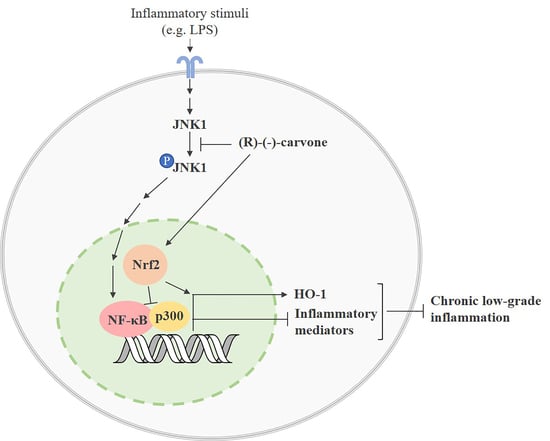
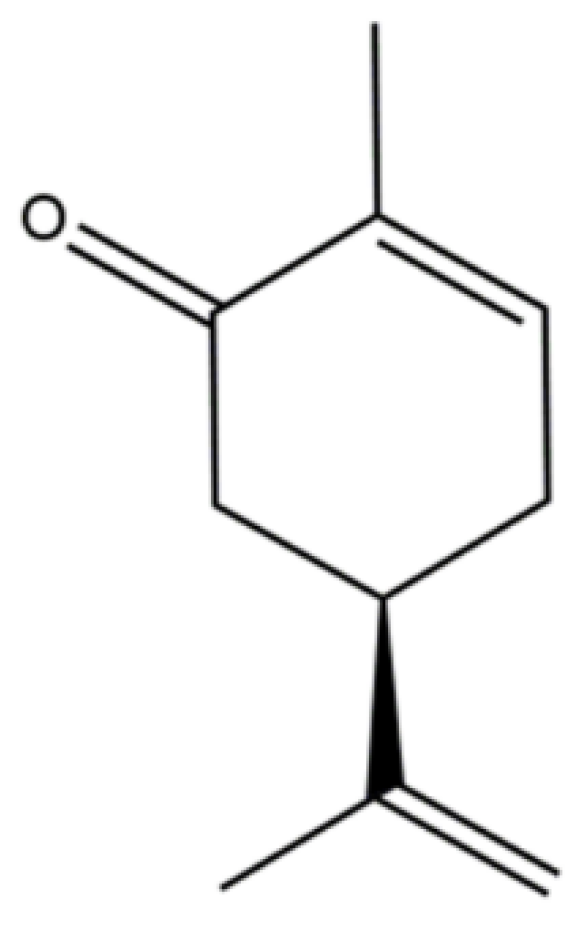
Molecular Mechanisms Underlying the Anti-Inflammatory Properties of (R)-(-)-Carvone: Potential Roles of JNK1, Nrf2 and NF-κB
Abstract
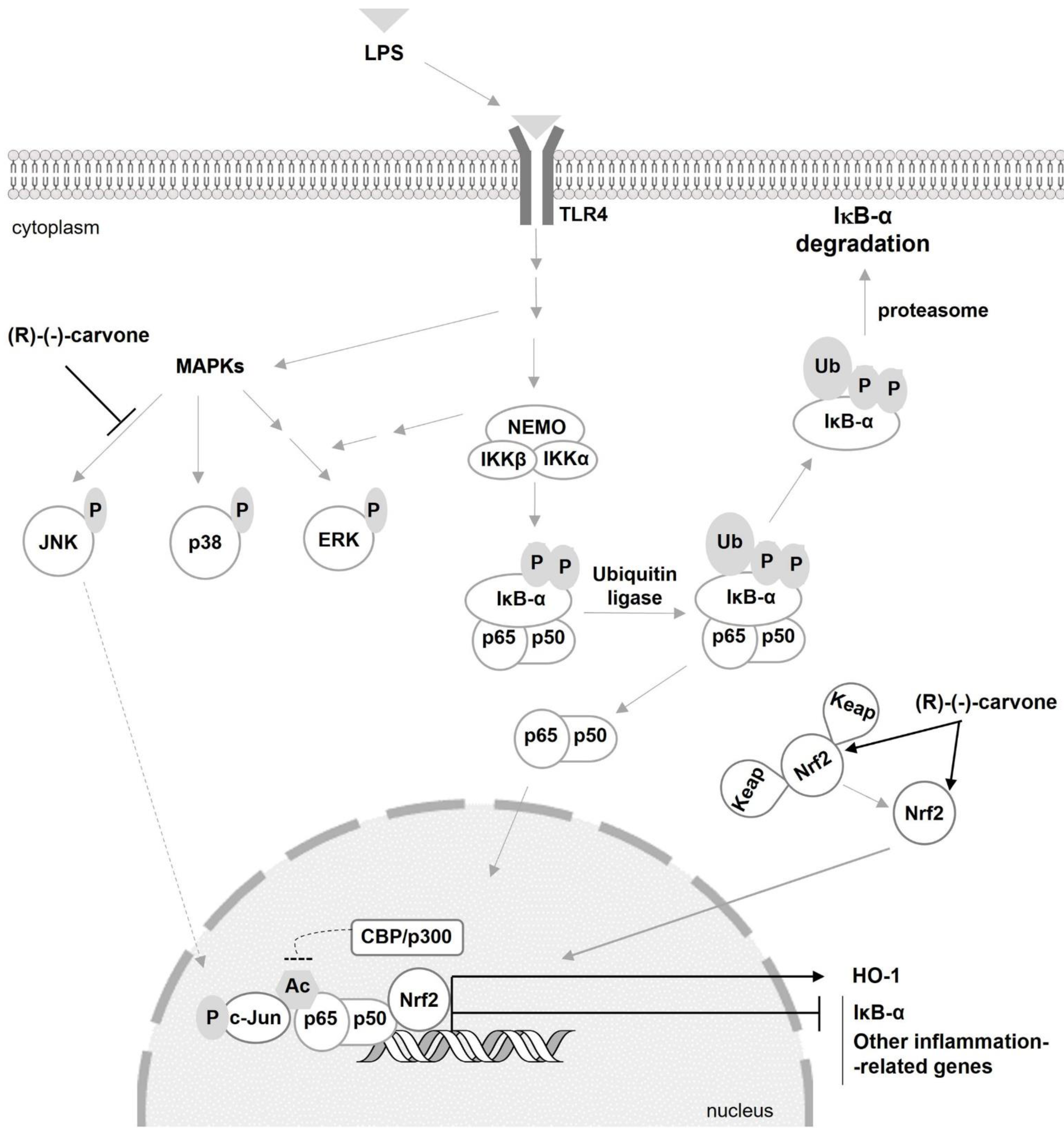
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Preparation of Cell Extracts
2.3. Western Blot
2.4. Immunocytochemistry
2.5. SIRT1 Activity Assay
2.6. Statistical Analysis
3. Results
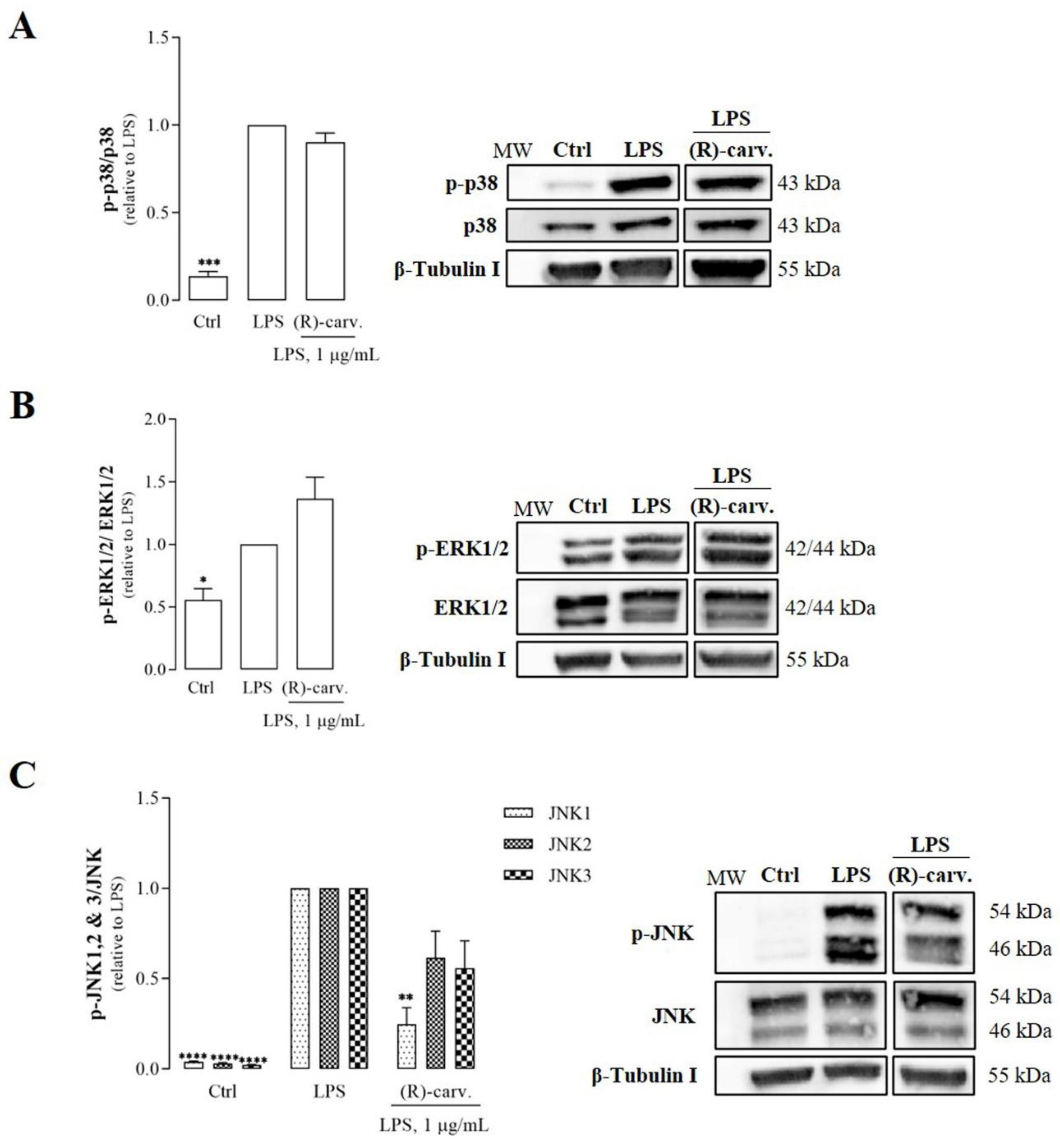
3.1.(R)-(-)-Carvone Inhibits LPS-Induced Phosphorylation of JNK1, but Not That of Other JNK Isoforms, p38 and ERK1/2
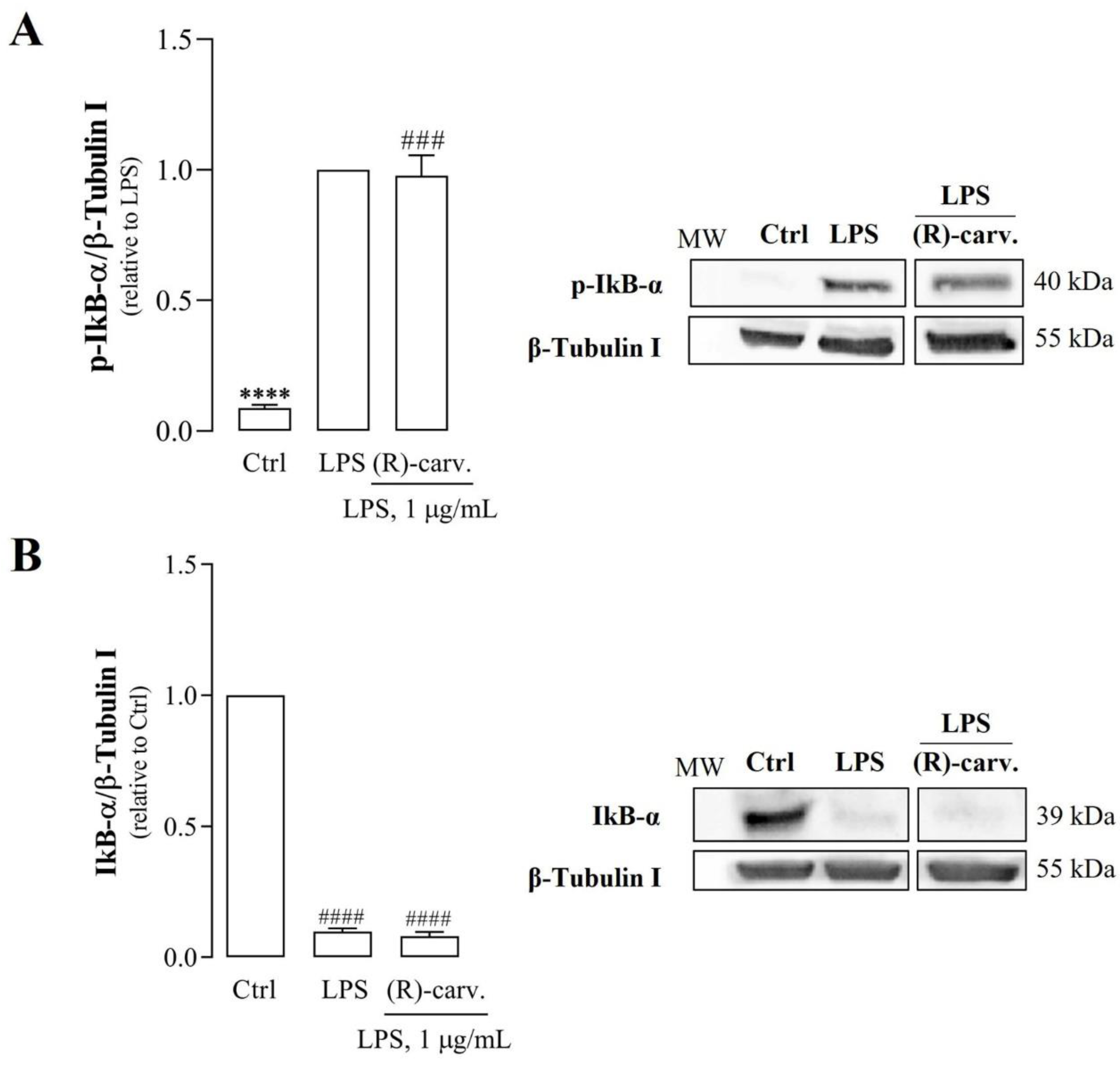
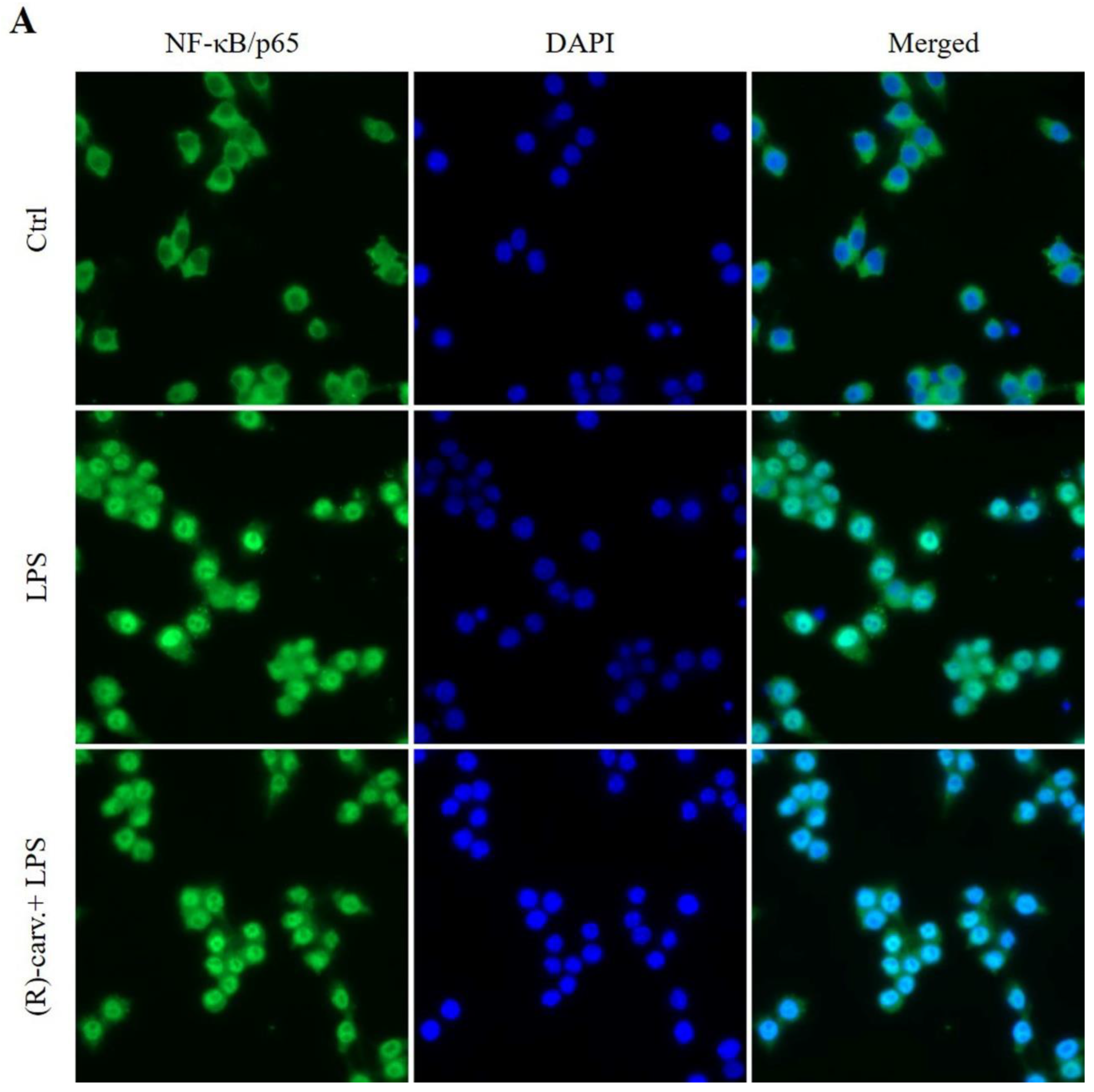
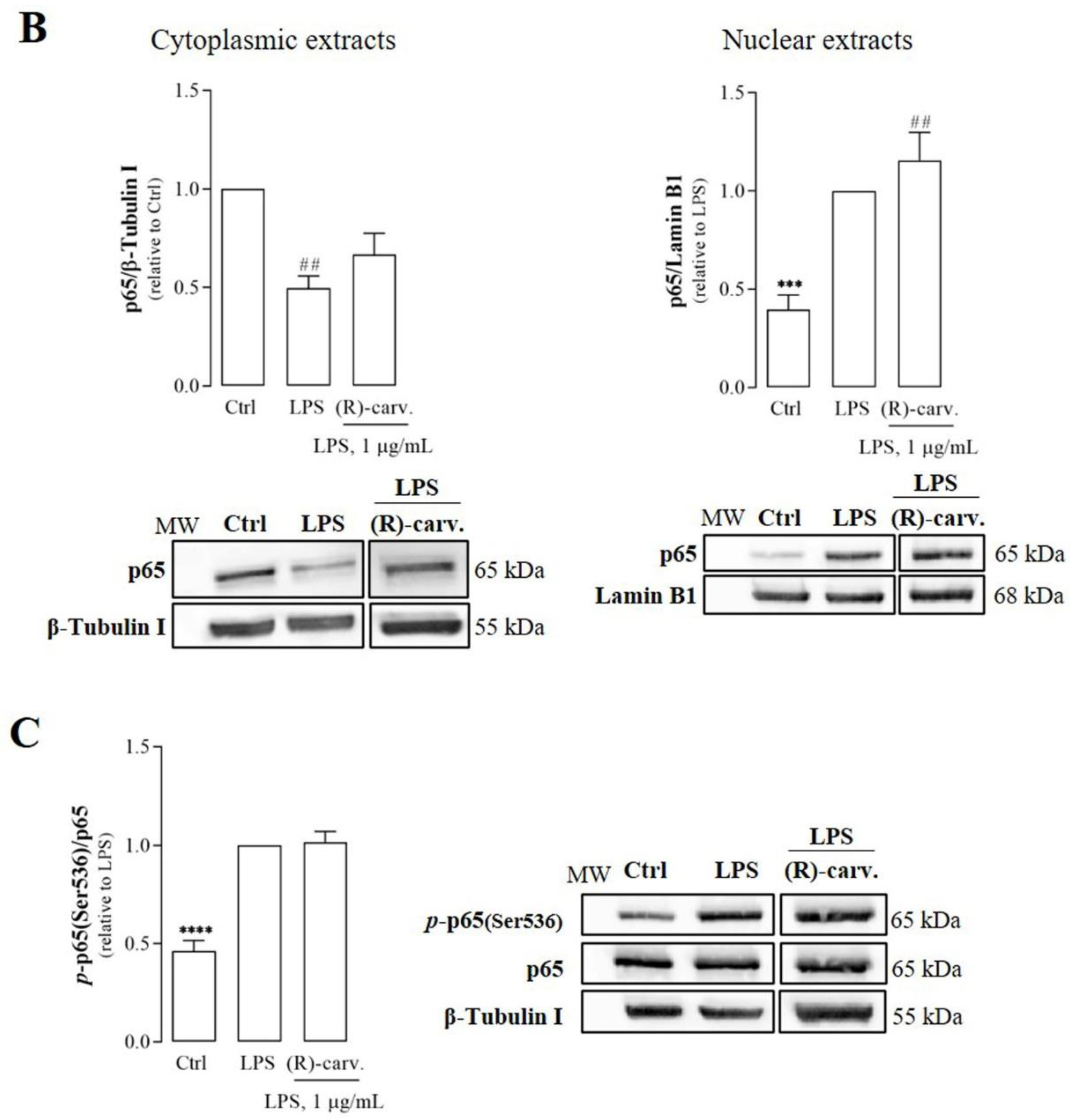
3.2.(R)-(-)-Carvone Does Not Interfere with the Canonical Activation Pathway and Nuclear Translocation of NF-κB
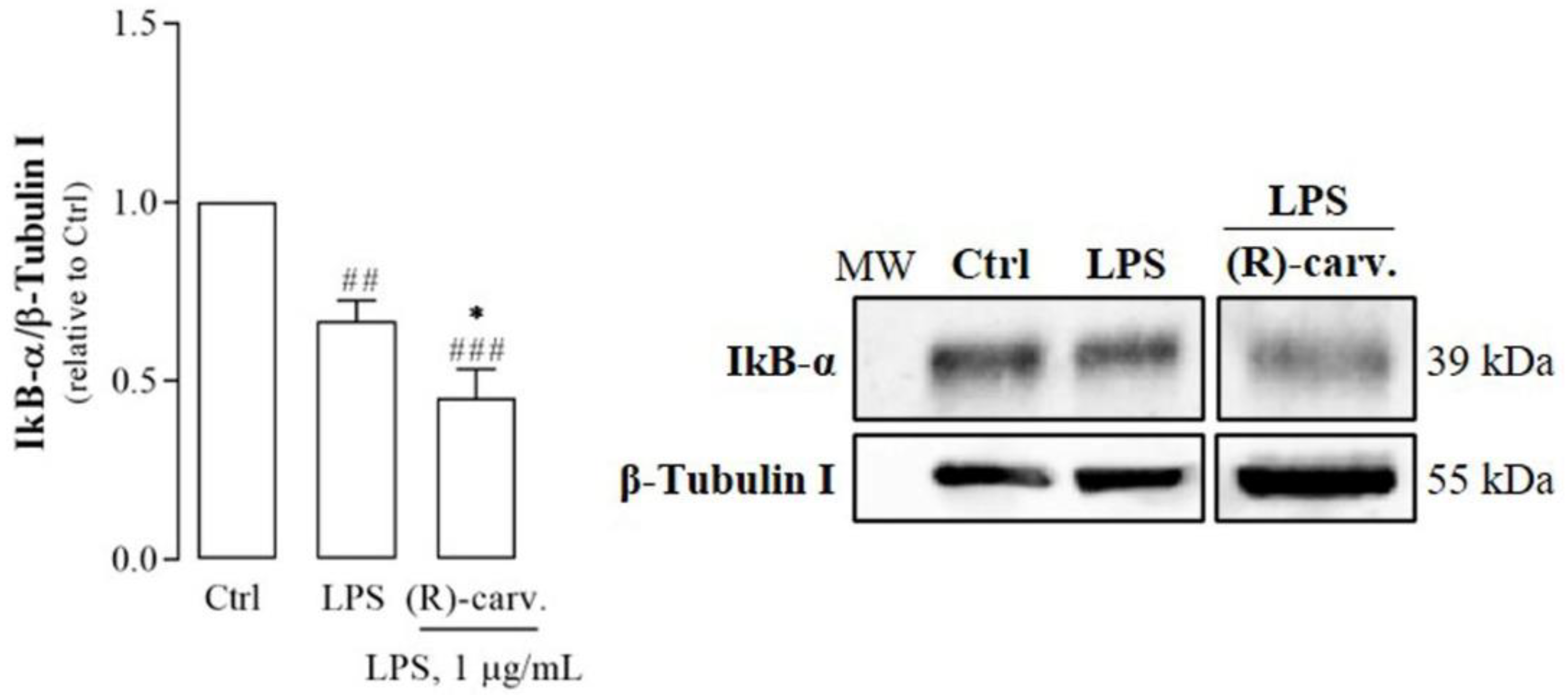
3.3. (R)-(-)-Carvone Inhibits IκB-α Resynthesis
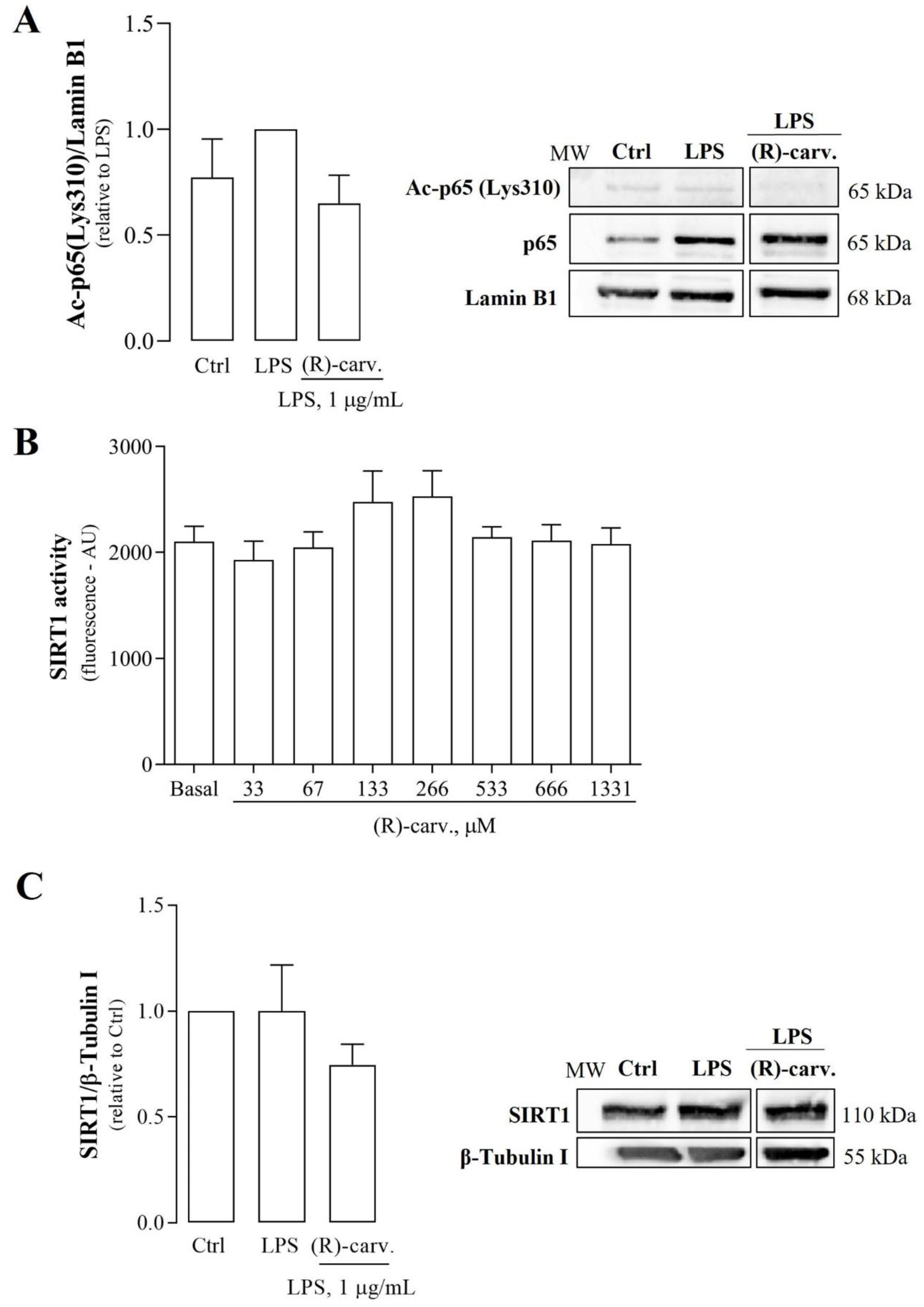
3.4. (R)-(-)-Carvone Tends to Decrease LPS-Induced Acetylation of NF-κB/p65 at Lys310 Independently of SIRT1 Activity and Expression
3.5. (R)-(-)-Carvone Promotes Nrf2 Nuclear Translocation and the Expression of its Target Gene, Heme Oxygenase-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azab, A.; Nassar, A.; Azab, A. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- de Cássia da Silveira e Sá, R.; Andrade, L.; de Sousa, D. