Biopolymer-Based Nanosystems for siRNA Drug Delivery to Solid Tumors including Breast Cancer
Abstract
1. Introduction
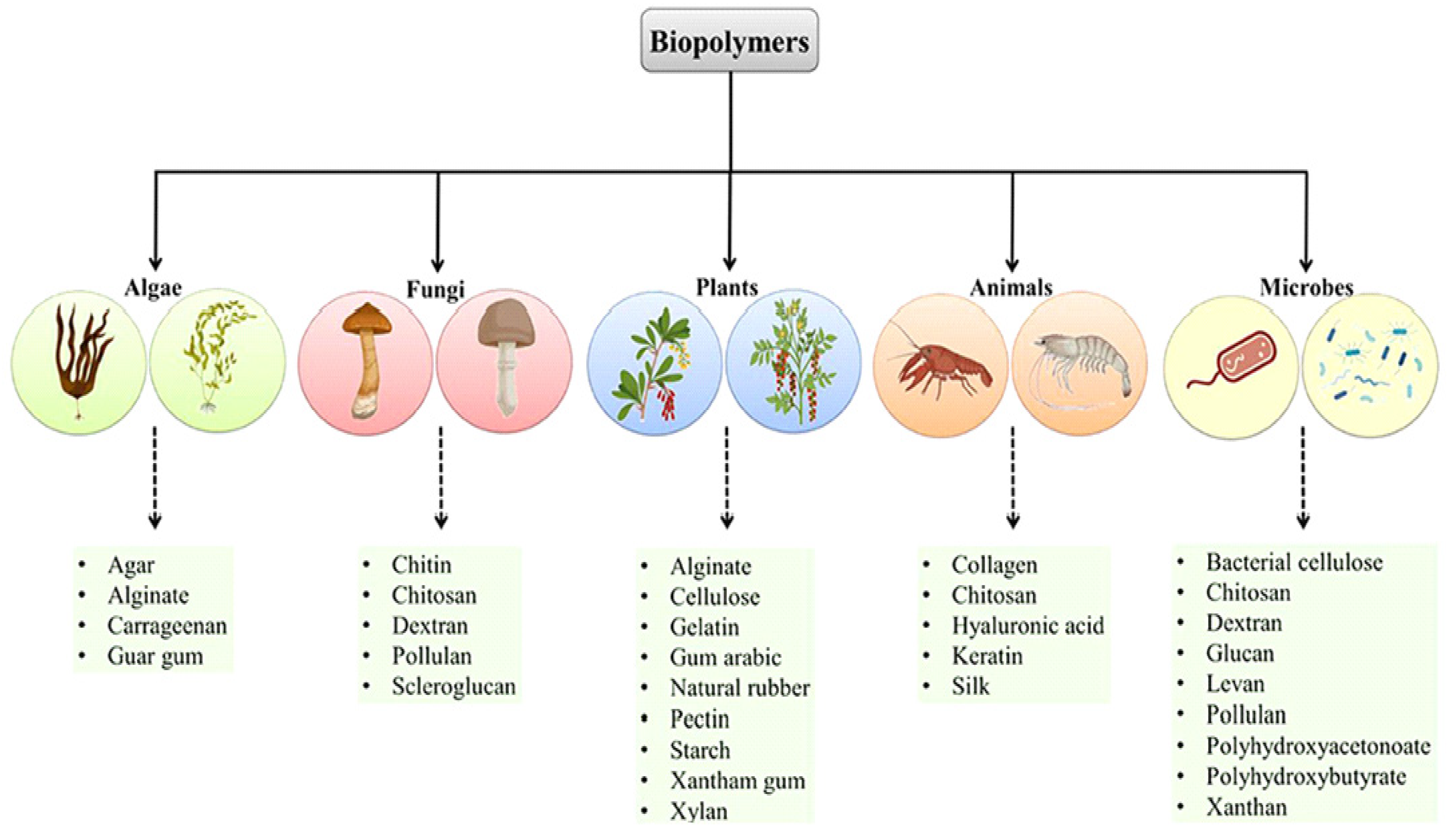
2. Sources of Biopolymers Utilized as Drug Delivery Vehicles
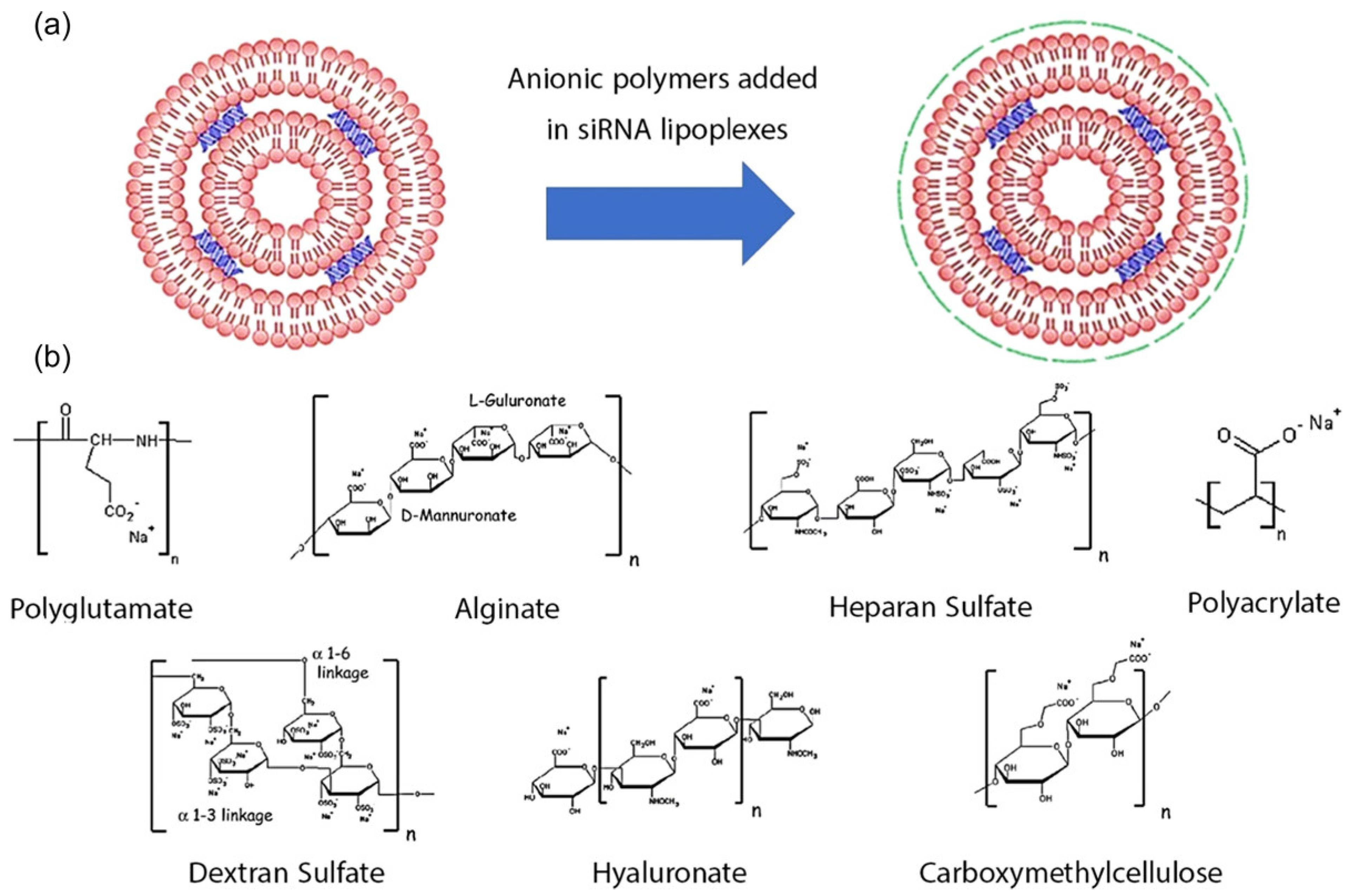
3. Biopolymer-Based Nanocarrier for siRNA Drug Delivery to Solid Tumors
4. Breast Cancer Therapy with Biopolymer-Based siRNA Nanoplatform
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byun, M.J.; Lim, J.; Kim, S.-N.; Park, D.-H.; Kim, T.-H.; Park, W.; Park, C.G. Advances in Nanoparticles for Effective Delivery of RNA Therapeutics. BioChip J. 2022, 16, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Liu, S.; Wong, H.L. Nanotoxicity: A key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine 2014, 9, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-generation vaccines: Nanoparticle-mediated DNA and mRNA delivery. Adv. Healthc. Mater. 2021, 10, e2001812. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles- from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Şenel, B.; Büyükköroğlu, G. Applications of Lipidic and Polymeric Nanoparticles for siRNA Delivery. In Antisense Therapy; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Cavallaro, G.; Sardo, C.; Craparo, E.F.; Porsio, B.; Giammona, G. Polymeric nanoparticles for siRNA delivery: Production and applications. Int. J. Pharm. 2017, 525, 313–333. [Google Scholar] [CrossRef]
- Ragelle, H.; Vandermeulen, G.; Préat, V. Chitosan-based siRNA delivery systems. J. Control. Release 2013, 172, 207–218. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Song, L.; Zhou, Z.; Sun, J.; Du, Y.; Lu, K.; Li, T.; Yin, A.; Xu, J.; et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J. Exp. Clin. Cancer Res. 2016, 35, 57. [Google Scholar] [CrossRef]
- Shakeran, Z.; Varshosaz, J.; Keyhanfar, M.; Mohammad-Beigi, H.; Rahimi, K.; Sutherland, D.S. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif. Cells Nanomed. Biotechnol. 2022, 50, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A mesoporous silica nanoparticle—PEI—Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-Step Synthesis of Amine-Functionalized Hollow Mesoporous Silica Nanoparticles as Efficient Antibacterial and Anticancer Materials. ACS Appl. Mater. Interfaces 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.B.; Zhang, Y. Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications. J. Biomed. Mater. Res. Part A 2005, 75A, 56–62. [Google Scholar] [CrossRef]
- Lv, G.; Qiu, L.; Liu, G.; Wang, W.; Li, K.; Zhao, X.; Lin, J. pH sensitive chitosan-mesoporous silica nanoparticles for targeted delivery of a ruthenium complex with enhanced anticancer effects. Dalton Trans. 2016, 45, 18147–18155. [Google Scholar] [CrossRef]
