Ocular Delivery of Therapeutic Proteins: A Review
Abstract
1. Introduction
| Molecule | Structure | Type | KD VEGF165 (pM) Equilibrium Dissociation Constant | Molecular Weight Mw (kDa) | Standard Dose (IVT) (mg/mL) | T1/2 Vitreous (days) | Indication | Target | References |
|---|---|---|---|---|---|---|---|---|---|
| Brolucizumab |  | ScFv | 1.6 | 26 | 6/0.005 | 2.94 to 13.4 | DR, DME, nAMD | VEGF-A | [11] |
| Ranibizumab (Lucentis®) |  | Monoclonal antibody fragment (Fab) | 46–172 | 48 | 0.5/0.005 | 1.4 to 7.19 | DR, DME, AMD | All isoforms of VEGF-A | [12,13,14,15] |
| Aflibercept |  | Fc Fusion protein fused with VEFR 1 domain 2 and VEGFR 2 domain 3 | 0.49 | 97–115 | 2/0.005 | 1.5 to 5.5 | DR, DME, AMD | VEGF-A, B and PlGF | [16,17] |
| Bevacizumab (Avastin®) |  | Monoclonal antibody | 58–1100 | 149 | 1.25/0.005 | 4.3 to 11.67 | DR, DME, AMD | All isoforms of VEGF-A | [18,19,20,21,22,23,24,25] |
| Abicipar pegol (Allergan®) |  | Akyrin repeat protein (recombinant protein) coupled with PEG | 486 fM | 34 | 2/0.005 | >13 | nAMD | VEGF-A165 | [26,27] |
| Faricimab |  | Monoclonal antibody | - | 150 | 6/0.005 | 2.83 | nAMD, DME | VEGF-A and Angiopoietin-2 | [28,29] |
| Conbercept (Lumitin®, Sichuan) |  | Fc Fusion protein fused with VEFR 1 domain 2 and VEGFR 2 domain 3 & 4 | 0.5 | 143 | 0.5/0.005 | 4.24 | AMD | VEGF-B and PlGF | [30] |
| Pegaptanib (Macugen®) |  | Aptamer (pegylated oligonucleotide | 200 | 40 | 0.3/0.009 | 12 | DR, DME, nAMD | VEGF165 | [31,32,33,34] |
2. Routes of Ocular Drug Administration
2.1. Intraocular
- (a)
- Intravitreal
- (b)
- Subretinal
- (c)
- Suprachoroidal
2.2. Periocular
3. Ocular Barriers and Approaches to Ocular Administration
3.1. Ocular Barriers
3.1.1. Tissue Conditions
3.1.2. Physicochemical Characteristics of Drug Molecules
3.1.3. Viscosity and pH of the Formulation
3.1.4. Protein Binding
3.1.5. Enzymatic Degradation
3.2. Use of Penetration Enhancers
4. Conjugation Approaches
4.1. Conjugation with Ligands
4.2. Conjugation with Lipid Derivatives
4.3. Conjugation with Melanin
4.4. Conjugation with Hyaluronan
4.5. Conjugation with Polyethylene Glycol
5. Formulation Approaches
5.1. Hydrogels
- (a)
- Hydrogels
- (b)
- Combined Hydrogel Systems
5.2. Particulate Carrier Systems
5.2.1. Microcarriers
5.2.2. Nanocarriers
Polymeric Nanoparticles
Micelles
Dendrimers
Lipid-Based Nanocarriers
- (a)
- Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
- (b)
- Niosomes
- (c)
- Liposomes
5.3. Microbubbles Technology
5.4. Nanofibers and Amphiphiles
5.5. Nanowafers
5.6. Cell-Penetrating Peptides
5.7. Encapsulated Cell Technology
5.8. Iontophoresis
5.9. Ocular Microneedles
5.10. Injectable Implants
| Delivery System | Material | Molecule | Remarks | References |
|---|---|---|---|---|
| Microparticles | PLGA | Anti-VEGF aptamer EYE001 | In vitro drug sustained release up to 20 days. | [178] |
| Microparticles | PLA nanoparticles in porous PLGA microparticles | Bevacizumab | In vivo sustained release after intravitreal injection in rats. | [214] |
| Microparticles | PLGA | Bevacizumab | In vitro drug sustained release for up to 91 days from the microparticles. | [284] |
| Microparticles | PLGA | Bevacizumab | In vivo sustained release after intravitreal injection to rabbit. | [285] |
| Microparticles | Silicon dioxide | Bevacizumab | In vitro sustained release for up to 165 days. From the porous silicon dioxide microparticles. | [286] |
| Microparticles/Nanoparticles | PLGA-albumin | Bevacizumab | In vivo and ex vivo rabbit vitreous injection showed sustained release for up to 165 days from the developed PLGA-albumin microparticles (~197 nm). | [287] |
| Nanoparticles | HSA-PEG | Apatinib | Reduced leakage in vascular tissues with significant inhibition of hyperpermeability in streptozotocin-induced diabetic mice after intravitreal injection of apatinib-HAS-PEG nanoparticles. | [288] |
| Nanoparticles | Albuminated PLGA | Bevacizumab | Sustained release with antibody protection obtained with its stability intravitreally for about 8 weeks with vitreous concentration maintained above 500 ng/mL from the coumarin-6-loaded albuminated-PLGA-NPs. | [289] |
| Nanoparticles | CS-PLGA | Bevacizumab | Sustained and effective delivery of bevacizumab to posterior ocular tissues after subconjunctival administration and more reduction in VEGF level in retina than the topical or intravitreal administration. | [290] |
| Nanoparticles | CS-HA, Zinc sulphate | Bevacizumab | Sustained release for up to two months with reduced CNV from CS-loaded bevacizumab nanoparticles containing implants, when administered intravitreally. | [291] |
| Nanoparticles | PLGA | Connexin43mimetic peptide | Improved light sensitivity and suppression of inflammatory areas showing high concentration in ganglion cell layer and choroid within half an hour after intravitreal injection. | [292] |
| Nanoparticles | PLGA | Bevacizumab | Enhanced antiangiogenic effect and reduced toxicity of tissues after intravitreal injection in vivo | [293] |
| Nanoparticles | ALBUMIN | Connexin43 mimetic peptide | Significant enhancement in protection against degradation and high retention in vitro with expression of CD44 cells in both retina and choroid. | [294] |
| Nanoparticles | CHITOSAN | Bevacizumab | VEGF expression inhibition after intravitreal injection. | [295] |
| Nanoparticles | PLA/PLA-PEO | C16Y Peptide | Sustained release and prolonged effect on suppression of CNV from NPs due to significant permeation to targeted tissues and reduced toxicity as compared to simple peptide solution after intravitreal injection. | [296] |
| Nanoparticles | 12-7NH-12, DOPE, DPPC | Cy5-DNA | Concentration of drug found within 4 h postinjection intravitreally from nanoparticles located in NFL. | [297] |
| Nanoparticles | Chitosan | pDNA | Effective transfection in INL, IPL, and RGC layers after intravitreal injection. | [298] |
| Nanoparticles | PLGA | pDNA, shRNA | GFP expression effect is persistent with significant reduction in CNV, lesion thickness, and retinal damage for 4 weeks after intravitreal injection. | [299] |
| Nanoparticles | PLGA | pDNA | Significant reduction of vascular leakage CNV induced diabetic rats without tissue damage and effective K5 expression in the retinal layer for up to 4 weeks after intravitreal injection. | [300] |
| Nano-balls | bPEI | siRNA | Sustained release, longer retention with effective concentration in choroid and RPE target tissues for up to 2 weeks after intravitreal injection. | [301] |
| Nanoparticles | DOTAP, DOPE | pDNA | Transfection effect is increased significantly up to 6-fold in RPE as well as 2-fold in in vitro after intravitreal injection. | [302] |
| Lipid-nanoparticles | DOTAP, Cholesterol, PEG-DSPE | siRNA | VEGFR1 expression inhibited in ARPE-19 cell-lines showing no tissue damage and reduced CNV areas after intravitreal injection. | [303] |
| SLN | Precirol (ATO5), DOTAP, Tween 80 Dextran, HA | pDNA | High transfection efficiency in both PR and INL showing improvement in retinal structure after 2 weeks after intravitreal injection. | [304] |
| NLC | Monolaurin, Monostearin, Glyceryl tripalmitate, Palmitin Glyceryl stearate | Sorafenib | Demonstrated excellent physicochemical properties and good tolerance, sustained release, and enhanced ocular bioavailability in CNV after topical administration. | [305] |
| NLC | Glyceryl monostearate, lipoplysaccharides | Dasatinib | Observed sustained release, reduced ocular toxicity, and facilitated penetration into cornea via topical administration with effective inhibition of CNV. | [306] |
| Dendrimer | Lecithin | Anti-VEGF Plus Oligonucleotide 1 | Topical delivery of dendrimers plus ODN-1 to the eyes of rats and inhibited laser-induced CNV for up to 6 months. | [307] |
| Niosomes | DOTAP, squalene, Polysorbate 80 | pDNA | Persistent protein expression after transfection intravitreally for at least 1 month after injection. Protection against enzymatic digestion, providing broad surface transfection in inner layer of retina with no cytotoxicity. | [224] |
| Liposomes | DOTMA, Cholesterol, DOPE | pDNA | Highest transfection effect with lower quantity of plasmid DNA in liposomes reached peak level within 3 days after intravitreal injection. | [228] |
| Liposomes | PC, Cholesterol, PEG-DSPE | Oligonucleotide | Sustained release in vitreous and retina–choroid from intravitreal injection of liposomal oligonucleotides showing protective effect against enzymatic degradation after intravitreal injection. | [229] |
| Liposomes | DPPC, EPC, Cholesterol | Bevacizumab | Significant improvement in mean residence time of bevacizumab after intravitreal injection. | [308] |
