-Mediated Ligand Dissociation: Renaissance for Biological Activity of N-Heterocyclic-[Ru(η6-arene)Cl2] Drug Candidates. Chem. Eur. J. 2013, 19, 14768–14772. [Google Scholar] [CrossRef] [PubMed]
- Kljun, J.; Scott, A.J.; Lanišnik Rižner, T.; Keiser, J.; Turel, I. Synthesis and Biological Evaluation of Organoruthenium Complexes with Azole Antifungal Agents. First Crystal Structure of a Tioconazole Metal Complex. Organometallics 2014, 33, 1594–1601. [Google Scholar] [CrossRef]
- Simpson, P.V.; Nagel, C.; Bruhn, H.; Schatzschneider, U. Antibacterial and Antiparasitic Activity of Manganese(I) Tricarbonyl Complexes with Ketoconazole, Miconazole, and Clotrimazole Ligands. Organometallics 2015, 34, 3809–3815. [Google Scholar] [CrossRef]
- Stevanović, N.L.; Kljun, J.; Aleksic, I.; Bogojevic, S.S.; Milivojevic, D.; Veselinovic, A.; Turel, I.; Djuran, M.I.; Nikodinovic-Runic, J.; Glišić, B.Đ. Clinically used antifungal azoles as ligands for gold(iii) complexes: The influence of the Au(iii) ion on the antimicrobial activity of the complex. Dalton Trans. 2022, 51, 5322–5334. [Google Scholar] [CrossRef]
- Mendes, S.S.; Marques, J.; Mesterházy, E.; Straetener, J.; Arts, M.; Pissarro, T.; Reginold, J.; Berscheid, A.; Bornikoel, J.; Kluj, R.M.; et al. Synergetic Antimicrobial Activity and Mechanism of Clotrimazole-Linked CO-Releasing Molecules. ACS Bio. Med. Chem. Au 2022, 2, 419–436. [Google Scholar] [CrossRef]
- Cortat, Y.; Nedyalkova, M.; Schindler, K.; Kadakia, P.; Demirci, G.; Nasiri Sovari, S.; Crochet, A.; Salentinig, S.; Lattuada, M.; Steiner, O.M.; et al. Computer-Aided Drug Design and Synthesis of Rhenium Clotrimazole Antimicrobial Agents. Antibiotics 2023, 12, 619. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B 2015, 136, 651–658. [Google Scholar] [CrossRef]
- Sanchez-Delgado, R.A.; Lazardi, K.; Rincon, L.; Urbina, J.A.; Hubert, A.J.; Noels, A.N. Toward a novel metal-based chemotherapy against tropical diseases. 1. Enhancement of the efficacy of clotrimazole against Trypanosoma cruzi by complexation to ruthenium in RuCl2(clotrimazole)2. J. Med. Chem. 1993, 36, 2041–2043. [Google Scholar] [CrossRef]
- Sánchez-Delgado, R.A.; Navarro, M.; Lazardi, K.; Atencio, R.; Capparelli, M.; Vargas, F.; Urbina, J.A.; Bouillez, A.; Noels, A.F.; Masi, D. Toward a novel metal based chemotherapy against tropical diseases 4. Synthesis and characterization of new metal-clotrimazole complexes and evaluation of their activity against Trypanosoma cruzi. Inorg. Chim. Acta 1998, 275-276, 528–540. [Google Scholar] [CrossRef]
- Martínez, A.; Carreon, T.; Iniguez, E.; Anzellotti, A.; Sánchez, A.; Tyan, M.; Sattler, A.; Herrera, L.; Maldonado, R.A.; Sánchez-Delgado, R.A. Searching for New Chemotherapies for Tropical Diseases: Ruthenium–Clotrimazole Complexes Display High in Vitro Activity against Leishmania major and Trypanosoma cruzi and Low Toxicity toward Normal Mammalian Cells. J. Med. Chem. 2012, 55, 3867–3877. [Google Scholar] [CrossRef]
- Colina-Vegas, L.; Lima Prado Godinho, J.; Coutinho, T.; Correa, R.S.; de Souza, W.; Cola Fernandes Rodrigues, J.; Batista, A.A.; Navarro, M. Antiparasitic activity and ultrastructural alterations provoked by organoruthenium complexes against Leishmania amazonensis. New J. Chem. 2019, 43, 1431–1439. [Google Scholar] [CrossRef]
- Soba, M.; Scalese, G.; Pérez-Díaz, L.; Gambino, D.; Machado, I. Application of microwave plasma atomic emission spectrometry in bioanalytical chemistry of bioactive rhenium compounds. Talanta 2022, 244, 123413. [Google Scholar] [CrossRef] [PubMed]
- Soba, M.; Scalese, G.; Casuriaga, F.; Pérez, N.; Veiga, N.; Echeverría, G.A.; Piro, O.E.; Faccio, R.; Pérez-Díaz, L.; Gasser, G.; et al. Multifunctional organometallic compounds for the treatment of Chagas disease: Re(i) tricarbonyl compounds with two different bioactive ligands. Dalton Trans. 2023, 52, 1623–1641. [Google Scholar] [CrossRef]
- Midlej, V.; Rubim, F.; Villarreal, W.; Martins-Duarte, É.S.; Navarro, M.; de Souza, W.; Benchimol, M. Zinc-clotrimazole complexes are effective against Trichomonas vaginalis. Parasitology 2019, 146, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, E.; Sánchez, A.; Vasquez, M.A.; Martínez, A.; Olivas, J.; Sattler, A.; Sánchez-Delgado, R.A.; Maldonado, R.A. Metal–drug synergy: New ruthenium(II) complexes of ketoconazole are highly active against Leishmania major and Trypanosoma cruzi and nontoxic to human or murine normal cells. J. Biol. Inorg. Chem. 2013, 18, 779–790. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo-França, J.A.; Granado, R.; Silva, S.T.d.M.; dos Santos-Silva, G.; Scapin, S.; Borba-Santos, L.P.; Rozental, S.; de Souza, W.; Martins-Duarte Érica, S.; Barrias, E.; et al. Synthesis and Biological Activity of Novel Zinc-Itraconazole Complexes in Protozoan Parasites and Sporothrix spp. Antimicrob. Agents Chemother. 2020, 64, e01919–e01980. [Google Scholar] [CrossRef]
- de Azevedo-França, J.A.; Barrias, E.; Franco, C.H.J.; Villarreal, W.; Vieira, E.G.; Ferreira, A.M.D.C.; de Souza, W.; Navarro, M. Promising fluconazole based zinc(II) and copper(II) coordination polymers against Chagas disease. J. Inorg. Biochem. 2022, 233, 111834. [Google Scholar] [CrossRef]