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.F.; Cruz, M.T.; Gualillo, O. Editorial: The Physiology of Inflammation—The Final Common Pathway to Disease. Front. Physiol. 2018, 9, 1741. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-Inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the Anti-Inflammatory, Anti-Catabolic and pro-Anabolic Effects of E-Caryophyllene, Myrcene and Limonene in a Cell Model of Osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Differential Effects of the Essential Oils of Lavandula Luisieri and Eryngium Duriaei Subsp. Juresianum in Cell Models of Two Chronic Inflammatory Diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef]
- Sousa, C.; Leitão, A.J.; Neves, B.M.; Judas, F.; Cavaleiro, C.; Mendes, A.F. Standardised Comparison of Limonene-Derived Monoterpenes Identifies Structural Determinants of Anti-Inflammatory Activity. Sci. Rep. 2020, 10, 7199. [Google Scholar] [CrossRef]
- Sousa, C.; Neves, B.M.; Leitão, A.J.; Mendes, A.F. Elucidation of the Mechanism Underlying the Anti-Inflammatory Properties of (S)-(+)-Carvone Identifies a Novel Class of Sirtuin-1 Activators in a Murine Macrophage Cell Line. Biomedicines 2021, 9, 777. [Google Scholar] [CrossRef]
- Sousa, C.; Ribeiro, M.; Rufino, A.T.; Leitão, A.J.; Mendes, A.F. Assessment of Cell Line Competence for Studies of Pharmacological GPR30 Modulation. J. Recept. Signal Transduct. 2017, 37, 181–188. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-Activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-ΚB, IκB, and IKK: Integral Components of Immune System Signaling. In Structural Immunology; Springer: Singapore, 2019; pp. 207–226. [Google Scholar]
