The Novel Peptide Chm-273s Has Therapeutic Potential for Metabolic Disorders: Evidence from In Vitro Studies and High-Sucrose Diet and High-Fat Diet Rodent Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Drug Candidates
2.2. Animals, Treatments, and Tissue Processing
2.3. Feed
2.4. Cell Culture
2.5. RNA Isolation and Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR) Protocol
2.6. Immunohistochemistry of Phosphorylated STAT3 (p-STAT3) in Mouse Hypothalamus
2.7. Serum Glucose, Insulin, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
2.8. WB Analysis
2.9. Evaluation of the Proinflammatory Cytokine Tumor Necrosis Factor Alpha (TNFα) in Serum
2.10. Free-Feeding Food Intake Experiment in Mice
2.11. OGTT
2.12. Histology
2.13. Statistical Analysis
3. Results
3.1. In Vitro Screening for Novel Regulatory Peptides to Treat Metabolic Disorders
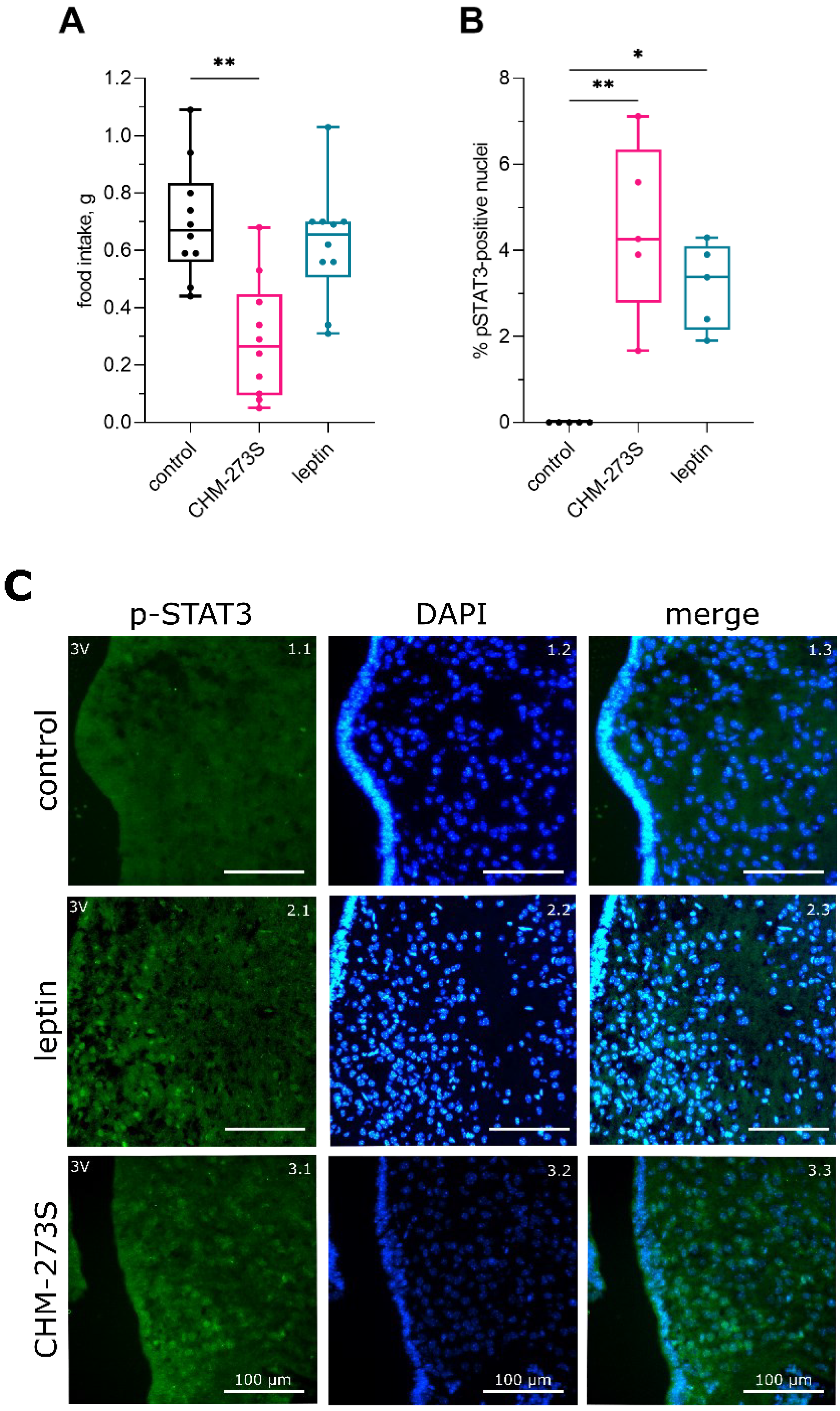
3.2. Free-Feeding Food Intake and Hypothalamic STAT3 Activity Regulation by CHM-273S
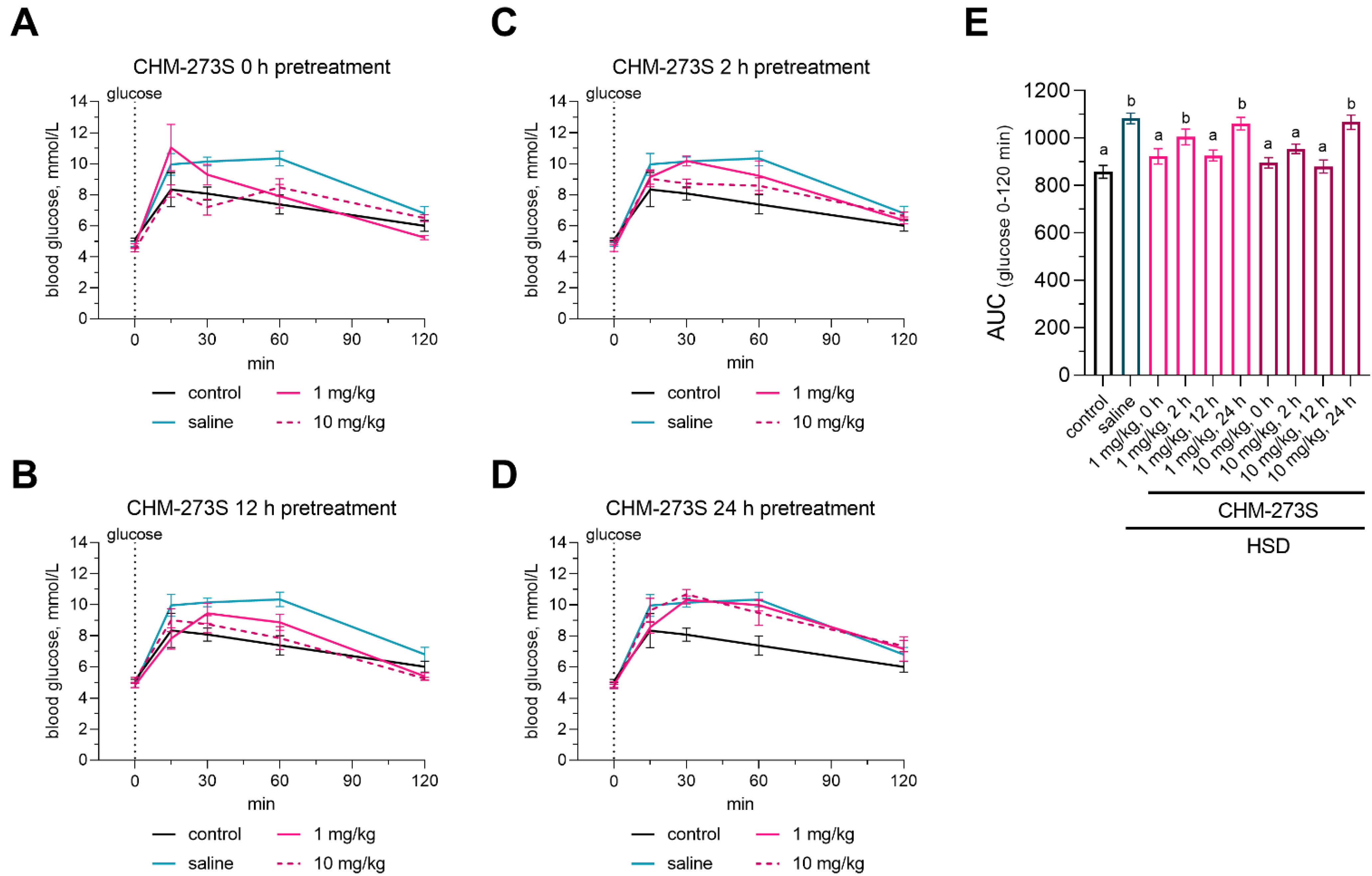
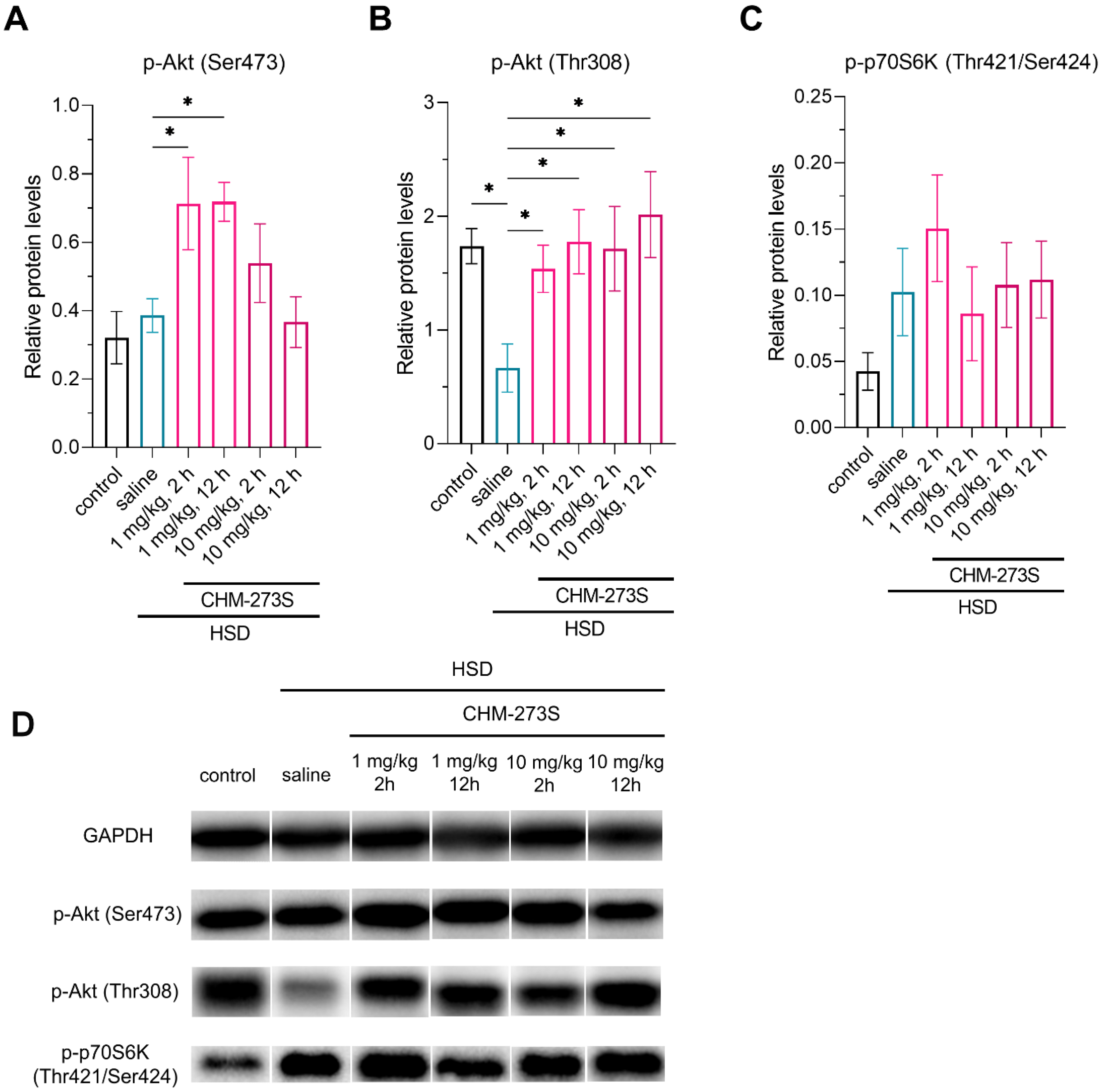
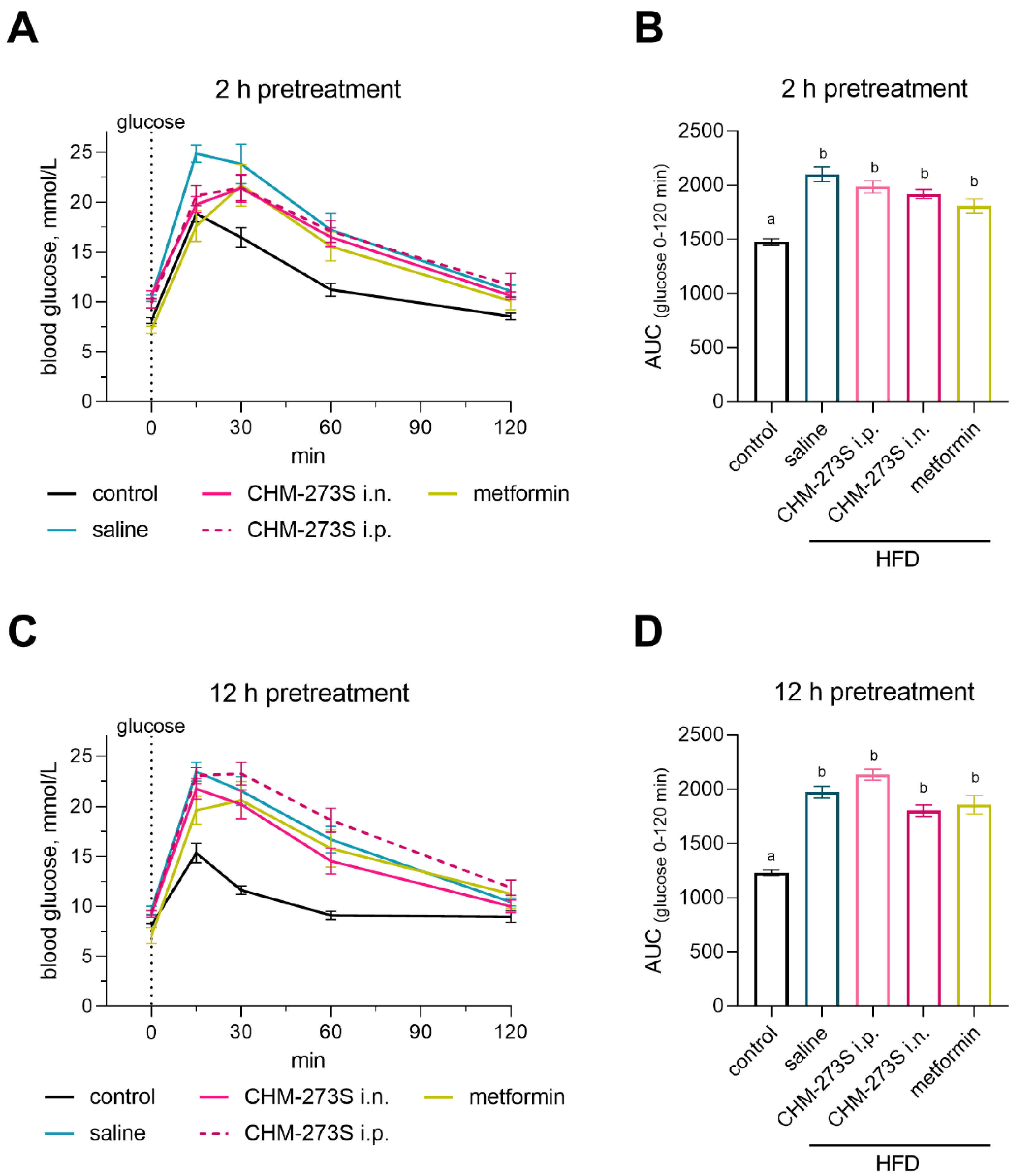
3.3. Glucose Tolerance, Insulin Resistance, and Liver Insulin Signaling Alterations Induced by the HSD and the Acute CHM-273S Treatment Effects