- Nhavene, E.P.F.; Andrade, G.F.; Faria, J.A.Q.A.; Gomes, D.A.; de Sousa, E.M.B. Biodegradable Polymers Grafted onto Multifunctional Mesoporous Silica Nanoparticles for Gene Delivery. Chemengineering 2018, 2, 24. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, Z.-K.; Zhu, Q.-L.; Rong, X.-H.; Liang, C.-L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.-H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surfaces B: Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Soliman, O.Y.; Alameh, M.G.; De Cresenzo, G.; Buschmann, M.D.; Lavertu, M. Efficiency of Chitosan/Hyaluronan-Based mRNA Delivery Systems In Vitro: Influence of Composition and Structure. J. Pharm. Sci. 2020, 109, 1581–1593. [Google Scholar] [CrossRef]
- Sargazi, S.; Arshad, R.; Ghamari, R.; Rahdar, A.; Bakhshi, A.; Karkan, S.F.; Ajalli, N.; Bilal, M.; Díez-Pascual, A.M. siRNA-based nanotherapeutics as emerging modalities for immune-mediated diseases: A preliminary review. Cell Biol. Int. 2022, 46, 1320–1344. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schafer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. pH-sensitive chitosan-heparin nanoparticles for effective delivery of genetic drugs into epithelial cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Ponce, N.T.; Banerjee, A.; Bandopadhyay, R.; Rajendran, S.; Lichtfouse, E. Green polymeric nanomaterials for the photocatalytic degradation of dyes: A review. Environ. Chem. Lett. 2020, 18, 1569–1580. [Google Scholar] [CrossRef]
- Farshchi, E.; Pirsa, S.; Roufegarinejad, L.; Alizadeh, M.; Rezazad, M. Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2–Ag nanoparticles. Carbohydr Polym. 2019, 216, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kolangare, I.M.; Isloor, A.M.; Karim, Z.A.; Kulal, A.; Ismail, A.F.; Inamuddin; Asiri, A. M. Antibiofouling hollow-fiber membranes for dye rejection by embedding chitosan and silver-loaded chitosan nanoparticles. Environ. Chem. Lett. 2019, 17, 581–587. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Bae, J.; Park, S.J.; Shin, D.S.; Lee, J.; Park, S.; Kim, H.J.; Kwon, O.S. A dual functional conductive hydrogel containing titania@polypyrrolecyclodextrin hybrid nanotubes for capture and degradation of toxic chemical. Biochip J. 2021, 15, 162–170. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Erdene-Ochir, T.; Ganbold, T.; Zandan, J.; Han, S.; Borjihan, G.; Baigude, H. Alkylation enhances biocompatibility and siRNA delivery efficiency of cationic curdlan nanoparticles. Int. J. Biol. Macromol. 2020, 143, 118–125. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes—Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-F.; Zuo, F.-F.; Yin, B.-C.; Ye, B.-C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT. Biomater. Sci. 2022, 10, 1582–1590. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Rai, A.; Groot, M.; Jin, Y. Exosome-Mediated Small RNA Delivery: A Novel Therapeutic Approach for Inflammatory Lung Responses. Mol. Ther. 2018, 26, 2119–2130. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- Shoari, A.; Tooyserkani, R.; Tahmasebi, M.; Löwik, D.W.P.M. Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade. Pharmaceutics 2021, 13, 1391. [Google Scholar] [CrossRef]
