Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticles
2.2.1. NP Formulation
2.2.2. Surface Modification with HA12
2.2.3. Chemico-Physical Characterization
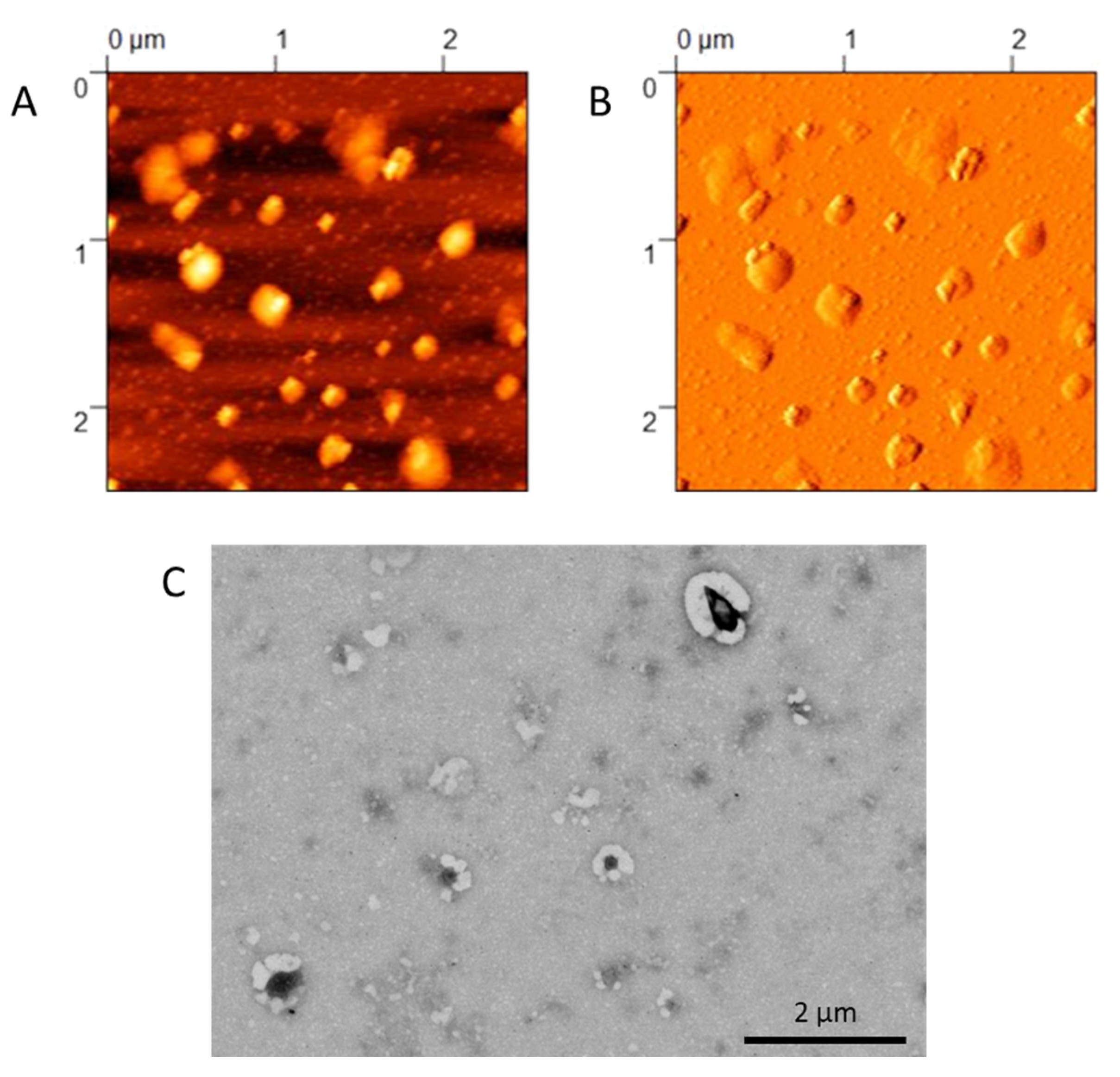
2.2.4. Morphological Characterization
2.2.5. Residual Poloxamer 188
2.3. In Vivo Biodistribution
2.4. In Vitro Tests
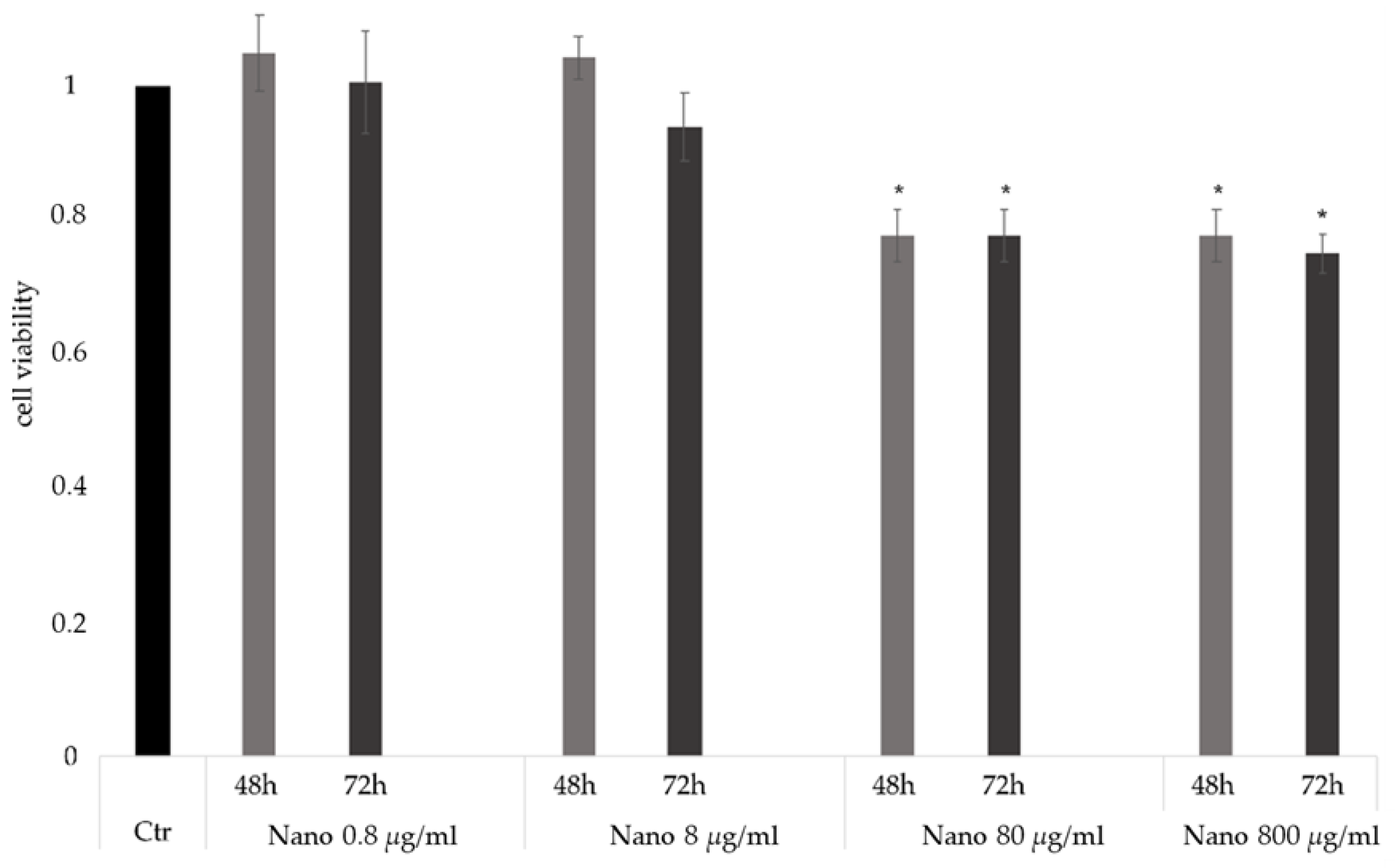
2.4.1. Cell Viability
2.4.2. Cell Uptake Studies
2.5. Thermosensitive Hydrogel (TSH)
2.6. Synthetic Vitreous (SV)
2.7. Rheological Measurements
2.8. NP Diffusion through TSH
2.9. Statistical Analysis
3. Results
3.1. Formulation and Characterization of NPs
3.2. In Vivo Biodistribution and Microglia Volocalization
3.3. In Vitro Uptake Studies
3.4. Formulation of the TSH
3.5. Mobility of NPs through the TSH in Synthetic Vitreous
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zetterberg, M. Age-related eye disease and gender. Maturitas 2016, 83, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K. The Prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Investig. Opthalmology Vis. Sci. 2013, 54, ORSF5–ORSF13. [Google Scholar] [CrossRef] [PubMed]
- Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Incidence, individual, and macro level risk factors of severe binocular visual impairment and blindness in persons aged 50 and older. PLoS ONE 2021, 16, e0251018. [Google Scholar] [CrossRef] [PubMed]
- Himawan, E.; Ekström, P.; Buzgo, M.; Gaillard, P.; Stefánsson, E.; Marigo, V.; Loftsson, T.; Paquet-Durand, F. Drug delivery to retinal photoreceptors. Drug Discov. Today 2019, 24, 1637–1643. [Google Scholar] [CrossRef]
- Vander Poorten, E.; Riviere, C.N.; Abbott, J.J.; Bergeles, C.; Nasseri, M.A.; Kang, J.U.; Sznitman, R.; Faridpooya, K.; Iordachita, I. 36 - Robotic Retinal Surgery. In Handbook of Robotic and Image-Guided Surgery; Abedin-Nasab, M.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 627–672. ISBN 978-0-12-814245-5. [Google Scholar]
- Loriga, B.; Di Filippo, A.; Tofani, L.; Signorini, P.; Caporossi, T.; Barca, F.; De Gaudio, A.R.; Rizzo, S.; Adembri, C. Postoperative pain after vitreo-retinal surgery is influenced by surgery duration and anesthesia conduction. Minerva Anestesiol. 2019, 85, 731–737. [Google Scholar] [CrossRef]
- Trapani, I.; Auricchio, A. Has retinal gene therapy come of age? From bench to bedside and back to bench. Hum. Mol. Genet. 2019, 28, R108–R118. [Google Scholar] [CrossRef]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 846782. [Google Scholar] [CrossRef]