| Liposomes | EPC-Chol and DPC-Cholesterol | Bevacizumab | Showed sustained release of drug for up to 42 days after intravitreal injection. | [221] |
| Liposomes | PC-PS(Cholesterol)Toc | Bevacizumab-(annexin) | Liposomes showed 100 nm of size prepared with dehydration–rehydration technique and coated with annexin after intravitreal injection. | [220] |
| Liposomes | PC, Cholesterol | Vasoactive intestinal peptide | Reduced inflammation significantly with prolonged protection of peptide up to 14 days in vivo after intravitreal injection. | [225] |
| Biodegradable Implants | Molded hydrogel matrix | Bevacizumab | Implants prepared using PRINT technology from molded hydrogel showed sustained release of bevacizumab for 2 months after intravitreal administration. | [159] |
| Biodegradable implants | PCL | Ranibizumab | Film device containing nanopores on PCL provided sustained release of ranibizumab for 3 months after intravitreal injection. | [309] |
| Nonbiodegradable implants | Programmable micropump device | Ranibizumab | Micropump for posterior segment prepared using nonbiodegradable polymers showed long-term release of ranibizumab after intravitreal administration. | [310] |
| Implants | Port delivery system | Ranibizumab | Semipermeable refillable membrane providing long-term release of ranibizumab for 1 year after intravitreal administration. | [311] |
| Non- biodegradable implants | NT-503 | VEGFR-Fc | Increased specific VEGFR binding observed with encapsulating cell showing continuous production of therapeutic proteins for two years after intravitreal administration. | [312] |
| Verisome IB20089 | Biodegradable implant with liquid gel | Triamcinolone/Ranibizumab | The formed spherules provided long term sustained release up to 1 year after intravitreal injection. | [313] |
| Microsphere in hydrogel | PNIPAAm | Ranibizumab and aflibercept | Sustained in vitro release for up to 196 days from thermosensitive hydrogel after intravitreal injection. | [180] |
| In situ hydrogel | HA(DEX) | Bevacizumab | Hydrogel formed by chemical crosslinking showed sustained release for up to 6 months in vivo administered intravitreal in rabbit model. | [146] |
| Hydrogel | Alginate(Chitosan) hydrogel/PLGA microspheres | Bevacizumab/Ranibizumab | Sustained release from intravitreally administered hydrogel observed for both bevacizumab and ranibizumab for up to 3 months. | [158] |
| Hydrogel | PLGA-mPEG | Bevacizumab | Sustained release for up to 1 month via intravitreal route in rabbits. | [159] |
| Hydrogel | PEOz(PCL)PEOz | Bevacizumab | Sustained release for up to 20 days in vitro. | [160] |
| Silk based hydrogels | Silk fibroin | Bevacizumab | Sustained release of bevacizumab from intravitreally administered hydrogel for 90 days in vitro as well as in vivo in Dutch-belted rabbits. | [161] |
| In situ gel | PCM(HEMA) | Bevacizumab | Provided in vivo retention for up to 2 months in SD rats via suprachoroidal route. | [162] |
| Hydrogel | PNIPAAm | Insulin | Sustained release of insulin for up to 30 days in vitro. | [163] |
| Hydrogel | PLGA–PEG | Ovalbumin (model protein) | Provided sufficient protein concentration for up to 14 days in ocular tissues via subconjunctival route. | [164] |
| Hydrogel | ESHU | Bevacizumab | Sustained release for up to 9 weeks via intravitreal route in rabbits. | [166] |
| Hydrogel | PEG-(ESHU) | Bevacizumab | Showed good in vitro and in vivo biocompatibility after intravitreal injection. | [167] |
| Hydrogel | PNIPAAm | Bevacizumab/Ranibizumab | Provided good mechanical properties, biocompatibility from thermosensitive hydrogel after intravitreal injection. | [170] |
| Diels-alder hydrogels | PEG Macromonomers | Bevacizumab | Provided mechanical stability and long-term release for up to 6 weeks from the chemically crosslinked hydrogel after intravitreal injection. | [314] |
| Encapsulated cells | Polysulfone | CNTF | Continued clinical trials. | [315] |
| Retinal cells | HAS | Connexin43 mimetic peptide | Sustained release and prolonged retention with suppression of RGC and inflammation observed when intravitreally injected NPs encapsulating Cx43 MP were evaluated in a rat model of retinal ischemia-reperfusion injury. | [316] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Ramanunny, A.K.; Awasthi, A.; Singh, S.K.; Khursheed, R.; Corrie, L.; Chitranshi, N.; Gupta, V.K.; et al. Recent advances in intraocular and novel drug delivery systems for the treatment of diabetic retinopathy. Expert Opin. Drug Del. 2021, 18, 553–576. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Vision Loss Expert Group. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [PubMed]
- Claudio, F.; Francesco, B.; Michele, R.; Giovanni, A. Intravitreal Therapy for Diabetic Macular Edema: An Update. J. Ophthalmol. 2021, 2021, 6654168. [Google Scholar] [CrossRef]
- Aiello, L.P. The potential role of PKC b in diabetic retinopathy and macular edema. Survey Ophthalmol. 2002, 47 (Suppl. S2), 263–269. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Y.; Li, X.; Han, W.; Zheng, J.; Yang, G.; Xu, A. Combination of Intrastromal and Intracameral Injections of Amphotericin B in the Treatment of Severe Fungal Keratitis. J. Ophthalmol. 2016, 2016, 3436415. [Google Scholar] [CrossRef]
- Patel, P.B.; Shastri, D.H.; Shelat, P.K.; Shukla, A.K. Ophthalmic drug delivery system: Challenges and approaches. Syst. Rev. Pharm. 2010, 1, 113–120. [Google Scholar]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Narayana, S.; Ahmed, M.G.; Gowda, J.B.H.; Shetty, P.K.; Nasrine, A.; Thriveni, M.; Noushida, N.; Sanjana, A. Recent advances in ocular drug delivery systems and targeting VEGF receptors for management of ocular angiogenesis: A comprehensive review. Future J. Pharm. Sci. 2021, 7, 186. [Google Scholar] [CrossRef]
- Karasavvidou, E.M.; Tranos, P.; Panos, G.D. Brolucizumab for the Treatment of Degenerative Macular Conditions: A Review of Clinical Studies. Drug Des. Devel. Ther. 2022, 16, 2659–2680. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; Group, M.S. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012, 154, 682–686.e682. [Google Scholar] [CrossRef]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics, and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef]
- Krohne, T.U.; Eter, N.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 2008, 146, 508–512. [Google Scholar] [CrossRef]
- Ahn, S.J.; Hong, H.K.; Na, Y.M.; Park, S.J.; Ahn, J.; Oh, J.; Chung, J.Y.; Park, K.H.; Woo, S.J. Use of Rabbit Eyes in Pharmacokinetic Studies of Intraocular Drugs. J. Vis. Exp. 2016, 113, e53878. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Carlton, M.M.; Knopp, M.V.; Hinkle, G.H. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2011, 5, 5899–5903. [Google Scholar] [CrossRef]
- Shelke, N.B.; Kadam, R.; Tyagi, P.; Rao, V.R.; Kompella, U.B. Intravitreal poly(L-lactide) microparticles sustain retinal and choroidal delivery of TG-0054, a hydrophilic drug intended for neovascular diseases. Drug Deliv. Transl. Res. 2011, 1, 76–90. [Google Scholar] [CrossRef]
- Sinapis, C.I.; Routsias, J.G.; Sinapis, A.I.; Sinapis, D.I.; Agrogiannis, G.D.; Pantopoulou, A.; Theocharis, S.E.; Baltatzis, S.; Patsouris, E.; Perrea, D. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin. Ophthalmol. 2011, 5, 697–704. [Google Scholar] [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Krohne, T.U.; Holz, F.G. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina 2011, 31, 1877–1884. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research Application Number 761125Orig1s000. Clinical Pharmacology Reviews. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000ClinPharmR.pdf (accessed on 30 June 2022).
- Ferro Desideri, L.; Traverso, C.E.; Nicolò, M. Abicipar pegol: An investigational anti-VEGF agent for the treatment of wet age-related macular degeneration. Expert Opin. Investig. Drugs. 2020, 29, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Faricimab: An investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin. Investig. Drugs. 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, S.; Wang, E.; Men, P.; Zhai, S. Conbercept for Treatment of Neovascular Age-Related Macular Degeneration and Visual Impairment due to Diabetic Macular Edema or Pathologic Myopia Choroidal Neovascularization: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 696201. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, B. Pegaptanib for the treatment of age-related macular degeneration. Exp. Eye Res. 2006, 83, 615–619. [Google Scholar] [CrossRef]