- Jones, S.W.; Christison, R.; Bundell, K.; Voyce, C.J.; Brockbank, S.M.V.; Newham, P.; Lindsay, M.A. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 2005, 145, 1093–1102. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhu, D.; Shi, K.; Ma, C.; Zhang, W.; Rocchi, P.; Jiang, L.; Liu, X. Self-assembly of amphiphilic phospholipid peptide dendrimer-based nanovectors for effective delivery of siRNA therapeutics in prostate cancer therapy. J. Control. Release 2020, 322, 416–425. [Google Scholar] [CrossRef]
- Strand, M.S.; Krasnick, B.A.; Pan, H.; Zhang, X.; Bi, Y.; Brooks, C.; Wetzel, C.; Sankpal, N.; Fleming, T.; Goedegebuure, S.P.; et al. Precision delivery of RAS-inhibiting siRNA to KRAS driven cancer via peptide-based nanoparticles. Oncotarget 2019, 10, 4761–4775. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Lv, J.; Cheng, Y. Design of polymers for siRNA delivery: Recent progress and challenges. VIEW 2021, 2, 20200026. [Google Scholar] [CrossRef]
- Peer, D.; Park, E.J.; Morishita, Y.; Carman, C.V.; Shimaoka, M. Systemic Leukocyte-Directed siRNA Delivery Revealing Cyclin D1 as an Anti-Inflammatory Target. Science 2008, 319, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, D.; Kazane, S.; Javahishvili, T.; Tian, F.; Song, F.; Sellers, A.; Barnett, B.; Schultz, P.G. Synthesis of Bispecific Antibodies using Genetically Encoded Unnatural Amino Acids. J. Am. Chem. Soc. 2013, 135, 13885. [Google Scholar] [CrossRef]
- Kohata, A.; Hashim, P.K.; Okuro, K.; Aida, T. Transferrin-Appended Nanocaplet for Transcellular siRNA Delivery into Deep Tissues. J. Am. Chem. Soc. 2019, 141, 2862–2866. [Google Scholar] [CrossRef]
- Khan, O.F.; Kowalski, P.S.; Doloff, J.C.; Tsosie, J.K.; Bakthavatchalu, V.; Winn, C.B.; Haupt, J.; Jamiel, M.; Langer, R.; Anderson, D.G. Endothelial siRNA delivery in nonhuman primates using ionizable low–molecular weight polymeric nanoparticles. Sci. Adv. 2018, 4, eaar8409. [Google Scholar] [CrossRef]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Yu, M.; Mahmoudi, M.; Rasmussen, J.; Ayyash, D.; Zhou, Y.; et al. Tumor Microenvironment-Responsive Multistaged Nanoplatform for Systemic RNAi and Cancer Therapy. Nano Lett. 2017, 17, 4427–4435. [Google Scholar] [CrossRef]
- Yu, D.; Khan, O.F.; Suvà, M.L.; Dong, B.; Panek, W.K.; Xiao, T.; Wu, M.; Han, Y.; Ahmed, A.U.; Balyasnikova, I.V.; et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA 2017, 114, E6147–E6156. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Kauffman, K.J.; Xing, Y.; Shaw, T.E.; Mir, F.F.; Dlott, C.C.; Langer, R.; Anderson, D.G.; Wang, E.T. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl. Acad. Sci. USA 2017, 114, 2060–2065. [Google Scholar] [CrossRef]
- Srivastava, A.; Gowda, D.V.; Madhunapantula, S.V.; Shinde, C.G.; Iyer, M. Mucosal vaccines: A paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS 2015, 123, 275–288. [Google Scholar] [CrossRef]