- Aziz, S.G.; Elroby, S.A.; Jedidi, A.; Babgi, B.A.; Alshehri, N.S.; Hussien, M.A. Synthesis, characterization, computational study, DNA binding and molecular docking studies of chromium (III) drug-based complexes. J. Mol. Struct. 2020, 1215, 128283. [Google Scholar] [CrossRef]
- Gagini, T.; Colina-Vegas, L.; Villarreal, W.; Borba-Santos, L.P.; de Souza Pereira, C.; Batista, A.A.; Kneip Fleury, M.; de Souza, W.; Rozental, S.; Costa, L.A.S.; et al. Metal–azole fungistatic drug complexes as anti-Sporothrix spp. agents. New J. Chem. 2018, 42, 13641–13650. [Google Scholar] [CrossRef]
- de Azevedo-França, J.A.; Borba-Santos, L.P.; de Almeida Pimentel, G.; Franco, C.H.J.; Souza, C.; de Almeida Celestino, J.; de Menezes, E.F.; dos Santos, N.P.; Vieira, E.G.; Ferreira, A.M.D.C.; et al. Antifungal promising agents of zinc(II) and copper(II) derivatives based on azole drug. J. Inorg. Biochem. 2021, 219, 111401. [Google Scholar] [CrossRef]
- Stevanović, N.L.; Glišić, B.Đ.; Vojnovic, S.; Wadepohl, H.; Andrejević, T.P.; Đurić, S.Ž.; Savić, N.D.; Nikodinovic-Runic, J.; Djuran, M.I.; Pavic, A. Improvement of the anti-Candida activity of itraconazole in the zebrafish infection model by its coordination to silver(I). J. Mol. Struct. 2021, 1232, 130006. [Google Scholar] [CrossRef]
- Starosta, R.; de Almeida, R.F.M.; Puchalska, M.; Białońska, A.; Panek, J.J.; Jezierska, A.; Szmigiel, I.; Suchodolski, J.; Krasowska, A. New anticandidal Cu(i) complexes with neocuproine and ketoconazole derived diphenyl(aminomethyl)phosphane: Luminescence properties for detection in fungal cells. Dalton Trans. 2020, 49, 8528–8539. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, Y.; Du, M. Synthesis, crystal structures and in vitro anti-fungal activities of two silver(I) coordination polymers with fluconazole. Inorg. Chim. Acta 2007, 360, 3182–3188. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Tang, G.-M.; Wang, Y.-T.; Cui, Y.-Z.; Ng, S.W. Copper-based metal coordination complexes with Voriconazole ligand: Syntheses, structures and antimicrobial properties. J. Solid State Chem. 2018, 259, 19–27. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; Zhao, M.; Tian, R.; Zhang, B.; Qi, Y. In Vitro Anticandidal Activity and Mechanism of a Polyoxovanadate Functionalized by Zn-Fluconazole Complexes. Molecules 2018, 23, 1122. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, N.L.; Aleksic, I.; Kljun, J.; Skaro Bogojevic, S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B.Đ. Copper(II) and Zinc(II) Complexes with the Clinically Used Fluconazole: Comparison of Antifungal Activity and Therapeutic Potential. Pharmaceuticals 2021, 14, 24. [Google Scholar] [CrossRef]
- Newbould, B.B. The Future of Drug Discovery. In Trends and Changes in Drug Research and Development: Proceedings of the Society for Drug Research 20th Anniversary Meeting held at the Pharmaceutical Society of Great Britain, London, UK, 26 September 1986; Walker, B.C., Walker, S.R., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 105–114. [Google Scholar]
- Sánchez-Delgado, R.A.; Navarro, M.; Pérez, H.; Urbina, J.A. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases. 2. Synthesis and Antimalarial Activity in Vitro and in Vivo of New Ruthenium− and Rhodium−Chloroquine Complexes. J. Med. Chem. 1996, 39, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Pérez, H.; Sánchez-Delgado, R.A. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases. 3. Synthesis and Antimalarial Activity in Vitro and in Vivo of the New Gold−Chloroquine Complex [Au(PPh3)(CQ)]PF6. J. Med. Chem. 1997, 40, 1937–1939. [Google Scholar] [CrossRef]
- Navarro, M.; Lehmann, T.; Cisneros-Fajardo, E.J.; Fuentes, A.; Sánchez-Delgado, R.A.; Silva, P.; Urbina, J.A. Toward a novel metal-based chemotherapy against tropical diseases.: Part 5. Synthesis and characterization of new Ru(II) and Ru(III) clotrimazole and ketoconazole complexes and evaluation of their activity against Trypanosoma cruzi. Polyhedron 2000, 19, 2319–2325. [Google Scholar] [CrossRef]
- Navarro, M.; Cisneros-Fajardo, E.J.; Lehmann, T.; Sánchez-Delgado, R.A.; Atencio, R.; Silva, P.; Lira, R.; Urbina, J.A. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases. 6. Synthesis and Characterization of New Copper(II) and Gold(I) Clotrimazole and Ketoconazole Complexes and Evaluation of Their Activity against Trypanosoma cruzi. Inorg. Chem. 2001, 40, 6879–6884. [Google Scholar] [CrossRef]
- Navarro, M.; Vásquez, F.; Sánchez-Delgado, R.A.; Pérez, H.; Sinou, V.; Schrével, J. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases. 7. Synthesis and in Vitro Antimalarial Activity of New Gold−Chloroquine Complexes. J. Med. Chem. 2004, 47, 5204–5209. [Google Scholar] [CrossRef]
- Strasberg Rieber, M.; Anzellotti, A.; Sánchez-Delgado, R.A.; Rieber, M. Tumor apoptosis induced by ruthenium(II)-ketoconazole is enhanced in nonsusceptible carcinoma by monoclonal antibody to EGF receptor. Int. J. Cancer. 2004, 112, 376–384. [Google Scholar] [CrossRef]