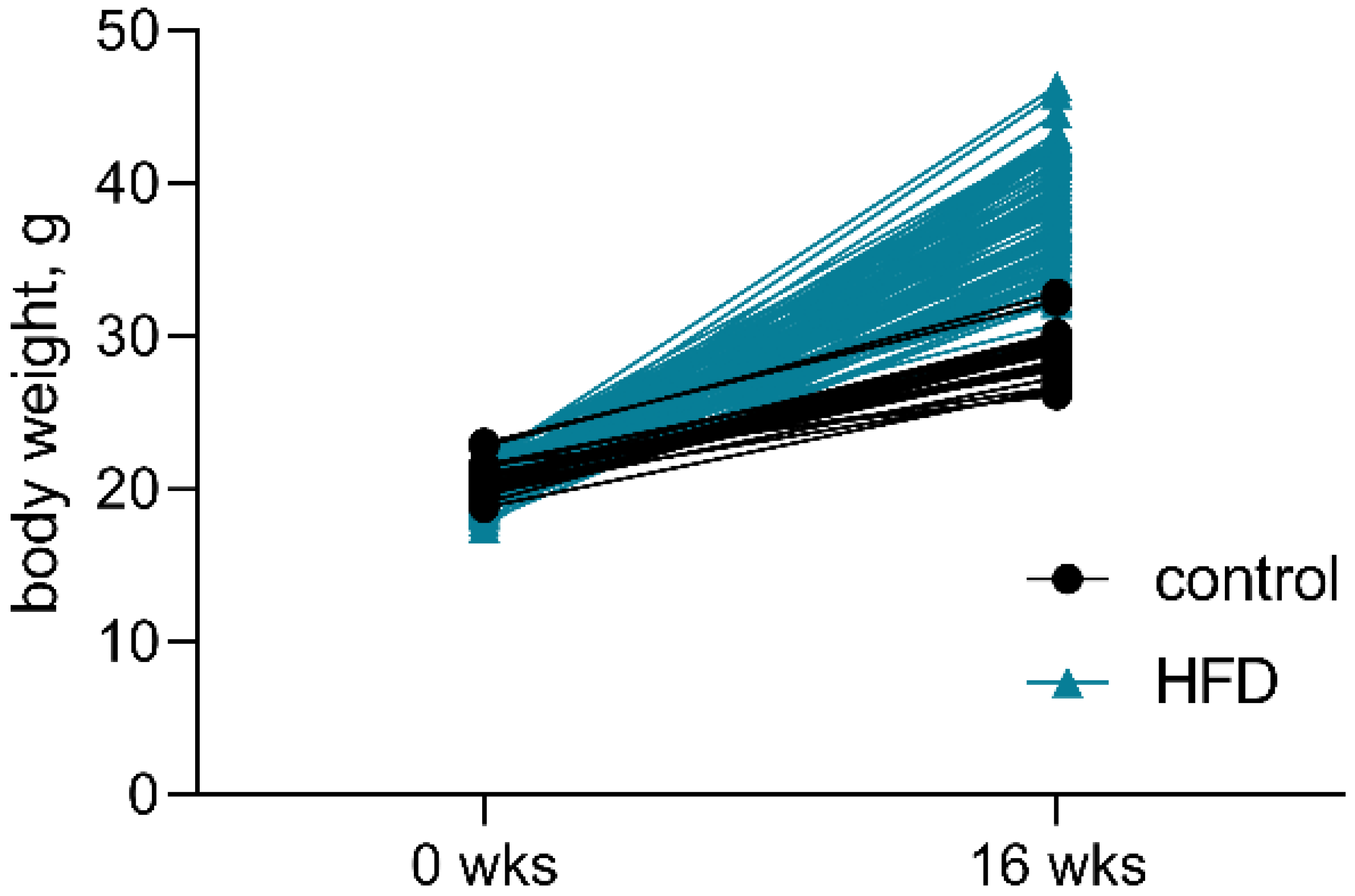
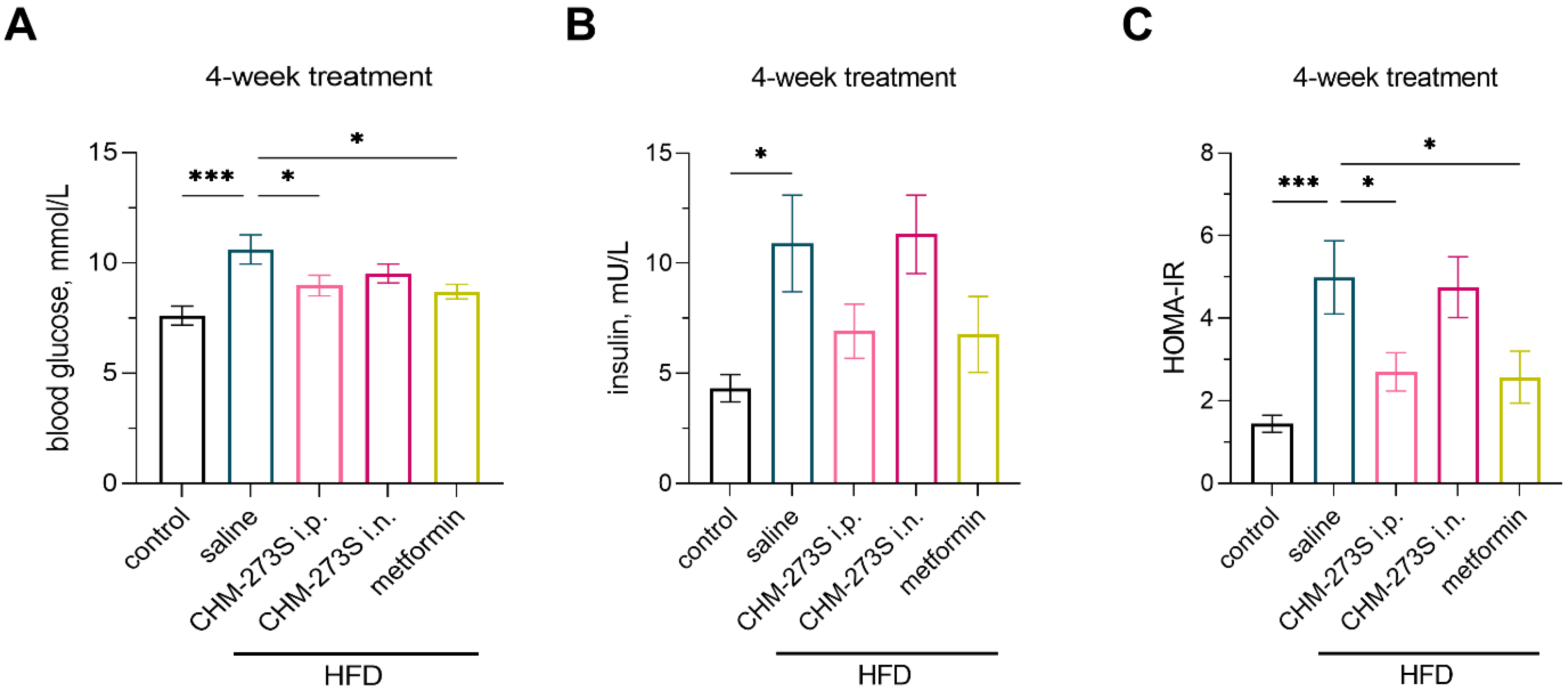
3.4. Glucose Tolerance, Insulin Resistance, and Systemic Inflammation in HFD-Fed Mice and the Effects of Chronic CHM-273S Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, R.J.; A Murray, B. Bioactive peptides and lactic fermentations. Int. J. Dairy Technol. 2006, 59, 118–125. [Google Scholar] [CrossRef]
- Schellekens, H.; Nongonierma, A.B.; Clarke, G.; van Oeffelen, W.E.; FitzGerald, R.J.; Dinan, T.G.; Cryan, J. Milk protein-derived peptides induce 5-HT2C-mediated satiety in vivo. Int. Dairy J. 2014, 38, 55–64. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2014, 18, 49–65. [Google Scholar] [CrossRef]
- Howick, K.; Wallace-Fitzsimons, S.E.; Kandil, D.; Chruścicka, B.; Calis, M.; Murphy, E.; Murray, B.A.; Fernandez, A.; Barry, K.M.; Kelly, P.M.; et al. A Dairy-Derived Ghrelinergic Hydrolysate Modulates Food Intake In Vivo. Int. J. Mol. Sci. 2018, 19, 2780. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Weidner, A.; Dharsono, T.; Boulier, A.; Wilhelm, M.; Schön, C. Postprandial Effects of a Proprietary Milk Protein Hydrolysate Containing Bioactive Peptides in Prediabetic Subjects. Nutrients 2019, 11, 1700. [Google Scholar] [CrossRef]