- Mitchell, J.P.; Carmody, R.J. NF-ΚB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.-Y. IKKβ Plays an Essential Role in the Phosphorylation of RelA/P65 on Serine 536 Induced by Lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. IκB Kinases Phosphorylate NF-ΚB P65 Subunit on Serine 536 in the Transactivation Domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.-D.; Lamb, A.; Chen, L.-F. Posttranslational Modifications of NF-ΚB: Another Layer of Regulation for NF-ΚB Signaling Pathway. Cell. Signal. 2010, 22, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.D.; Ryseck, R.-P.; Attar, R.M.; Dambach, D.; Bravo, R. Functional Redundancy of the Nuclear Factor ΚB Inhibitors IκBα and IκBβ. J. Exp. Med. 1998, 188, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Mu, Y.; Greene, W.C. Acetylation of RelA at Discrete Sites Regulates Distinct Nuclear Functions of NF-KappaB. EMBO J. 2002, 21, 6539–6548. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-ΚB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Zheng, G.; Kenney, P.; Lam, L. Anethofuran, Carvone, and Limonene: Potential Cancer Chemoprotective Agents from Dill Weed Oil and Caraway Oil. Planta Med. 1992, 58, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; De Long, M.J.; Prochaska, H.J. Identification of a Common Chemical Signal Regulating the Induction of Enzymes That Protect against Chemical Carcinogenesis. Proc. Natl. Acad. Sci. USA 1988, 85, 8261–8265. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, J.; Weinstein, S.L.; Lem, L.; DeFranco, A.L. Activation of C-Jun N-Terminal Kinase in Bacterial Lipopolysaccharide-Stimulated Macrophages. Proc. Natl. Acad. Sci. USA 1996, 93, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Xia, Y. C-Jun, at the Crossroad of the Signaling Network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.U.; Wesche, H. Summary and Comparison of the Signaling Mechanisms of the Toll/Interleukin-1 Receptor Family. Biochim. Biophys. Acta-Mol. Cell Res. 2002, 1592, 265–280. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS Induction of Gene Expression in Human Monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Wolter, S.; Doerrie, A.; Weber, A.; Schneider, H.; Hoffmann, E.; von der Ohe, J.; Bakiri, L.; Wagner, E.F.; Resch, K.; Kracht, M. C-Jun Controls Histone Modifications, NF-ΚB Recruitment, and RNA Polymerase II Function To Activate the Ccl2 Gene. Mol. Cell. Biol. 2008, 28, 4407–4423. [Google Scholar] [CrossRef]
- Marques, F.M.; Figueira, M.M.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. In Vitro Anti-Inflammatory Activity of Terpenes via Suppression of Superoxide and Nitric Oxide Generation and the NF-ΚB Signalling Pathway. Inflammopharmacology 2019, 27, 281–289. [Google Scholar] [CrossRef]