- Navarro, M.; Colmenares, I.; Correia, H.; Hernández, A.; Ching, Y.; Millán, Y.; Ojeda, L.E.; Velásquez, M.; Fraile, G. In vitro Activities of Transition Metal Derivatives of Ketoconazole and Clotrimazole against a Wild Type Strain of Saccharomyces cerevisiae in Absence or Presence of Human Neutrophils. Arzneimittelforschung 2004, 54, 752–756. [Google Scholar] [CrossRef]
- Navarro, M.; Peña, N.P.; Colmenares, I.; González, T.; Arsenak, M.; Taylor, P. Synthesis and characterization of new palladium–clotrimazole and palladium–chloroquine complexes showing cytotoxicity for tumor cell lines in vitro. J. Inorg. Biochem. 2006, 100, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Higuera-Padilla, A.R.; Arsenak, M.; Taylor, P. Synthesis, characterization, DNA interaction studies and anticancer activity of platinum–clotrimazole complexes. Transit. Met. Chem. 2009, 34, 869–875. [Google Scholar] [CrossRef]
- Iniguez, E.; Varela-Ramirez, A.; Martínez, A.; Torres, C.L.; Sánchez-Delgado, R.A.; Maldonado, R.A. Ruthenium-Clotrimazole complex has significant efficacy in the murine model of cutaneous leishmaniasis. Acta Trop. 2016, 164, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Halim, H.F.; Nour El-Dien, F.A.; Mohamed, G.G.; Mohmed, N.A. Metalloantibiotics: Synthesis and Antimicrobial Activity of Clotrimazole Metal Chelates, Spectroscopic, and Thermal Characterization. Synth. React. Inorg. M. 2011, 41, 544–554. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Chmel, N.P.; Zimmerman, M.T.; Barrón-Sosa, L.R.; Garino, C.; Salassa, L.; Rodger, A.; Brumaghim, J.L.; Gracia-Mora, I.; Barba-Behrens, N. Redox-active and DNA-binding coordination complexes of clotrimazole. Dalton Trans. 2015, 44, 3673–3685. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef]
- Heeres, J.; Backx, L.J.J.; Mostmans, J.H.; Van Cutsem, J. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J. Med. Chem. 1979, 22, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Sánchez-Delgado, R. Spectroscopic Study of the Interactions of Ruthenium-Ketoconazole Complexes of Known Antiparasitic Activity with Human Serum Albumin and Apotransferrin. J. Mex. Chem. Soc. 2013, 57, 169–174. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9, 16214. [Google Scholar] [CrossRef]
- Han, H.; Song, Y.; Hou, H.; Fan, Y.; Zhu, Y. A series of metal–organic polymers assembled from MCl2 (M = Zn, Cd, Co, Cu): Structures, third-order nonlinear optical and fluorescent properties. Dalton Trans. 2006, 16, 1972–1980. [Google Scholar] [CrossRef]
- Han, H.; Zhang, S.; Hou, H.; Fan, Y.; Zhu, Y. Fe(Cu)-Containing Coordination Polymers: Syntheses, Crystal Structures, and Applications as Benzyl Alcohol Oxidation Catalysts. Eur. J. Inorg. Chem. 2006, 2006, 1594–1600. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, C.W.; Xia, Z.N. Synthesis and characterization of metal(II)–fluconazole complexes: Chain-like structure and photoluminescence. J. Mol. Struct. 2007, 837, 48–57. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Hu, C.; Gao, W. Solvent-induced supramolecular isomers: Two dimensional coordination polymers constructed by Cu(II) and fluconazole. Inorg. Chem. Commun. 2007, 10, 575–579. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, T.; Tang, W.; Wu, F.; Gao, W.; Hu, C. Anion-directed assembly: Framework conversion in dimensionality and photoluminescence. J. Solid State Chem. 2007, 180, 1476–1488. [Google Scholar] [CrossRef]
- Li; Lan, Y.-Q.; Ma, J.-F.; Yang, J.; Wang, X.-H.; Su, Z.-M. Syntheses and Structures of Organic−Inorganic Hybrid Compounds Based on Metal−Fluconazole Coordination Polymers and the β-Mo8O26 Anion. Inorg. Chem. 2007, 46, 8283–8290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ling, Y.; Peng, F.; Du, M. Anion-tuned self-assembly of zinc(II)–fluconazole complexes: Crystal structures, luminescent and thermal properties. J. Mol. Struct. 2007, 829, 161–167. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Wang, Q.; Du, M. Metal-directed assembly of 1-D and 2-D coordination polymers with fluconazole and dicyanamide co-ligand. Inorg. Chim. Acta 2007, 360, 1970–1976. [Google Scholar] [CrossRef]
- Lan, Y.-Q.; Li, S.-L.; Shao, K.-Z.; Wang, X.-L.; Su, Z.-M. Construction of different dimensional inorganic–organic hybrid materials based on polyoxometalates and metal–organic units via changing metal ions: From non-covalent interactions to covalent connections. Dalton Trans. 2008, 29, 3824–3835. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Lan, Y.-Q.; Ma, J.-F.; Yang, J.; Liu, J.; Fu, Y.-M.; Su, Z.-M. Inorganic–organic hybrid materials with different dimensions constructed from copper–fluconazole metal–organic units and Keggin polyanion clusters. Dalton Trans. 2008, 15, 2015–2025. [Google Scholar] [CrossRef]
- Zhang, E.; Hou, H.; Han, H.; Fan, Y. Syntheses, crystal structures of a series of copper(II) complexes and their catalytic activities in the green oxidative coupling of 2,6-dimethylphenol. J. Organomet. Chem. 2008, 693, 1927–1937. [Google Scholar] [CrossRef]
