SmartFilm Tablets for Improved Oral Delivery of Poorly Soluble Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
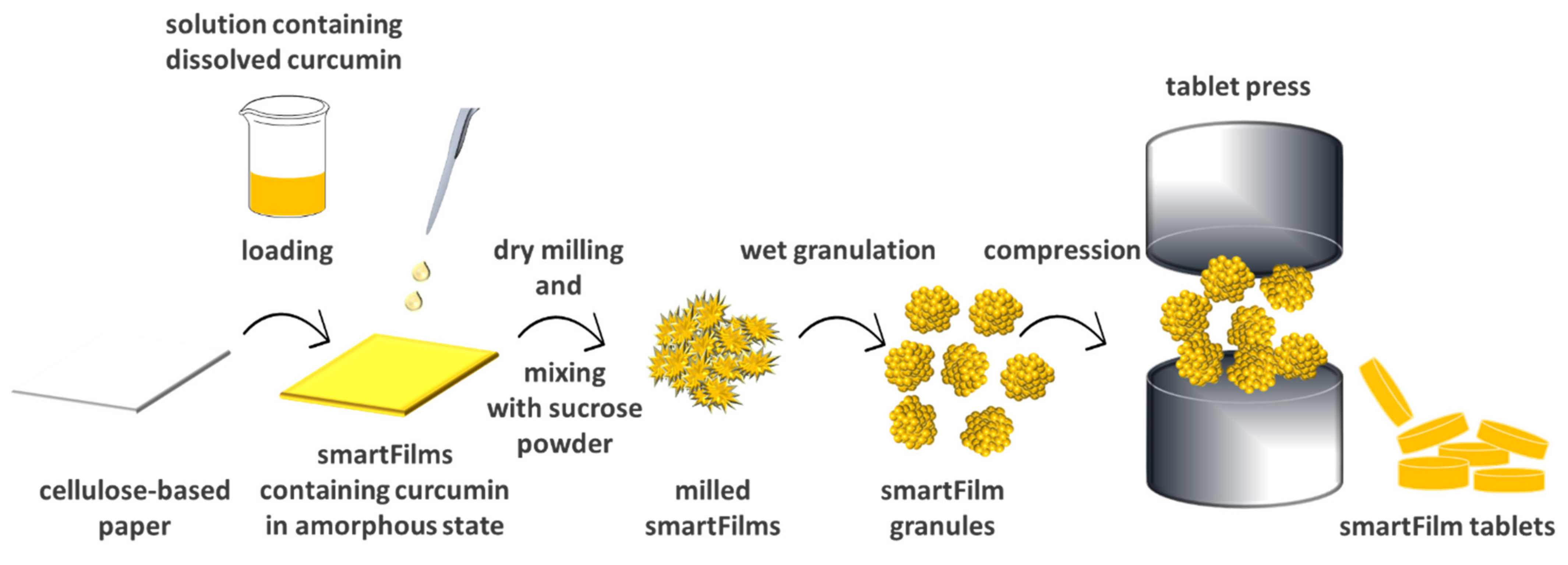
2.2.1. Production of Curcumin-Loaded smartFilms and smartFilm Granules
2.2.2. Characterization of Curcumin-Loaded smartFilm Granules
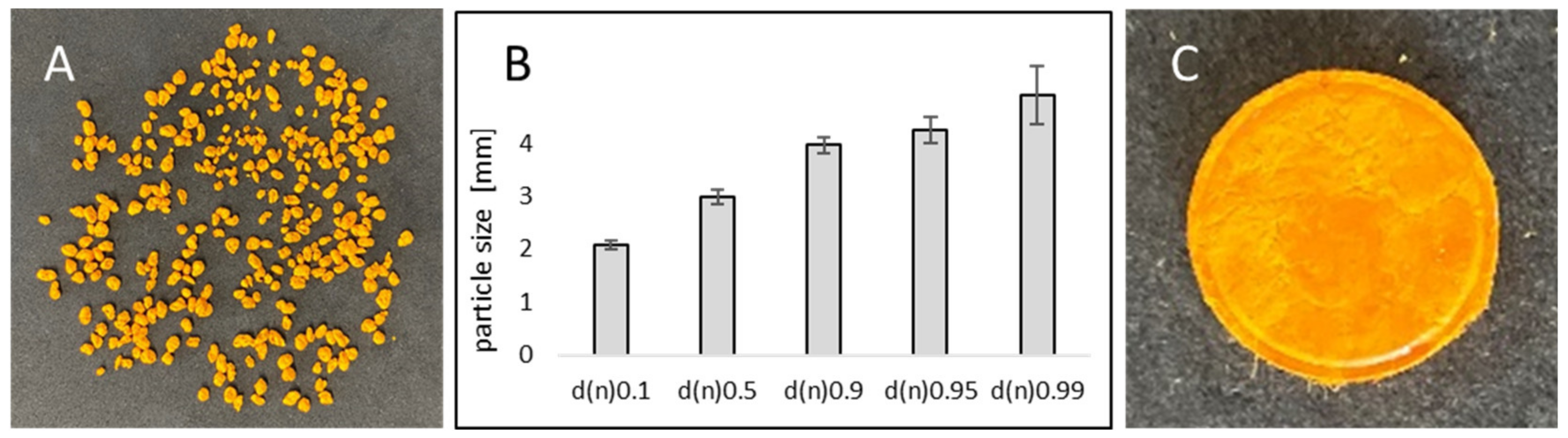
Determination of Particle Size and Shape
Determination of Bulk and Tapped Density
Angle of Repose
2.2.3. Production of Curcumin-Loaded smartFilm Tablets
2.2.4. Characterization of Curcumin-Loaded smartFilm Tablets
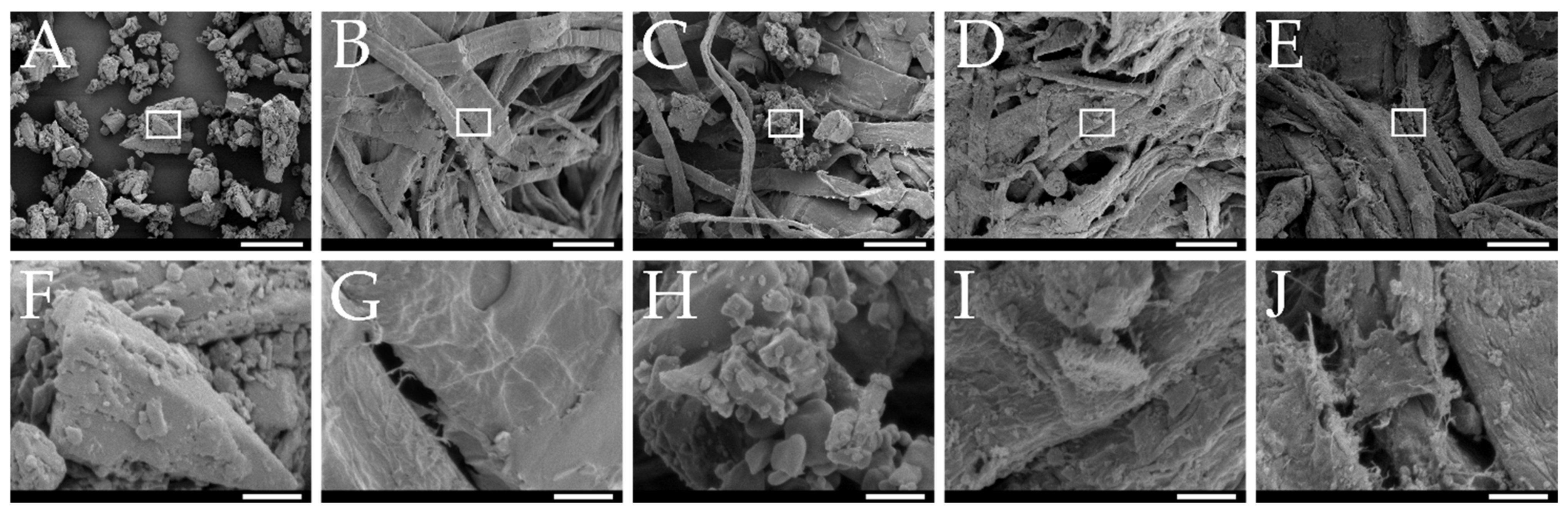
Macroscopic and Microscopic Analysis
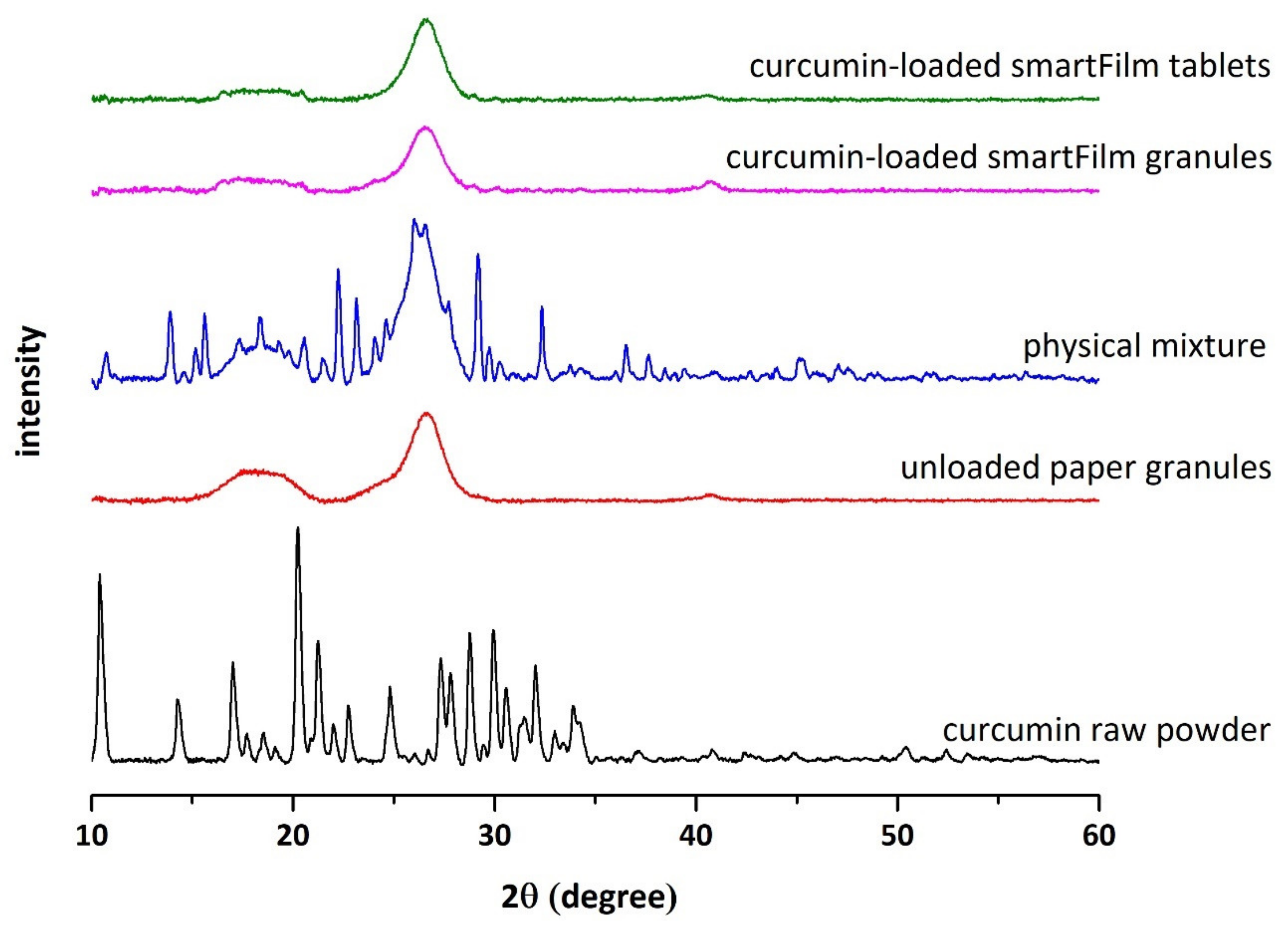
Determination of Crystalline State of Curcumin
Determination of Tablet Thickness and Mass Uniformity
Determination of Friability
Resistance to Crushing
Disintegration
Content Uniformity
Dissolution
2.2.5. Determination of Intestinal Permeability
Pre-Digestion of the Formulations
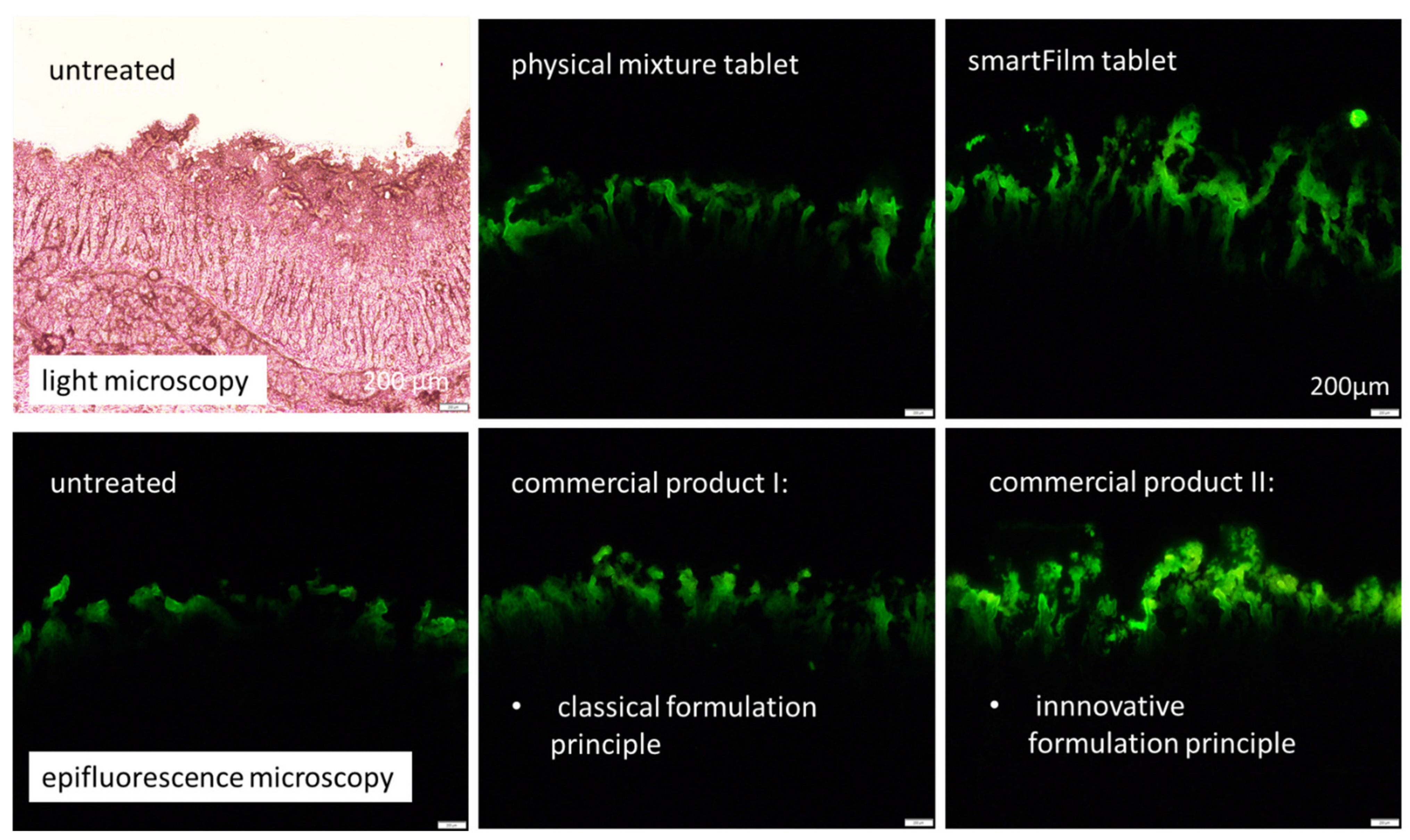
Digital Image Analysis
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Production and Characterization of Curcumin-Loaded smartFilm Granules