- Mendes, A.F.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Differential Roles of Hydrogen Peroxide and Superoxide in Mediating IL-1-Induced NF-?B Activation and INOS Expression in Bovine Articular Chondrocytes. J. Cell. Biochem. 2003, 88, 783–793. [Google Scholar] [CrossRef]
- Ferreira Mendes, A.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Hydrogen Peroxide Mediates Interleukin-1β-Induced AP-1 Activation in Articular Chondrocytes: Implications for the Regulation of INOS Expression. Cell Biol. Toxicol. 2003, 19, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting Innate Immunity Protein Kinase Signalling in Inflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef]
- Nam, N.-H. Naturally Occurring NF-KB Inhibitors. Mini-Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A. Intersection of Inflammation and Herbal Medicine in the Treatment of Osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-M.; Guan, P.; Luo, L.-F.; Qin, L.-Y.; Wang, N.; Zhao, Y.-S.; Ji, E.-S. Resveratrol Protects against CIH-Induced Myocardial Injury by Targeting Nrf2 and Blocking NLRP3 Inflammasome Activation. Life Sci. 2020, 245, 117362. [Google Scholar] [CrossRef]
- Sousa, C.; Mendes, A.F. Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules 2022, 12, 921. [Google Scholar] [CrossRef]
- Abbas, M.A.; Oriquat, G.A.; Abbas, M.M.; Al-Najjar, B.O.; Kandil, Y.I. Hypolipidaemic and Insulin Secretagogue Activities of (R)-(−)-Carvone. Malaysian J. Med. Sci. 2020, 27, 39–52. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(-)-Carvone Attenuated Doxorubicin Induced Cardiotoxicity In Vivo and Potentiated Its Anticancer Toxicity In Vitro. Balkan Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, J.; da Silva Brandi, J.; Ferreira Costa, H.; Carla de Paula Medeiros, K.; Alves Leite, J.; Pergentino de Sousa, D.; Regina Piuvezam, M. Carvone Enantiomers Differentially Modulate IgE-Mediated Airway Inflammation in Mice. Int. J. Mol. Sci. 2020, 21, 9209. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Liu, F.-C.; Hung, L.-F.; Wu, W.-L.; Chang, D.-M.; Huang, C.-Y.; Lai, J.-H.; Ho, L.-J. Chondroprotective Effects and Mechanisms of Resveratrol in Advanced Glycation End Products-Stimulated Chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, J.; Sun, K.; Guo, J.; Yao, X.; Wang, G.; Hou, L.; Xu, J.; Guo, J.; Guo, F. Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway. Front. Pharmacol. 2022, 13, 791376. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin Protects against Osteoarthritis via Nrf2: A Guardian of Cartilage Homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Y. Curcumin Reduces Inflammation in Knee Osteoarthritis Rats through Blocking TLR4 /MyD88/NF-κB Signal Pathway. Drug Dev. Res. 2019, 80, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol Ameliorates Inflammatory Damage and Protects against Osteoarthritis in a Rat Model of Osteoarthritis. Mol. Med. Rep. 2017, 17, 1493–1498. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-ΚB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol Suppresses LPS-Induced pro-Inflammatory Activation in RAW 264.7 Macrophages through ERK1/2 and NF-KB Pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]

