Systematic Evaluation of Voriconazole Pharmacokinetic Models without Pharmacogenetic Information for Bayesian Forecasting in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Data and Patients
- (1)
- Patients treated with VRC for a possible, probable or proven invasive aspergillosis according to EORTC/MSG consensus [16].
- (2)
- Patients aged ≥18 years.
- (3)
- Patients with at least two measured VRC concentrations.
- (4)
- Patients with full dosing history available.
2.2. Model Selection/Literature Research
2.3. Model Evaluation
3. Results
3.1. Data and Patients
3.2. Population Pharmacokinetic Models
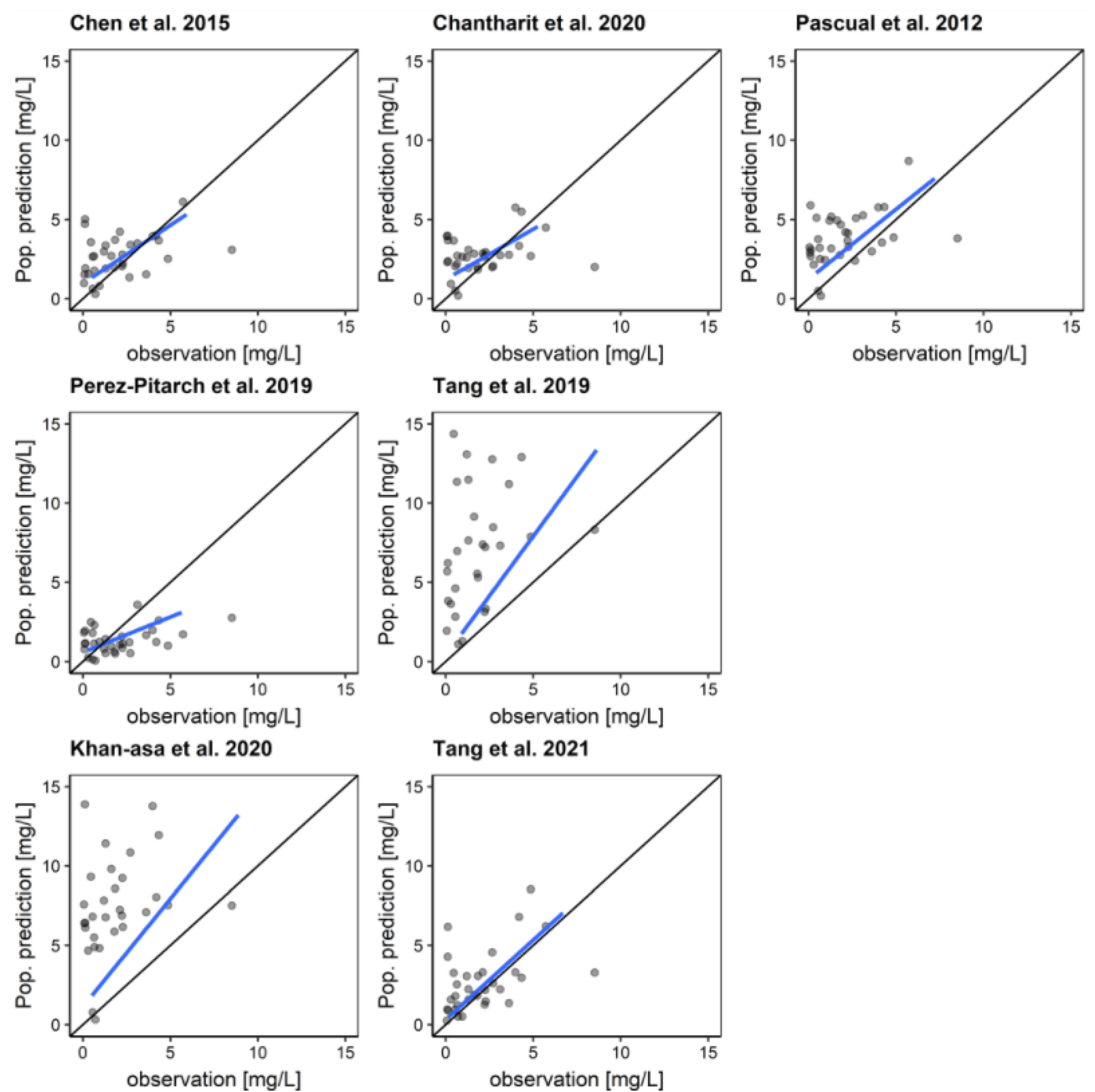
3.3. Model Evaluation
3.4. Comparison of a Priori and Bayesian Predictions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatelon, J.; Cortegiani, A.; Hammad, E.; Cassir, N.; Leone, M. Choosing the Right Antifungal Agent in ICU Patients. Adv. Ther. 2019, 36, 3308–3320. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Hope, W.W. Therapy for Fungal Diseases: Opportunities and Priorities. Trends Microbiol. 2010, 18, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Csajka, C.; Buclin, T.; Bolay, S.; Bille, J.; Calandra, T.; Marchetti, O. Challenging Recommended Oral and Intravenous Voriconazole Doses for Improved Efficacy and Safety: Population Pharmacokinetics-Based Analysis of Adult Patients with Invasive Fungal Infections. Clin. Infect. Dis. 2012, 55, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Roffey, S.J.; Cole, S.; Comby, P.; Gibson, D.; Jezequel, S.G.; Nedderman, A.N.R.; Smith, D.A.; Walker, D.K.; Wood, N. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 2003, 31, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic Drug Monitoring (TDM) of Antifungal Agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole Therapeutic Drug Monitoring in Patients with Invasive Mycoses Improves Efficacy and Safety Outcomes. Clin. Infect. Dis. 2008, 46, 201–211. [Google Scholar] [CrossRef]
- Dolton, M.J.; McLachlan, A.J. Voriconazole Pharmacokinetics and Exposure-Response Relationships: Assessing the Links between Exposure, Efficacy and Toxicity. Int. J. Antimicrob. Agents 2014, 44, 183–193. [Google Scholar] [CrossRef]
- Park, W.B.; Kim, N.-H.; Kim, K.-H.; Lee, S.H.; Nam, W.-S.; Yoon, S.H.; Song, K.-H.; Choe, P.G.; Kim, N.J.; Jang, I.-J.; et al. The Effect of Therapeutic Drug Monitoring on Safety and Efficacy of Voriconazole in Invasive Fungal Infections: A Randomized Controlled Trial. Clin. Infect. Dis. 2012, 55, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Guilhaumou, R.; Blin, O.; Velly, L.; Marsot, A. External Evaluation of Population Pharmacokinetic Models for Continuous Administration of Meropenem in Critically Ill Adult Patients. Eur. J. Clin. Pharmacol. 2020, 76, 1281–1289. [Google Scholar] [CrossRef]