- Lan, Y.-Q.; Li, S.-L.; Shao, K.-Z.; Wang, X.-L.; Hao, X.-R.; Su, Z.-M. Combination of POMs and deliberately designed macrocations: A rational approach for synthesis of POM-pillared metal–organic framework. Dalton Trans. 2009, 6, 940–947. [Google Scholar] [CrossRef]
- Ling, Y.; Zhang, L.; Li, J.; Fan, S.-S.; Du, M. Thiocyanate-induced conformational transformation of a flexible fluconazole ligand in Cd(II) coordination polymers. CrystEngComm 2009, 12, 604–611. [Google Scholar] [CrossRef]
- Ling, Y.; Zhang, L.; Li, J.; Hu, A.X. Three-fold-Interpenetrated Diamondoid Coordination Frameworks with Torus Links Constructed by Tetranuclear Building Blocks. Cryst. Growth Des. 2009, 9, 2043–2046. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.-W.; Ge, Y.-Y.; Ling, Y.; Ouyang, X.-P.; Li, J.; Du, M. Anion-directed supramolecular assembly of cobalt(II)-fluconazole coordination polymers: Structural diversity, fluorescent and magnetic properties. Inorganica Chim. Acta 2010, 363, 866–876. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, Y.; Li, D.-J.; Li, J.; Du, M. Synthesis, crystal structures and properties of novel zinc(II) and cadmium(II) polymeric and cyclic bimetallic complexes with fluconazole and dicarboxylate co-ligands. Inorganica Chim. Acta 2010, 363, 1031–1038. [Google Scholar] [CrossRef]
- Pan, G.-H.; Tang, J.-N.; Xu, W.-J.; Liang, P.; Huang, Z.-J. Synthesis, Structure and Properties of Two Novel 2D Zinc(II) Coordination Polymers based on Fluconazole and Benzene Carboxylic Acid. Bull. Korean Chem. Soc. 2013, 34, 3867–3870. [Google Scholar] [CrossRef][Green Version]
- Pan, G.-H.; Tang, J.-N.; Yin, X.-H.; Tian, W.-M.; Huang, Z.-J. Synthesis, Structure and Properties of a 2D Cadmium(II) Coordination Polymer Based on Fluconazole and Isophthalate Ligands. Z. Naturforsch. B 2013, 68, 1333–1339. [Google Scholar] [CrossRef]
- Pan, G.-H.; Tang, J.-N.; Yin, X.-H.; Li, P.-F.; Huang, Z.-J. Synthesis, crystal structure, and properties of cobalt, zinc, and manganese coordination polymers based on fluconazole. J. Coord. Chem. 2014, 67, 1962–1979. [Google Scholar] [CrossRef]
- Pan, G.-H.; Xu, S.-H.; Xu, W.-J.; Liang, P.; Tian, W.-M.; Huang, Z.-J. Synthesis, Crystal Structure and Fluorescence Properties of Three 1-D Coordination Polymers Based on Fluconazole. J. Chem. Crystallogr. 2014, 44, 312–319. [Google Scholar] [CrossRef]
- Pan, G.-H.; Yin, X.-H.; Xu, W.-J.; Liang, P.; Tian, W.-M.; Huang, Z.-J. Synthesis, Crystal Structure, and Properties of Two Zinc Tubular Coordination Polymers Based on Fluconazole. Mol. Cryst. Liq. Cryst. 2014, 605, 155–164. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Lai, J.-C.; Fan, Z.-L.; Zhang, W.-Q.; Zhang, G.-F.; Cui, D.; Gao, Z.-W. Diversity of Coordination Modes, Structures, and Properties of Chiral Metal–Organic Coordination Complexes of the Drug Voriconazole. Eur. J. Inorg. Chem. 2015, 2015, 5281–5290. [Google Scholar] [CrossRef]
- Lin, C.J.; Zhou, L.X.; Niu, Q.J.; Zheng, Y.Q.; Zhu, H.L.; Zhang, B.B. Three metal-organic polymers assembled from Cd(II)–fluconazole: Syntheses, crystal structures, and characterization. J. Struct. Chem. 2016, 57, 155–166. [Google Scholar] [CrossRef]
- Lupetti, A.; Welling, M.M.; Mazzi, U.; Nibbering, P.H.; Pauwels, E.K. Technetium-99m labelled fluconazole and antimicrobial peptides for imaging of Candida albicans and Aspergillus fumigatus infections. Eur. J. Nucl. Med. Mol. Imaging. 2002, 29, 674–679. [Google Scholar] [CrossRef]
- Paula Cormick, M.; Rovera, M.; Durantini, E.N. Synthesis, spectroscopic properties and photodynamic activity of a novel Zn(II) phthalocyanine substituted by fluconazole groups. J. Photochem. Photobiol. A 2008, 194, 220–229. [Google Scholar] [CrossRef]
- Nagaj, J.; Starosta, R.; Szczepanik, W.; Barys, M.; Młynarz, P.; Jeżowska-Bojczuk, M. The Cu(II)-fluconazole complex revisited. Part I: Structural characteristics of the system. J. Inorg. Biochem. 2012, 106, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ząbek, A.; Nagaj, J.; Grabowiecka, A.; Dworniczek, E.; Nawrot, U.; Młynarz, P.; Jeżowska-Bojczuk, M. Activity of fluconazole and its Cu(II) complex towards Candida species. Med. Chem. Res. 2015, 24, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Tang, G.-M.; Wang, Y.-T.; Cui, Y.-Z. Synthesis, spectroscopic studies, antimicrobial activity, and crystal structure of a Zn(II) complex based on Voriconazole. J. Coord. Chem. 2017, 70, 189–200. [Google Scholar] [CrossRef]
- Ali, M.; Ahmed, M.; Ahmed, S.; Ali, S.; Perveen, S.; Mumtaz, M.; Haider, S.; Nazim, U. Fluconazole and its interaction with metal (II) complexes: SEM, Spectroscopic and antifungal studies. Pak. J. Pharm. Sci. 2017, 30, 187–194. [Google Scholar] [PubMed]

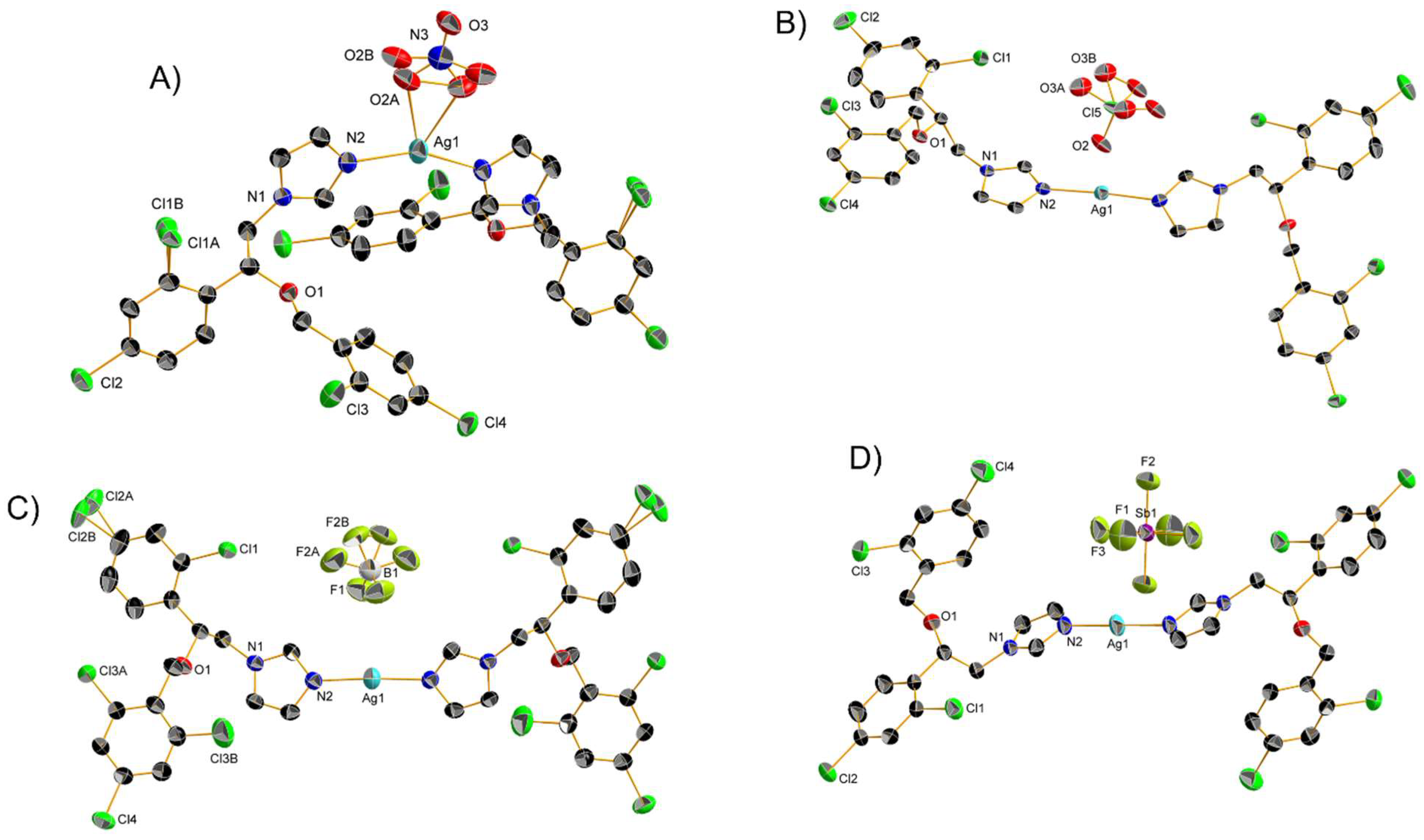
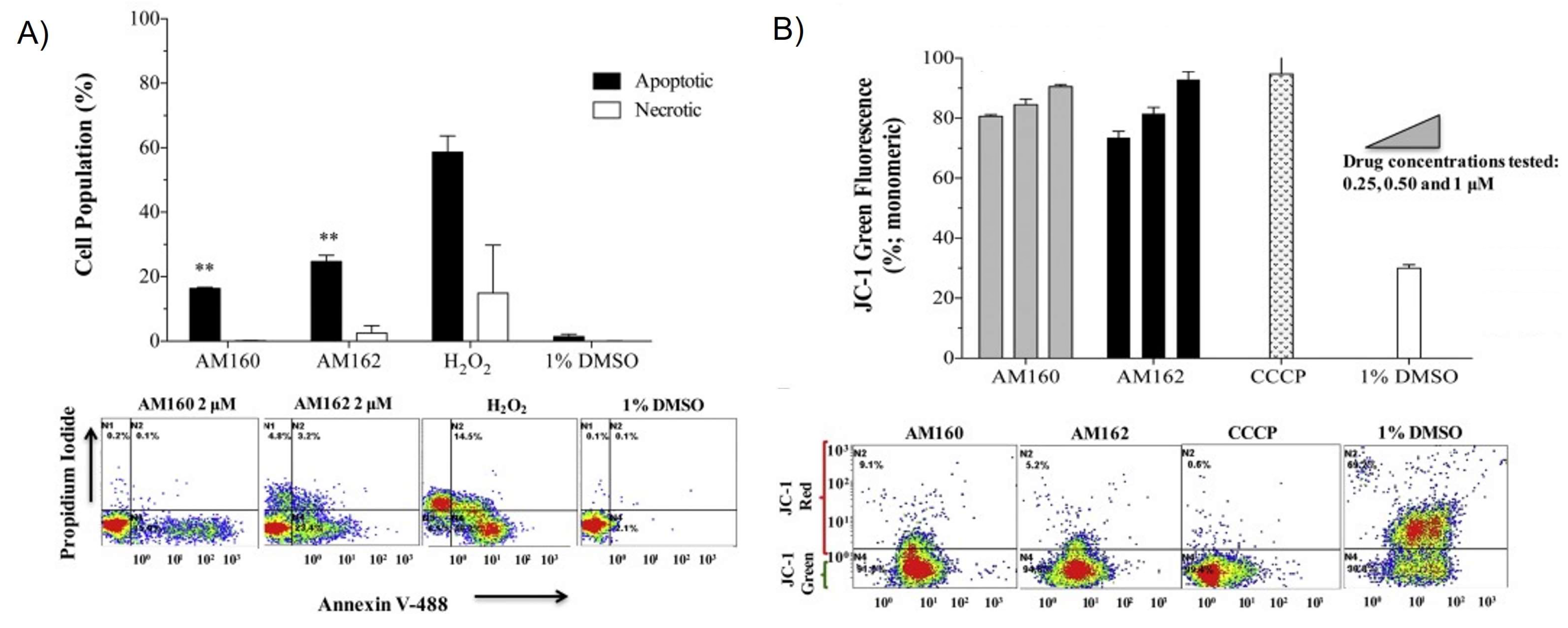
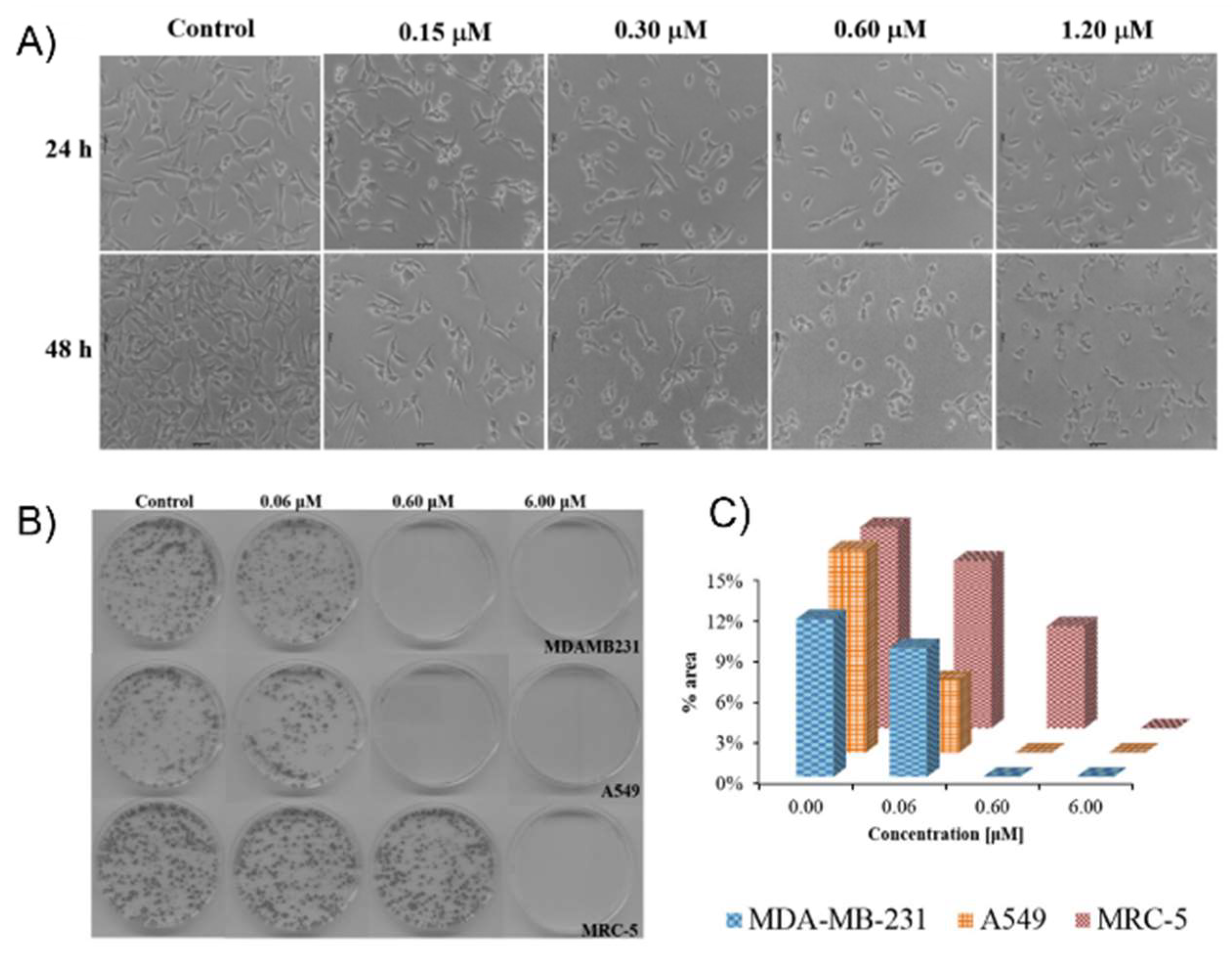
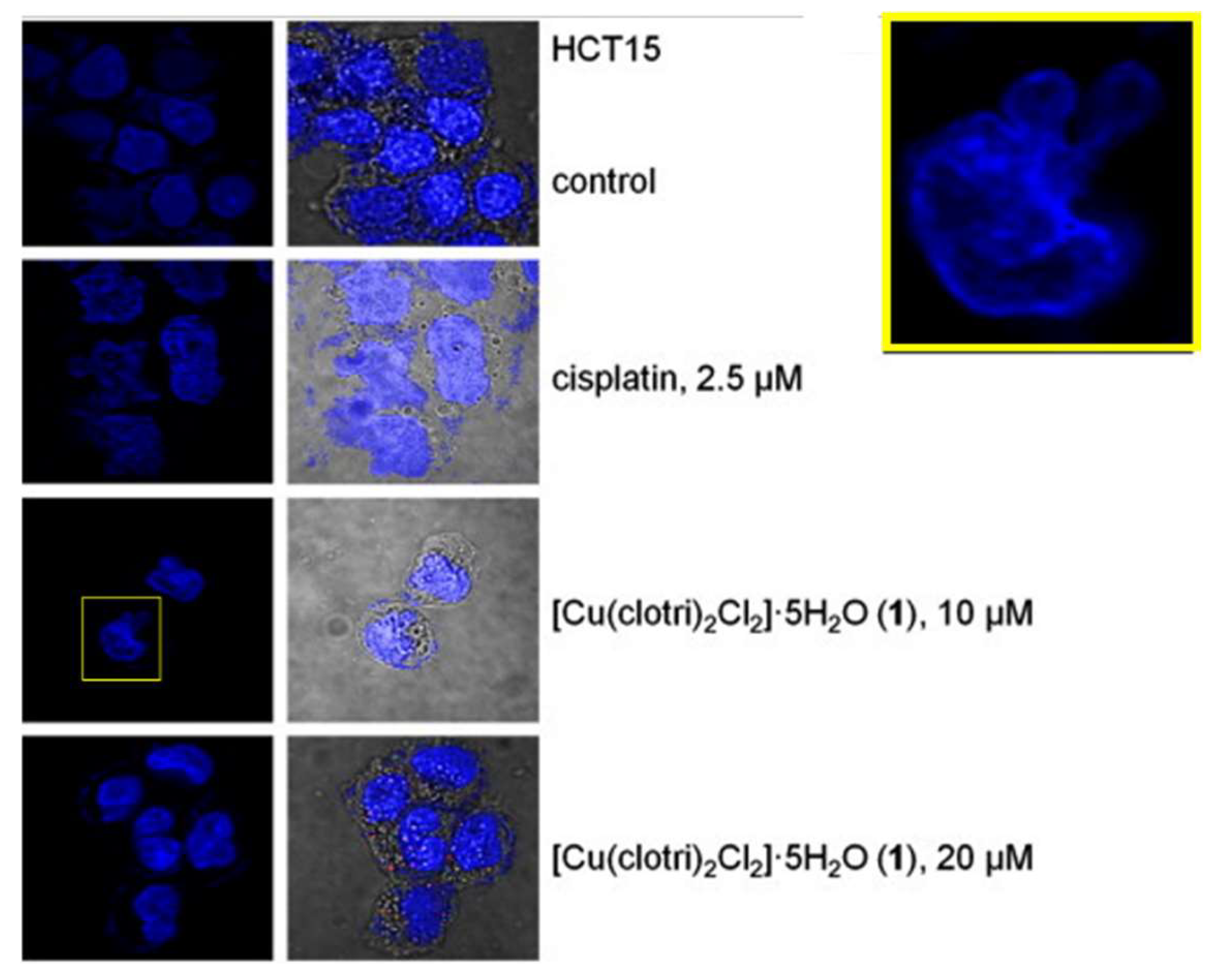
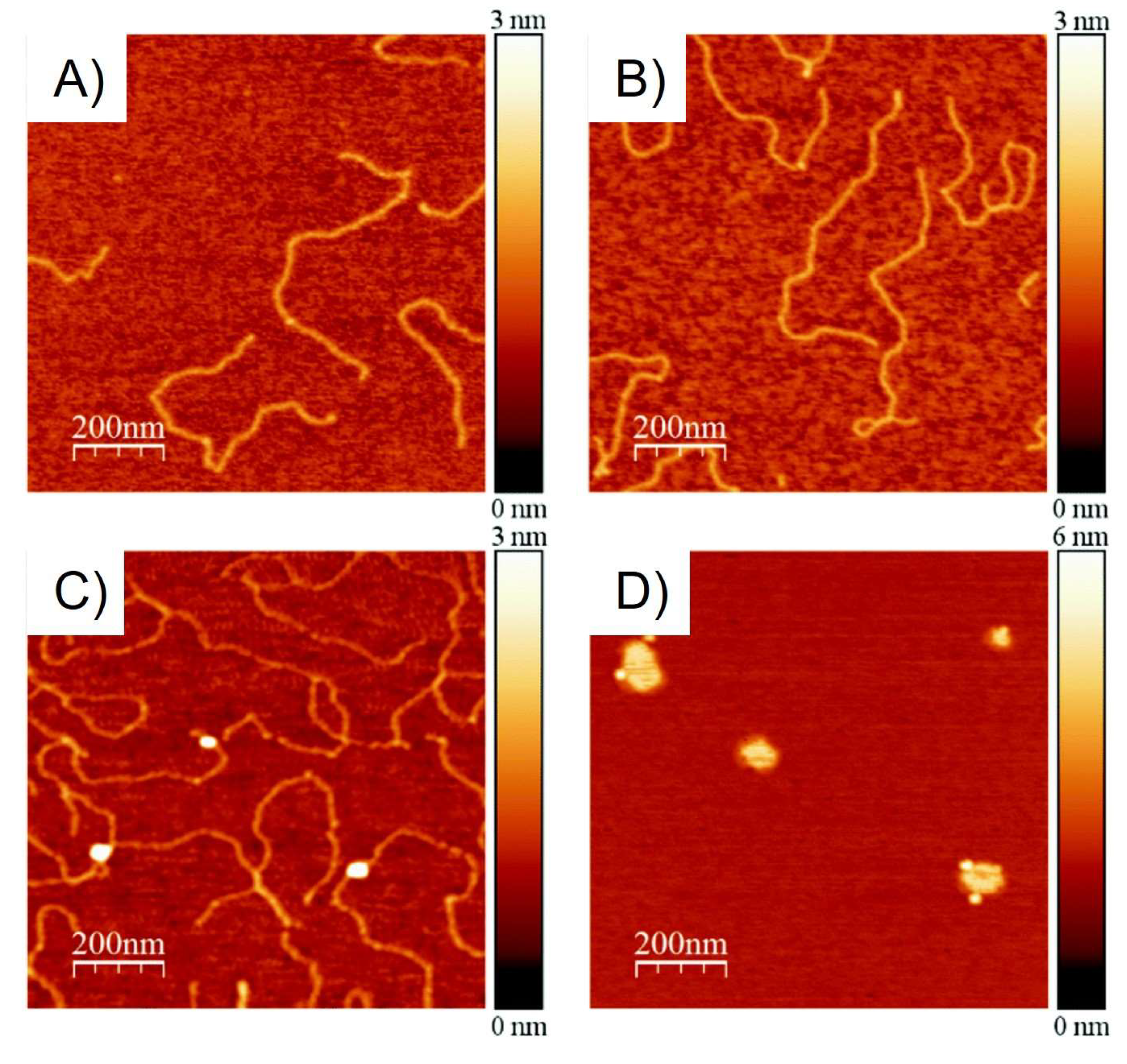
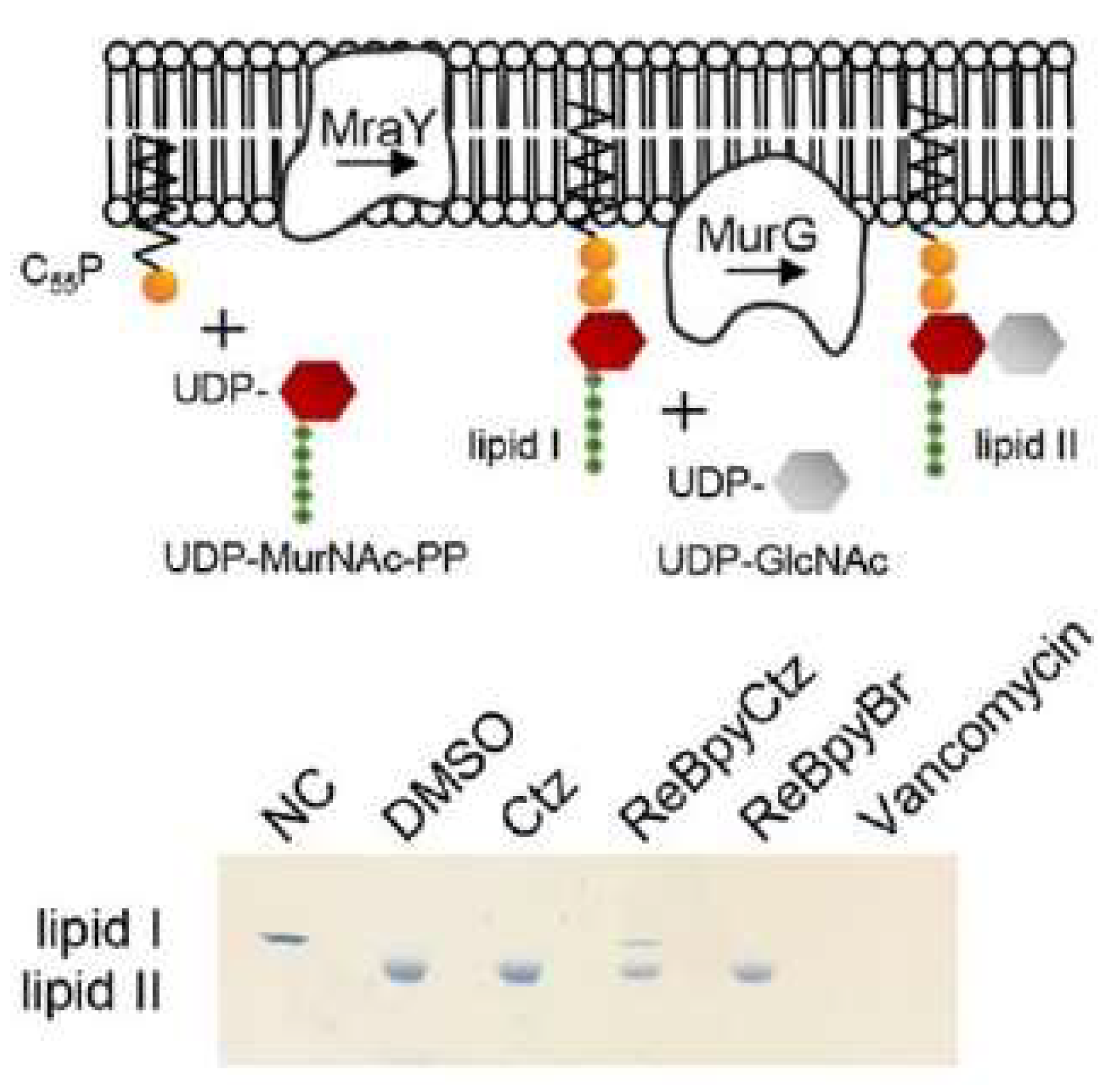
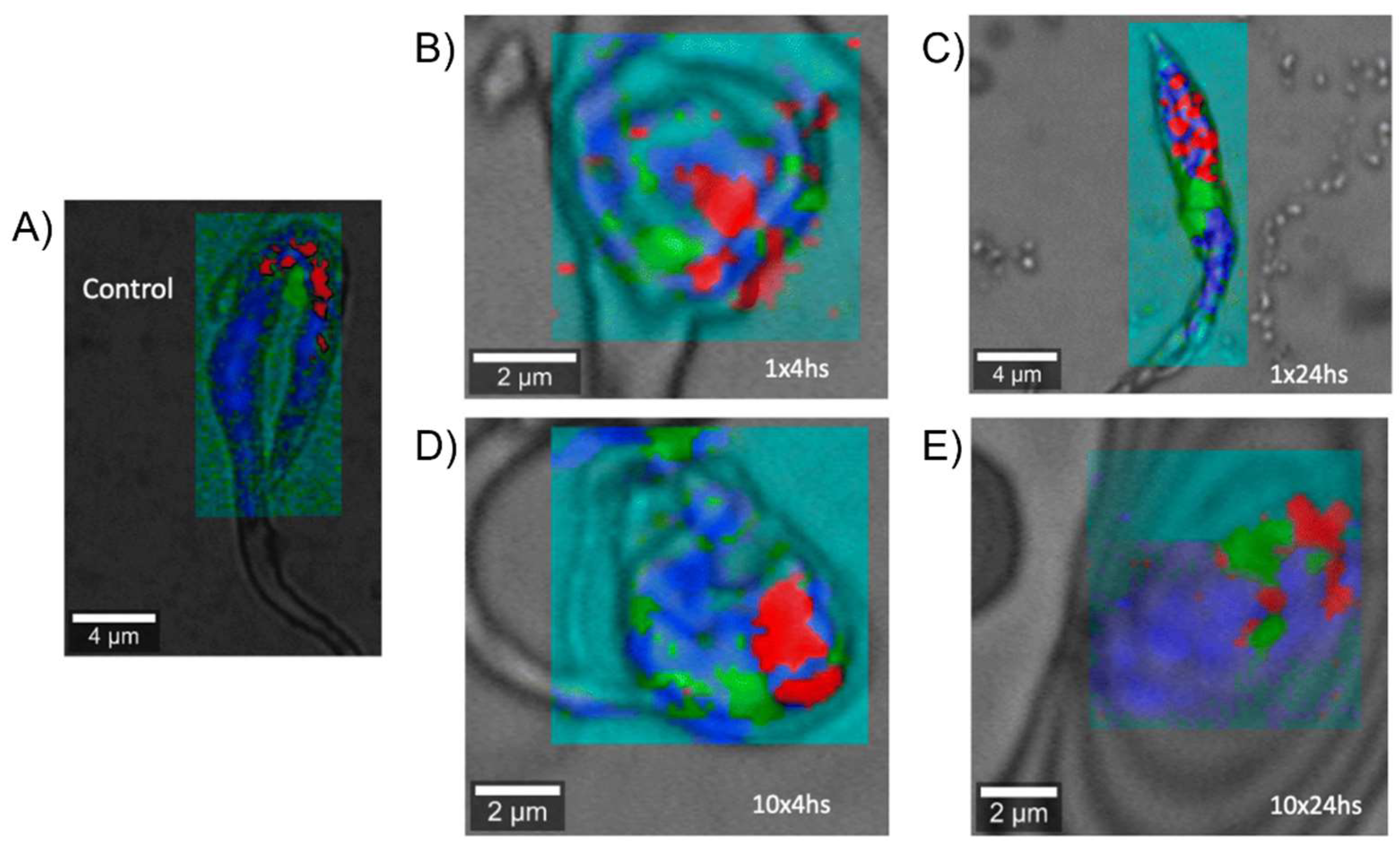
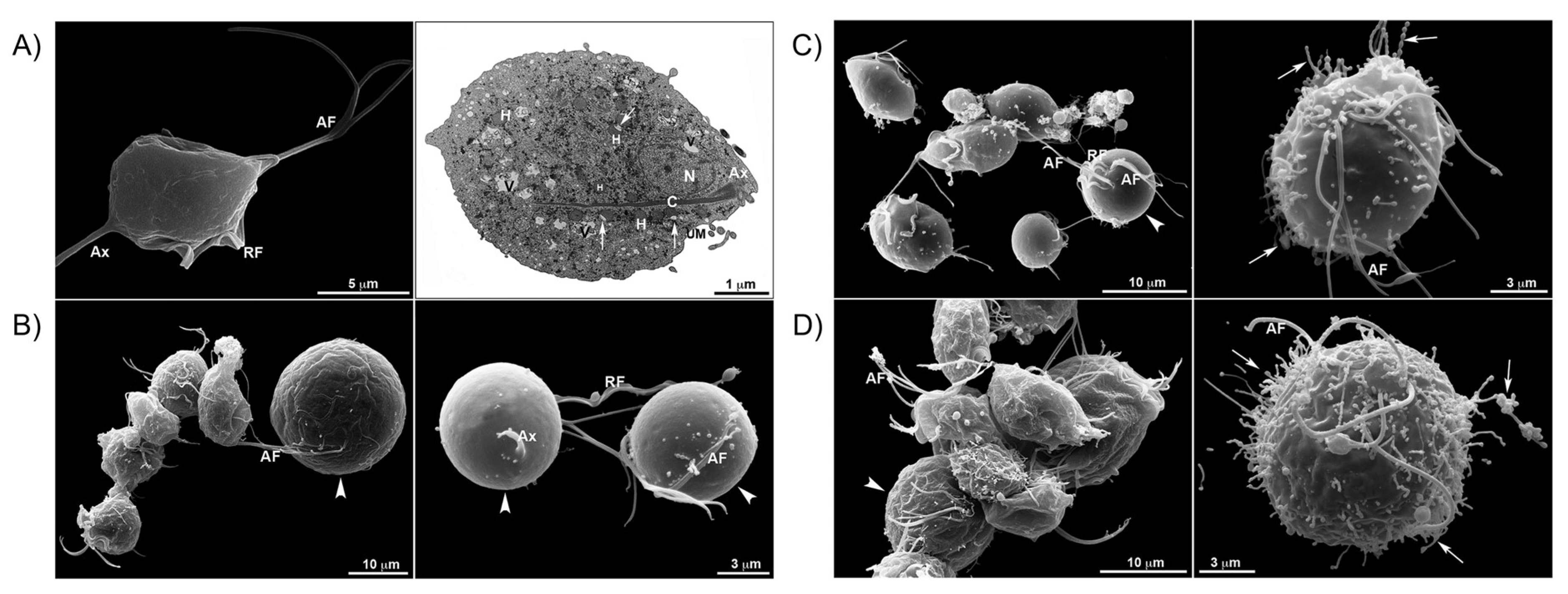
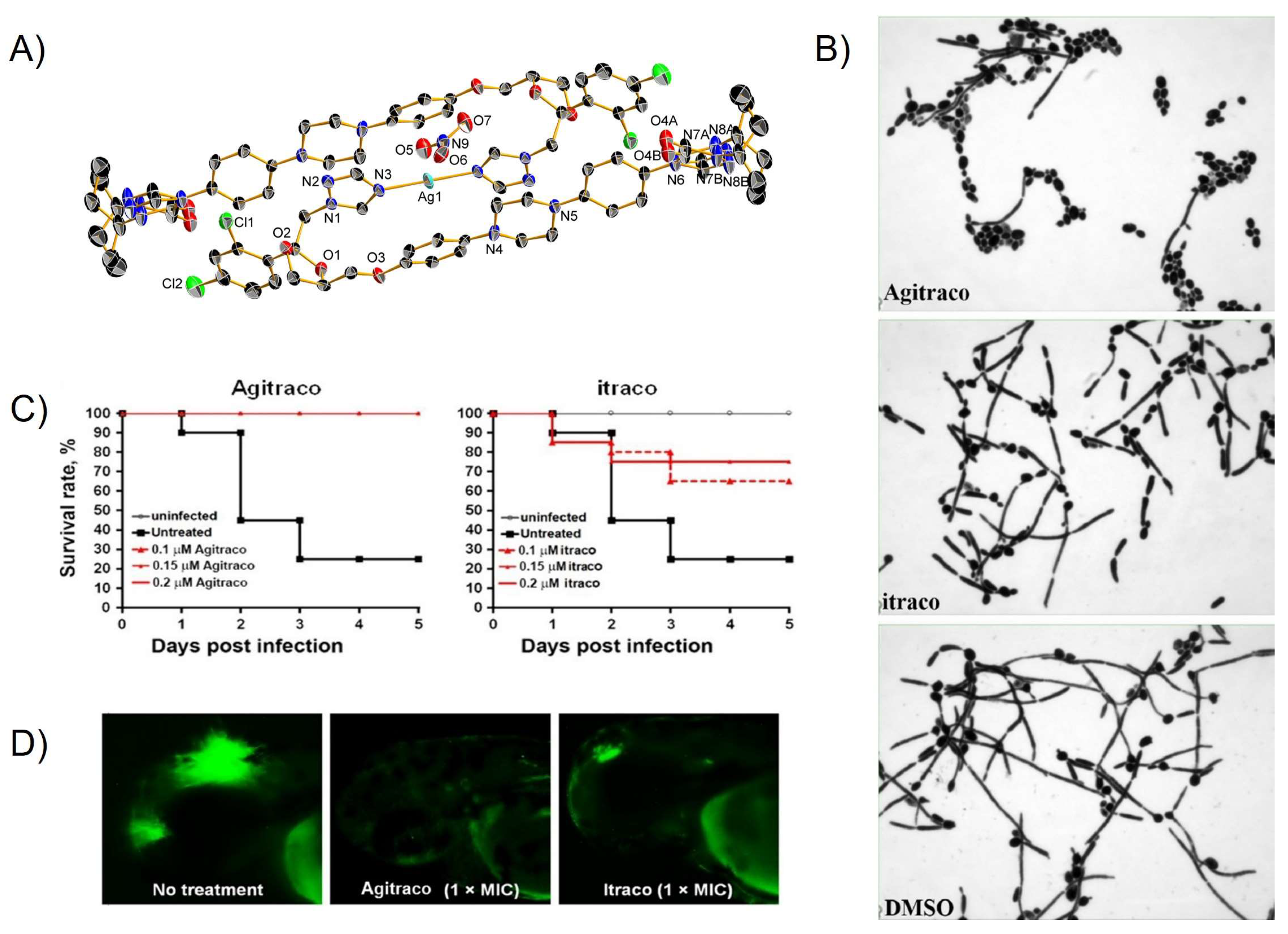

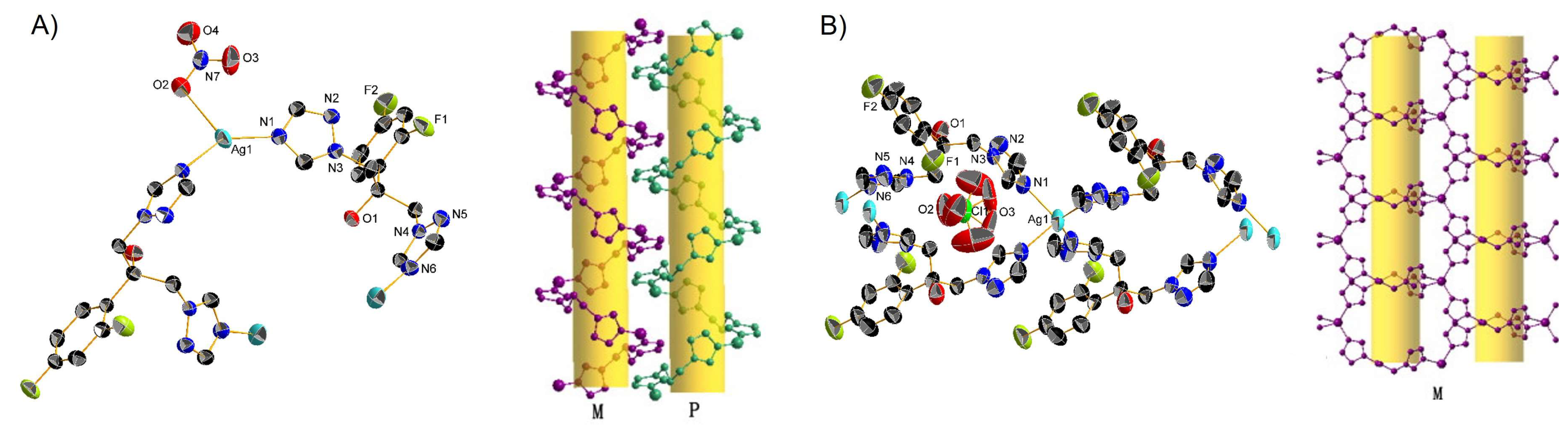
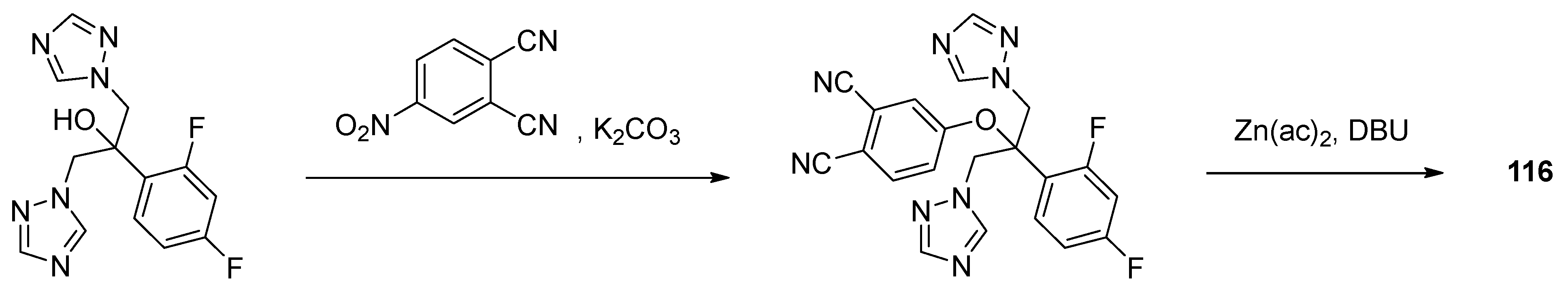
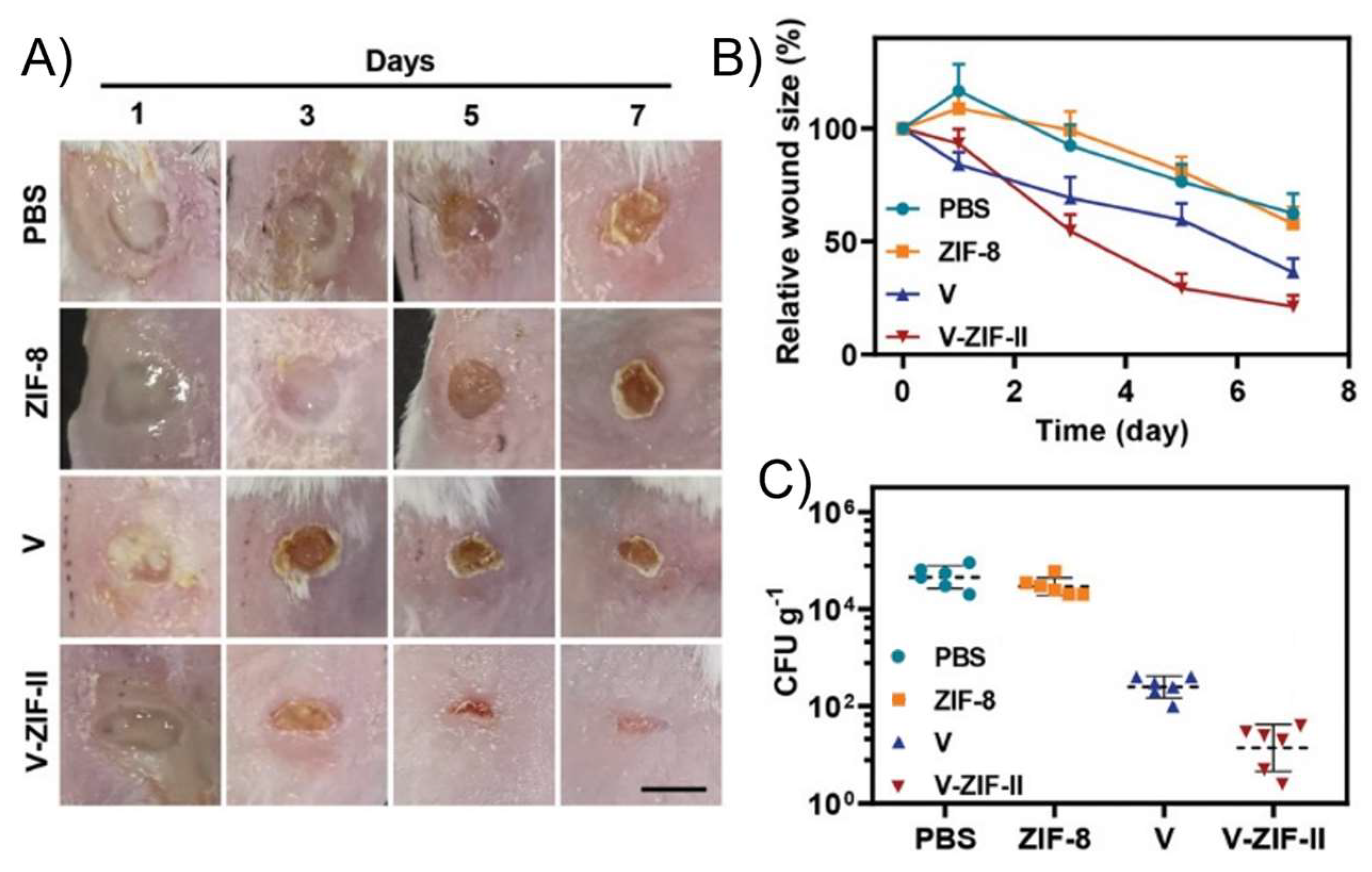