- Kourlas, H.; Schiller, D.S. Pegaptanib sodium for the treatment of neovascular age-related macular degeneration: A review. Clin. Ther. 2006, 28, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn, H.W.; Bhavsar, A.R., Jr. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.; Adamis, A.P. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann. N. Y. Acad. Sci. 2006, 1082, 151–171. [Google Scholar] [CrossRef]
- Chen, W.; Yung, B.C.; Qian, Z.; Chen, X. Improving Long-Term Subcutaneous Drug Delivery by Regulating Material-Bioenvironment Interaction. Adv. Drug Deliv. Rev. 2018, 127, 20–34. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for Enhancing Oral Bioavailability of Peptides and Proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef]
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef]
- Brown, L.R. Commercial Challenges of Protein Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 29–42. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sadeghi, A.; Sarkhel, S.; Hagström, M.; Bahrpeyma, S.; Toropainen, E.; Auriola, S.; Urtti, A. Release of functional dexamethasone by intracellular enzymes: A modular peptide-based strategy for ocular drug delivery. J. Control. Release 2020, 327, 584–594. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef]
- Lin, J. Pharmacokinetics of Biotech Drugs: Peptides, Proteins and Monoclonal Antibodies. Curr. Drug Metab. 2009, 10, 661–691. [Google Scholar] [CrossRef]
- Ahmed, I.; Patton, T.F. Disposition of Timolol and Inulin in the Rabbit Eye Following Corneal versus Non-Corneal Absorption. Int. J. Pharm. 1987, 38, 9–21. [Google Scholar] [CrossRef]
- Donovan, M.D.; Flynn, G.L.; Amidon, G.L. Absorption of Polyethylene Glycols 600 Through 2000: The Molecular Weight Dependence of Gastrointestinal and Nasal Absorption. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1990, 7, 863–868. [Google Scholar]
- Shen, W.; Matsui, T. Intestinal Absorption of Small Peptides: A Review. Int. J. Food Sci. Technol. 2019, 54, 1942–1948. [Google Scholar] [CrossRef]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.Y.; Nance, E.A.; Yang, J.C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle Diffusion in, and Microrheology of, the Bovine Vitreous Ex Vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Heys, J.J.; Barocas, V.H.; Randolph, T.W. Permeability and Diffusion in Vitreous Humor: Implications for Drug Delivery. Pharm. Res. 2000, 17, 664–669. [Google Scholar] [CrossRef]
- Balachandran, R.K.; Barocas, V.H. Contribution of Saccadic Motion to Intravitreal Drug Transport: Theoretical Analysis. Pharm. Res. 2011, 28, 1049–1064. [Google Scholar] [CrossRef]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef]
- Nakano, M.; Lockhart, C.M.; Kelly, E.J.; Rettie, A.E. Ocular Cytochrome P450s and Transporters: Roles in Disease and Endobiotic and Xenobiotic Disposition. Drug Metab. Biophys. J. Rev. 2014, 46, 247–260. [Google Scholar] [CrossRef]
- Urtti, A. Nanostructures Overcoming the Ocular Barrier: Drug Delivery Strategies; Chapter 4.2.; Royal Society of Chemistry: London, UK, 2012; pp. 190–204. [Google Scholar]
- DiCarlo, J.E.; Mahajan, V.B.; Tsang, S.H. Gene therapy and genome surgery in the retina. J. Clin. Investig. 2018, 128, 2177–2188. [Google Scholar] [CrossRef]
- Sørensen, N.B. Subretinal Surgery: Functional and Histological Consequences of Entry into the Subretinal Space. Acta Ophthalmol. 2019, 97, 1–23. [Google Scholar] [CrossRef]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design Principles of Ocular Drug Delivery Systems: Importance of Drug Payload, Release Rate, and Material Properties. Drug Discov. Today. 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, B.; Mejlachowicz, D.; Behar-Cohen, F. Ocular Barriers and Their Influence on Gene Therapy Products Delivery. Pharmaceutics 2022, 14, 998. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, X.X.; Jiang, Y.R.; Bai, X.B.; Wu, B.D.; Dong, J.Q. Diffusion of macromolecule through retina after experimental branch retinal vein occlusion and estimate of intraretinal barrier. Curr. Drug Metab. 2007, 8, 151–156. [Google Scholar] [CrossRef]
- Jackson, T.L.; Antcliff, R.J.; Hillenkamp, J.; Marshall, J. Human retinal molecular weight exclusion limit and estimate of species variation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Blessing, C.I.; Charis, R.; Roel, F.M.; Mio, T.; Mei, C.; Wim, E.H. Hyaluronic Acid-PEG-Based Diels–Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 1525–7797. [Google Scholar]
- Burgalassi, S.; Monti, D.; Nicosia, N.; Tampucci, S.; Terreni, E.; Vento, A.; Chetoni, P. Freeze-dried matrices for ocular administration of bevacizumab: A comparison between subconjunctival and intravitreal administration in rabbits. Drug Deliv. Transl. Res. 2018, 8, 461–472. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clini. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye. Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Sidman, R.L.; Li, J.; Lawrence, M.; Hu, W.; Musso, G.F.; Giordano, R.J.; Cardo-Vila, M.; Pasqualini, R.; Arap, W. The peptidomimetic Vasotide targets two retinal VEGF receptors and reduces pathological angiogenesis in murine and nonhuman primate models of retinal disease. Sci. Transl. Med. 2015, 7, 309. [Google Scholar] [CrossRef]
- Pescina, S.; Ferrari, G.; Govoni, P.; Macaluso, C.; Padula, C.; Santi, P.; Nicoli, S. In-vitro permeation of bevacizumab through human sclera: Effect of iontophoresis application. J. Pharm. Pharmacol. 2010, 62, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Swami, R.; Shahiwala, A. Impact of physiochemical properties on pharmacokinetics of protein therapeutics. Eur. J. Drug Metab. Pharmacokinetics. 2013, 38, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.T.; Baker, K.; Yoshida, M.; Qiao, S.W.; Aveson, V.G.; Lencer, W.I.; Blumberg, R.S. Neonatal Fc receptor: From immunity to therapeutics. J. Clin. Immunol. 2010, 30, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E. Ophthalmic drug delivery systems for the treatment of retinal diseases: Basic research to clinical applications. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Durairaj, C.; Lin, T.; Liu, Y.; Burke, J. Ocular pharmacokinetics of intravitreally administered brimonidine and dexamethasone in animal models with and without blood-retinal barrier breakdown. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1056–1066. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Con. Rel. 2014, 190, 172–181. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef]
- Raghava, S.; Hammond, M.; Ub, K. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1). Mediat. Inflamm. 2013, 2013, 975032. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Garg, N.K.; Lunde, E.; Han, K.Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef]
- Vinores, S.A. Pegaptanib in the treatment of wet, age-related macular degeneration. Int. J. Nanomed. 2006, 1, 263–268. [Google Scholar]
- Joseph, M.; Trinh, H.M.; Cholkar, K.; Pal, D.; Mitra, A.K. Recent perspectives on the delivery of biologics to back of the eye. Expert Opin. Drug Deliv. 2016, 14, 631–645. [Google Scholar] [CrossRef]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles, Wiley interdisciplinary reviews. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Waisbourd, M.; Ben-Artsi, E.; Levinger, E.; Barak, A.; Daniels, T.; Csaky, K.; Loewenstein, A.; Barequet, I.S. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 331–337. [Google Scholar]
- Sharma, Y.R.; Venkatesh, P.; Gogia, V. Aflibercept—How does it compare with other Anti-VEGF Drugs? Aust. J. Clin. Ophthalmol. 2014, 1, 1–8. [Google Scholar]
- Neri, P.; Lettieri, M.; Fortuna, C.; Zucchi, M.; Manoni, M.; Celani, S.A. Giovannini, Adalimumab (humira) in ophthalmology: A review of the literature. Middle East Afr. J. Ophthalmol. 2010, 17, 290–296. [Google Scholar] [CrossRef]
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic monoclonal antibodies in ophthalmology. Prog. Ret. Eye Res. 2009, 28, 117–144. [Google Scholar] [CrossRef] [PubMed]
- Khawli, L.A.; Goswami, S.; Hutchinson, R.; Kwong, Z.W.; Yang, J.; Wang, X.; Yao, Z.; Sreedhara, A.; Cano, T.; Tesar, D. Charge variants in IgG1: Isolation, characterization, In vitro binding properties and pharma-cokinetics in rats. MAbs 2010, 2, 613–624. [Google Scholar] [CrossRef]
- Maurice, D.M.; Watson, P.G. The distribution and movement of serum albumin in the cornea. Exp. Eye Res. 1965, 4, 355–363. [Google Scholar] [CrossRef]
- Kim, J.H.; Green, K.; Martinez, M.; Paton, D. Solute permeability of the corneal endothelium and Descemet’s membrane. Exp. Eye Res. 1971, 12, 231–238. [Google Scholar] [CrossRef]