3.2. Production and Characterization of Curcumin-Loaded smartFilm Tablets
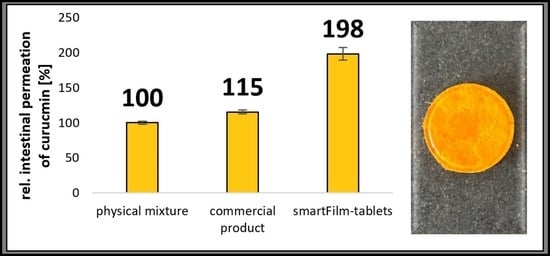
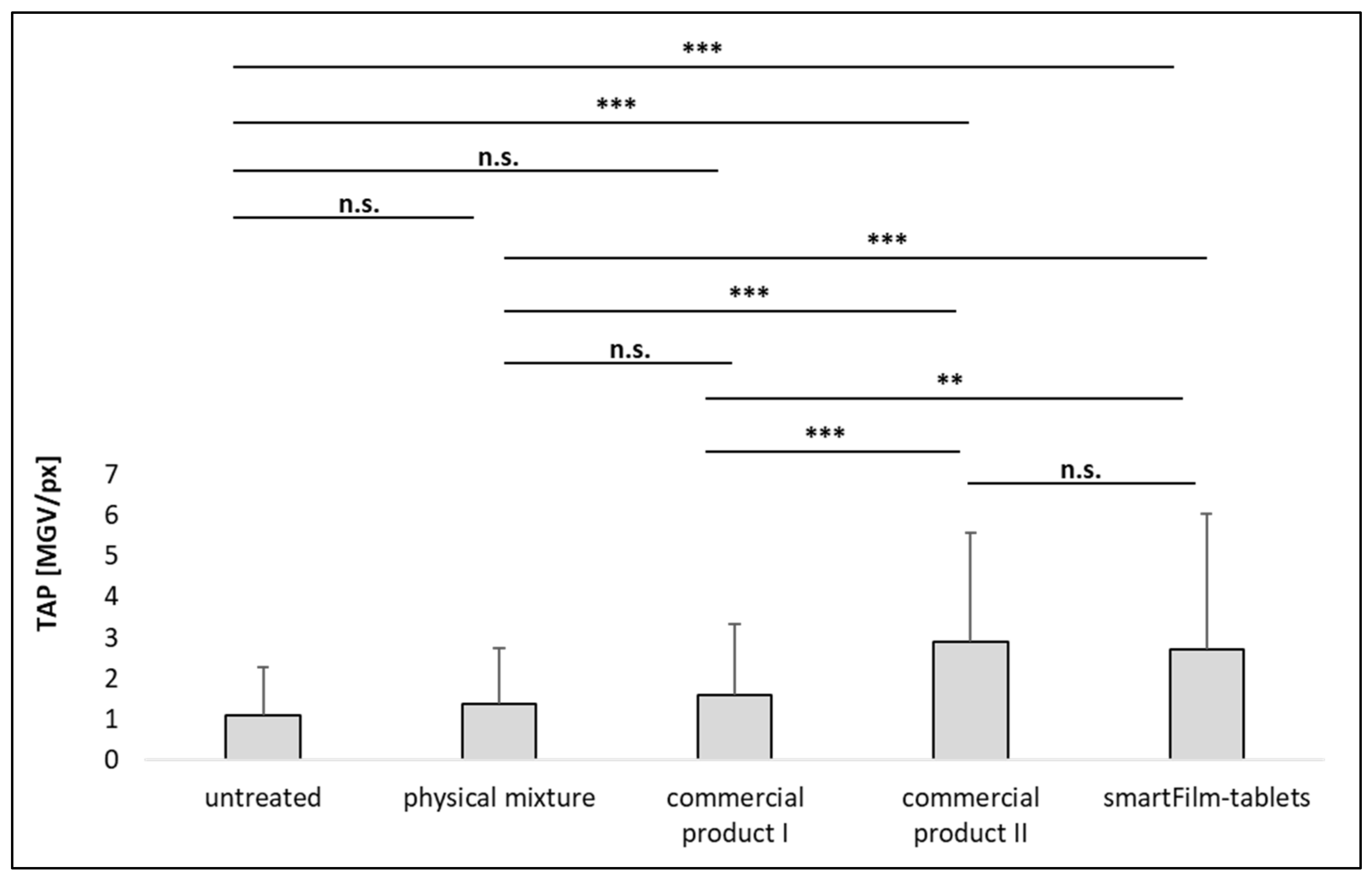
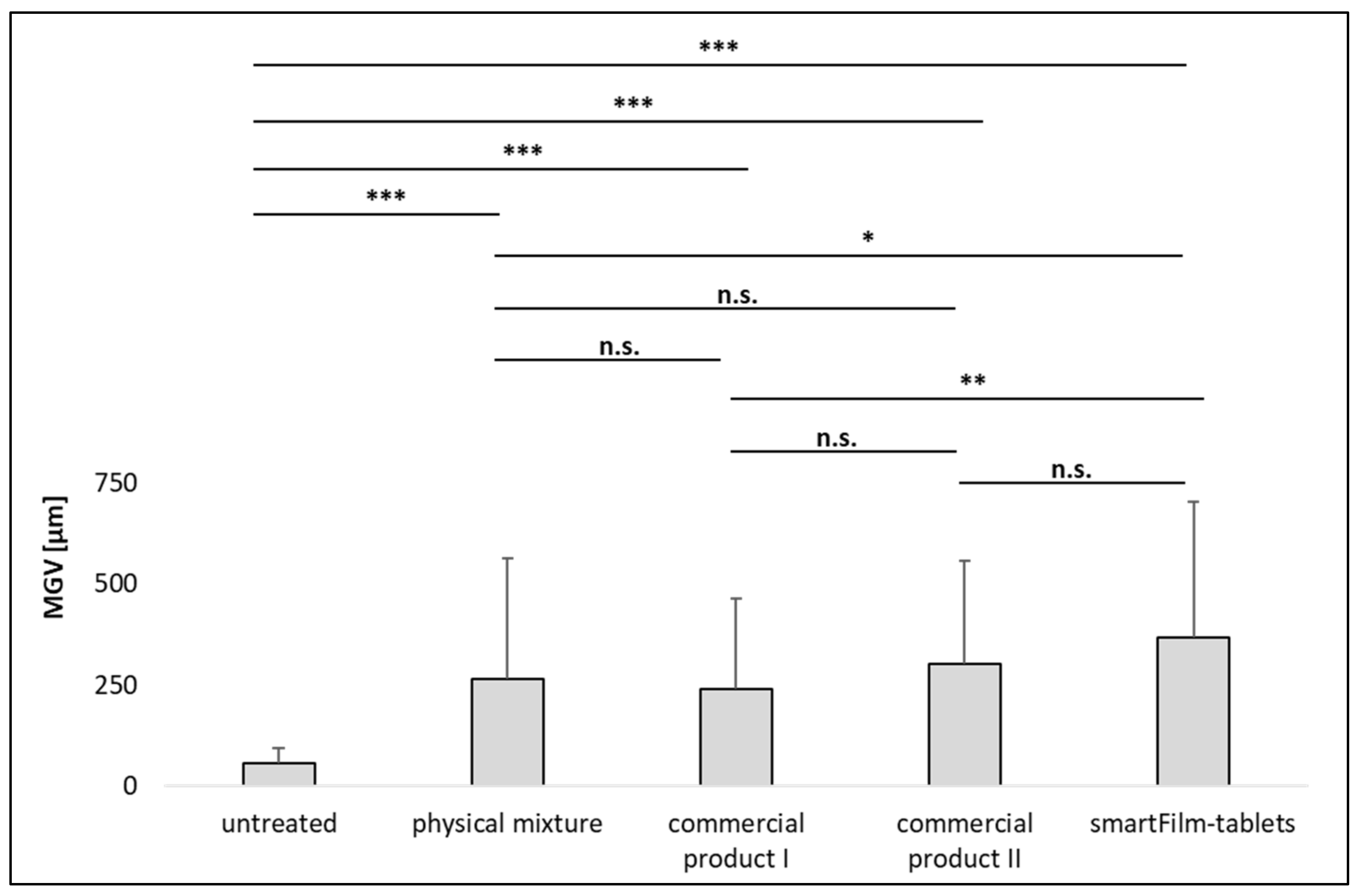
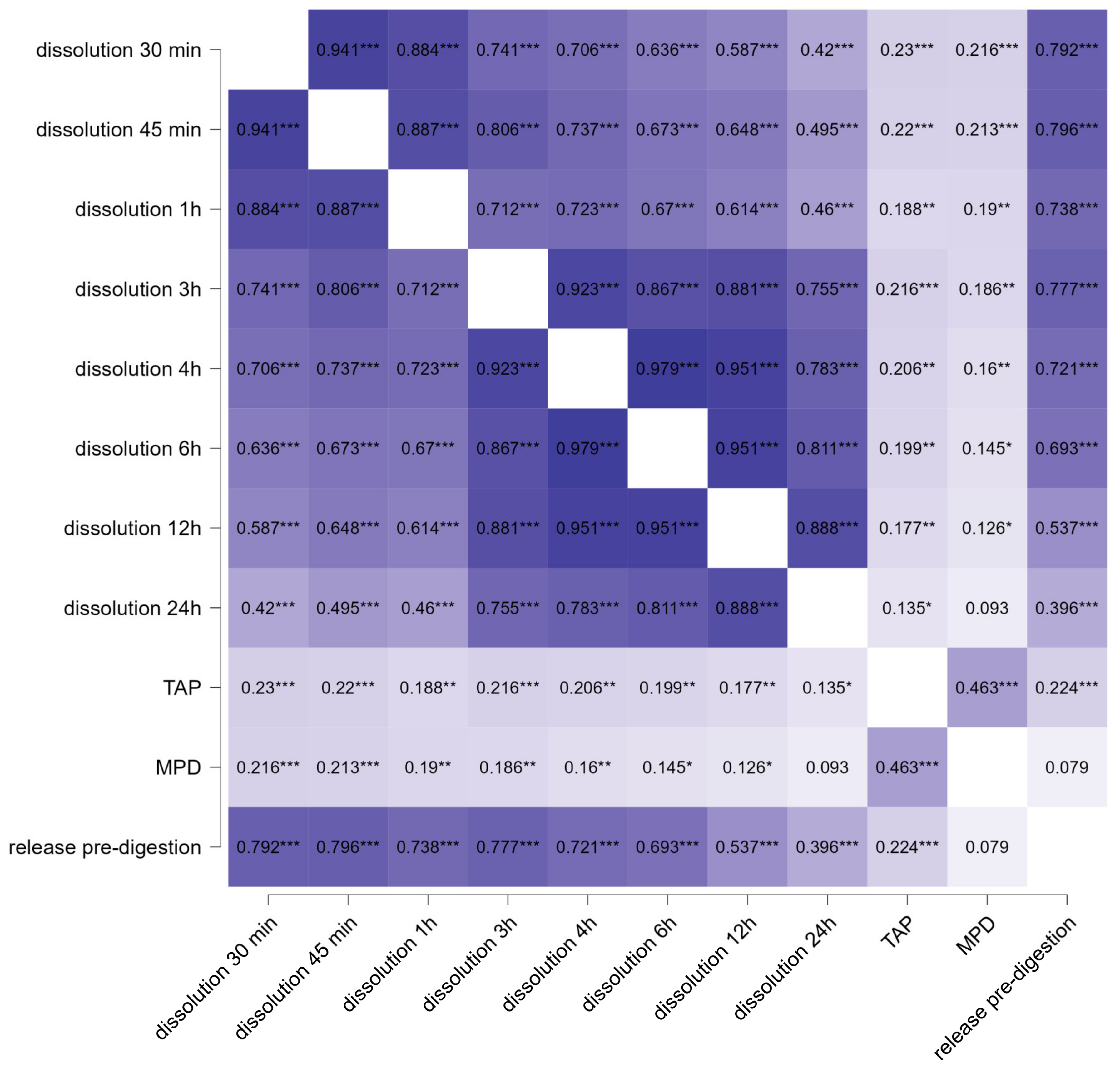
3.3. Determination of Intestinal Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract-Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.O.; Marques, M.B.D.; Kato, K.C.; Carneiro, G. Nanonization techniques to overcome poor water-solubility with drugs. Expert Opin. Drug Discov. 2020, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Fathi, H.A.; Allam, A.; Elsabahy, M.; Fetih, G.; El-Badry, M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf. B 2018, 162, 236–245. [Google Scholar] [CrossRef]
- Lemke, S.; Strätling, E.J. SmartFilms-oral and peroral films for optimized delivery of nanoparticulate or amorphous drugs. In Proceedings of the Controlled Release Society Local Chapter, Saarbrücken, Germany, 7 March 2016. [Google Scholar]
- Lemke, S.; Strätling, E.J.; Welzel, H.P. Cellulosefaserbasierte Trägermatrices (smartFilms) zur Applikation von Inhaltsstoffen Sowie Deren Herstellung. German Patent Application DE102016000541A1, 20 July 2017. [Google Scholar]