- Parmar, R.G.; Poslusney, M.; Busuek, M.; Williams, J.M.; Garbaccio, R.; Leander, K.; Walsh, E.; Howell, B.; Sepp-Lorenzino, L.; Riley, S.; et al. Novel Endosomolytic Poly(amido amine) Polymer Conjugates for Systemic Delivery of siRNA to Hepatocytes in Rodents and Nonhuman Primates. Bioconjugate Chem. 2014, 25, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.-H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Iqbal, S.; Shen, S.; Luo, Y.L.; Yang, X.; Wang, J. Development of "CLAN" Nanomedicine for Nucleic Acid Therapeutics. Small 2019, 15, e1900055. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, S.; Zhang, Y.; Peng, Q.; Li, H.; Wang, P.; Fu, X.; Lei, X.; Qin, A.; Yu, X. Homotypic Targeting Delivery of siRNA with Artificial Cancer Cells. Adv. Health Mater. 2020, 9, 1900772. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Feng, C.; Lee, A.; Yin, J.; Chung, R.; Park, J.B.; Rizos, H.; Tao, W.; Zheng, M.; et al. Charge Conversional Biomimetic Nanocomplexes as a Multifunctional Platform for Boosting Orthotopic Glioblastoma RNAi Therapy. Nano Lett. 2020, 20, 1637. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Ruan, M.; Liu, W.; Song, R.; Dai, J.; Xue, W. Worm-Like Biomimetic Nanoerythrocyte Carrying siRNA for Melanoma Gene Therapy. Small 2018, 14, e1803002. [Google Scholar] [CrossRef]
- Cho, S.K.; Kwon, Y.J. Simultaneous gene transduction and silencing using stimuli-responsive viral/nonviral chimeric nanoparticles. Biomaterials 2012, 33, 3316–3323. [Google Scholar] [CrossRef]
- Hong, C.A.; Cho, S.K.; Edson, J.A.; Kim, J.; Ingato, D.; Pham, B.; Chuang, A.; Fruman, D.A.; Kwon, Y.J. Viral/Nonviral Chimeric Nanoparticles To Synergistically Suppress Leukemia Proliferation via Simultaneous Gene Transduction and Silencing. ACS Nano 2016, 10, 8705–8714. [Google Scholar] [CrossRef]
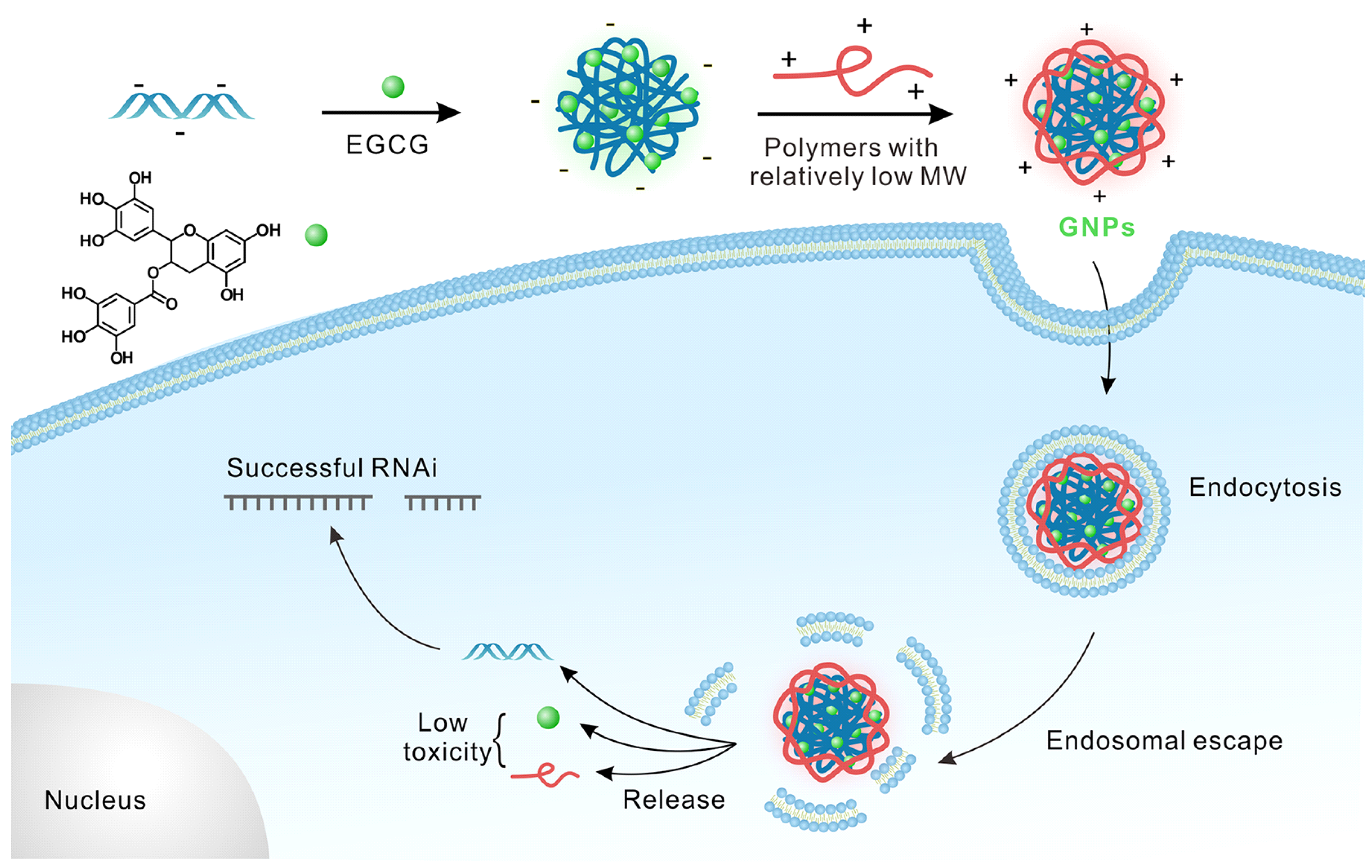
- Shen, W.; Wang, R.; Fan, Q.; Gao, X.; Wang, H.; Shen, Y.; Li, Y.; Cheng, Y. Natural Polyphenol Inspired Polycatechols for Efficient siRNA Delivery. CCS Chem. 2020, 2, 146–157. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Q.; Shen, Y.; Gao, X.; Li, L.; Yan, Y.; Wang, H.; Cheng; Y. Green Tea Catechin Dramatically Promotes RNAi Mediated by LowMolecular-Weight Polymers. ACS Cent. Sci. 2018, 4, 1326–1333. [Google Scholar] [CrossRef]
- Choi, K.Y.; Correa, S.; Min, J.; Li, J.; Roy, S.; Laccetti, K.H.; Dreaden, E.; Kong, S.; Heo, R.; Roh, Y.H.; et al. Binary Targeting of siRNA to Hematologic Cancer Cells In Vivo Using Layer-by-Layer Nanoparticles. Adv. Funct. Mater. 2019, 29, 1900018. [Google Scholar] [CrossRef]
- Hattab, D.; Bakhtiar, A. Bioengineered siRNA-Based Nanoplatforms Targeting Molecular Signaling Pathways for the Treatment of Triple Negative Breast Cancer: Preclinical and Clinical Advancements. Pharmaceutics 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, S.; Gore, K.R. Advances in siRNA therapeutics and synergistic effect on siRNA activity using emerging dual ribose modifications. RNA Biol. 2022, 19, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, R.; Pei, X.; Chai, M.; Sun, L.; Huang, Y.; Wang, J.; Barth, S.; Yu, F.; He, H. Antibody–siRNA conjugates (ARC): Emerging siRNA drug formulation. Med. Drug Discov. 2022, 15, 100128. [Google Scholar] [CrossRef]
- Islam, F.; Ward, E.M.; Sung, H. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, S.; Khademi, A.; Khazaei, M. Natural and herbal compounds targeting breast cancer, a review based on cancer stem cells. Iran J. Basic Med. Sci. 2020, 23, 970–983. [Google Scholar] [CrossRef]