- Olsen, T.W.; Edelhauser, H.F.; Lim, J.I.; Geroski, D.H. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1893–1903. [Google Scholar]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Drug delivery to the retina: Challenges and opportunities. Expert Opin. Biol. Ther. 2003, 3, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of protein pharmaceuticals: An update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Liddell, M.R.; Wen, H. Effective electrophoretic mobilities and charges of anti-VEGF proteins determined by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2011, 55, 603–607. [Google Scholar] [CrossRef]
- Kaja, S.; Hilgenberg, J.D.; Everett, E.; Olitsky, S.E.; Gossage, J.; Koulen, P. Effects of dilution and prolonged storage with preservative in a polyethylene container on Bevacizumab (Avastin) for topical delivery as a nasal spray in anti-hereditary hemorrhagic telangiectasia and related therapies. Hum. Antibodies 2011, 20, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gregoritza, M.; Messmann, V.; Abstiens, K.; Brandl, F.P.; Goepferich, A.M. Controlled antibody release from degradable thermoresponsive hydrogels cross-linked by Diels-Alder chemistry. Biomacromolecules 2017, 18, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Lang, J.; Rodeheaver, D.; Missel, P.; Roehrs, R.; Chowhan, M. Design and Evaluation of Ophthalmic Pharmaceutical Products, Modern Pharmaceutics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ali, Y.; Lehmussaari, K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1258–1268. [Google Scholar] [CrossRef]
- Subrizi, A.; Toropainen, E.; Ramsay, E.; Airaksinen, A.J.; Kaarniranta, K.; Urtti, A. Oxidative stress protection by exogenous delivery of rhHsp70 chaperone to the retinal pigment epithelium (RPE), a possible therapeutic strategy against RPE degeneration. Pharm. Res. 2015, 32, 211–221. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Rousseau, F. Protein aggregation: A rescue by chaperones. Nat. Chem. Biol. 2016, 12, 58–59. [Google Scholar] [CrossRef]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic Analyses of the Vitreous Humour. Mediat. Inflamm. 2012, 2012, 148039. [Google Scholar] [CrossRef]
- Murthy, K.R.; Goel, R.; Subbannayya, Y.; Jacob, H.K.C.; Murthy, P.R.; Manda, S.S.; Patil, A.H.; Sharma, R.; Sahasrabuddhe, N.A.; Parashar, A.; et al. Proteomic Analysis of Human Vitreous Humor. Clin. Proteom. 2014, 11, 29. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Burke, L.; Micans, P.; Richer, S.P. N-Acetylcarnosine Sustained Drug Delivery Eye Drops to Control the Signs of Ageless Vision: Glare Sensitivity, Cataract Amelioration and Quality of Vision Currently Available Treatment for the Challenging 50,000-Patient Population. Clin. Interv. Aging 2009, 4, 31–50. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; Minnaert, A.K.; De Smedt, S.C.; Remaut, K. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Curr. Eye Res. 2019, 44, 465–475. [Google Scholar] [CrossRef]
- Boye, S.L.; Bennett, A.; Scalabrino, M.L.; McCullough, K.T.; Van Vliet, K.; Choudhury, S.; Ruan, Q.; Peterson, J.; Agbandje-McKenna, M.; Boye, S.E. Impact of Heparan Sulfate Binding on Transduction of Retina by Recombinant Adeno-Associated Virus Vectors. J. Virol. 2016, 90, 4215–4231. [Google Scholar] [CrossRef]
- Bisht, R.; Rupenthal, I.D.; Sreebhavan, S.; Jaiswal, J.K. Development of a Novel Stability Indicating RP-HPLC Method for Quantification of Connexin43 Mimetic Peptide and Determination of Its Degradation Kinetics in Biological Fluids. J. Pharm. Anal. 2017, 7, 365–373. [Google Scholar] [CrossRef]
- Stampfli, H.F.; Quon, C.Y. Polymorphic metabolism of flestolol and other ester containing compounds by a carboxylesterase in New Zealand white rabbit blood and cornea. Res. Commun. Mol. Pathol. Pharmacol. 1995, 88, 87–97. [Google Scholar] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.; Sonali, N.; Moreno, M.; Nirmal, J.; Fernandez, A.A.; Venkatraman, S.; Agrawal, R. Protein delivery to the back of the eye: Barriers, carriers and stability of anti-VEGF proteins. Drug Discov. Today. 2017, 22, 416–423. [Google Scholar] [CrossRef]
- Vaughan-Thomas, A.; Gilbert, S.J.; Duance, V.C. Elevated levels of proteolytic enzymes in the aging human vitreous. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3299–3304. [Google Scholar]
- Pescosolido, N.; Barbato, A.; Pascarella, A.; Giannotti, R.; Genzano, M.; Nebbioso, M. Role of Protease-Inhibitors in Ocular Diseases. Molecules 2014, 19, 20557–20569. [Google Scholar] [CrossRef]
- Jwala, J.; Boddu, S.H.S.; Shah, S.; Sirimulla, S.; Pal, D.; Mitra, A.K. Ocular Sustained Release Nanoparticles Containing Stereoiso-meric Dipeptide Prodrugs of Acyclovir. J. Ocul. Pharmacol. Ther. 2011, 27, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, L.H.; Kalashnikov, V.V.; Thompson, L.W.; Yamshchikov, G.V.; Pierce, R.A.; Galavotti, H.S.; Engelhard, V.H.; Slingluff, C.L. Terminal Modifications Inhibit Proteolytic Degradation of an Immunogenic MART-127-35 Peptide: Implications for Peptide Vaccines. Int. J. Cancer 1999, 83, 326–334. [Google Scholar] [CrossRef]
- Werle, M.; Bernkop-Schnürch, A. Strategies to Improve Plasma Half Life Time of Peptide and Protein Drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Attar, M.; Shen, J.; Ling, K.H.J.; Tang-Liu, D. Ophthalmic Drug Delivery Considerations at the Cellular Level: Drug-Metabolising Enzymes and Transporters. Expert Opin. Drug Deliv. 2005, 2, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, M.L.; Masferrer, J.; Dunn, M.W.; Mcgiff, J.C.; Abraham, N.G. Cytochrome P450, Drug Metabolizing Enzymes and Arachidonic Acid Metabolism in Bovine Ocular Tissues. Curr. Eye Res. 1987, 6, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, S. Lysosomal Enzymes in Ocular Tissues and Diseases. Surv. Ophthalmol. 1983, 27, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, J.; Ludwig, A. Ocular Drug Delivery: Nanomedicine Applications. Nanomedicine 2007, 2, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug. Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Traynor, K. Aflibercept approved for macular degeneration. Am. J. Health Sys. Pharm. 2012, 69, 6. [Google Scholar] [CrossRef]
- Ng, E.W.; Shima, D.T.; Calias, P.; Cunningham, E.T.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Theodossiadis, P.G.; Markomichelakis, N.N.; Sfikakis, P.P. Tumor necrosis factor antagonists: Preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007, 27, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Harooni, M.; Freilich, J.M.; Abelson, M.; Refojo, M. Efficacy of hyaluronidase in reducing increases in intraocular pressure related to the use of viscoelastic substances. Arch. Ophthalmol. 1998, 116, 1218–1221. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Pardridge, W.M. Widespread expression of an exogenous gene in the eye after intravenous administration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3075–3080. [Google Scholar]
- Chandola, C.; Casteleijn, M.G.; Chandola, U.M.; Gopalan, L.N.; Urtti, A.; Neerathilingam, M. CD44 Aptamer Mediated Cargo Delivery to Lysosomes of Retinal Pigment Epithelial Cells to Prevent Age-Related Macular Degeneration. Biochem. Biophys. Rep. 2019, 18, 100642. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine as a Suitable Building Block for the Preparation of Hybrid Materials and Their Potential Applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef] [PubMed]
- Tadayoni, R.; Sararols, L.; Weissgerber, G.; Verma, R.; Clemens, A.; Holz, F.G. Brolucizumab: A Newly Developed Anti-VEGF Molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Patil, P.N. An explanation for the long duration of mydriatic effect of atropine in eye. Investig. Ophthalmol. 1976, 15, 671–673. [Google Scholar]
- Rimpelä, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; del Amo, E.M. Implications of Melanin Binding in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Bernstein, H.; Zvaifler, N.; Rubin, M.; Mansour, A.M. The Ocular Deposition of Chloroquine. Investig. Ophthalmol. 1963, 2, 384–392. [Google Scholar]
- Robbie, S.J.; von Leithner, P.L.; Ju, M.; Lange, C.A.; King, A.G.; Adamson, P.; Lee, D.; Sychterz, C.; Coffey, P.; Ng, Y.S.; et al. Assessing a Novel Depot Delivery Strategy for Noninvasive Administration of VEGF/PDGF RTK Inhibitors for Ocular Neovascular Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Machinaga, N.; Ashley, G.W.; Reid, R.; Yamasaki, A.; Tanaka, K.; Nakamura, K.; Yabe, Y.; Yoshigae, Y.; Santi, D.V. A Controlled Release System for Long-Acting Intravitreal Delivery of Small Molecules. Transl. Vis. Sci. Technol. 2018, 7, 21. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, C.S. The Pharmacology of PEGylation: Balancing PD with PK to Generate Novel Therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.; Nashed, Y.; Mitra, A. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. Curr. Eye. Res. 2003, 26, 151–163. [Google Scholar] [CrossRef]