- Thomson, A.H.; Whiting, B. Bayesian Parameter Estimation and Population Pharmacokinetics. Clin. Pharmacokinet. 1992, 22, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Zhang, D.; You, H.; Dong, Y.; Liu, Y.; Du, Q.; Sun, D.; Zhang, T.; Dong, Y. Therapeutic Drug Monitoring Coupled with Bayesian Forecasting Could Prevent Vancomycin-Associated Nephrotoxicity in Renal Insufficiency Patients: A Prospective Study and Pharmacoeconomic Analysis. Ther. Drug Monit. 2020, 42, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Bertolissi, M.; di Silvestre, A.; Poz, D.; Giordano, F.; Furlanut, M. TDM Coupled with Bayesian Forecasting Should Be Considered an Invaluable Tool for Optimizing Vancomycin Daily Exposure in Unstable Critically Ill Patients. Int. J. Antimicrob. Agents 2002, 20, 326–332. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, C.; Li, Z.; Pan, Y.; Liu, M.; Jiao, Z. Can Population Pharmacokinetics of Antibiotics Be Extrapolated? Implications of External Evaluations. Clin. Pharmacokinet. 2021, 60, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Schuster, C.; Paal, M.; Lindner, J.; Zoller, M.; Liebchen, U.; Scharf, C.; Vogeser, M. Isotope Dilution LC-Orbitrap-HRMS with Automated Sample Preparation for the Simultaneous Quantification of 11 Antimycotics in Human Serum. J. Pharm. Biomed. Anal. 2019, 166, 398–405. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Some Suggestions for Measuring Predictive Performance. J. Pharmacokinet. Biopharm. 1981, 9, 503–512. [Google Scholar] [CrossRef]
- Broeker, A.; Nardecchia, M.; Klinker, K.P.; Derendorf, H.; Day, R.O.; Marriott, D.J.; Carland, J.E.; Stocker, S.L.; Wicha, S.G. Towards Precision Dosing of Vancomycin: A Systematic Evaluation of Pharmacometric Models for Bayesian Forecasting. Clin. Microbiol. Infect. 2019, 25, 1286.e1–1286.e7. [Google Scholar] [CrossRef]
- Hara, M.; Masui, K.; Eleveld, D.J.; Struys, M.M.R.F.; Uchida, O. Predictive Performance of Eleven Pharmacokinetic Models for Propofol Infusion in Children for Long-Duration Anaesthesia. Br. J. Anaesth. 2017, 118, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pitarch, A.; Guglieri-Lopez, B.; Ferriols-Lisart, R.; Pérez, A.; Ezquer-Garín, C.; Hernández-Boluda, J.C.; Piñana, J.L.; Navarro, D.; Solano, C.; Alós-Almiñana, M. Pharmacokinetic/Pharmacodynamic Analysis of Voriconazole Against Candida spp. and Aspergillus spp. in Allogeneic Stem Cell Transplant Recipients. Ther. Drug Monit. 2019, 41, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, H.; Liang, F.; Meng, D.; Rui, J.; Yin, X.; Zhang, T.; Xiao, X.; Cai, S.; Liu, X.; et al. Population Pharmacokinetics in China: The Dynamics of Intravenous Voriconazole in Critically Ill Patients with Pulmonary Disease. Biol. Pharm. Bull. 2015, 38, 996–1004. [Google Scholar] [CrossRef]