- Meyer, C.H.; Krohne, T.U.; Issa, P.C.; Liu, Z.; Holz, F.G. Routes for drug delivery to the eye and retina: Intravitreal injections. Retinal Pharmacother. 2016, 55, 63–70. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular drug delivery to the retina: Current innovations and future perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Causin, P.; Malgaroli, F. Mathematical and numerical methods for modeling drug delivery to the posterior segment of the eye. J. Ophthalmic Res. Ocul. Care 2017, 1, 4–11. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; Rinaldi, A.; Ottonelli, I.; Caraffi, R.; De Benedictis, C.A.; Sauer, A.K.; Tosi, G.; Vandelli, M.A.; Ruozi, B.; Grabrucker, A.M. Glioblastoma multiforme selective nanomedicines for improved anti-cancer treatments. Pharmaceutics 2022, 14, 1450. [Google Scholar] [CrossRef]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Passoni, A.; Favagrossa, M.; Duskey, J.T.; Bombaci, M.; Vandelli, M.A.; Colombo, L.; Bagnati, R.; et al. Insights into kinetics, release, and behavioral effects of brain-targeted hybrid nanoparticles for cholesterol delivery in Huntington’s disease. J. Control. Release 2021, 330, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 nanoparticles for blood–brain barrier crossing: Proof-of-Concept study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; Ottonelli, I.; Rinaldi, A.; Parmeggiani, I.; Zambelli, B.; Wang, L.Z.; Prud’homme, R.K.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Tween® preserves enzyme activity and stability in PLGA nanoparticles. Nanomaterials 2021, 11, 2946. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; da Ros, F.; Ottonelli, I.; Zambelli, B.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Enzyme stability in nanoparticle preparations Part 1: Bovine serum albumin improves enzyme function. Molecules 2020, 25, 4593. [Google Scholar] [CrossRef]
- Kim, H.M.; Ha, S.; Hong, H.K.; Hwang, Y.; Kim, P.; Yang, E.; Chung, J.Y.; Park, S.; Park, Y.J.; Park, K.H.; et al. Intraocular distribution and kinetics of intravitreally injected antibodies and nanoparticles in rabbit eyes. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef]
- Martens, T.F.; Remaut, K.; Deschout, H.; Engbersen, J.F.J.; Hennink, W.E.; van Steenbergen, M.J.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J. Control. Release 2015, 202, 83–92. [Google Scholar] [CrossRef]
- Eriksen, A.Z.; Brewer, J.; Andresen, T.L.; Urquhart, A.J. The diffusion dynamics of PEGylated liposomes in the intact vitreous of the ex vivo porcine eye: A fluorescence correlation spectroscopy and biodistribution study. Int. J. Pharm. 2017, 522, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.; Chen, F.; Goldberg, J.L.; Sabel, B.A. Nanomedicine and drug delivery to the retina: Current status and implications for gene therapy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1477–1507. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.; Kostyushev, D.; Zamyatnin, A.A.; Parodi, A. Nanomedicine for treating diabetic retinopathy vascular degeneration. Int. J. Transl. Med. 2021, 1, 18. [Google Scholar] [CrossRef]