- Pudlarz, A.; Szemraj, J. Nanoparticles as Carriers of Proteins, Peptides and Other Therapeutic Molecules. Open Life Sci. 2018, 13, 285–298. [Google Scholar] [CrossRef]
- Peyman, G.A.; Ganiban, G.J. Delivery systems for intraocular routes. Adv. Drug Deliv. Rev. 1995, 16, 107–123. [Google Scholar] [CrossRef]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef]
- Lambert, G.; Guilatt, R.L. Current ocular drug delivery challenges. Drug Dev. Rep. Ind. Overv. Details 2005, 33, 1–2. [Google Scholar]
- Lang, J.C. Recent developments in ophthalmic drug delivery: Conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Kim, S.H.; GalbáN, C.J.; Lutz, R.J.; Dedrick, R.L.; Csaky, K.G.; Lizak, M.J.; Wang, N.S.; Tansey, G.; Robinson, M.R. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Investig. Ophthalmol. Vis. Sci. 2007, 48, 808–814. [Google Scholar] [CrossRef]
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomed. 2019, 14, 45–55. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: A 6-month in vivo study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Bae, K.H.; Wang, L.S.; Kurisawa, M. Injectable biodegradable hydrogels: Progress and challenges. J. Mater. Chem. B 2013, 1, 5371. [Google Scholar] [CrossRef] [PubMed]
- Franssen, O.; Vandervennet, L.; Roders, P.; Hennink, W.E. Degradable dextran hydrogels: Controlled release of a model protein from cylinders and microspheres. J. Control. Release. 1999, 60, 211–221. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Censi, R.; Vermonden, T.; Van, M.J. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release. 2009, 140, 230–236. [Google Scholar] [CrossRef]
- Kirchhof, S.; Abrami, M.; Messmann, V. Diels-alder hydrogels for controlled antibody release: Correlation between mesh size and release rate. Mol. Pharm. 2015, 12, 3358–3368. [Google Scholar] [CrossRef]
- Shastri, D.H.; Patel, L.D.; Parikh, R.K. Studies on in situ hydrogel: A smart way for safe and sustained ocular drug delivery. J. Young Pharm. 2010, 2, 116–120. [Google Scholar] [CrossRef]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Effects of sterilization on poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. A 2008, 87, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Saher, O.; Ghorab, D.M.; Mursi, N.M. Preparation and in vitro/in vivo evaluation of antimicrobial ocular in situ gels containing a disappearing preservative for topical treatment of bacterial conjunctivitis. Pharm. Dev. Technol. 2016, 21, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Janet, T.; Stuart, W.; Kevin, H.; Gary, O.; Gabe, F.; Nicole, M.; Tomas, N.; Benjamin, M.; Benjamin, Y. In-vitro release of Bevacizumab from hydrogel-based drug delivery systems. Investig. Ophthalmol. Vis. Sci. 2015, 56, 222. [Google Scholar]
- Blessing, C.I.; Arto, U.; Wim, E.H.; Tina, V. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Hu, C.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Controlled release bevacizumab in thermoresponsive hydrogel found to inhibit angiogenesis. Biomed. Mater. Eng. 2014, 24, 1941–1950. [Google Scholar] [CrossRef]
- Wang, C.H.; Hwang, Y.S.; Chiang, P.R.; Shen, C.R.; Hong, W.H.; Hsiue, G.H. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules 2012, 13, 40–48. [Google Scholar] [CrossRef]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti- VEGF) therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Tyagi, P.; Barros, M.; Stansbury, J.W.; Kompella, U.B. Light activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. [Google Scholar] [CrossRef]
- Misra, G.P.; Singh, R.S.J.; Aleman, T.S.; Jacobson, S.G.; Gardner, T.W.; Lowe, T.L. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials 2009, 30, 6541–6547. [Google Scholar] [CrossRef]
- Rieke, E.R.; Amaral, J.; Becerra, S.P.; Lutz, R.J. Sustained subconjunctival protein delivery using a thermosetting gel delivery system. J. Ocul. Pharmacol. Ther. 2010, 26, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, A.R.; Christian, W.; Katie, B.R. Polymer based sustained drug delivery to the ocular posterior segment: Barriers and future opportunities for the treatment of neovascular pathologies. Adv. Drug Deliv. Rev. 2022, 187, 114–342. [Google Scholar] [CrossRef] [PubMed]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Wu, W.; Wang, Y. A functionalizable reverse thermal gel based on a polyurethane/PEG block copolymer. Biomaterials 2011, 32, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Park, C.G.; Huh, B.K.; Cho, M.O.; Khatun, Z.; Li, Z.; Kang, S.W.; Choy, Y.B.; Huh, K.M. Thermosensitive hexanoyl glycol chitosan-based ocular delivery system for glaucoma therapy. Acta Biomater. 2016, 39, 124–132. [Google Scholar] [CrossRef]
- Joseph, M.; Patel, S.P.; AGRAHARI, V.; Mitra, A.K. Pentablock Copolymer nano formulation for controlled Ocular Delivery of Protein Therapeutics. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4629. [Google Scholar]
- Alexander, A.; Ajazuddin, J.; Khan, J.; Saraf, S. Polyethylene glycol (PEG)-Poly(N- isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Cohen, S.; Yoshioka, T.; Lucarelli, M.; Hwang, L.H.; Langer, R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991, 8, 713–720. [Google Scholar] [CrossRef]
- Ron, E.; Turek, T.; Mathiowitz, E.; Chasin, M.; Hageman, M.; Langer, R. Controlled release of polypeptides from polyanhydrides. Proc. Natl. Acad. Sci. USA 1993, 90, 4176–4180. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.A.; Klumb, L.A.; Herberger, J.D.; Nguyen, X.C.; Harrell, R.A.; Zordich, M. Poly(lactide-co-glycolide) microsphere formulations of darbepoetin alfa: Spray drying is an alternative to encapsulation by spray-freeze drying. Pharm. Res. 2004, 21, 500–506. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Mandal, A.; Gokulgandhi, M.; Patel, S.; Mitra, A.K. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from PLGA microparticles. Int. J. Pharm. 2015, 489, 237–245. [Google Scholar] [CrossRef]
- Carrasquillo, K.G.; Ricker, J.A.; Rigas, I.K.; Miller, J.W.; Gragoudas, E.S.; Adamis, A.P. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Investig. Ophthalmol. Vis. Sci. 2003, 44, 290–299. [Google Scholar] [CrossRef]
- Cook, G.P.; Burgess, L.; Wing, J.; Dowie, T.; Calias, P.; Shima, D.T.; Campbell, K.; Allison, D.; Volker, S.; Schmidt, P. Preparation and Characterization of Pegaptanib Sustained Release Microsphere Formulations for Intraocular Application. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5123. [Google Scholar]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended In vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Mandal, A.; Patel, S.; Mitra, A.K. Extended release microparticle-in-gel formulation of octreotide: Effect of polymer type on acylation of peptide during In vitro release. Int. J. Pharm. 2015, 496, 676–688. [Google Scholar] [CrossRef]
- Mahlumba, P.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications for Ocular Delivery. Molecules 2016, 21, 2. [Google Scholar] [CrossRef]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuetn, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef]
- Yang, F.; Pan, Y.F.; Wang, Z.Y.; Yang, Y.Q.; Zhao, Y.M.; Liang, S.Z.; Zhang, Y.M. Preparation and characteristics of interferon-alpha poly(lactic-co-glycolic acid) microspheres. J. Microencap. 2010, 27, 133–141. [Google Scholar] [CrossRef]
- Pan, C.K.; Durairaj, C.; Kompella, U.B.; Agwu, O.; Oliver, S.C.; Quiroz-Mercado, H.; Mandava, N.; Olson, J.L. Comparison of long-acting bevacizumab formulations in the treatment of choroidal neovascularization in a rat model. J. Ocul. Pharmacol. Ther. 2011, 27, 219–224. [Google Scholar] [CrossRef]
- Ramos, T.I.; Villacis-Aguirre, C.A.; Vispo, N.S.; Padilla, L.S.; Santana, S.P.; Parra, N.C.; Alonso, J.R.T. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics 2021, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Luaces-Rodríguez, A.; Mondelo, G.C.; Zarra-Ferro, I.; Barcia, M.; Aguiar, P.; Fernández-Ferreiro, A.; Otero-Espinar, F. Intravitreal anti-VEGF drug delivery systems for age-related macular degeneration. Int. J. Pharm. 2019, 573, 118767. [Google Scholar] [CrossRef] [PubMed]
- Harish Prashanth, K.V.; Tharanathan, R.N. Depolymerized products of chitosan as potent inhibitors of tumor-induced angiogenesis. Biochim. Et Biophys. Acta 2005, 1722, 22–29. [Google Scholar] [CrossRef]