| Protein | Source | Clonality | Dilution | Supplier | Catalogue/Lot Number |
|---|---|---|---|---|---|
| phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc., Danvers, MA, USA | 9101/27 |
| p44/42 MAPK (ERK1/2) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | 9102/26 |
| phospho-p38 MAPK (Thr180/Tyr182) | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | 9211/ 21 |
| p38 MAPK | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | 9212/17 |
| phospho-SAPK/JNK (Thr183/Tyr185) | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | 4668/11 |
| SAPK/JNK | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc. | 9252/17 |
| phospho-IκB-α (Ser32/36) | mouse | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | 9246/14 |
| IκB-α | rabbit | polyclonal | 1:1000 | Cell Signaling Technology, Inc., Danvers, MA, USA | 9242/9 |
| NF-κB p65 (D14E12) XP(R) | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | 8242/4 |
| phospho- NF-κB p65 (Ser536) | rabbit | monoclonal | 1:1000 | Cell Signaling Technology, Inc. | 3033/14 |
| acetyl-NF-κB p65 (Lys310) | rabbit | polyclonal | 1:750 | Cell Signaling Technology, Inc. | 3045/2 |
| Sirtuin-1 | rabbit | polyclonal | 1:1000 | Sigma-Aldrich Co. | 07-131/2736563 |
| Nrf2 (C-20) | rabbit | polyclonal | 1:500 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | sc-722/A1612 |