- Tang, D.; Song, B.-L.; Yan, M.; Zou, J.-J.; Zhang, M.; Zhou, H.-Y.; Wang, F.; Xiao, Y.-W.; Xu, P.; Zhang, B.-K.; et al. Identifying Factors Affecting the Pharmacokinetics of Voriconazole in Patients with Liver Dysfunction: A Population Pharmacokinetic Approach. Basic Clin. Pharmacol. Toxicol. 2019, 125, 34–43. [Google Scholar] [CrossRef]
- Tang, D.; Yan, M.; Song, B.-L.; Zhao, Y.-C.; Xiao, Y.-W.; Wang, F.; Liang, W.; Zhang, B.-K.; Chen, X.-J.; Zou, J.-J.; et al. Population Pharmacokinetics, Safety and Dosing Optimization of Voriconazole in Patients with Liver Dysfunction: A Prospective Observational Study. Br. J. Clin. Pharmacol. 2021, 87, 1890–1902. [Google Scholar] [CrossRef]
- Khan-Asa, B.; Punyawudho, B.; Singkham, N.; Chaivichacharn, P.; Karoopongse, E.; Montakantikul, P.; Chayakulkeeree, M. Impact of Albumin and Omeprazole on Steady-State Population Pharmacokinetics of Voriconazole and Development of a Voriconazole Dosing Optimization Model in Thai Patients with Hematologic Diseases. Antibiotics 2020, 9, 574. [Google Scholar] [CrossRef]
- Chantharit, P.; Tantasawat, M.; Kasai, H.; Tanigawara, Y. Population Pharmacokinetics of Voriconazole in Patients with Invasive Aspergillosis: Serum Albumin Level as a Novel Marker for Clearance and Dosage Optimization. Ther. Drug Monit. 2020, 42, 872–879. [Google Scholar] [CrossRef]
- Han, K.; Bies, R.; Johnson, H.; Capitano, B.; Venkataramanan, R. Population Pharmacokinetic Evaluation with External Validation and Bayesian Estimator of Voriconazole in Liver Transplant Recipients. Clin. Pharmacokinet. 2011, 50, 201–214. [Google Scholar] [CrossRef]
- Han, K.; Capitano, B.; Bies, R.; Potoski, B.A.; Husain, S.; Gilbert, S.; Paterson, D.L.; McCurry, K.; Venkataramanan, R. Bioavailability and Population Pharmacokinetics of Voriconazole in Lung Transplant Recipients. Antimicrob. Agents Chemother. 2010, 54, 4424–4431. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Huang, F.; Tong, L.; Xia, Y.; Wu, J.; Li, J.; Hu, X.; Liang, T.; Liu, X.; Zhong, G.; et al. Pharmacokinetics of Intravenous Voriconazole in Patients with Liver Dysfunction: A Prospective Study in the Intensive Care Unit. Int. J. Infect. Dis. 2020, 93, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of Voriconazole Plasma Concentrations on Treatment Response in Critically Ill Patients. J. Clin. Pharm. Ther. 2019, 44, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Fujimoto, Y.; Kanbayashi, Y.; Ikawa, K.; Taniwaki, M. Pharmacokinetic–Pharmacodynamic Analysis of Voriconazole in Japanese Patients with Hematological Malignancies. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Garnett, C.E.; Gobburu, J.V.S.; Bhattaram, V.A.; Brar, S.; Earp, J.C.; Jadhav, P.R.; Krudys, K.; Lesko, L.J.; Li, F.; et al. Impact of Pharmacometric Analyses on New Drug Approval and Labelling Decisions. Clin. Pharmacokinet. 2011, 50, 627–635. [Google Scholar] [CrossRef]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are Vancomycin Trough Concentrations Adequate for Optimal Dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef]