- Ornik, J.; Knoth, D.; Koch, M.; Keck, C.M. Terahertz-spectroscopy for non-destructive determination of crystallinity of L-tartaric acid in smartFilms® and tablets made from paper. Int. J. Pharm. 2020, 581, 119253. [Google Scholar] [CrossRef]
- Subrahmanyeswari, C.D.; Prasanth, Y.; Sameeda, R. Formulation and development of efavirenz tablets by paper technique using co-solvency method. Int. J. Curr. Pharm. Res. 2019, 11, 87–92. [Google Scholar] [CrossRef]
- Stumpf, F.; Keck, C.M. Tablets made from paper. Int. J. Pharm. 2018, 548, 812–819. [Google Scholar] [CrossRef]
- Abdelkader, A.; Moos, C.; Pelloux, A.; Pfeiffer, M.; Alter, C.; Kolling, S.; Keck, C.M. Granulation as a reliable approach for large scale production of paper tablets. Pharmaceuticals 2022. submitted. [Google Scholar]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Carvalho Henriques, M.; Faustino, M.A.F.; Santos Braga, S. Curcumin innovative delivery forms: Paving the ‘Yellow Brick Road’of antitumoral phytotherapy. Appl. Sci. 2020, 10, 8990. [Google Scholar] [CrossRef]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhöfer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, 2100613. [Google Scholar] [CrossRef]