| Complex | Cancerous Cell Line | IC50 Value (µM) wAA | IC50 Value (µM) of the AA (Ratio) | IC50 Value (µM) of Cisplatin (Ratio) | IC50 Value (µM) against Healthy Cell Lines (SI) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | HCT15 | 6.78 | >516 (>71.1) | 10.46 (1.543) | [54] | |||||||
| 6 | HeLa | 27.067 | >516 (>19.1) | 200.8 (7.419) | [54] | |||||||
| 9 | HeLa | 15.14 | >258 (>17.0) | 18.43 (1.217) | [55] | |||||||
| 18 | MCF7 | 1.9 | 12.5 b (6.6) | [56] | ||||||||
| MCF7 a | 1.4 | 15.3 b (11) | ||||||||||
| LNCaP | 4.2 | 8.6 b (2.0) | ||||||||||
| LNCaPa | 0.4 | 10.4 b (30) | ||||||||||
| PC3 | 13.6 | 18.4 b (1.35) | ||||||||||
| PC3a | 1.8 | 15.1 b (8.4) | ||||||||||
| DLD1 | 8.1 | 42.5 b (5.2) | ||||||||||
| DLD1 a | 3.2 | 43.4 b (14) | ||||||||||
| 19 | MCF7 | 20.8 | 12.5 b (0.601) | [56] | ||||||||
| MCF7 a | 2 | 15.3 b (8) | ||||||||||
| LNCaP | 18.5 | 8.6 b (0.46) | ||||||||||
| LNCaP a | 0.6 | 10.4 b (20) | ||||||||||
| PC3 | 98.4 | 18.4 b (0.187) | ||||||||||
| PC3 a | 5.2 | 15.1 b (2.9) | ||||||||||
| DLD1 | 57.2 | 42.5 b (0.743) | ||||||||||
| DLD1a | 5.70 | 43.4 b (7.61) | ||||||||||
| 20 | HepG2 | 10.5 | 25.5 c (2.43) | [57] | ||||||||
| MCF7 | 5.96 | 19.0 d (3.19) | ||||||||||
| 21 | Balb/c3T3 | [58] | ||||||||||
| HepG2 | 0.92 | >1 (>1) | >1 (>1) | 1.1 (1.2) | ||||||||
| 0.28 e | >1 (>4) | <0.0001 (<0.0004) | 0.50 (1.8) | |||||||||
| 22 | Balb/c3T3 | [59] | ||||||||||
| HepG2 | 0.90 | >1 (>1) | >1 (>1) | 1.1 (1.2) | ||||||||
| 0.24 e | >1 (>4) | <0.0001 (<0.0004) | 0.48 (2.0) | |||||||||
| 37 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| HeLa | 36.9 f | 94.3 (2.56) | 26.1 | (0.707) | 42.9 | (1.16) | 5.6 | (0.15) | ||||
| LNCaP | 5.3 f | 15.4 (2.9) | (4.9) | (8.1) | (1.1) | |||||||
| YT | 5.2 f | 16.7 (3.2) | (5.0) | (8.2) | (1.1) | |||||||
| 41 | A2780 | 0.54 | 5.7 (11) | 1.9 (3.5) | [61] | |||||||
| HeLa | 3.6 | 18.4 (5.1) | 7.00 (1.9) | |||||||||
| MCF7 | 5.4 | 20.5 (3.8) | 28 (5.2) | |||||||||
| 42 | L929 | MRC5 | [62] | |||||||||
| A549 | 0.61 | 14.47 (24) | 14.42 (24) | 1.15 | (1.9) | 1.16 | (1.9) | |||||
| DU145 | 5.13 | 15.82 (3.08) | 2.33 (0.454) | (0.224) | (0.226) | |||||||
| MDAMB231 | 0.63 | 10.11 (16) | 2.44 (3.9) | (1.8) | (1.8) | |||||||
| 43 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| 22Rv1 | 7.2 f | 19.2 (2.7) | NA (not active) | 39.0 | (5.4) | 9.6 | (1.3) | |||||
| HeLa | 76.4 f | 94.3 (1.23) | (0.510) | (0.13) | ||||||||
| LNCaP | 4.8 f | 15.4 (3.2) | (8.1) | (2.0) | ||||||||
| YT | 7.2 f | 16.7 (2.3) | (5.4) | (1.3) | ||||||||
| 44 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| LNCaP | 10 f | 15.4 (1.5) | NA | 67.0 | (6.7) | 9.0 | (0.90) | |||||
| YT | 8.6 f | 16.7 (1.9) | (7.8) | (1.0) | ||||||||
| 53 | HCT15 | 13 | 52.3 (4.0) | 4.6 (0.35) | [63] | |||||||
| HeLa | 7.8 | 12.4 (1.6) | 5.2 (0.67) | |||||||||
| PC3 | 9.8 | 12.3 (1.3) | 19.0 (1.9) | |||||||||
| 54 | HCT15 | 14.4 | 52.3 (3.63) | 4.6 (0.32) | [63] | |||||||
| HeLa | 10.5 | 12.4 (1.18) | 5.2 (0.50) | |||||||||
| 71 | L929 | [64] | ||||||||||
| A549 | 2.11 | 14.47 (6.86) | 14.42 (6.83) | 2.61 | (1.24) | |||||||
| DU145 | 3.64 | 15.82 (4.35) | 2.33 (0.640) | (0.717) | ||||||||
| MCF7 | 3.83 | 16.80 (4.39) | 13.98 (3.65) | (0.681) | ||||||||
| 72 | L929 | [64] | ||||||||||
| A549 | 0.47 | 14.47 (31) | 14.42 (31) | 0.48 | (1.0) | |||||||
| DU145 | 3.32 | 15.82 (4.77) | 2.33 (0.702) | (0.14) | ||||||||
| MCF7 | 1.68 | 16.80 (10.0) | 13.98 (8.32) | (0.29) | ||||||||
| 73 | L929 | [64] | ||||||||||
| A549 | 4.79 | 14.47 (3.02) | 14.42 (3.01) | 5.89 | (1.23) | |||||||
| DU145 | 7.40 | 15.82 (2.14) | 2.33 (0.315) | (0.796) | ||||||||
| MCF7 | 13.28 | 16.80 (1.265) | 13.98 (1.053) | (0.444) | ||||||||
| 77 | ARPE19 | [65] | ||||||||||
| BE | 8.5 | 0.7 (0.08) | 32.5 | (3.8) | ||||||||
| MiaPaCa2 | 4.9 | 2.8 (0.57) | (6.6) | |||||||||
| Panc10.05 | 1.9 | 1.7 (0.89) | (17) | |||||||||
| 81 | ARPE19 | [65] | ||||||||||
| BE | 3.6 | 0.7 (0.2) | 8.7 (2.4) | |||||||||
| 82 | ARPE19 | [65] | ||||||||||
| BE | 18.0 | 0.7 (0.04) | >50 | (>2.8) | ||||||||
| MiaPaCa2 | 11.7 | 2.8 (0.24) | (>4.3) | |||||||||
| Panc10.05 | 7.1 | 1.7 (0.24) | (>7.0) | |||||||||
| 83 | ARPE19 | [65] | ||||||||||
| BE | 9.0 | 0.7 (0.08) | 41.2 | (4.6) | ||||||||
| MiaPaCa2 | 8.6 | 2.8 (0.33) | (4.8) | |||||||||
| Panc10.05 | 6.5 | 1.7 (0.26) | (6.3) | |||||||||
| 84 | ARPE19 | BHK21 | [65,66] | |||||||||
| BE | 3.3 | 0.7 (0.2) | 24.2 | (7.3) | 23.0 | |||||||
| B16F1 | 19.3 | 48 g (2.5) | 5.4 (0.28) | (1.2) | ||||||||
| CT26WT | 12.2 | 46.2 g (3.79) | 6.0 (0.49) | (1.9) | ||||||||
| 85 | ARPE19 | [65] | ||||||||||
| BE | 3.1 | 0.7 (0.2) | 26.0 (8.4) | |||||||||
| 86 | BHK21 | [66] | ||||||||||
| B16F1 | 14 | 48 g (3.4) | 5.4 (0.39) | 37.6 | (2.7) | |||||||
| CT26WT | 15 | 46.2 g (3.1) | 6.0 (0.40) | (2.5) | ||||||||
| 87 | HepG2 | 8.27 | 25.5 c (3.08) | [57] | ||||||||
| MCF7 | 6.97 | 19.0 d (2.73) | ||||||||||
| 89 | A2780 | 6.1 | 10.5 (1.7) | [67] | ||||||||
| A549 | 19.0 | 38.8 (2.04) | ||||||||||
| SW480 | 38.3 | 45.1 (1.18) | ||||||||||
| 94 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| 22Rv1 | 21.4 f | 24.2 (1.13) | NA | 6.7 | (0.31) | 5.2 | (0.24) | |||||