- Hanafi-Bojd, M.Y.; Ansari, L.; Malaekeh-Nikouei, B. Codelivery of anticancer drugs and siRNA by mesoporous silica nanoparticles. Ther. Deliv. 2016, 7, 649–655. [Google Scholar] [CrossRef]
- Sun, P.; Huang, W.; Jin, M.; Wang, Q.; Fan, B.; Kang, L.; Gao, Z. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int. J. Nanomed. 2016, 11, 4931–4945. [Google Scholar] [CrossRef] [PubMed]
- Baghani, L.; Noroozi Heris, N.; Khonsari, F.; Dinarvand, S.; Dinarvand, M.; Atyabi, F. Trimethyl-Chitosan Coated Gold Nanoparticles Enhance Delivery, Cellular Uptake and Gene Silencing Effect of EGFR-siRNA in Breast Cancer Cells. Front. Mol. Biosci. 2022, 9, 871541. [Google Scholar] [PubMed]
- Cui, L.; Zheng, R.; Lui, W.; Shen, P.; Tang, Y.; Luo, J.; Zhang, W.; Jia, G.; Wang, Y.; Zhao, S.; et al. Preparation of chitosan silicon dioxide/BCSG1 siRNA nanoparticles to enhance therapeutic efficacy in breast cancer cells. Mol. Med. Rep. 2018, 17, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van Der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kozlovskaya, V.; Zhang, Z.; Xing, C.; Zaharias, S.; Dolmat, M.; Qian, S.; Zhang, J.; Warram, J.M.; Yang, E.S.; et al. Poly(N-vinylpyrrolidone)-block-Poly(dimethylsiloxane)-block-Poly(N-vinylpyrrolidone) Triblock Copolymer Polymersomes for Delivery of PARP1 siRNA to Breast Cancers. ACS Appl. Bio Mater. 2022, 5, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A Synthetic Lethal Therapeutic Approach: Poly(ADP) Ribose Polymerase Inhibitors for the Treatment of Cancers Deficient in DNA Double-Strand Break Repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef]
- Telli, M.L.; Ford, J.M. Novel Treatment Approaches for Triple-Negative Breast Cancer. Clin. Breast Cancer 2010, 10, E16–E22. [Google Scholar] [CrossRef]
- Zimmer, A.S.; Gillard, M.; Lipkowitz, S.; Lee, J.-M. Update on PARP Inhibitors in Breast Cancer. Curr. Treat. Options Oncol. 2018, 19, 21. [Google Scholar] [CrossRef]
- Lee, A.; Djamgoz, M.B. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef]
- Loibl, S.; O'Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Timms, K.M.; Abkevich, V.; Hughes, E.; Neff, C.; Reid, J.; Morris, B.; Kalva, S.; Potter, J.; Tran, T.V.; Chen, J.; et al. Association of BRCA1/2 Defects with Genomic Scores Predictive of DNA Damage Repair Deficiency Among Breast Cancer Subtypes. Breast Cancer Res. 2014, 16, 475. [Google Scholar] [CrossRef]
- Garufi, G.; Palazzo, A.; Tortora, G.; Bria, E.; Carbognin, L. Homologous Repair Deficiency Status and Response to Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: The Best Current Biomarker to Select the Most Appropriate Treatment? J. Cancer Sci. Clin. Ther. 2021, 5, 124–133. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Z.; Yin, X.; Fu, S.; Deng, X. Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 4517. [Google Scholar] [CrossRef]
- Lu, S.; Morris, V.B.; Labhasetwar, V. Effectiveness of Small Interfering RNA Delivery via Arginine-Rich Polyethylenimine-Based Polyplex in Metastatic and Doxorubicin-Resistant Breast Cancer Cells. J. Pharmacol. Exp. Ther. 2019, 370, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Rietwyk, S.; Peer, D. Next-Generation Lipids in RNA Interference Therapeutics. ACS Nano 2017, 11, 7572–7586. [Google Scholar] [CrossRef] [PubMed]