- McGregor, R.A.; Poppitt, S.D. Milk protein for improved metabolic health: A review of the evidence. Nutr. Metab. 2013, 10, 46. [Google Scholar] [CrossRef]
- Gregersen, S.; Bystrup, S.; Overgaard, A.; Jeppesen, P.B.; Thorup, A.C.S.; Jensen, E.; Hermansen, K. Effects of Whey Proteins on Glucose Metabolism in Normal Wistar Rats and Zucker Diabetic Fatty (ZDF) Rats. Rev. Diabet. Stud. 2013, 10, 252–269. [Google Scholar] [CrossRef]
- Neder Morato, P.; Lollo, P.C.B.; Moura, C.S.; Batista, T.M.; Camargo, R.L.; Carneiro, E.M.; Amaya-Farfan, J. Whey Protein Hydrolysate Increases Translocation of GLUT-4 to the Plasma Membrane Independent of Insulin in Wistar Rats. PLoS ONE 2013, 8, e71134. [Google Scholar] [CrossRef]
- Gaudel, C.; Nongonierma, A.B.; Maher, S.; Flynn, S.; Krause, M.; Murray, B.A.; Kelly, P.M.; Baird, A.W.; Fitzgerald, R.J.; Newsholme, P. A Whey Protein Hydrolysate Promotes Insulinotropic Activity in a Clonal Pancreatic β-Cell Line and Enhances Glycemic Function in ob/ob Mice. J. Nutr. 2013, 143, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fu, Z.; Li, X.; Hong, H.; Zhan, X.; Guo, X.; Luo, Y.; Tan, Y. Whey Protein Hydrolysate Alleviated Atherosclerosis and Hepatic Steatosis by Regulating Lipid Metabolism in ApoE-/- Mice Fed a Western Diet. Food Research International 2022, 157, 111419. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Takezoe, S.; Kitaura, N.; Sutani, T.; Miyazaki, H.; Aoi, W. A milk casein hydrolysate-derived peptide enhances glucose uptake through the AMP-activated protein kinase signalling pathway in skeletal muscle cells. Exp. Physiol. 2021, 106, 496–505. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Mercer, A.; Mawhinney, H.; Pulinilkunnil, T.; Udenigwe, C.C.; Kienesberger, P.C. Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells. Nutrients 2020, 12, 425. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Funct. 2013, 4, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.; Brodkorb, A.; Giblin, L. Dairy-derived peptides for satiety. J. Funct. Foods 2020, 66, 103801. [Google Scholar] [CrossRef]
- Ezquerra, E.A.; Vázquez, J.M.C.; Barrero, A.A. Obesity, Metabolic Syndrome, and Diabetes: Cardiovascular Implications and Therapy. Rev. Española De Cardiol. 2008, 61, 752–764. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215866 (accessed on 20 June 2022).