- Devoldere, J.; Wels, M.; Peynshaert, K.; Dewitte, H.; De Smedt, S.C.; Remaut, K. The obstacle course to the inner retina: Hyaluronic acid-coated lipoplexes cross the vitreous but fail to overcome the inner limiting membrane. Eur. J. Pharm. Biopharm. 2019, 141, 161–171. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Brown, M.B.; Cook, M.T.; Chau, D.Y.S. Diffusion through the ex vivo vitreal body–bovine, porcine, and ovine models are poor surrogates for the human vitreous. Int. J. Pharm. 2018, 550, 207–215. [Google Scholar] [CrossRef]
- Romeo, A.; Bonaccorso, A.; Carbone, C.; Lupo, G.; Daniela Anfuso, C.; Giurdanella, G.; Caggia, C.; Randazzo, C.; Russo, N.; Luca Romano, G.; et al. Melatonin Loaded Hybrid Nanomedicine: DoE approach, optimization and in vitro study on diabetic retinopathy model. Int. J. Pharm. 2022, 627, 122195. [Google Scholar] [CrossRef]
- Xie, L.; Yue, W.; Ibrahim, K.; Shen, J. A Long-Acting curcumin nanoparticle/in situ hydrogel composite for the treatment of uveal melanoma. Pharmaceutics 2021, 13, 1335. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Djoudi, A.; Molina-Peña, R.; Ferreira, N.; Ottonelli, I.; Tosi, G.; Garcion, E.; Boury, F. Hyaluronic acid scaffolds for loco-regional therapy in nervous system related disorders. Int. J. Mol. Sci. 2022, 23, 12174. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, B.; Sajkiewicz, P.; Kolbuk, D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural Eng. 2018, 15, 051002. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2014, 23, 748–770. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Biswas, A.; Kumar, A.; Yadav, A.; Maiti, P.; Jewrajka, S.K. Gold nanoparticle promoted formation and biological properties of injectable hydrogels. Biomacromolecules 2020, 21, 3782–3794. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Nah, H.R.; Lee, S.J.; Ko, W.-K.; Lee, J.S.; Moon, H.-J.; Bang, J.B.; Hwang, Y.-S.; Reis, R.L.; et al. Injectable hydrogel composite containing modified gold nanoparticles: Implication in bone tissue regeneration. Int. J. Nanomed. 2018, 13, 7019–7031. [Google Scholar] [CrossRef]