- Magriotis, P.A. Recent progress toward the asymmetric synthesis of carbon-substituted piperazine pharmacophores and oxidative related heterocycles. RSC Med. Chem. 2020, 11, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Girase, P.S.; Dhawan, S.; Kumar, V.; Shinde, S.R.; Palkar, M.B.; Karpoormath, R. An appraisal of anti-mycobacterial activity with structure-activity relationship of piperazine and its analogues: A review. Eur. J. Med. Chem. 2021, 210, 112967. [Google Scholar] [CrossRef]
- Ni, H.; Hatit, M.Z.C.; Zhao, K.; Loughrey, D.; Lokugamage, M.P.; Peck, H.E.; Del Cid, A.; Muralidharan, A.; Kim, Y.; Santangelo, P.J.; et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat. Commun. 2022, 13, 4766. [Google Scholar] [CrossRef]
- Ashique, S.; Almohaywi, B.; Haider, N.; Yasmin, S.; Hussain, A.; Mishra, N.; Garg, A. siRNA-based nanocarriers for targeted drug delivery to control breast cancer. Adv. Cancer Biol. Metastasis 2022, 4, 100047. [Google Scholar] [CrossRef]
- Hafiz, M.M.; Mazatulikhma, M.Z.; Faiz, F.M.; Saifulaman, M.M. Targeted RNAi of the mitogen-activated protein kinase pathway genes in acute myeloid leukemia cells. Sains Malays. 2013, 42, 1131–1137. [Google Scholar]
- Kamaruzman, N.I.; Tiash, S.; Ashaie, M.; Chowdhury, E.H. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Li, T.; Xu, M.; Chen, Y.; Wu, C.; Dang, X.; Liu, Y. Multifunctional Core/Shell Nanoparticles Cross-linked Polyetherimide-folic Acid as Efficient Notch-1 siRNA Carrier for Targeted Killing of Breast Cancer. Sci. Rep. 2014, 4, 7072. [Google Scholar] [CrossRef]
- Subramanian, K.; Kanwar, J.R.; Athalya, P.K.; Janakiraman, N.; Khetan, V.; Kanwar, R.K.; Eluchuri, S.; Krishnakumar, S. EpCAM aptamer mediated cancer cell specific delivery of EpCAM siRNA using polymeric nanocomplex. J. Biomed. Sci. 2015, 22, 4. [Google Scholar] [CrossRef]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic Administration of siRNA with Anti-HB-EGF Antibody-Modified Lipid Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, W.; Xia, Y.; Sun, J.; Chen, H.; Li, B.; Zhang, D.; Qian, W.; Meng, Y.; Deng, L.; et al. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials 2011, 32, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjugate Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yang, J.; Di Jia, M.A.M.; Auguste, D.T. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics 2016, 6, 1–13. [Google Scholar] [CrossRef]
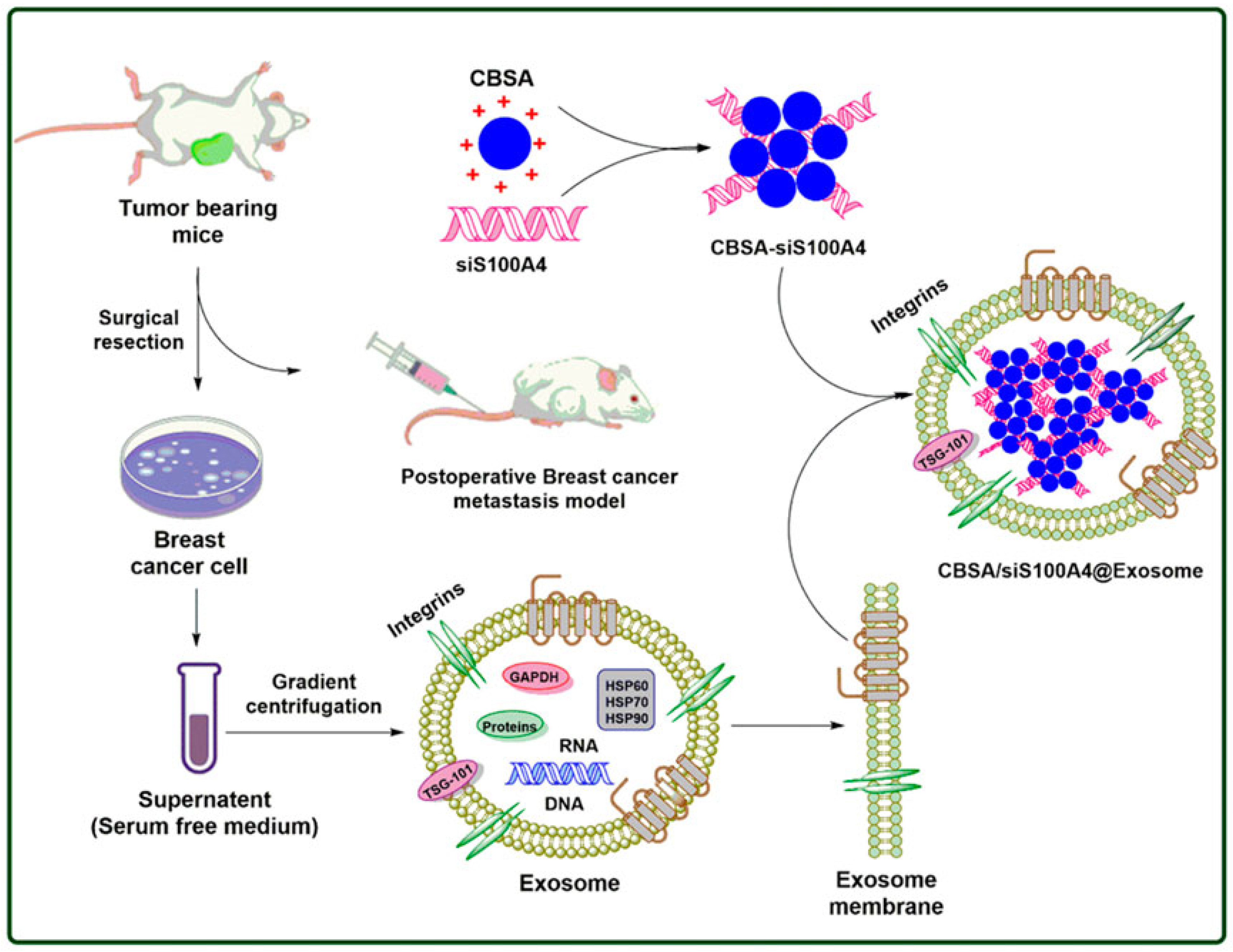
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Chopra, H.; Singh, I.; Dua, K.; Gautam, R.K. Insights on prospects of nano-siRNA based approaches in treatment of Cancer. Front. Pharmacol. 2022, 13, 985670. [Google Scholar] [CrossRef]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef]
- Keam, B.; Kim, S.; Ahn, Y.-O.; Kim, T.M.; Lee, S.-H.; Kim, D.-W.; Heo, D.S. In vitro anticancer activity of PI3K alpha selective inhibitor BYL719 in head and neck cancer. Anticancer. Res. 2015, 35, 175–182. [Google Scholar]