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. New Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Yao, F.; MacKenzie, R.G. Obesity Drug Update: The Lost Decade? Pharmaceuticals 2010, 3, 3494–3521. [Google Scholar] [CrossRef]
- Markham, A. Setmelanotide: First Approval. Drugs 2021, 81, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Szilvásy-Szabó, A.; Farkas, E.; Ruska, Y.; Pyke, C.; Knudsen, L.B.; Fekete, C. Glucagon-Like Peptide-1 Regulates the Proopiomelanocortin Neurons of the Arcuate Nucleus both Directly and Indirectly via Presynaptic Action. Neuroendocrinology 2020, 111, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Boccia, L.; Gamakharia, S.; Coester, B.; Whiting, L.; Lutz, T.A.; Le Foll, C. Amylin brain circuitry. Peptides 2020, 132, 170366. [Google Scholar] [CrossRef]
- Gao, Z.; Hwang, D.; Bataille, F.; Lefevre, M.; York, D.; Quon, M.J.; Ye, J. Serine Phosphorylation of Insulin Receptor Substrate 1 by Inhibitor κB Kinase Complex. J. Biol. Chem. 2002, 277, 48115–48121. [Google Scholar] [CrossRef]
- White, M.F. Insulin Signaling in Health and Disease. Science 2003, 302, 1710–1711. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Lin, X.; Taguchi, A.; Park, S.; A Kushner, J.; Li, F.; Li, Y.; White, M.F. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J. Clin. Investig. 2004, 114, 908–916. [Google Scholar] [CrossRef]
- Zander, M.; Madsbad, S.; Madsen, J.L.; Holst, J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet 2002, 359, 824–830. [Google Scholar] [CrossRef]
- Rui, L.; Yuan, M.; Frantz, D.; Shoelson, S.; White, M.F. SOCS-1 and SOCS-3 Block Insulin Signaling by Ubiquitin-mediated Degradation of IRS1 and IRS2. J. Biol. Chem. 2002, 277, 42394–42398. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; González-Abuín, N.; Blay, M.; Ardévol, A. Chapter 16—Dietary Proanthocyanidin Modulation of Pancreatic β Cells: Molecular Aspects. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 197–210. ISBN 978-0-12-801585-8. [Google Scholar]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2015, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Melanson, E.L.; Wyatt, H.T. Dietary Fat Intake and Regulation of Energy Balance: Implications for Obesity. J. Nutr. 2000, 130, 284S–288S. [Google Scholar] [CrossRef]
- Schrauwen, P.; Westerterp, K.R. The role of high-fat diets and physical activity in the regulation of body weight. Br. J. Nutr. 2000, 84, 417–427. [Google Scholar] [CrossRef]
- Jéquier, E. Pathways to obesity. Int. J. Obes. Relat. Metab. Disord. 2002, 26 (Suppl. 2), S12–S17. [Google Scholar] [CrossRef]
- French, S.; Robinson, T. Fats and food intake. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 629–634. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2012, 15, 798–808. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef]
- Inui, A. Obesity—A chronic health problem in cloned mice? Trends Pharmacol. Sci. 2003, 24, 77–80. [Google Scholar] [CrossRef]
- Seluanov, A.; Vaidya, A.; Gorbunova, V. Establishing Primary Adult Fibroblast Cultures From Rodents. J. Vis. Exp. 2010, 44, 2033. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.T.; Yao, X.X.; Zhao, F.L.; Zou, X.H.; Yang, W.; Cui, R.J.; Li, B.J. Role of leptin in the regulation of food intake in fasted mice. J. Cell. Mol. Med. 2020, 24, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Cady, G.; Landeryou, T.; Garratt, M.; Kopchick, J.J.; Qi, N.; Garcia-Galiano, D.; Elias, C.F.; Myers, M.G.; Miller, R.A.; Sandoval, D.A.; et al. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LepRb) expressing neurons. Mol. Metab. 2017, 6, 393–405. [Google Scholar] [CrossRef]
- Vasanji, Z.; Cantor, E.J.F.; Juric, D.; Moyen, M.; Netticadan, T. Alterations in cardiac contractile performance and sarcoplasmic reticulum function in sucrose-fed rats is associated with insulin resistance. Am. J. Physiol. Physiol. 2006, 291, C772–C780. [Google Scholar] [CrossRef]
- Dutta, K.; Podolin, D.A.; Davidson, M.B.; Davidoff, A.J. Cardiomyocyte Dysfunction in Sucrose-Fed Rats Is Associated With Insulin Resistance. Diabetes 2001, 50, 1186–1192. [Google Scholar] [CrossRef]
- Wold, L.E.; Dutta, K.; Mason, M.M.; Ren, J.; Cala, S.E.; Schwanke, M.L.; Davidoff, A.J. Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J. Mol. Cell. Cardiol. 2005, 39, 297–307. [Google Scholar] [CrossRef]
- Gerbaix, M.; Metz, L.; Ringot, E.; Courteix, D. Visceral fat mass determination in rodent: Validation of dual-energy x-ray absorptiometry and anthropometric techniques in fat and lean rats. Lipids Health Dis. 2010, 9, 140. [Google Scholar] [CrossRef]