- Baumann, B.; Wittig, R.; Lindén, M. Mesoporous silica nanoparticles in injectable hydrogels: Factors influencing cellular uptake and viability. Nanoscale 2017, 9, 12379–12390. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv. Mater. 2019, 31, e1805730. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Huh, H.W.; Zhao, L.; Kim, S.Y. Biomineralized biomimetic organic/inorganic hybrid hydrogels based on hyaluronic acid and poloxamer. Carbohydr. Polym. 2015, 126, 130–140. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lai, P.-L.; Lin, Y.-K.; Peng, S.; Lee, L.-Y.; Chen, C.-N.; Chu, I.-M. A poloxamer-polypeptide thermosensitive hydrogel as a cell scaffold and sustained release depot. Polym. Chem. 2016, 7, 2976–2985. [Google Scholar] [CrossRef]
- Huang, L.; Kutluer, M.; Adani, E.; Comitato, A.; Marigo, V. New in vitro cellular model for molecular studies of retinitis pigmentosa. Int. J. Mol. Sci. 2021, 22, 6440. [Google Scholar] [CrossRef]
- Tan, E.; Ding, X.-Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef]
- Belletti, D.; Grabrucker, A.M.; Pederzoli, F.; Menrath, I.; Vandelli, M.A.; Tosi, G.; Duskey, T.J.; Forni, F.; Ruozi, B. Hybrid nanoparticles as a new technological approach to enhance the delivery of cholesterol into the brain. Int. J. Pharm. 2018, 543, 300–310. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.P.; Lan-Chun-Fung, Y.L.; Pritchard, J.G. determination of poly(vinyl alcohol) via its complex with boric acid and iodine. Anal. Chim. Acta 1979, 104, 153–160. [Google Scholar] [CrossRef]
- Ottonelli, I.; Duskey, J.T.; Genovese, F.; Pederzoli, F.; Caraffi, R.; Valenza, M.; Tosi, G.; Vandelli, M.A.; Ruozi, B. Quantitative comparison of the protein corona of nanoparticles with different matrices. Int. J. Pharm. 2022, 4, 100136. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Maeda, T.; Bereta, G.; Okano, K.; Golczak, M.; Sumaroka, A.; Roman, A.J.; Cideciyan, A.V.; Jacobson, S.G.; Palczewski, K. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem. 2011, 286, 10551–10567. [Google Scholar] [CrossRef]
- Sanges, D.; Comitato, A.; Tammaro, R.; Marigo, V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. 2006, 103, 17366–17371. [Google Scholar] [CrossRef]
- Huang, L.; Himawan, E.; Belhadj, S.; García, R.O.P.; Durand, F.P.; Schipper, N.; Buzgo, M.; Simaite, A.; Marigo, V. Efficient delivery of hydrophilic small molecules to retinal cell lines using gel core-containing solid lipid nanoparticles. Pharmaceutics 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.S.; Shenoy, S.K.; Suk, J.S.; Hanes, J.S.; Rupenthal, I.D. Validation of hyaluronic acid-agar-based hydrogels as vitreous humor mimetics for in vitro drug and particle migration evaluations. Eur. J. Pharm. Biopharm. 2020, 148, 118–125. [Google Scholar] [CrossRef]