| Heme oxygenase-1 | mouse | monoclonal | 1:1000 | Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA | MA 1-112/TK2665301 |
| β-tubulin I | mouse | monoclonal | 1:20,000 | Sigma-Aldrich Co. | T7816/052M4835 |
| lamin B1 | rabbit | polyclonal | 1:1000 | Abcam, Cambridge, UK | ab16048/ GR48958-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, C.; Neves, B.M.; Leitão, A.J.; Mendes, A.F. Molecular Mechanisms Underlying the Anti-Inflammatory Properties of (R)-(-)-Carvone: Potential Roles of JNK1, Nrf2 and NF-κB. Pharmaceutics 2023, 15, 249. https://doi.org/10.3390/pharmaceutics15010249
Sousa C, Neves BM, Leitão AJ, Mendes AF. Molecular Mechanisms Underlying the Anti-Inflammatory Properties of (R)-(-)-Carvone: Potential Roles of JNK1, Nrf2 and NF-κB. Pharmaceutics. 2023; 15(1):249. https://doi.org/10.3390/pharmaceutics15010249
Chicago/Turabian StyleSousa, Cátia, Bruno Miguel Neves, Alcino Jorge Leitão, and Alexandrina Ferreira Mendes. 2023. "Molecular Mechanisms Underlying the Anti-Inflammatory Properties of (R)-(-)-Carvone: Potential Roles of JNK1, Nrf2 and NF-κB" Pharmaceutics 15, no. 1: 249. https://doi.org/10.3390/pharmaceutics15010249
APA StyleSousa, C., Neves, B. M., Leitão, A. J., & Mendes, A. F. (2023). Molecular Mechanisms Underlying the Anti-Inflammatory Properties of (R)-(-)-Carvone: Potential Roles of JNK1, Nrf2 and NF-κB. Pharmaceutics, 15(1), 249. https://doi.org/10.3390/pharmaceutics15010249