- Mistry, A.M.; Swick, A.G.; Romsos, D.R. Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. J. Nutr. 1997, 127, 2065–2072. [Google Scholar] [CrossRef]
- Sadagurski, M.; Nofech-Mozes, S.; Weingarten, G.; White, M.F.; Kadowaki, T.; Wertheimer, E. Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology. J. Cell. Physiol. 2007, 213, 519–527. [Google Scholar] [CrossRef]
- Sadagurski, M.; Weingarten, G.; Rhodes, C.J.; White, M.F.; Wertheimer, E. Insulin Receptor Substrate 2 Plays Diverse Cell-specific Roles in the Regulation of Glucose Transport. J. Biol. Chem. 2005, 280, 14536–14544. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Illario, M.; Rusciano, M.R.; Gragnaniello, G.; Di Spigna, G.; Leggiero, E.; Pastore, L.; Fenzi, G.; Rossi, G.; Vitale, M. Insulin stimulates fibroblast proliferation through calcium-calmodulin-dependent kinase II. Cell Cycle 2009, 8, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, A.V.; Sukhanova, I.A.; Zlobin, A.S.; Gedzun, V.R.; Pavshintsev, V.V.; Vasileva, E.V.; Zalevsky, A.O.; Doronin, I.I.; Mitkin, N.A.; Golovin, A.V.; et al. In silico Screening and Behavioral Validation of a Novel Peptide, LCGA-17, With Anxiolytic-Like Properties. Front. Neurosci. 2021, 15, 705590. [Google Scholar] [CrossRef]
- Malyshev, A.V.; Sukhanova, I.A.; Ushakova, V.M.; Zorkina, Y.A.; Abramova, O.V.; Morozova, A.Y.; Zubkov, E.A.; Mitkin, N.A.; Pavshintsev, V.V.; Doronin, I.I.; et al. Peptide LCGA-17 Attenuates Behavioral and Neurochemical Deficits in Rodent Models of PTSD and Depression. Pharmaceuticals 2022, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- West, J.A.; Tsakmaki, A.; Ghosh, S.S.; Parkes, D.G.; Grønlund, R.V.; Pedersen, P.J.; Maggs, D.; Rajagopalan, H.; Bewick, G.A. Chronic peptide-based GIP receptor inhibition exhibits modest glucose metabolic changes in mice when administered either alone or combined with GLP-1 agonism. PLoS ONE 2021, 16, e0249239. [Google Scholar] [CrossRef] [PubMed]
- Hampe, L.; Xu, C.; Harris, P.W.R.; Chen, J.; Liu, M.; Middleditch, M.; Radjainia, M.; Wang, Y.; Mitra, A.K. Synthetic peptides designed to modulate adiponectin assembly improve obesity-related metabolic disorders: De-signed Peptides Counter Obesity-Related Disorders. J. Cereb. Blood Flow Metab. 2017, 174, 4478–4492. [Google Scholar] [CrossRef]
- Dholakia, J.; Prabhakar, B.; Shende, P. Strategies for the delivery of antidiabetic drugs via intranasal route. Int. J. Pharm. 2021, 608, 121068. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef]
- Banks, W.A.; During, M.J.; Niehoff, M.L. Brain Uptake of the Glucagon-Like Peptide-1 Antagonist Exendin(9-39) after Intranasal Administration. J. Pharmacol. Exp. Ther. 2004, 309, 469–475. [Google Scholar] [CrossRef]
- Rykalina, N.V.; Askerova, E.V.; Bulushova, N.V.; Kozlov, D.G. Intranasal Human Recombinant Modified Glucagon-Like Peptide-1: High Antihyperglycemic Activity and Duration of Action in Mice. Bull. Exp. Biol. Med. 2020, 169, 53–56. [Google Scholar] [CrossRef]
- Kageyama, H.; Shiba, K.; Hirako, S.; Wada, N.; Yamanaka, S.; Nogi, Y.; Takenoya, F.; Nonaka, N.; Hirano, T.; Inoue, S.; et al. Anti-obesity effect of intranasal administration of galanin-like peptide (GALP) in obese mice. Sci. Rep. 2016, 6, 28200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bartell, S.M.; Baile, C.A.; Chen, B.; Podolsky, R.H.; McIndoe, R.A.; She, J.-X. Hepatic Gene Expression Profiling Reveals Key Pathways Involved in Leptin-Mediated Weight Loss in ob/ob Mice. PLoS ONE 2010, 5, e12147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Camacho, R.; Schwartz, G.J.; Leroith, D. Intracerebroventricular Leptin Infusion Improves Glucose Homeostasis in Lean Type 2 Diabetic MKR Mice via Hepatic Vagal and Non-Vagal Mechanisms. PLoS ONE 2011, 6, e17058. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bradbury, M.W.; Berk, P.D. Leptin and Insulin Modulate Nutrient Partitioning and Weight Loss in ob/ob Mice through Regulation of Long-Chain Fatty Acid Uptake by Adipocytes. J. Nutr. 2003, 133, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Banas, S.M.; Rouch, C.; Kassis, N.; Markaki, E.M.; Gerozissis, K. A Dietary Fat Excess Alters Metabolic and Neuroendocrine Responses Before the Onset of Metabolic Diseases. Cell. Mol. Neurobiol. 2008, 29, 157–168. [Google Scholar] [CrossRef]