| DU145 | 8.1 f | 28.6 (3.5) | (0.83) | (0.64) | ||||||||
| HeLa | 47.8 f | 66.7 (1.40) | (0.14) | (0.11) | ||||||||
| LNCaP | 4.3 f | 27.9 (6.5) | (1.6) | (1.2) | ||||||||
| 95 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| 22Rv1 | 7.4 f | 24.2 (3.3) | 61.6 | (8.3) | 66.4 | (9.0) | 25.3 | (3.4) | ||||
| HeLa | 61.4 f | 66.7 (1.09) | (1.00) | (1.08) | (0.412) | |||||||
| 96 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| 22Rv1 | 14.7 f | 24.2 (1.65) | 312.6 | (21.3) | 171 | (11.6) | 39.2 | (2.67) | ||||
| LNCaP | 6.6 f | 27.9 (4.2) | (47) | (26) | (5.9) | |||||||
| 97 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| HeLa | 33.3 f | 66.7 (2.00) | 113.1 | (3.40) | 12.3 | (0.369) | 37.3 | (1.12) | ||||
| LNCaP | 6.8 f | 27.9 (4.1) | (17) | (1.8) | (5.5) | |||||||
| 98 | HaCaT | MCF10A | MEFs | [60] | ||||||||
| LNCaP | 7.4 f | 27.9 (3.8) | NA | NA | 212.5 | (29) | ||||||
| YT | <4 f | 4.9 (>1) | (>50) | |||||||||
| 99 | L929 | MRC5 | [60] | |||||||||
| A549 | 0.64 | 41.85 (65) | 14.42 (23) | 1.09 | (1.7) | 1.80 | (2.8) | |||||
| DU145 | 4.45 | 47.54 (10.7) | 2.33 (0.524) | (0.245) | (0.404) | |||||||
| MDAMB231 | 0.62 | 10.26 (17) | 2.44 (3.9) | (1.8) | (2.9) | |||||||
| 110 | BHK21 | [66] | ||||||||||
| B16F1 | 2.8 | 4.3 g (1.5) | 5.4 (1.9) | 26.3 | (9.4) | |||||||
| CT26WT | 16.1 | 45.4 g (2.82) | 6.0 (0.37) | (1.63) | ||||||||
| 111 | BHK21 | [66] | ||||||||||
| B16F1 | 4.4 | 4.3 g (0.98) | 5.4 (1.2) | 48.4 | (11) | |||||||
| CT26WT | 31.4 | 45.4 g (1.45) | 6.0 (0.19) | (1.54) | ||||||||
| 123 | KB31 | 26.08 | >100 (>3.834) | [68] | ||||||||
| SNB19 | 55.60 | >100 (>1.799) | ||||||||||
| 129 | L929 | MRC5 | [62] | |||||||||
| A549 | 2.94 | >100 (>34.0) | 14.42 (4.90) | 2.00 | (0.680) | 2.02 | (0.687) | |||||
| DU145 | 3.90 | >100 (>25.6) | 2.33 (0.597) | (0.513) | (0.518) | |||||||
| MDAMB231 | 2.35 | >100 (>42.6) | 2.44 (1.04) | (0.851) | (0.860) | |||||||
| Complex | Bacterial Pathogen | MIC Value (µM) wAA | MIC Value (µM) of the AA (Ratio) | Control Salt | MIC Value (µM) of the Control Salt (Ratio a) | References | |
|---|---|---|---|---|---|---|---|
| G+ | G− | ||||||
| 16 | E. coli | 20 | >40 (>2.0) | [73] | |||
| E. faecium | 1.25 | 10 (8.0) | |||||
| E. faekalis | 2.5 | 10 (4.0) | |||||
| S. aureus | 1.25 | 5 (4) | |||||
| S. epidermis | 1.25 | 2.5 (2.0) | |||||
| Y. pestis | 10 | >40 (>4.0) | |||||
| Y. pseudotuberculosa | 20 | >40 (>2.0) | |||||
| 21 | M. luteus | 0.98 | 1.18 (1.2) | AgNO3 | 11.40 (23) | [59] | |
| 22 | P. mirabilis | 120.4 | >1000 (>8.306) | AgClO4 | 151.21 (2.512) | [59] | |
| 25 | E. coli | 36.5 | >450 (>12.3) | [Au(imz)Cl3] | 67.5 (1.85) | [74] | |
| P. aeruginosa (PA14) | 18.3 | >450 (24.6) | 67.5 (3.69) | ||||
| S. aureus (ATCC 259233) | 18.3 | 225 (12.3) | 67.5 (3.69) | ||||
| S. aureus (MRSA) | 9.1 | 14.1 (1.5) | 67.5 (7.4) | ||||
| 26 | E. coli | 36.2 | 516 (14.3) | [Au(imz)Cl3] | 67.5 (1.86) | [74] | |
| P. aeruginosa (PA14) | 18.1 | 516 (28.6) | 67.5 (3.73) | ||||
| S. aureus (ATCC 259233) | 72.4 | 258 (3.56) | 67.5 (0.932) | ||||
| S. aureus (MRSA) | 18.1 | 32.2 (1.78) | 67.5 (3.73) | ||||
| 29 | E. coli | 30.9 | >580 (>18.8) | [Au(imz)Cl3] | 67.5 (2.18) | [74] | |
| P. aeruginosa (PA14) | 38.6 | >580 (>15.0) | 67.5 (1.75) | ||||
| S. aureus (ATCC 259233) | 19.3 | 290 (15.0) | 67.5 (3.50) | ||||
| S. aureus (MRSA) | 19.3 | 36.3 (1.88) | 67.5 (3.50) | ||||
| 55 | E. faecium | 2.5 | 20 (8.0) | [73] | |||
| E. faekalis | 2.5 | 40 (16) | |||||
| S. aureus | 0.625 | 20 (32) | |||||
| S. epidermis | 0.625 | 2.5 (4.0) | |||||
| 56 | S. aureus | 3 | 10 (3) | [75] | |||
| 57 | A. baumannii | 9 | >93 (>10) | [Re(CO)3(bpy)Br] | >63 (>7) | [75,76] | |
| B. subtilis | 0.27 | 6 (20) | >63 (>230) | ||||
| E. aerogenes | 35 | >93 (>2.7) | >63 (>1.8) | ||||
| E. coli | 17 | >93 (>5.5) | >63 (>3.7) | ||||
| E. faecium | 2 | 20 (10) | >63 (>30) | ||||
| K. pneumoniae | 35 | >93 (>2.7) | >63 (>1.8) | ||||
| P. aeruginosa | 35 | >93 (>2.7) | >63 (>1.8) | ||||
| S. aureus (ATCC 29213) | 0.27 | 20 (74) | >63 (>230) | ||||
| S. aureus (NCTC 8325) | 0.27 | 10 (37) | >63 (>230) | ||||
| S. aureus (MRSA) | 0.5 | 10 (20) | >63 (>100) | ||||
| S. aureus | 4 | ||||||
| 9 b | |||||||
| S. aureus (MRSA) | 4 | ||||||
| 9 b | |||||||
| 58 | S. aureus | 0.6 | 10 (20) | [75] | |||
| 59 | S. aureus | 0.6 | 10 (20) | [75] | |||
| 66 | S. aureus | 8 | [76] | ||||
| >8 b | |||||||
| S. aureus (MRSA) | 8 | ||||||
| >8 b | |||||||
| 67 | S. aureus | 8 | [76] | ||||
| >8 b | |||||||
| S. aureus (MRSA) | 8 | ||||||
| >8 b | |||||||
| 68 | S. aureus | 7 | [76] | ||||
| 7 b | |||||||
| S. aureus (MRSA) | 7 | ||||||
| 7 b | |||||||
| 70 | S. aureus | 22.7 | 90.62 (3.99) | [77] | |||
| S. aureus (MRSA) | 36.34 | 90.62 (2.494) | |||||
| 70 c | S. aureus | 7.3 | 302 (41) | [77] | |||
| S. aureus (MRSA) | 7.3 | 302 (41) | |||||
| 71 | M. tuberculosis | 10.52 | 70.25 (6.678) | [64] | |||
| 72 | M. tuberculosis | 10.30 | 70.25 (6.820) | [64] | |||
| 73 | M. tuberculosis | 18.53 | 70.25 (3.791) | [64] | |||
| 100 | E. faecium | 10 | >40 (>4.0) | [73] | |||
| E. faekalis | 20 | >40 (>2.0) | |||||
| S. aureus | 2.5 | 40 (16) | |||||