- Khalili, H.; Lee, R.W.; Khaw, P.T.; Brocchini, S.; Dick, A.D.; Copland, D.A. An anti-TNF-alpha antibody mimetic to treat ocular inflammation. Sci. Rep. 2016, 6, 36905. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotech. 2010, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, K.; McPhie, P.; Minton, A.P. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol. 2003, 326, 1227–1237. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comp. Struct. Biotech. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Vinciguerra, B.; Cortinas, A.B.; Thapa, L.S. Supramolecular PEGylation of biopharmaceuticals. Proc. Natl. Acad. Sci. USA 2016, 113, 14189–14194. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Robinson, S.B.; Csaky, K.G. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm. Res. 2009, 26, 329–337. [Google Scholar] [CrossRef]
- Hayashi, A.; Naseri, A.; Pennesi, M.E.; de Juan, E., Jr. Subretinal delivery of immunoglobulin G with gold nanoparticles in the rabbit eye. Jap. J. Ophthalmol. 2009, 53, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef]
- Swed, A.; Cordonnier, T.; Fleury, F.; Boury, F. Protein Encapsulation into PLGA Nanoparticles by a Novel Phase Separation Method Using Non-Toxic Solvents. J. Nanomed. Nanotechnol. 2014, 5, 6. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Wang, Y.; Song, C. Pharmacokinetics, and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int. J. Nanomed. 2009, 4, 175–183. [Google Scholar] [CrossRef]
- Carroll, R.; Bhatia, D.; Geldenhuys, W.; Bhatia, R.; Miladore, N.; Bishayee, A.; Sutariya, V. Brain-targeted delivery of tempol-loaded nanoparticles for neurological disorders. J. Drug Target. 2010, 18, 665–674. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers. 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Poly (Lactic-co-Glycolic Acid) Nanoparticles and Nanoliposomes for Protein Delivery in Targeted Therapy: A Comparative In Vitro Study. Polymers 2020, 12, 2566. [Google Scholar] [CrossRef]
- Feczko, T.; Toth, J.; Dosa, G.; Gyenis, J. Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Process. 2011, 50, 757–765. [Google Scholar] [CrossRef]
- Li, H.; Tran, V.V.; Hu, Y.; Mark Saltzman, W.; Barnstable, C.J.; Tombran-Tink, J. A PEDF N- terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp. Eye Res. 2006, 83, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, K.K.; Park, K.; Hu, Y.; Xu, X.; Zheng, Z.; Tyagi, P.; Kompella, U.B.; Ma, J.X. Anti- inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6230–6237. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, R.; Lee, K.; Tyagi, P.; Ding, L.; Kompella, U.B.; Chen, J.; Xu, X.; Ma, J.X. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler. Thromb. Vas. Bio. 2015, 35, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V.; Grant, M.B. Wnting out ocular neovascularization: Using nanoparticle delivery of very-low density lipoprotein receptor extracellular domain as Wnt pathway inhibitor in the retina. Arterioscler. Thromb. Vas. Bio. 2015, 35, 1046–1047. [Google Scholar] [CrossRef]
- Agrahari, V.; Hung, W.T.; Christenson, L.K.; Mitra, A.K. Composite Nano-formulation Therapeutics for Long-Term Ocular Delivery of Macromolecules. Mol. Pharm. 2016, 13, 2912–2922. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. WIREs Nanomed. Nanobiotechnol. 2017, 10, e1473. [Google Scholar] [CrossRef]
- Kelly, S.J.; Hirani, A.; Shahidadpury, V.; Solanki, A.; Halasz, K.; Varghese Gupta, S.; Madow, B.; Sutariya, V. Aflibercept Nanoformulation Inhibits VEGF Expression in Ocular In vitro Model: A Preliminary Report. Biomedicines 2018, 6, 92. [Google Scholar] [CrossRef]
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol. Pharm. 2013, 10, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathama, A.; Somavarapu, S. PLGA microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol. Pharm. 2016, 13, 2923–2940. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.S.; Kim, K.S.; Yang, J.A.; Joo, C.K.; Hahn, S.K. Flt1 peptide-hyaluronate conjugate micelle-like nanoparticles encapsulating genistein for the treatment of ocular neovascularization. Acta Biomater. 2012, 8, 3932–3940. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed. Nanotech. Bio. Med. 2016, 12, 2149–2160. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Normando, E.M.; Guo, L.; Turner, A.; Nizari, S.; O’Shea, P.; Moss, S.E.; Somavarapu, S.; Cordeiro, M.F. Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small 2014, 10, 1575–1584. [Google Scholar] [CrossRef]
- Abrishami, M.; Zarei-Ghanavati, S.; Soroush, D.; Rouhbakhsh, M.; Jaafari, M.R.; Malaekeh-Nikouei, B. Preparation, characterization, and in vivo evaluation of nanoliposomes- encapsulated bevacizumab (avastin) for intravitreal administration. Retina 2009, 29, 699–703. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Lema, M.I.; Otero-Espinar, F.J. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar]
- Puras, G.; Martínez-Navarrete, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Avilés-Trigueros, M.; et al. Protamine/DNA/niosome ternary nonviral vectors for gene delivery to the retina: The role of protamine. Mol. Pharm. 2015, 12, 3658–3671. [Google Scholar] [CrossRef]
- Camelo, S.; Lajavardi, L.; Bochot, A.; Goldenberg, B.; Naud, M.; Fattal, E.; Behar-Cohen, F.; De Kozak, Y. Ocular and systemic bio-distribution of rhodamine-conjugated liposomes loaded with VIP injected into the vitreous of Lewis rats. Mol. Vis. 2007, 13, 2263–2274. [Google Scholar]
- Bochot, A.; Fattal, E. Liposomes for intravitreal drug delivery: A state of the art. J. Control. Release 2012, 161, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug. Deliv. 2011, 2011, 863734. [Google Scholar] [CrossRef]
- Kawakami, S.; Harada, A.; Sakanaka, K.; Nishida, K.; Nakamura, J.; Sakaeda, T.; Ichikawa, N.; Nakashima, M.; Sasaki, H. In vivo gene transfection via intravitreal injection of cationic liposome/plasmid DNA complexes in rabbits. Int. J. Pharm. 2004, 278, 255–262. [Google Scholar] [CrossRef]
- Bochot, A.; Fattal, E.; Boutet, V.; Deverre, J.R.; Jeanny, J.C.; Chacun, H.; Couvreur, P. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 253–259. [Google Scholar]
- Ben-Arzi, A.; Ehrlich, R.; Neumann, R. Retinal Diseases: The Next Frontier in Pharmacodelivery. Pharmaceutics 2022, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release. 2017, 48, 96–116. [Google Scholar] [CrossRef]
- Patel, S.P.; Vaishya, R.; Patel, A.; Agrahari, D.; Mitra, A.K. Optimization of novel pentablock copolymer based composite formulation for sustained delivery of peptide/protein in the treatment of ocular diseases. J. Microencapsul. 2016, 33, 103–113. [Google Scholar] [CrossRef]
- Senturk, B.; Cubuk, M.O.; Ozmen, M.C.; Aydin, B.; Guler, M.O.; Tekinay, A.B. Inhibition of VEGF mediated corneal neovascularization by anti-angiogenic peptide nanofibers. Biomaterials 2016, 107, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Vaishya, R.; Pal, D.; Mitra, A.K. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS PharmSciTech. 2015, 16, 327–343. [Google Scholar] [CrossRef]
- Patel, S.P.; Vaishya, R.; Mishra, G.P.; Tamboli, V.; Pal, D.; Mitra, A.K. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. J. Drug Deliv. 2014, 2014, 40174. [Google Scholar] [CrossRef]
- Acharya, G.; Yuan, X.; Marcano, D.; Shin, C.; Hua, X. Nanowafer drug delivery to treat corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5032. [Google Scholar]
- Roman, V.M.; Peter, W.J.M.; Fraser, S.; Vitaliy, V.K. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics. 2019, 11, 321. [Google Scholar] [CrossRef]
- Young, K.H.; Yum, Y.S.; Jang, G.; Ahn, D.R. Discovery of a non-cationic cell penetrating peptide derived from membrane-interacting human proteins and its potential as a protein delivery carrier. Sci. Rep. 2015, 5, 11719. [Google Scholar] [CrossRef] [PubMed]
- De Cogan, F.; Hill, L.J.; Lynch, A.; Morgan-Warren, P.J.; Lechner, J.; Berwick, M.R.; Peacock, A.F.A.; Chen, M.; Scott, R.A.H.; Xu, H.; et al. Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, H.; Lin, S.; Qu, J.; Xiao, J.; Huang, Y.; Xiao, Y.; Fu, X.; Yang, Y.; Li, X. Cell-Penetrating Peptide TAT-Mediated Delivery of Acidic FGF to Retina and Protection against Ischemia-Reperfusion Injury in Rats. J. Cell. Mol. Med. 2010, 14, 1998–2005. [Google Scholar] [CrossRef]