- Ornik, J.; Heidrich, L.; Schesny, R.; Castro-Camus, E.; Keck, C.M.; Koch, M. Non-destructive crystallinity assessment of indomethacin in tablets made from smartFilms® using terahertz time-domain spectroscopy. Sci. Rep. 2022, 12, 6099. [Google Scholar] [CrossRef]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved dermal and transdermal delivery of curcumin with smartfilms and nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Stumpf, F. Tabletten aus Papier–Tablets Made from Paper–Zur Oralen Applikation Schwerlöslicher Wirkstoffe. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2019. [Google Scholar]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2021, 329, 598–613. [Google Scholar] [CrossRef]
- Chopra, R.; Michael Newton, J.; Alderborn, G.; Podczeck, F. Preparation of pellets of different shape and their characterization. Pharm. Dev. Technol. 2001, 6, 495–503. [Google Scholar] [CrossRef]
- C.H. Beck. European Pharmacopoeia, 8th ed.; Pharmaceutical Technical Procedures; C.H. Beck: Nördlingen, Germany, 2016. [Google Scholar]
- Aslani, A.; Jahangiri, H. Formulation, characterization and physicochemical evaluation of ranitidine effervescent tablets. Adv. Pharm. Bull. 2013, 3, 315–322. [Google Scholar] [CrossRef]
- Sitterberg, J.; Ozcetin, A.; Ehrhardt, C.; Bakowsky, U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 74, 2–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Widmer, G.; Tzipori, S. A pig model of the human gastrointestinal tract. Gut Microbes 2013, 4, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keck, C.M.; Abdelkader, A.; Pelikh, O.; Wiemann, S.; Kaushik, V.; Specht, D.; Eckert, R.W.; Alnemari, R.M.; Dietrich, H.; Brüßler, J. Assessing the Dermal Penetration Efficacy of Chemical Compounds with the Ex-Vivo Porcine Ear Model. Pharmaceutics 2022, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Chaiprateep, E.; Dietrich, H.; Sengupta, S. Influence of Mechanical Skin Treatments on Dermal Penetration Efficacy of Active Ingredients. Pharmaceutics 2022, 14, 1788. [Google Scholar] [CrossRef]
- Pelikh, O.; Pinnapireddy, S.R.; Keck, C.M. Dermal penetration analysis of curcumin in an ex-vivo porcine ear model using epifluorescence microscopy and digital image processing. Skin Pharmacol. Physiol. 2021, 34, 281–299. [Google Scholar] [CrossRef]