| S. epidermis | 2.5 | 40 (16) | |||||
| 133 | E. coli | 76.6 | 572 (7.47) | [Au(imz)Cl3] | 67.5 (0.881) | [74] | |
| P. aeruginosa (PA14) | 76.6 | 572 (7.47) | 67.5 (0.881) | ||||
| S. aureus (ATCC 259233) | 153 | 572 (3.74) | 67.5 (0.441) | ||||
| S. aureus (MRSA) | 76.6 | 572 (7.47) | 67.5 (0.881) | ||||
| Complex | Kinetoplastid | IC50 Value (µM) wAA | IC50 Value (µM) of the AA (Ratio) | IC50 Value (µM) against Healthy Cell Lines (SI) | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 293T | J774.1 | [73] | ||||||||
| L. major | 1.8 | 42.6 (24) | 1.9 | (1.1) | 1.8 | (1.0) | |||||
| T. brucei | 0.4 | 17.5 (40) | (4.8) | (4.5) | |||||||
| 27 | Vero | [78] | |||||||||
| T. cruzi a | 0.2 b | 3 (10) | NA (not active) | [79] | |||||||
| T. cruzi c | 0.01 b | 0.01 (1) | |||||||||
| 37 | Human osteoblast | [80] | |||||||||
| L. major d | 0.015 e | 1.6 (110) | >7.5 | (>500) | |||||||
| L. major c | 0.029 f | >1 (>30) | |||||||||
| T. cruzi a | 0.1 e | 5.8 (60) | (>70) | ||||||||
| 40 | Human osteoblast | [80] | |||||||||
| L. major d | 0.45 e | 1.6 (3.6) | 6.55 | (15) | |||||||
| T. cruzi a | 2.9 e | 5.8 (2.0) | (2.3) | ||||||||
| 41 | Murine macrophages | [61] | |||||||||
| T. brucei | 0.6 | >25 (>40) | 1.9 | (3) | |||||||
| T. cruzi a | 0.25 | 1.8 (7.2) | (7.6) | ||||||||
| 42 | L. amazonensis d | 0.57 | 0.87 (1.5) | [81] | |||||||
| L. amazonensis c | 0.02580 | ||||||||||
| 55 | 293T | J774.1 | [73] | ||||||||
| L. major | 2.2 | 64.6 (29) | <1.25 | (<0.57) | 1.9 | (0.86) | |||||
| T. brucei | 0.5 | 17.4 (30) | (<2) | (4) | |||||||
| 61 | Vero | [82,83] | |||||||||
| T. cruzi a | 2.43 | 5.10 | |||||||||
| T. cruzi a | 3.48 | 22.2 (6.38) | (1.47) | ||||||||
| T. cruzi g | 0.61 | 10.2 (17) | (8.4) | ||||||||
| 62 | Vero | [83] | |||||||||
| T. cruzi a | 9.42 | 22.2 (2.36) | 3.20 | (0.340) | |||||||
| T. cruzi g | 1.28 | 10.2 (7.97) | (2.50) | ||||||||
| 63 | Vero | [83] | |||||||||
| T. cruzi a | 7.53 | 22.2 (2.95) | 6.50 | (0.863) | |||||||
| T. cruzi g | 1.11 | 10.2 (9.19) | (5.86) | ||||||||
| 64 | Vero | [83] | |||||||||
| T. cruzi a | 8.48 | 22.2 (2.62) | 14.0 | (1.65) | |||||||
| T. cruzi g | 2.26 | 10.2 (4.51) | (6.19) | ||||||||
| 65 | Vero | [83] | |||||||||
| T. cruzi a | 8.43 | 22.2 (2.63) | 12.8 | (1.52) | |||||||
| T. cruzi g | 2.79 | 10.2 (3.66) | (4.59) | ||||||||
| 74 | HFF | [84] | |||||||||
| T. vaginalis | 9.8 | 17.2 (1.8) | 56.8 (5.8) | ||||||||
| 94 | Hs27 | IPφ | RAW264.7 | U2OS | [85] | ||||||
| L. major d | 0.8 e | 1.9 (2) | >120 | (>100) | >3.5 | (>4) | >7.5 | (>9) | 120 | (100) | |
| T. cruzi a | 1.39 e | 1.5 (1.1) | (>86.3) | (>2.5) | (>5.4) | (86.3) | |||||
| 99 | L. amazonensis d | 0.15 | >3.00 (>20) | [81] | |||||||
| L. amazonensis c | 0.01553 | ||||||||||
| 100 | 293T | J774.1 | [73] | ||||||||
| L. major | 2.0 | 66.0 (33) | 6.3 | (3.1) | 8.2 | (4.1) | |||||
| T. brucei | 0.7 | 20.5 (30) | (10) | (10) | |||||||
| 103 | Raw264.7 | LLCMK2 | HFF | [86] | |||||||
| L. amazonensis d | 0.008 | 0.044 (5) | |||||||||
| L. amazonensis c | 0.000336 | 0.14538 (433) | 1.60 (4760) | ||||||||
| T. cruzi a | 0.00662 | 0.0870 (13.1) | |||||||||
| T. cruzi c | 0.00228 | 0.0023 (1.0) | 0.73782 (324) | ||||||||
| T. gondii | 0.0744 | 0.1623 (2.18) | >10.00 (>134) | ||||||||
| 104 | Raw264.7 | LLCMK2 | HFF | [86] | |||||||
| L. amazonensis d | 0.0032 | 0.044 (14) | |||||||||
| L. amazonensis c | 0.06614 | 0.14538 (2.198) | 1.20 (18.1) | ||||||||
| T. cruzi a | 0.0026 | 0.0870 (33) | |||||||||
| T. cruzi c | 0.0018 | 0.0023 (1.3) | 1.011 (560) | ||||||||
| T. gondii | 0.120 | 0.1623 (1.35) | >10.00 (>83.3) | ||||||||
| 123 | HFFL | [87] | |||||||||
| T. cruzi a | 3.56 | 10.6 (2.98) | 107 | (30.1) | |||||||
| T. cruzi c | 12 | 24.3 (2.0) | (8.9) | ||||||||
| 124 | HFFL | [87] | |||||||||
| T. cruzi a | 1.7 | 10.6 (6.2) | 860 | (510) | |||||||
| T. cruzi c | 10 | 24.3 (2.4) | (86) | ||||||||
| T. cruzi g | 6.30 | 7.04 (1.12) | (137) | ||||||||
| 125 | HFFL | [87] | |||||||||
| T. cruzi a | 1.9 | 10.6 (5.6) | 171 | (90) | |||||||
| T. cruzi c | 13 | 24.3 (1.9) | (13) | ||||||||
| 127 | HFFL | [87] | |||||||||
| T. cruzi a | 2.54 | 10.6 (4.17) | 760 | (299) | |||||||
| T. cruzi c | 13 | 24.3 (1.9) | (58) | ||||||||
| 128 | HFFL | [87] | |||||||||
| T. cruzi a | 3.0 | 10.6 (3.5) | >1000 | (>330) | |||||||
| T. cruzi c | 9.90 | 24.3 (2.45) | (>101) | ||||||||
| Complex | Fungus | MIC Value (µM) wAA | MIC Value (µM) of the AA (Ratio) | Control Salt | MIC Value (µM) of the Control Salt (Ratio a) | References |
|---|---|---|---|---|---|---|
| 21 | C. albicans | 2.0 | 4.69 (2.3) | AgNO3 | 11.40 (11) | [59] |
| C. glabrata | 3.90 | 4.69 (1.20) | 11.40 (5.85) | |||
| 22 | C. albicans | 3.76 | 4.69 (1.25) | AgClO4 | 9.42 (5.01) | [59] |
| C. glabrata | 3.76 | 4.69 (1.25) | 9.42 (5.01) | |||
| 23 | C. albicans | 3.80 | 4.69 (1.23) | AgBF4 | 20.10 (10.6) | [59] |
| C. glabrata | 3.80 | 4.69 (1.23) | 10.05 (5.29) | |||
| 24 | C. albicans | 3.32 | 4.69 (1.41) | AgSbF6 | 11.37 (6.85) | [59] |
| C. glabrata | 1.7 | 4.69 (2.8) | 11.37 (13) | |||
| 25 | C. albicans | 4.6 | 7.0 (1.5) | [Au(imz)Cl3] | 135 (29) | [74] |