- Thorens, B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes. Metab. 2011, 13 (Suppl. 1), 82–88. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ramos, E.; González-Rodríguez, Á.; Canelles, S.; Baquedano, E.; Frago, L.M.; Revuelta-Cervantes, J.; Gómez-Ambrosi, J.; Frühbeck, G.; Chowen, J.A.; Argente, J.; et al. Differential Insulin Receptor Substrate-1 (IRS1)-Related Modulation of Neuropeptide Y and Proopiomelanocortin Expression in Nondiabetic and Diabetic IRS2−/− Mice. Endocrinology 2012, 153, 1129–1140. [Google Scholar] [CrossRef]
- Kitamura, T.; Feng, Y.; Kitamura, Y.I.; Chua, S.C., Jr.; Xu, A.W.; Barsh, G.S.; Rossetti, L.; Accili, D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 2006, 12, 534–540. [Google Scholar] [CrossRef]
- Belgardt, B.F.; Husch, A.; Rother, E.; Ernst, M.B.; Wunderlich, F.T.; Hampel, B.; Klöckener, T.; Alessi, D.; Kloppenburg, P.; Brüning, J.C. PDK1 Deficiency in POMC-Expressing Cells Reveals FOXO1-Dependent and -Independent Pathways in Control of Energy Homeostasis and Stress Response. Cell Metab. 2008, 7, 291–301. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Ader, M.; Stefanovski, D.; Richey, J.M.; Kim, S.P.; Kolka, C.M.; Ionut, V.; Kabir, M.; Bergman, R.N. Failure of Homeostatic Model Assessment of Insulin Resistance to Detect Marked Diet-Induced Insulin Resistance in Dogs. Diabetes 2014, 63, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Fraulob, J.C.; Ogg-Diamantino, R.; Fernandes-Santos, C.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J. Clin. Biochem. Nutr. 2010, 46, 212–223. [Google Scholar] [CrossRef]
- Sun, W.; Bi, Y.; Liang, H.; Cai, M.; Chen, X.; Zhu, Y.; Li, M.; Xu, F.; Yu, Q.; He, X.; et al. Inhibition of obesity-induced hepatic ER stress by early insulin therapy in obese diabetic rats. Endocrine 2010, 39, 235–241. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Zierath, J.R.; Kane, S.; Krook, A.; Lienhard, G.E.; Wallberg-Henriksson, H. Insulin-Stimulated Phosphorylation of the Akt Substrate AS160 Is Impaired in Skeletal Muscle of Type 2 Diabetic Subjects. Diabetes 2005, 54, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, Y.; Liu, Y.X.; Ping, J.; Shou, Q.Y.; Chen, F.M.; Ruo, R. Metformin improves hepatic IRS2/PI3K/Akt signaling in insulin-resistant rats of NASH and cirrhosis. J. Endocrinol. 2016, 229, 133–144. [Google Scholar] [CrossRef]
- Frøsig, C.; Jensen, T.; Jeppesen, J.; Pehmøller, C.; Treebak, J.T.; Maarbjerg, S.J.; Kristensen, J.; Sylow, L.; Alsted, T.J.; Schjerling, P.; et al. AMPK and Insulin Action—Responses to Ageing and High Fat Diet. PLoS ONE 2013, 8, e62338. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, Y.; Hernández, M.A.S.; Han, Y.; Liu, R.; Park, S.W. BRD7 deficiency leads to the development of obesity and hyperglycemia. Sci. Rep. 2019, 9, 5327. [Google Scholar] [CrossRef]
- Kim, T.; Holleman, C.L.; Nason, S.; Arble, D.M.; Ottaway, N.; Chabenne, J.; Loyd, C.; Kim, J.-A.; Sandoval, D.; Drucker, D.J.; et al. Hepatic Glucagon Receptor Signaling Enhances Insulin-Stimulated Glucose Disposal in Rodents. Diabetes 2018, 67, 2157–2166. [Google Scholar] [CrossRef]
- Tsuboi, K.; Mizukami, H.; Inaba, W.; Baba, M.; Yagihashi, S. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses development of neuropathy in diabetic rodents: Effects on peripheral sensory nerve function, structure and molecular changes. J. Neurochem. 2016, 136, 859–870. [Google Scholar] [CrossRef]
- Tremblay, F.; Gagnon, A.; Veilleux, A.; Sorisky, A.; Marette, A. Activation of the Mammalian Target of Rapamycin Pathway Acutely Inhibits Insulin Signaling to Akt and Glucose Transport in 3T3-L1 and Human Adipocytes. Endocrinology 2005, 146, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Korsheninnikova, E.; Van Der Zon, G.C.M.; Voshol, P.J.; Janssen, G.M.; Havekes, L.M.; Grefhorst, A.; Kuipers, F.; Reijngoud, D.-J.; A Romijn, J.; Ouwens, M.; et al. Sustained activation of the mammalian target of rapamycin nutrient sensing pathway is associated with hepatic insulin resistance, but not with steatosis, in mice. Diabetologia 2006, 49, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Sugimoto, K.; Yamada, K.; Morishige, J.-I.; Ushijima, K.; Fujimura, A.; Nagata, N.; Ando, H. Chronic Treatment with Metformin Has No Disrupting Effect on the Hepatic Circadian Clock in Mice. Medicina 2022, 58, 293. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Hassan, N.M.; Alhossary, A.A.; Mu, Y.; Kwoh, C.-K. Protein-Ligand Blind Docking Using QuickVina-W With Inter-Process Spatio-Temporal Integration. Sci. Rep. 2017, 7, 15451. [Google Scholar] [CrossRef]