- Ottonelli, I.; Duskey, J.T.; Rinaldi, A.; Grazioli, M.V.; Parmeggiani, I.; Vandelli, M.A.; Wang, L.Z.; Prud’homme, R.K.; Tosi, G.; Ruozi, B. Microfluidic technology for the production of hybrid nanomedicines. Pharmaceutics 2021, 13, 1495. [Google Scholar] [CrossRef]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Wakebayashi, D.; Nishiyama, N.; Itaka, K.; Miyata, K.; Yamasaki, Y.; Harada, A.; Koyama, H.; Nagasaki, Y.; Kataoka, K. Polyion complex micelles of pdna with acetal-poly(ethylene glycol)-poly(2-(dimethylamino)ethyl methacrylate) block copolymer as the gene carrier system: Physicochemical properties of micelles relevant to gene transfection efficacy. Biomacromolecules 2004, 5, 2128–2136. [Google Scholar] [CrossRef]
- Nickerson, C.S.; Karageozian, H.L.; Park, J.; Kornfield, J.A. The mechanical properties of the vitreous humor. Investig. Ophthalmol. Vis. Sci. 2004, 45, 37. [Google Scholar]
- Nickerson, C.S.; Park, J.; Kornfield, J.A.; Karageozian, H. Rheological properties of the vitreous and the role of hyaluronic acid. J. Biomech. 2008, 41, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.A.; Ghorab, M.; Mahmoud, A.A.; Abdel Hady, M. PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech 2017, 18, 2517–2528. [Google Scholar] [CrossRef]
- Zhang, E.; Zhukova, V.; Semyonkin, A.; Osipova, N.; Malinovskaya, Y.; Maksimenko, O.; Chernikov, V.; Sokolov, M.; Grigartzik, L.; Sabel, B.A.; et al. Release kinetics of fluorescent dyes from PLGA nanoparticles in retinal blood vessels: In Vivo monitoring and ex vivo localization. Eur. J. Pharm. Biopharm. 2020, 150, 131–142. [Google Scholar] [CrossRef]
- Meikle, T.G.; Drummond, C.J.; Conn, C.E.; Meikle, T.G.; Drummond, C.J.; Conn, C.E. Microfluidic synthesis of rifampicin loaded PLGA nanoparticles and the effect of formulation on their physical and antibacterial properties. Aust. J. Chem. 2019, 73, 151–157. [Google Scholar] [CrossRef]
- Markowski, A.; Jaromin, A.; Migdał, P.; Olczak, E.; Zygmunt, A.; Zaremba-Czogalla, M.; Pawlik, K.; Gubernator, J. Design and development of a new type of hybrid PLGA/lipid nanoparticle as an ursolic acid delivery system against pancreatic ductal adenocarcinoma cells. Int. J. Mol. Sci. 2022, 23, 5536. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases: Nanocarrier mediated retinal drug delivery. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Laradji, A.; Karakocak, B.B.; Kolesnikov, A.V.; Kefalov, V.J.; Ravi, N. Hyaluronic acid-based gold nanoparticles for the topical delivery of therapeutics to the retina and the retinal pigment epithelium. Polymers 2021, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, K.; Zhang, H.; Liu, Q.; Wang, J.; Cao, L.; Li, W.; Wang, K.; Hong, Z. DOTAP-incorporated PEG-PLGA nanoparticles for efficient in vitro and in vivo gene delivery. J. Biomed. Nanotechnol. 