- Kaushik, V.; Ganashalingam, Y.; Schesny, R.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Massage and Skin Hydration on Dermal Penetration Efficacy of Nile Red from Petroleum Jelly-An Unexpected Outcome. Pharmaceutics 2021, 13, 2190. [Google Scholar] [CrossRef]
- Kaushik, V.; Keck, C.M. Influence of mechanical skin treatment (massage, ultrasound, microdermabrasion, tape stripping and microneedling) on dermal penetration efficacy of chemical compounds. Eur. J. Pharm. Biopharm. 2021, 169, 29–36. [Google Scholar] [CrossRef]
- Keck, C.M.; Specht, D.; Brüßler, J. Influence of lipid matrix composition on biopharmaceutical properties of lipid nanoparticles. J. Control. Release 2021, 338, 149–163. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image processing and analysis in Java. Astrophysics Source Code Library: Cambridge, MA, USA, 2012; ascl: 1206.013. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- JASP Team. JASP; Version 0.162; JASP Team: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor and Francis: Hoboken, NJ, USA, 2013; ISBN 9781134742707. [Google Scholar]
- Chen, H.; Wang, C.; Liu, S.; Sun, C.C. Development of piroxicam mini-tablets enabled by spherical cocrystallization. Int. J. Pharm. 2020, 590, 119953. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Des Rieux, A.; Vandermeulen, G.; Préat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. In vitro-in vivo correlation for wet-milled tablet of poorly water-soluble cilostazol. J. Control. Release 2008, 130, 29–37. [Google Scholar] [CrossRef]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef]
- Lu, Y.; Kim, S.; Park, K. In vitro-in vivo correlation: Perspectives on model development. Int. J. Pharm. 2011, 418, 142–148. [Google Scholar] [CrossRef]
- Mackie, A.R.; Round, A.N.; Rigby, N.M.; Macierzanka, A. The role of the mucus barrier in digestion. Food Dig. 2012, 3, 8–15. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery–understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Particle-Assisted Dermal Penetration-A Simple Formulation Strategy to Foster the Dermal Penetration Efficacy. Pharmaceutics 2022, 14, 1039. [Google Scholar] [CrossRef]