| C. glabrata | 9.1 | 56.2 (6.2) | 135 (15) | |||
| C. parapsilosis | 2.5 | 3.9 (1.6) | 135 (54) | |||
| 26 | C. albicans | 0.6 | 2.3 (4) | [Au(imz)Cl3] | 135 (200) | [74] |
| C. auris | 3.6 | 8.1 (2.2) | 270 (75) | |||
| C. glabrata | 9.0 | 64.5 (7.2) | 135 (15) | |||
| C. krusei | 1.5 | 32.2 (21) | 67.5 (45) | |||
| 29 | C. auris | 1.9 | 9.1 (4.8) | [Au(imz)Cl3] | 270 (140) | [74] |
| C. glabrata | 4.8 | 9.1 (1.9) | 135 (28) | |||
| C. krusei | 0.4 | 1.4 (3) | 67.5 (200) | |||
| C. parapsilosis | 1.4 | 10.2 (7.3) | 135 (96) | |||
| 35 | S. schenckii | 0.001 | 0.005 (5) | [Au(PPh3)Cl] | 0.002 (2) | [89] |
| 0.001 b | 0.020 (20) | 0.008 (8) | ||||
| 51 | C. albicans | 0.1 c | 0.5 (5) | [90] | ||
| C. neoformans | 0.250 c | 0.5 (2) | ||||
| S. brasiliensis | 0.250 c | 0.5 (2) | ||||
| 74 | C. albicans | 0.50 c | 0.5 (1) | [90] | ||
| C. neoformans | 0.50 c | 0.5 (1) | ||||
| S. brasiliensis | 0.06 c | 0.5 (8) | ||||
| 76 | C. albicans | 0.250 c | 0.5 (2) | [90] | ||
| C. neoformans | 0.50 c | 0.5 (1) | ||||
| S. brasiliensis | 0.06 c | 0.5 (8) | ||||
| 92 | S. schenckii | 0.0002 | 0.0009 (4) | [Au(PPh3)Cl] | 0.002 (10) | [89] |
| 0.001 b | >0.040 (>40) | 0.008 (8) | ||||
| 93 | S. schenckii | 0.004 | 0.0009 (0.2) | [Cu(PPh3)2(NO3)] | >0.02 (>10) | [89] |
| 0.008 b | >0.040 (>5) | >0.02 (>5) | ||||
| 103 | S. brasiliensis | 0.2 | 0.6 (3) | ZnCl2 | >20 (>200) | [86] |
| S. schenckii | 0.2 | 1.25 (6) | >20 (>200) | |||
| 5.0 b | >20 (>4.0) | >20 (>8.0) | ||||
| 104 | S. brasiliensis | 0.6 | 0.6 (1) | Zn(ac)2 | >20 (>70) | [86] |
| S. schenckii | 0.32 | 1.25 (3.9) | >20 (>120) | |||
| 105 | C. albicans | 0.38 | 0.44 (1.2) | [91] | ||
| C. glabrata | 0.78 | 1.77 (2.3) | ||||
| C. parapsilosis | 0.38 | 0.44 (1.2) | ||||
| 106 | C. albicans d | 0.39 | 3.13 f (8.0) | [92] | ||
| C. albicans e | 0.78 | 50 f (64) | ||||
| 107 | C. albicans d | 0.39 | 3.13 f (8.0) | [92] | ||
| C. albicans e | 0.78 | 50 f (64) | ||||
| 108 | C. albicans | 0.1 c | 0.25 (2) | [90] | ||
| C. neoformans | <0.008 c | 0.06 (>7) | ||||
| S. brasiliensis | 0.030 c | 0.03 (1) | ||||
| 109 | C. albicans | 0.06 c | 0.25 (4) | [90] | ||
| C. neoformans | <0.008 c | 0.06 (>7) | ||||
| S. brasiliensis | 0.030 c | 0.03 (1) | ||||
| 112 | A. niger | 10 g | >2090 (>210) | [93] | ||
| C. albicans | 0.53 g | 0.82 (1.5) | ||||
| M. mucedo | 10 g | 65 (6.5) | ||||
| P. uniculosum | 21 g | 2090 (100) | ||||
| R. tolonifer | 10 g | 65 (6.5) | ||||
| S. cerevisiae | 4 g | >210 (>50) | ||||
| 113 | A. niger | 24 g | >2090 (>87) | [93] | ||
| C. albicans | 0.61 g | 0.82 (1.3) | ||||
| M. mucedo | 10 g | 65 (6.5) | ||||
| P. uniculosum | 24 g | 2090 (87) | ||||
| R. tolonifer | 10 g | 65 (6.5) | ||||
| S. cerevisiae | 5 g | >210 (>40) | ||||
| 114 | C. albicans | 2 i | 46 (20) | Ag(ClO4) | 10 (10) | [50] |
| C. albicans h | 4 i | 20 (5) | 10 (5) | |||
| C. albicans h | 4 i | >180 (>40) | 5 (2) | |||
| C. albicans h | 4 i | 46 (10) | 10 (5) | |||
| C. albicans h | 4 i | 180 (40) | 10 (5) | |||
| 115 | C. albicans | 1 i | 46 (50) | Ag(NO3) | 10 (20) | [50] |
| C. albicans h | 2 i | 20 (10) | 6 (6) | |||
| C. albicans h | 2 i | >180 (>90) | 3 (3) | |||
| C. albicans h | 2 i | 46 (20) | 6 (6) | |||
| C. albicans h | 1 i | 180 (200) | 3 (6) | |||
| 118 | A. flavus | 0.1 | 0.6 (6) | [94] | ||
| A. niger | 0.1 | 0.6 (6) | ||||
| 119 | A. flavus | 0.1 | 0.6 (6) | [94] | ||
| A. niger | 0.1 | 0.6 (6) | ||||
| C. neoformans | 0.1 | 0.6 (6) | ||||
| 122 | C. albicans (HL 3968) | 2 c | 10 (5) | [95] | ||
| 20 g | 100 (5) | |||||
| C. albicans (HL 973) | 4 c | 52 (10) | ||||
| 8 g | 210 (30) | |||||
| 124 | C. krusei | 7.42 | 40.81 (5.50) | [96] | ||
| C. parapsilosis | 2.09 | 5.71 (2.73) | ||||
| 131 | C. albicans | 0.23 | 0.29 (1.3) | ZIF-8 | NA (not active) | [51] |
| 0.91 b | 2.2 (2.4) | |||||
| C. albicans j | 0.029 | 0.29 (10) | ||||
| 0.11 b | 2.2 (20) | |||||
| 132 | C. albicans | 0.52 | 0.29 (0.56) | ZIF-8 | NA | [51] |
| 2.1 b | 2.2 (1.0) | |||||
| C. albicans j | 0.066 | 0.29 (4.4) | ||||
| 0.26 b | 2.2 (8.5) | |||||
| 133 | C. albicans | 4.8 | 35.8 (7.5) | [Au(imz)Cl3] | 135 (28) | [74] |
| C. auris | 19.1 | 572 (29.9) | 270 (14.1) | |||
| C. glabrata | 76.6 | 572 (7.47) | 135 (1.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortat, Y.; Zobi, F. Resurgence and Repurposing of Antifungal Azoles by Transition Metal Coordination for Drug Discovery. Pharmaceutics 2023, 15, 2398. https://doi.org/10.3390/pharmaceutics15102398
Cortat Y, Zobi F. Resurgence and Repurposing of Antifungal Azoles by Transition Metal Coordination for Drug Discovery. Pharmaceutics. 2023; 15(10):2398. https://doi.org/10.3390/pharmaceutics15102398
Chicago/Turabian StyleCortat, Youri, and Fabio Zobi. 2023. "Resurgence and Repurposing of Antifungal Azoles by Transition Metal Coordination for Drug Discovery" Pharmaceutics 15, no. 10: 2398. https://doi.org/10.3390/pharmaceutics15102398
APA StyleCortat, Y., & Zobi, F. (2023). Resurgence and Repurposing of Antifungal Azoles by Transition Metal Coordination for Drug Discovery. Pharmaceutics, 15(10), 2398. https://doi.org/10.3390/pharmaceutics15102398