2018, 14, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, E.; Vandervelden, C.; Fakhari, A.; Qian, J.; Berkland, C.; Gehrke, S.H. Design of hollow hyaluronic acid cylinders for sustained intravitreal protein delivery. J. Pharm. Sci. 2018, 107, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, A.; Kamali, N.; Shiri, F.; Majd, M.H. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cells Nanomed. Biotechnol. 2018, 46, 500–509. [Google Scholar] [CrossRef]
- Gómez-Mariscal, M.; Puerto, B.; Muñoz-Negrete, F.J.; de Juan, V.; Rebolleda, G. Acute and chronic optic nerve head biomechanics and intraocular pressure changes in patients receiving multiple intravitreal injections of anti-VEGF. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2221–2231. [Google Scholar] [CrossRef]
- Pacella, E.; Loffredo, L.; Malvasi, M.; Trovato Battagliola, E.; Messineo, D.; Pacella, F.; Arrico, L. Effects of repeated intravitreal injections of dexamethasone implants on intraocular pressure: A 4-Year study. Clin. Ophthalmol. 2020, 14, 3611–3617. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Lin, Q.; Boo, Y.J.; Ow, V.; Zhao, X.; Wong, D.S.L.; Lim, J.Y.C.; Xue, K.; Su, X.; et al. Injectable PTHF-based thermogelling polyurethane implants for long-term intraocular application. Biomater. Res. 2022, 26, 70. [Google Scholar] [CrossRef]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-hydrogel composites: From molecular interactions to macroscopic behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Xia, S.; Muhoza, B.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Sodium carboxymethyl cellulose modulates the stability of cinnamaldehyde-loaded liposomes at high ionic strength. Food Hydrocoll. 2019, 93, 10–18. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-responsive PLGA-PEG-PLGA hydrogels as novel injectable platforms for neuroprotective combined therapies in the treatment of retinal degenerative diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Neupane, Y.R.; Mehra, N.; Nematullah, M.; Khan, F.; Alam, O.; Iqubal, A.; Jain, G.K.; Kohli, K. Sirolimus loaded chitosan functionalized poly (lactic-co-glycolic acid) (PLGA) nanoparticles for potential treatment of age-related macular degeneration. Int. J. Biol. Macromol. 2021, 191, 548–559. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-hydrogel composite drug delivery system for potential ocular applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Taheri, S.L.; Rezazadeh, M.; Hassanzadeh, F.; Akbari, V.; Dehghani, A.; Talebi, A.; Mostafavi, S.A. Preparation, physicochemical, and retinal anti-angiogenic evaluation of poloxamer hydrogel containing dexamethasone/avastin-loaded chitosan-N-acetyl-L-cysteine nanoparticles. Int. J. Biol. Macromol. 2022, 220, 1605–1618. [Google Scholar] [CrossRef]