- Kim, M.J.; Lee, S.J.; Ryu, J.H.; Kim, S.H.; Kwon, I.C.; Roberts, T.M. Combination of KRAS gene silencing and PI3K inhibition for ovarian cancer treatment. J. Control. Release 2020, 318, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wen, W.; Yost, S.E.; Xing, Q.; Yan, J.; Han, E.S.; Mortimer, J.; Yim, J.H. Combination therapy with BYL719 and LEE011 is synergistic and causes a greater suppression of p-S6 in triple negative breast cancer. Sci. Rep. 2019, 9, 7509. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Ferreira, D.; Rodrigues, L.R. Exosome-based delivery of RNAi leads to breast cancer inhibition. J. Drug Deliv. Sci. Technol. 2022, 78, 103931. [Google Scholar] [CrossRef]
- Kaban, K.; Hinterleitner, C.; Zhou, Y.; Salva, E.; Kantarci, A.; Salih, H.; Märklin, M. Therapeutic Silencing of BCL-2 Using NK Cell-Derived Exosomes as a Novel Therapeutic Approach in Breast Cancer. Cancers 2021, 13, 2397. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Espinoza, A.; Mongayt, D.; Markman, J.L.; Elramsisy, A.; Phillips, H.W.; et al. Nanobiopolymer for Direct Targeting and Inhibition of EGFR Expression in Triple Negative Breast Cancer. PLoS ONE 2012, 7, e31070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Yang, C. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, K.; Zhang, G.; Jia, Z.; Yu, Y.; Guo, J.; Hua, Y.; Guo, F.; Li, X.; Zou, W.; et al. Enrichment of CD44 in Exosomes from Breast Cancer Cells Treated with Doxorubicin Promotes Chemoresistance. Front. Oncol. 2020, 10, 960. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Control. Release 2016, 243, 160–171. [Google Scholar] [CrossRef]
- Pillé, J.Y.; Li, H.; Blot, E.; Bertrand, J.-R.; Pritchard, L.-L.; Opolon, P.; Maksimenko, A.; Lu, H.; Vannier, J.-P.; Soria, J.; et al. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: Safety and e_cacy in xenografted aggressive breast cancer. Hum. Gene Ther. 2006, 17, 1019–1026. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Landry, B.; Mahdipoor, P.; Uludağ, H. Induction of Apoptosis by Survivin Silencing through siRNA Delivery in a Human Breast Cancer Cell Line. Mol. Pharm. 2011, 8, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.G.; Santos, A.O.; Bimbo, L.M.; Moura, V.; Ramalho, J.S.; De Lima, M.C.P.; Simões, S.; Moreira, J.N. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int. J. Pharm. 2012, 434, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Salva, E.; Kabasakal, L.; Eren, F.; Özkan, N.; Çakalağaoğlu, F.; Akbuğa, J. Local delivery of chitosan/VEGF siRNA nanoplexes reduces angiogenesis and growth of breast cancer in vivo. Nucleic Acid Ther. 2012, 22, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Cheng, W.; Li, S.; Wu, B.; Leng, X.; Xu, S.; Tian, J. Novel cell-penetrating peptide-loaded nanobubbles synergized with ultrasound irradiation enhance EGFR siRNA delivery for triple negative Breast cancer therapy. Colloids Surf. B Biointerfaces 2016, 146, 387–395. [Google Scholar] [CrossRef]
| siRNA Drugs | Delivery System | Target Gene | Disease | Company | Status/ Phase |
|---|---|---|---|---|---|