- Liu, C.; Tai, L.; Zhang, W.; Wei, G.; Pan, W.; Lu, W. Penetratin, a Potentially Powerful Absorption Enhancer for Noninvasive Intraocular Drug Delivery. Mol. Pharm. 2014, 11, 1218–1227. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Steinman, L.; Kim, D.T.; Fathman, C.G.; Rothbard, J.B. Polyarginine Enters Cells More Efficiently than Other Polycationic Homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, N.; Yu, H.; Mu, H.; He, B.; Hua, H.; Wang, A.; Sun, K. Topical Ocular Delivery to Laser-Induced Choroidal Neovascularization by Dual Internalizing RGD and TAT Peptide-Modified Nanoparticles. Int. J. Nanomed. 2017, 12, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Li, Z.; Sheng, J.; Zhang, X.; Feng, D.; Zhang, X.; Yin, F.; Wang, A.; Wang, F. Tat PTD-Endostatin-RGD: A Novel Protein with Anti-Angiogenesis Effect in Retina via Eye Drops. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Moellering, R.E.; Hilinski, G.J.; Kim, Y.W.; Grossmann, T.N.; Yeh, G.L. Towards understanding cell penetration by stapled peptides. Med. Chem. Comm. 2015, 6, 111–119. [Google Scholar] [CrossRef]
- Ho, J.; Uger, R.A.; Zwick, M.B.; Luscher, M.A.; Barber, B.H.; MacDonald, K.S. Conformational constraints imposed on a pan-neutralizing HIV-1 antibody epitope result in increased antigenicity but not neutralizing response. Vaccine 2005, 23, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Murakami, T.; Gotoh, N.; Fukuda, R.; Hashida, Y.; Hashida, M.; Tsujikawa, A.; Yoshimura, N. High-Density Lipoprotein Mutant Eye Drops for the Treatment of Posterior Eye Diseases. J. Control. Release 2017, 266, 301–309. [Google Scholar] [CrossRef]
- Tai, L.; Liu, C.; Jiang, K.; Chen, X.; Feng, L.; Pan, W.; Wei, G.; Lu, W. A Novel Penetratin-Modified Complex for Noninvasive Intraocular Delivery of Antisense Oligonucleotides. Int. J. Pharm. 2017, 529, 347–356. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, X.; Shen, Q.; Zhan, C.; Zhang, Y.; Xie, C.; Wei, G.; Lu, W. Discerning the Composition of Penetratin for Safe Penetration from Cornea to Retina. Acta Biomater. 2017, 63, 123–134. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. NT-501: An ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr. Opin. Mol. Ther. 2008, 10, 506–515. [Google Scholar]
- Kuno, N.; Fujii, S. Biodegradable intraocular therapies for retinal disorders: Progress to date. Drugs Aging 2010, 27, 117–134. [Google Scholar] [CrossRef]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts 2016, 6, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, M.; Annamalai, B.; Parsons, N.; Shuler, A.; Potts, J.; Rohrer, B. Encapsulated Cell Technology for the Delivery of Biologics to the Mouse Eye. J. Vis. Exp. 2020. [CrossRef] [PubMed]
- Annamalai, B.; Parsons, N.; Brandon, C.; Rohrer, B. The use of Matrigel combined with encapsulated cell technology to deliver a complement inhibitor in a mouse model of choroidal neovascularization. Mol. Vis. 2020, 26, 370–377. [Google Scholar] [PubMed]
- Raiskup-Wolf, F.; Eljarrat-Binstock, E.; Rehak, M.; Domb, A.; Frucht-Pery, J. Transcorneal and transscleral iontophoresis of the dexamethasone phosphate into the rabbit eye. Cesk. Slov. Oftalmol. 2007, 63, 360–368. [Google Scholar]
- Eljarrat-Binstock, E.; Orucov, F.; Frucht-Pery, J.; Pe’er, J.; Domb, A.J. Methylprednisolone delivery to the back of the eye using hydrogel iontophoresis. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2008, 24, 344–350. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J.; Orucov, F.; Dagan, A.; Frucht-Pery, J.; Pe’er, J. In vitro and in vivo evaluation of carboplatin delivery to the eye using hydrogel-iontophoresis. Curr. Eye Res. 2008, 33, 269–275. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J.; Frucht-Pery, J.; Pe’er, J. Methotrexate delivery to the eye using transscleral hydrogel iontophoresis. Curr. Eye Res. 2007, 32, 639–646. [Google Scholar] [CrossRef]
- Molokhia, S.; Papangkorn, K.; Butler, C.; Higuchi, J.W.; Brar, B.; Ambati, B.; Li, S.K.; Higuchi, W.I. Transscleral Iontophoresis for Noninvasive Ocular Drug Delivery of Macromolecules. J. Ocul. Pharmacol. Ther. 2020, 36, 247–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Yu, X.; Qi, Y.; Chen, Y.; Hu, Z.; Li, Z. A flexible device for ocular iontophoretic drug delivery. Biomicrofluidics 2016, 10, 011911. [Google Scholar] [CrossRef]
- Parkinson, T.M.; Ferguson, E.; Febbraro, S.; Bakhtyari, A.; King, M.; Mundasad, M. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 2003, 19, 145–151. [Google Scholar] [CrossRef]
- Vollmer, D.L.; Szlek, M.A.; Kolb, K.; Lloyd, L.B.; Parkinson, T.M. in vivo transscleral iontophoresis of amikacin to rabbit eyes. J. Ocul. Pharmacol. Ther. 2002, 18, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, B.E.; Raiskup, F.; Frucht, P.J.; Domb, A.J. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J. Control. Release 2005, 106, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.C.; Liang, X.H.; Brenda, F.B.; Rosanne, M.C. Antisense technology: A review. J. Bio. Chem. 2021, 296. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak; Osmałek, T.; Beilstein, J. Microneedle-based ocular drug delivery systems—Recent advances and challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef]
- Thakur, R.R.; Fallows, S.J.; McMillan, H.L.; Donnelly, R.F.; Jones, D.S. Microneedle- mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. J. Pharm. Pharmacol. 2014, 66, 584–595. [Google Scholar] [CrossRef]
- Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.M. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Procopio, A.; Lagreca, E.; Jamaledin, R.; La Manna, S.; Corrado, B.; Di Natale, C.; Onesto, V. Recent Fabrication Methods to Produce Polymer-Based Drug Delivery Matrices (Experimental and In Silico Approaches). Pharmaceutics 2022, 14, 872. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Adelli, G.R.; Hippalgaonkar, K.; Repka, M.A.; Majumdar, S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: Development, characterization, and In vitro evaluation. J. Ocu. Pharmacol. Ther. 2013, 29, 216–228. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Sakurai, E.; Tabata, Y.; Kimura, H. Intraocular sustained drug delivery using implantable polymeric devices. Adv. Drug Deliv. Rev. 2005, 57, 2033–2046. [Google Scholar] [CrossRef]
- Malcles, A.; Dot, C.; Voirin, N.; Vie, A.L.; Agard, E.; Bellocq, D.; Denis, P.; Kodjikian, L. Safety of intravitreal Dexamethasone implant (OZURDEX): The SAFODEX study. Incidence and Risk Factors of Ocular Hypertension. Retina 2017, 37, 1352–1359. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Chang, S.; Jiang, A.; Wang, J.; Cebulla, C.M. Intravitreal devices for the treatment of vitreous inflammation. Mediat. Inflamm. 2012, 2012, 126463. [Google Scholar] [CrossRef] [PubMed]
- Torriglia, A.; Valamanesh, F.; Behar-Cohen Francine, F. On the Retinal Toxicity of Intraocular Glucocorticoids. Biochem. Pharmacol. 2010, 80, 1878–1886. [Google Scholar] [CrossRef]
- Dugel, P.U.; Bandello, F.; Loewenstein, A. Dexamethasone Intravitreal Implant in the Treatment of Diabetic Macular Edema. Clin. Ophthalmol. 2015, 9, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lewis, H.; Foster, R.E.; Schwendeman, S.P. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J. Control. Release 1998, 55, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Bourges, J.L.; Bloquel, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Taban, M.; Lowder, C.Y.; Kaiser, P.K. Outcome of fluocinolone acetonide implant (Retisert) reimplantation for chronic noninfectious posterior uveitis. Retina 2008, 28, 1280–1288. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone Acetonide Uveitis Study, Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: Thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef]
- Ambati, J.; Gragoudas, E.S.; Miller, J.W.; You, T.T.; Miyamoto, K.; Delori, F.C.; Adamis, A.P. Transscleral delivery of bioactive protein to the choroid and retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1186–1191. [Google Scholar]
- Agarwal, P.; Rupenthal, I.D. Injectable implants for the sustained release of protein and peptide drugs. Drug Disc. Today 2013, 18, 337–349. [Google Scholar] [CrossRef]
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol. J. 2012, 6, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ji, Y.L.; Ma, X.; Wen, J.C.; Wei, W.; Huang, S.M. Pharmacokinetics, and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int. J. Ophthalmol. 2015, 8, 653–658. [Google Scholar] [PubMed]
- Freeman, W.R.; Sailor, M.; Chen, M.; Cheng, L. Nanostructured Porous Silicon Dioxide Microparticles as an Intravitreal Injectable Drug Delivery System for Avastin (Bevacizumab) Lasting Six Months. Investig. Ophthalmol. Vis. Sci. 2012, 53, 456. [Google Scholar]
- Varshochian, R.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Khoshayand, M.R.; Atyabi, F.; Sabzevari, A.; Esfahani, M.R.; Dinarvand, R. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur. J. Pharm. Sci. 2013, 50, 341–352. [Google Scholar] [CrossRef]