- Henriksson, E.; Huber, A.-L.; Soto, E.K.; Kriebs, A.; Vaughan, M.E.; Duglan, D.; Chan, A.B.; Papp, S.J.; Nguyen, M.; Afetian, M.E.; et al. The Liver Circadian Clock Modulates Biochemical and Physiological Responses to Metformin. J. Biol. Rhythm. 2017, 32, 345–358. [Google Scholar] [CrossRef]
- Caton, P.W.; Kieswich, J.; Yaqoob, M.M.; Holness, M.J.; Sugden, M.C. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes, Obes. Metab. 2011, 13, 1097–1104. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Ren, L.-W.; Zhan, P.; Yang, H.-Y.; Chai, D.-D.; Yu, Z.-W. Metformin exerts glucose-lowering action in high-fat fed mice via attenuating endotoxemia and enhancing insulin signaling. Acta Pharmacol. Sin. 2016, 37, 1063–1075. [Google Scholar] [CrossRef]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef]
- Hamdy, O.; Porramatikul, S.; Al-Ozairi, E. Bentham Science Publisher Bentham Science Publisher Metabolic Obesity: The Paradox Between Visceral and Subcutaneous Fat. Curr. Diabetes Rev. 2006, 2, 367–373. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, M.; Li, M.-D.; Zhang, K.; Zhang, B.; Wang, S.; Liu, Y.; Ni, W.; Ong, Q.; Mi, J.; et al. O-GlcNAc transferase inhibits visceral fat lipolysis and promotes diet-induced obesity. Nat. Commun. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Smith, B.K.; Mohammadi-Shemirani, P.; Morrow, M.R.; Gutgesell, R.M.; Lu, R.; Raphenya, A.R.; Kabiri, M.; McArthur, A.G.; et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 2019, 1, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2019, 578, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. Am. J. Physiol. Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef]
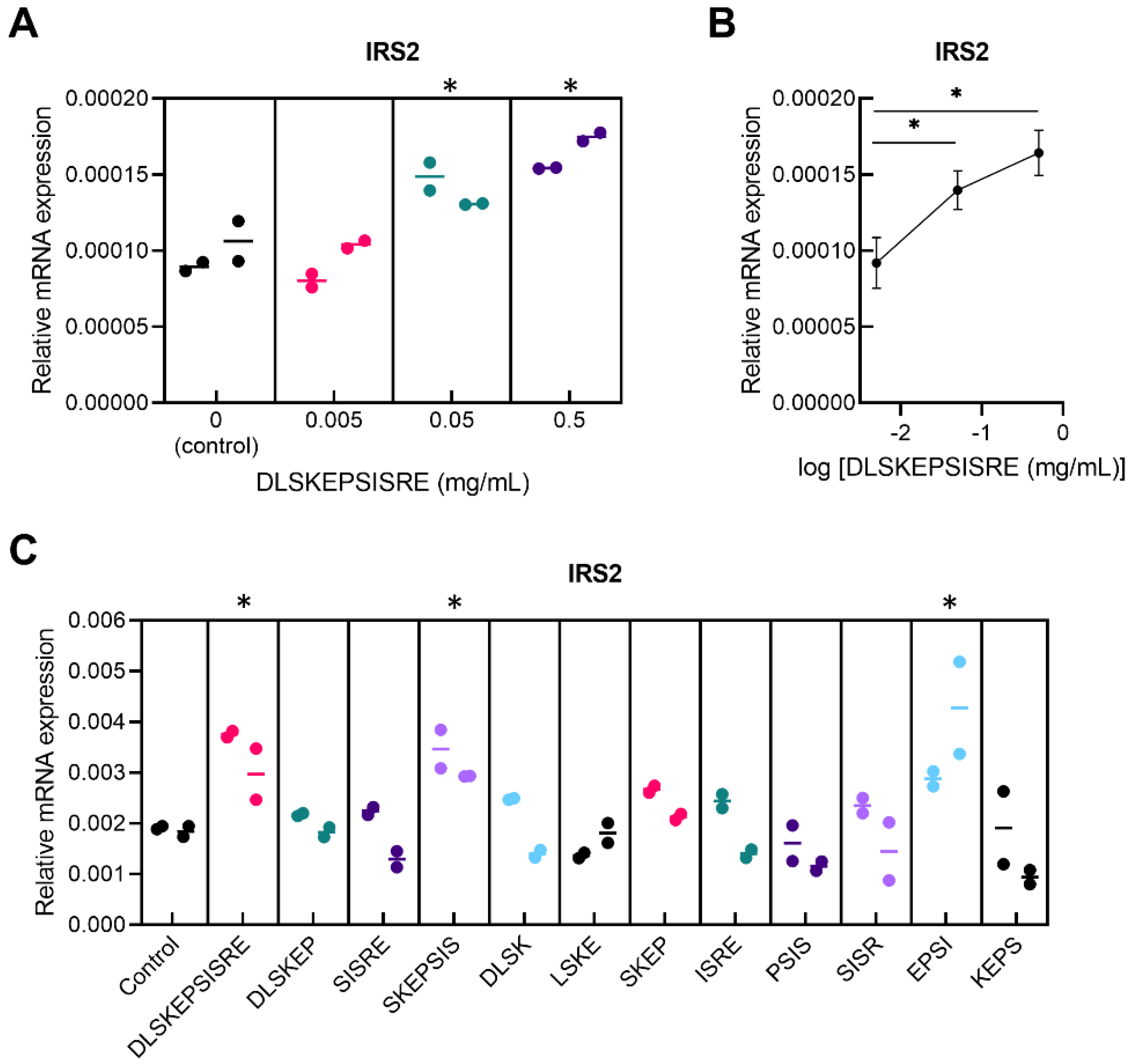



| Amino Acid Sequence | Source of Peptide | ||
|---|---|---|---|
| SKDIGSESTEDQAME | CasA1 | CHM-273 fragments | |
| REQLSTSEEN | CasA2 | Amino acid sequence | |
| NKKIEKF | CasB | DLSKEP | |
| VQVTSTAV | CasK | SISRE | |
| LIVTQTMKGLD | LacB | SKEPSIS (CHM-273L) | |
| WENGECAQK | LacB | DLSK | |
| DLSKEPSISRE (CHM-273) | GlyCam1 | LSKE | |
| NKPEDETHL | GlyCam1 | → | SKEP |
| FEVVKT | GlyCam1 | ISRE | |
| NLENTVK | GlyCam1 | PSIS | |
| AGGPGAPADPGRPT | PIGR | SISR | |
| SNVQSPD | OSTP | EPSI (CHM-273S) | |
| SHIESEEMHD | OSTP | KEPS | |
| HKSEEDKHL | OSTP |
| DAY OF EXPERIMENT | BODY WEIGHT (G) | BLOOD GLUCOSE (MMOL/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 5) | HSD (n = 45) | Control (n = 5) | HSD (n = 45) | |||||
| 0 | 402.2 | ±27.8 | 415.0 | ±33.6 | ||||
| 8 | 431.2 | ±26.0 | 434.3 | ±35.6 | 4.8 | ±0.2 | 4.7 | ±0.4 |
| 15 | 436.6 | ±30.2 | 451.5 | ±41.3 | 5 | ±0.7 | 4.7 | ±0.5 |
| 22 | 449 | ±25.6 | 452.1 | ±44.3 | 5.3 | ±0.6 | 5.4 ab | ±0.6 |
| 28 | 456.4 | ± 24.0 | 467.0 | ± 47.7 | 5.4 | ± 0.6 | 6.1 * abc | ± 0.5 |
| WEEKS | CONTROL (N = 25) | HFD (N = 100) | ||
|---|---|---|---|---|
| 0 | 20.5 | ±1 | 20.1 | ±1.1 |
| 16 | 28.9 | ±1.6 | 37.6 * | ±3.6 |
| % OF WEIGHT GAIN | 41% | ±7.8% | 87.5% | ±20.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitkin, N.A.; Pavshintcev, V.V.; Sukhanova, I.A.; Doronin, I.I.; Babkin, G.A.; Sadagurski, M.; Malyshev, A.V. The Novel Peptide Chm-273s Has Therapeutic Potential for Metabolic Disorders: Evidence from In Vitro Studies and High-Sucrose Diet and High-Fat Diet Rodent Models. Pharmaceutics 2022, 14, 2088. https://doi.org/10.3390/pharmaceutics14102088
Mitkin NA, Pavshintcev VV, Sukhanova IA, Doronin II, Babkin GA, Sadagurski M, Malyshev AV. The Novel Peptide Chm-273s Has Therapeutic Potential for Metabolic Disorders: Evidence from In Vitro Studies and High-Sucrose Diet and High-Fat Diet Rodent Models. Pharmaceutics. 2022; 14(10):2088. https://doi.org/10.3390/pharmaceutics14102088
Chicago/Turabian StyleMitkin, Nikita A., Vsevolod V. Pavshintcev, Iuliia A. Sukhanova, Igor I. Doronin, Gennady A. Babkin, Marianna Sadagurski, and Anton V. Malyshev. 2022. "The Novel Peptide Chm-273s Has Therapeutic Potential for Metabolic Disorders: Evidence from In Vitro Studies and High-Sucrose Diet and High-Fat Diet Rodent Models" Pharmaceutics 14, no. 10: 2088. https://doi.org/10.3390/pharmaceutics14102088
APA StyleMitkin, N. A., Pavshintcev, V. V., Sukhanova, I. A., Doronin, I. I., Babkin, G. A., Sadagurski, M., & Malyshev, A. V. (2022). The Novel Peptide Chm-273s Has Therapeutic Potential for Metabolic Disorders: Evidence from In Vitro Studies and High-Sucrose Diet and High-Fat Diet Rodent Models. Pharmaceutics, 14(10), 2088. https://doi.org/10.3390/pharmaceutics14102088