| Patisiran | LNP-siRNA | TTR | TTR-mediated amyloidosis | Alnylam | Approved, FDA 2018 |
| Givosiran | GalNAc-siRNA | ALAS1 | Acute hepatic porphyria | Alnylam | Approved, FDA 2019 |
| Lumasiran | GalNAc-siRNA | HAO1 | Primary heperoxaluria type 1 | Alnylam | Approved, FDA 2020 |
| Inclisiran | GalNAc-siRNA | PCSK9 | Hypercholesterolemia | Alnylam Novartis | Approved, FDA 2021 |
| Vutrisiran (ALN-TTRSCO2) | GalNAc-siRNA | TTR | TTR-mediated amyloidosis | Alnylam | III |
| Nedosiran (DCR-PHXC) | GalNAc-siRNA | LDHA | Primary heperoxaluria | Alnylam | III |
| Fitusiran (ALN-AT3SC) | GalNAc-siRNA | AT | Hemophilia A and B and rare blood disorder | Alnylam Sanofi Genzyme | III |
| Atu027 | Liposomal-siRNA, AtuPLEX | PKN3 | Solid tumors, advanced or metastatic pancreatic cancer | Silence Therapeutics | II completed |
| TMK-PLK1 | LNP-siRNA | PLK1 | Solid tumors, adrenal cortical carcinoma, hepatocellular carcinoma | Arbutus Biopharma | II completed |
| siG12D LODER | siG12D-LODER™ system and chemotherapy | G12D mutated KRAS | Pancreatic cancer, pancreatic ductal adenocarcinoma | Silenseed Ltd. | II ongoing |
| ALN-VSP02 | LNP-siRNA | VEGF, KSP | Solid tumors | Alnylam | I completed |
| Target | Nanoparticle | Cell Line | Reference |
|---|---|---|---|
| RhoA | Chitosan-coated polyisohexylcyanoacrylate (PIHCA) NPs | MDA-MB-231 | [111] |
| Survivin | Lipid substituted polymer | MDA-MB-231 | [112] |
| HB-EGF | Fab’s antibody-modified LNP | MDA-MB-231 | [94] |
| EGFR | Fab conjugated liposomal NPs | MDA-MB-231 | [95] |
| EGFR | PMLA-based nanobioconjugate | MDA-MB-468 | [106] |
| eGFP | F3-targeted liposomal NPs | MDA-MB-231 MDA-MB-435 | [113] |
| VEGF-A, VEGFR-1, VEGFR-2, and neuropilin-1 | Chitosan nanocomplexes | Breast tumor induced in rat using N-nitroso-N-methylurea | [114] |
| Lipocalin-2 | ICAM-1 conjugated liposomes | MDA-MB-231 | [97] |
| HR | EpCAM aptamer polyethylineimine | MCF-7 | [93] |
| EGFR | CPP-loaded nanobubbles | MDA-MB-231 | [115] |
| CD44 | Exosome | MCF-7 | [109] |
| PIK3CA | BJExo | MDA-MB-231, MDA-MB-453 | [104] |
| SUR, Bcl2 | Bovine milk exosomes | MCF-7, MDA-MB-231 | [33] |
| ABCB1 | PEG-L-arginine oligo(-alkylaminosiloxane)-PEI polyplex | MCF-7 ADR, MDA-MB-231 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.A.; Torchilin, V.P. Biopolymer-Based Nanosystems for siRNA Drug Delivery to Solid Tumors including Breast Cancer. Pharmaceutics 2023, 15, 153. https://doi.org/10.3390/pharmaceutics15010153
Subhan MA, Torchilin VP. Biopolymer-Based Nanosystems for siRNA Drug Delivery to Solid Tumors including Breast Cancer. Pharmaceutics. 2023; 15(1):153. https://doi.org/10.3390/pharmaceutics15010153
Chicago/Turabian StyleSubhan, Md Abdus, and Vladimir P. Torchilin. 2023. "Biopolymer-Based Nanosystems for siRNA Drug Delivery to Solid Tumors including Breast Cancer" Pharmaceutics 15, no. 1: 153. https://doi.org/10.3390/pharmaceutics15010153
APA StyleSubhan, M. A., & Torchilin, V. P. (2023). Biopolymer-Based Nanosystems for siRNA Drug Delivery to Solid Tumors including Breast Cancer. Pharmaceutics, 15(1), 153. https://doi.org/10.3390/pharmaceutics15010153