- Jeong, J.H.; Nguyen, H.K.; Lee, J.E.; Suh, W. Therapeutic effect of apatinib-loaded nanoparticles on diabetes-induced retinal vascular leakage. Int. J. Nanomed. 2016, 11, 3101–3109. [Google Scholar]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.-R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. A 2015, 103, 3148–3156. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan coated nanoparticles for efficient delivery of bevacizumab in the posterior ocular tissues via subconjunctival administration. Carbohydr. Polym. 2021, 267, 118217. [Google Scholar] [CrossRef]
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing Bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. A 2018, 106, 2261–2271. [Google Scholar] [CrossRef]
- Mat Nor, M.N.; Guo, C.X.; Rupenthal, I.D.; Chen, Y.-S.; Green, C.R.; Acosta, M.L. Sustained connexin43 mimetic peptide release from loaded nanoparticles reduces retinal and choroidal photodamage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 36–82. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of nanoencapsulation using poly (lactide-co- glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery to the retina. Mol. Pharm. 2017, 14, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, N.; Huang, X.; Cheng, J.-W.; Li, F.-Q.; Wei, R.-L.; Cai, J.-P. Effect of intravitreal injection of bevacizumab-chitosan nanoparticles on retina of diabetic rats. Int. J. Ophthalmol. 2014, 7, 1–7. [Google Scholar] [PubMed]
- Kim, H.; Saky, K.G. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Alqawlaq, S.; Sivak, J.; Huzil, J.T.; Ivanova, M.V.; Flanagan, J.G.; Beazely, M.A.; Foldvari, M. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: Towards non-invasive glaucoma gene therapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Zarate, J.; Aceves, M.; Murua, A.; Díaz, A.; Avilés-Triguero, M.; Fernández, E.; Pedraz, J. Low molecular weight oligochitosans for non-viral retinal gene therapy. Eur. J. Pharm. Biopharm. 2013, 83, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.-S.; Wu, H.; Zhang, Z.-X.; Cai, Y.; Hou, H.-Y.; Zhao, W.; Yang, X.-M.; Ma, J.-X. Inhibitory efficacy of hypoxia-inducible factor 1a short hairpin RNA plasmid DNA-loaded poly (D,L-lactide-co-glycolide) nanoparticles on choroidal neovascularization in a laser-induced rat model. Gene Ther. 2010, 17, 338–351. [Google Scholar] [CrossRef]
- Park, K.; Chen, Y.; Hu, Y.; Mayo, A.S.; Kompella, U.B.; Longeras, R.; Ma, J.-X. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes 2009, 58, 1902–1913. [Google Scholar] [CrossRef]
- Ryoo, N.-K.; Lee, J.; Lee, H.; Hong, H.K.; Kim, H.; Lee, J.B.; Woo, S.J.; Park, K.H.; Kim, H. Therapeutic effects of a novel siRNA-based anti-VEGF (siVEGF) nanoball for the treatment of choroidal neovascularization. Nanoscale 2017, 9, 15461–15469. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.; Liu, Y.; Li, D.; Zhang, H.; Yang, Y.; Qi, J.; Wang, H.; Gan, L. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: Improving transfection efficiency in retinal pigment epithelium. J. Pharm. Pharmacol. 2018, 70, 1139–1151. [Google Scholar] [CrossRef]
- Liu, H.-A.; Liu, Y.-L.; Ma, Z.-Z.; Wang, J.-C.; Zhang, Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4789–4794. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; del Pozo-Rodriguez, A.; Solinís, M.A.; Rodríguez, J.M.; Friedrich, U.; Torrecilla, J.; Weber, B.H.; Rodríguez-Gascón, A. Structural recovery of the retina in a retinoschisin- deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, J.; Xu, H.; Shi, J.; Liang, Z.; Zhang, R.; Lu, P.; Pu, G.; Zhao, N.; Zhang, J. Sorafenib-loaded nanostructured lipid carriers for topical ocular therapy of corneal neovascularization: Development, in vitro and in vivo study. Drug Deliv. 2022, 29, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Zhang, P. Dasatinib loaded nanostructured lipid carriers for effective treatment of corneal neovascularization. Biomater Sci. 2021, 9, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Marano, R.J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser induced CNV, distribution, uptake, and toxicity. Gene Ther. 2005, 12, 1544–1550. [Google Scholar] [CrossRef]
- Mu, H.; Wang, Y.; Chu, Y.; Jiang, Y.; Hua, H.; Chu, L.; Wang, K.; Wang, A.; Liu, W.; Li, Y.; et al. Multivesicular liposomes for sustained release of bevacizumab in treating laser-induced choroidal neovascularization. Drug. Deliv. 2018, 25, 1372–1383. [Google Scholar] [CrossRef]
- Lance, K.D.; Bernards, D.A.; Ciaccio, N.A.; Good, S.D.; Mendes, T.S. In vivo and in vitro sustained release of ranibizumab from a nanoporous thin-film device. Drug Deliv. Transl. Res. 2016, 6, 771–780. [Google Scholar] [CrossRef]
- Humayun, M.; Santos, A.; Altamirano, J.C.; Ribeiro, R.; Gonzalez, R.; de la Rosa, A.; Shih, J.; Pang, F.; Jiang, F.; Calvillo, P.; et al. Implantable Micropump for Drug Delivery in Patients with Diabetic Macular Edema. Transl. Vis. Sci. Tech. 2014, 3, 5. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef]
- Smith, J.; Ward, D.; Michaelides, M.; Moore, A.T.; Simpson, S. New and emerging technologies for the treatment of inherited retinal diseases: A horizon scanning review. Eye 2015, 29, 131–1140. [Google Scholar] [CrossRef]
- Lim, J.I.; Niec, M.; Wong, V. One year results of a phase 1 study of the safety and tolerability of combination therapy using sustained release intravitreal triamcinolone acetonide and ranibizumab for subfoveal neovascular AMD. Brit. J. Ophthalmol. 2015, 99, 618–623. [Google Scholar] [CrossRef]
- Kirchhof, S.; Gregoritza, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Brandl, F.P. Diels-Alder hydrogels with enhanced stability: First step toward controlled release of bevacizumab. Eur. J. Pharm. Biopharm. 2015, 96, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Green, C.R.; Rupenthal, I.D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery in the treatment of retinal ischaemia. Biomaterials 2018, 168, 10–23. [Google Scholar] [CrossRef] [PubMed]
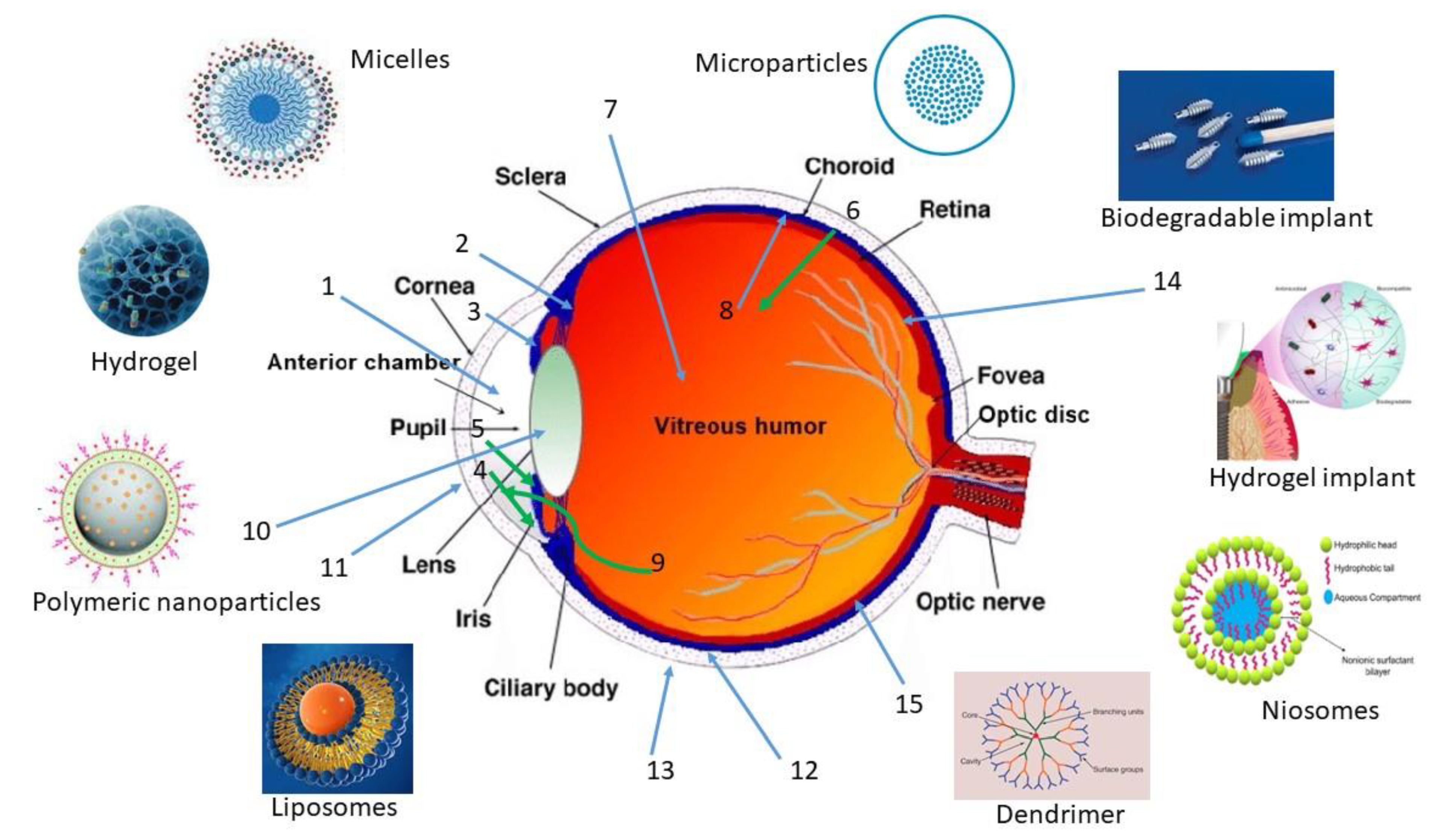
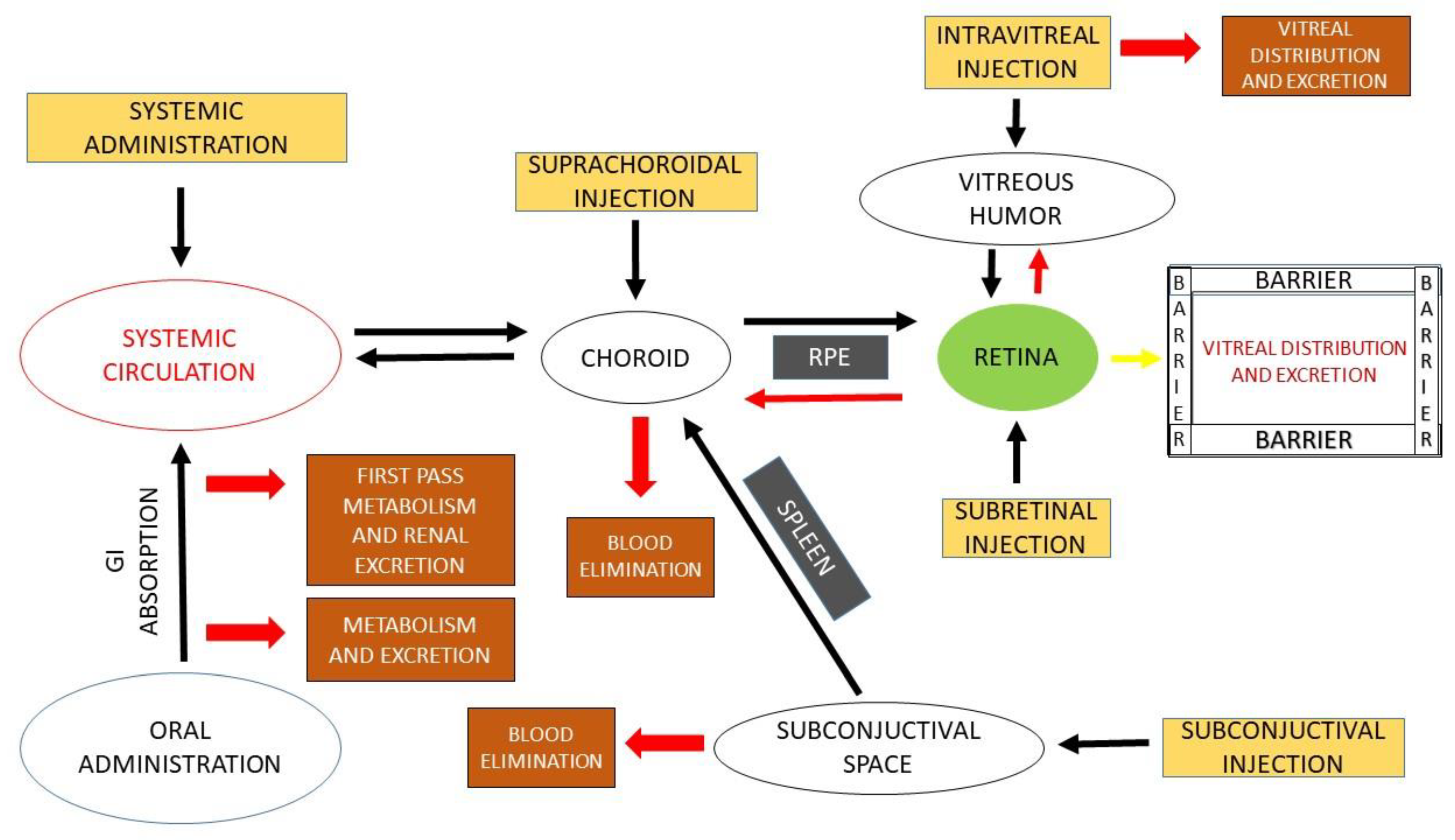
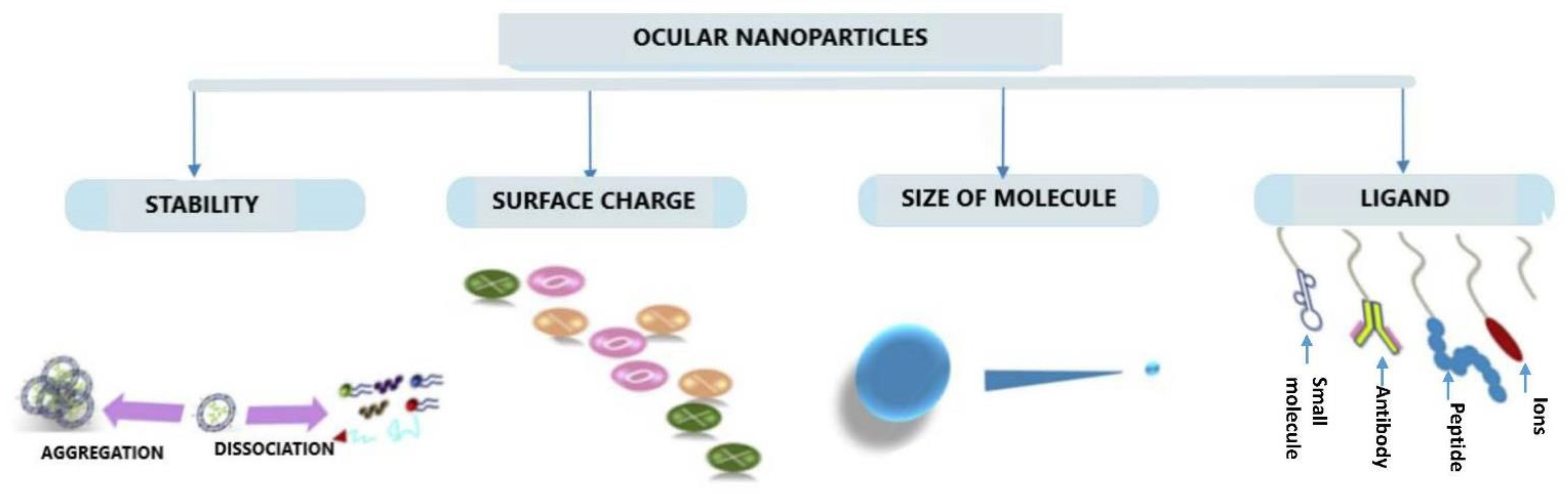
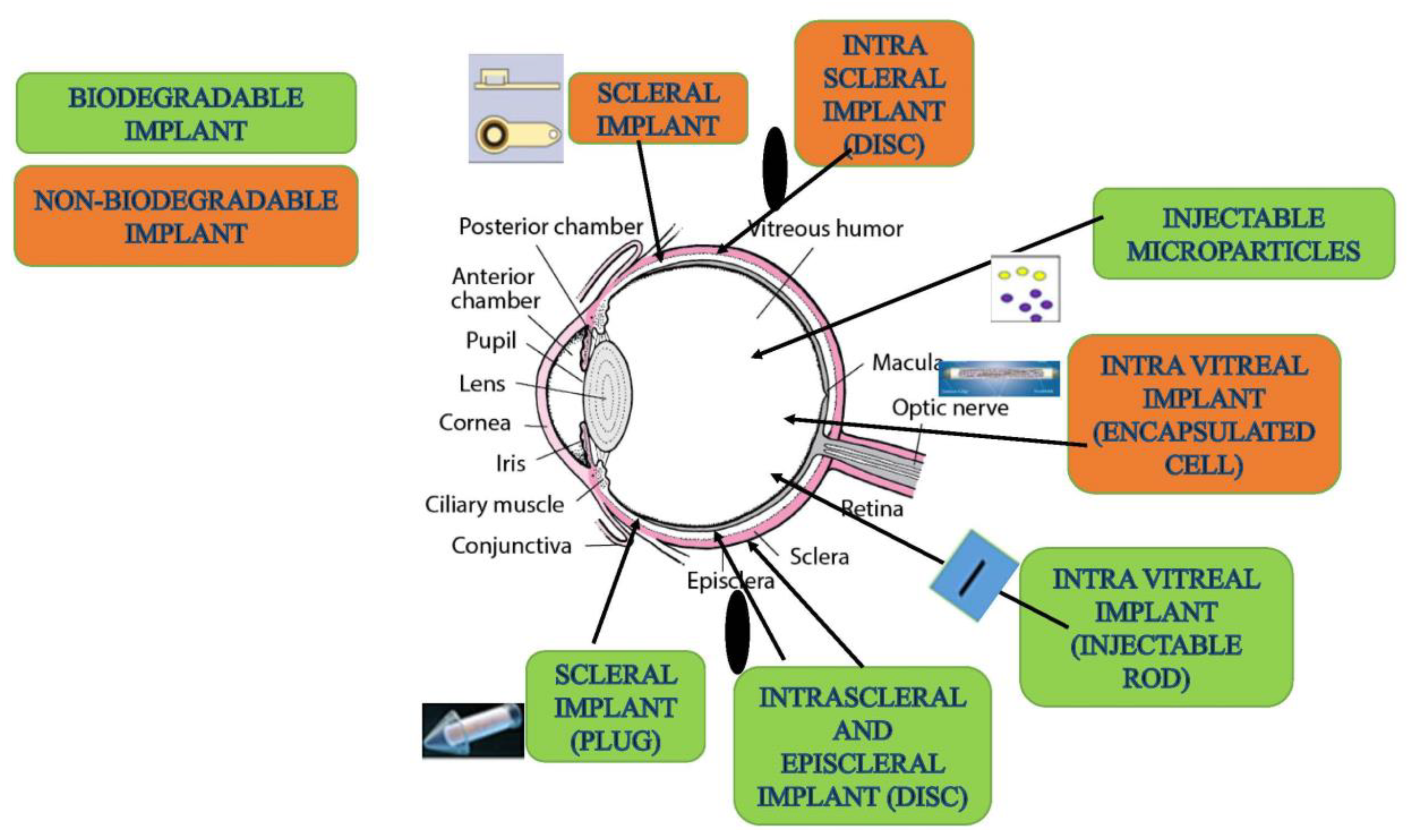
| Approaches | Remarks |
|---|---|
| Use of penetration enhancers | Increases corneal permeability. |
| Improve enzymatic resistance | Avoids degradation by enzymes present in ocular tissues. |
| Conjugation Approaches | |
| Conjugation with ligands | Tissue-specific delivery with minimal toxicity and minimal systemic exposures. |
| Conjugation with ligand, lipids, melanin, hyaluronan, and PEG | Improves half-lives or reduces immunogenicity, protects macromolecules, prevents proteolytic degradation, and provides long-term stability/stability. Improves membrane penetration. |
| Formulation Approaches | |
| Hydrogels | Protect molecules from degradation and provide long-term release. |
| Micro/Nanocarriers | Protect molecules from enzymatic degradation. Enhance permeation. Restrict drug release to the desired area of eye. Provide depot and prolonged retention of the formulation. Improve physical stability. Increase drug permeability. |
| Mucoadhesive polymeric system | Achieve site-specific drug delivery. Improves permeation. |
| Cell-Penetrating Peptides | Improve penetration overcoming ocular barriers and provide sustained release by preventing degradation and increasing the drug residence time. |
| Encapsulated Cell Technology | Enzyme protection, stability, long-term release. |
| Iontophoresis | Improves permeation, provides transfer of ionized molecules through biological membranes using low electric current to the ocular tissues. |
| Microneedles | Provide passive diffusion of therapeutics, overcoming the transport barriers of epithelial tissues, eliminating clearance by conjunctival mechanisms, and minimizing retinal damage. |
| Implants | Protect from degradation and provide depot with slow and sustained release at constant rate over extended period. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastri, D.H.; Silva, A.C.; Almeida, H. Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics 2023, 15, 205. https://doi.org/10.3390/pharmaceutics15010205
Shastri DH, Silva AC, Almeida H. Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics. 2023; 15(1):205. https://doi.org/10.3390/pharmaceutics15010205
Chicago/Turabian StyleShastri, Divyesh H., Ana Catarina Silva, and Hugo Almeida. 2023. "Ocular Delivery of Therapeutic Proteins: A Review" Pharmaceutics 15, no. 1: 205. https://doi.org/10.3390/pharmaceutics15010205
APA StyleShastri, D. H., Silva, A. C., & Almeida, H. (2023). Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics, 15(1), 205. https://doi.org/10.3390/pharmaceutics15010205