- Stockmann, C.; Hersh, A.L.; Roberts, J.K.; Bhongsatiern, J.; Korgenski, E.K.; Spigarelli, M.G.; Sherwin, C.M.T.; Frymoyer, A. Predictive Performance of a Vancomycin Population Pharmacokinetic Model in Neonates. Infect. Dis. Ther. 2015, 4, 187–198. [Google Scholar] [CrossRef]
- Brendel, K.; Dartois, C.; Comets, E.; Lemenuel-Diot, A.; Laveille, C.; Tranchand, B.; Girard, P.; Laffont, C.M.; Mentré, F. Are Population Pharmacokinetic and/or Pharmacodynamic Models Adequately Evaluated? Clin. Pharmacokinet. 2007, 46, 221–234. [Google Scholar] [CrossRef]
- Gastine, S.; Lehrnbecher, T.; Müller, C.; Farowski, F.; Bader, P.; Ullmann-Moskovits, J.; Cornely, O.A.; Groll, A.H.; Hempel, G. Pharmacokinetic Modeling of Voriconazole to Develop an Alternative Dosing Regimen in Children. Antimicrob. Agents Chemother. 2017, 62, e01194-17. [Google Scholar] [CrossRef]
- Farkas, A.; Daroczi, G.; Villasurda, P.; Dolton, M.; Nakagaki, M.; Roberts, J.A. Comparative Evaluation of the Predictive Performances of Three Different Structural Population Pharmacokinetic Models to Predict Future Voriconazole Concentrations. Antimicrob. Agents Chemother. 2016, 60, 6806–6812. [Google Scholar] [CrossRef]
- Chen, C.; Yang, T.; Li, X.; Ma, L.; Liu, Y.; Zhou, Y.; Ren, H.; Cui, Y. Population Pharmacokinetics of Voriconazole in Chinese Patients with Hematopoietic Stem Cell Transplantation. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 659–668. [Google Scholar] [CrossRef]
- Alihodzic, D.; Broeker, A.; Baehr, M.; Kluge, S.; Langebrake, C.; Wicha, S.G. Impact of Inaccurate Documentation of Sampling and Infusion Time in Model-Informed Precision Dosing. Front. Pharmacol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.-A.; Ng, K.; Pont, L.G.; McLachlan, A.J. Multicenter Study of Voriconazole Pharmacokinetics and Therapeutic Drug Monitoring. Antimicrob. Agents Chemother. 2012, 56, 4793–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| n | |
|---|---|
| Number of patients | 33 |
| 23 |
| 10 |
| Diseases | |
| 20 |
| 12 |
| 1 |
| Median (Range) | |
| Age (years) | 44 (26–67) |
| Weight (kg) | 71 (40–120) |
| Height (cm) | 175 (160–188) |
| BMI (kg/m2) | 22.4 (13.1–37.0) |
| Voriconazole plasma concentration sample 1 (mg/L) | 1.51 (0.08–8.89) |
| Voriconazole plasma concentration sample 2 (mg/L) | 1.62 (0.06–8.51) |
| SAPS II | 40 (23–77) |
| LBW (kg) | 45.9 (37.9–63.6) |
| IL6 (pg/mL) | 39 (3.4–415) |
| CRP (mg/dL) | 8.0 (0.3–46.6) |
| Creatinine (mg/dL) | 0.9 (0.3–3.1) |
| GFR-CKD-EPI (mL/min) | 109 (20–206) |