| N:O Ratio | Size (nm) | PDI | Z Potential (mV) |
|---|---|---|---|
| 1:0 † | 239 ± 18 | 0.16 ± 0.04 | +39 ± 2 |
| 1:0.025 | 306 ± 12 | 0.29 ± 0.05 | +38 ± 7 |
| 1:0.05 | >1000 | / | / |
| 1:0.1 | >1000 | / | / |
| 1:0.2 | >1000 | / | / |
| 1:0.5 | 846 ± 147 | 0.78 ± 0.11 | −30 ± 7 |
| 1:1 | 257 ± 6 | 0.21 ± 0.04 | −35 ± 5 |
| 1:2 | 298 ± 16 | 0.25 ± 0.07 | −38 ± 6 |
| HA330 | Tgel °C (SD) |
|---|---|
| 0.1% | 27.2 (2.0) |
| 0.25% | 24.2 (0.3) |
| 0.5% | 25.7 (3.0) |
| 0.75% | 23.1 (0.1) |
| 1% | 26.6 (1.9) |
| P407 | Tgel °C (SD) |
|---|---|
| 5% | >50 |
| 10% | 46.2 (3.1) |
| 15% | 25.7 (3.0) |
| [NPs] | Uncoated NPs | HA12-coated NPs | ||||
|---|---|---|---|---|---|---|
| Size nm (SD) | PDI (SD) | Tgel °C (SD) | Size nm (SD) | PDI (SD) | Tgel °C (SD) | |
| 4 mg/mL | 228 (5) | 0.10 (0.05) | 40.4 (2.4) | 224 (13) | 0.16 (0.02) | 36.6 (0.9) |
| 8 mg/mL | 239 (18) | 0.16 (0.04) | 34.3 (0.5) | 257 (6) | 0.21 (0.04) | 35.1 (1.1) |
| 12 mg/mL | 460 (114) | 0.25 (0.18) | 39.2 (10.3) | 361 (24) | 0.30 (0.05) | 38.8 (9.4) |
| Agarose | HA1100 | η (Pas) | G’ (Pa) |
|---|---|---|---|
| 0.5% w/v | 0.5% w/v | 11.0 ± 1.9 | 15.1 ± 3.2 |
| 0.1% w/v | 0.5% w/v | 5.1 ± 0.5 | 13.9 ± 2.3 |
| 0.1% w/v | 0.25% w/v | 0.5 ± 0.1 | 0.7 ± 0.1 |
| 0.05% w/v | 0.5% w/v | 0.7 ± 0.1 | 1.2 ± 0.2 |
| 0.05% w/v | 0.25% w/v | 0.1 ± 0.0 | 0.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottonelli, I.; Bighinati, A.; Adani, E.; Loll, F.; Caraffi, R.; Vandelli, M.A.; Boury, F.; Tosi, G.; Duskey, J.T.; Marigo, V.; et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics 2023, 15, 25. https://doi.org/10.3390/pharmaceutics15010025
Ottonelli I, Bighinati A, Adani E, Loll F, Caraffi R, Vandelli MA, Boury F, Tosi G, Duskey JT, Marigo V, et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics. 2023; 15(1):25. https://doi.org/10.3390/pharmaceutics15010025
Chicago/Turabian StyleOttonelli, Ilaria, Andrea Bighinati, Elisa Adani, François Loll, Riccardo Caraffi, Maria Angela Vandelli, Frank Boury, Giovanni Tosi, Jason Thomas Duskey, Valeria Marigo, and et al. 2023. "Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles" Pharmaceutics 15, no. 1: 25. https://doi.org/10.3390/pharmaceutics15010025
APA StyleOttonelli, I., Bighinati, A., Adani, E., Loll, F., Caraffi, R., Vandelli, M. A., Boury, F., Tosi, G., Duskey, J. T., Marigo, V., & Ruozi, B. (2023). Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics, 15(1), 25. https://doi.org/10.3390/pharmaceutics15010025