| Albumin (mg/dL) | 2.3 (1.8–3.0) |
| GOT (U/L) | 30 (8–7000) |
| GPT (U/L) | 29 (5–918) |
| Bilirubin (mg/dL) | 0.7 (0.2–7.1) |
| Mean | p (t-Test) | Variance | p (Fisher Variance) | |
|---|---|---|---|---|
| Chen et al., 2015 [24] | −0.3247 | 0.03 | 1.0122 | 1 |
| Chantharit et al., 2020 [28] | −0.0777 | 1 | 1.3098 | 0.286 |
| Khan-asa et al., 2020 [27] | −1.0333 | <0.001 | 0.7391 | 0.3416 |
| Pascual et al., 2012 [4] | −0.7567 | <0.001 | 1.0699 | 1 |
| Perez-Pitarch et al., 2019 [23] | 0.1031 | 1 | 3.2898 | <0.001 |
| Tang et al., 2019 [25] | −1.2824 | <0.001 | 0.9115 | 1 |
| Tang et al., 2021 [26] | −0.5025 | 0.04 | 2.5568 | <0.001 |
| rRMSE (%) | rBias (%) | ME (mg/L) | MAE (mg/L) | |
|---|---|---|---|---|
| A priori/Bayes | A priori/Bayes | A priori/Bayes | A priori/Bayes | |
| Chen et al., 2015 [24] | 96.9/85.0 | 48.2/4.0 | 0.67/−0.01 | 1.45/0.91 |
| Chantharit et al., 2020 [28] | 97.7/91.4 | 48.6/49.5 | 0.70/0.66 | 1.54/1.29 |
| Khan-asa et al., 2020 [28] | 139.1/115.9 | 124.6/95.6 | 8.01/3.10 | 8.11/3.21 |
| Pascual et al., 2012 [4] | 108.5/96.7 | 74.3/59.8 | 1.78/1.06 | 2.26/1.54 |
| Perez-Pitarch et al., 2019 [23] | 106.6/83.7 | −15.4/40.6 | −0.70/0.81 | 1.46/1.24 |
| Tang et al., 2019 [25] | 135.7/122.1 | 124.4/106.9 | 7.15/3.79 | 7.16/3.80 |
| Tang et al., 2021 [26] | 89.3/83.5 | 42.9/20.2 | 0.66/0.41 | 1.45/1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallee, S.; Scharf, C.; Schatz, L.M.; Paal, M.; Vogeser, M.; Irlbeck, M.; Zander, J.; Zoller, M.; Liebchen, U. Systematic Evaluation of Voriconazole Pharmacokinetic Models without Pharmacogenetic Information for Bayesian Forecasting in Critically Ill Patients. Pharmaceutics 2022, 14, 1920. https://doi.org/10.3390/pharmaceutics14091920
Kallee S, Scharf C, Schatz LM, Paal M, Vogeser M, Irlbeck M, Zander J, Zoller M, Liebchen U. Systematic Evaluation of Voriconazole Pharmacokinetic Models without Pharmacogenetic Information for Bayesian Forecasting in Critically Ill Patients. Pharmaceutics. 2022; 14(9):1920. https://doi.org/10.3390/pharmaceutics14091920
Chicago/Turabian StyleKallee, Simon, Christina Scharf, Lea Marie Schatz, Michael Paal, Michael Vogeser, Michael Irlbeck, Johannes Zander, Michael Zoller, and Uwe Liebchen. 2022. "Systematic Evaluation of Voriconazole Pharmacokinetic Models without Pharmacogenetic Information for Bayesian Forecasting in Critically Ill Patients" Pharmaceutics 14, no. 9: 1920. https://doi.org/10.3390/pharmaceutics14091920
APA StyleKallee, S., Scharf, C., Schatz, L. M., Paal, M., Vogeser, M., Irlbeck, M., Zander, J., Zoller, M., & Liebchen, U. (2022). Systematic Evaluation of Voriconazole Pharmacokinetic Models without Pharmacogenetic Information for Bayesian Forecasting in Critically Ill Patients. Pharmaceutics, 14(9), 1920. https://doi.org/10.3390/pharmaceutics14091920