- Rea, L.M.; Parker, R.A. Designing and Conducting Survey Research: A Comprehensive Guide, 4th ed.; Jossey-Bass: San Francisco, CA, USA, 2014; ISBN 9781118767009. [Google Scholar]
- Visser, M.R.; Baert, L.; Klooster, G.V.; Schueller, L.; Geldof, M.; Vanwelkenhuysen, I.; de Kock, H.; De Meyer, S.; Frijlink, H.W.; Rosier, J.; et al. Inulin solid dispersion technology to improve the absorption of the BCS Class IV drug TMC240. Eur. J. Pharm. Biopharm. 2010, 74, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhao, J.; Ding, Y.; Li, L. A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci. 2017, 485, 91–98. [Google Scholar] [CrossRef]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech 2012, 13, 159–166. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, A.; Preis, E.; Keck, C.M. SmartFilm Tablets for Improved Oral Delivery of Poorly Soluble Drugs. Pharmaceutics 2022, 14, 1918. https://doi.org/10.3390/pharmaceutics14091918
Abdelkader A, Preis E, Keck CM. SmartFilm Tablets for Improved Oral Delivery of Poorly Soluble Drugs. Pharmaceutics. 2022; 14(9):1918. https://doi.org/10.3390/pharmaceutics14091918
Chicago/Turabian StyleAbdelkader, Ayat, Eduard Preis, and Cornelia M. Keck. 2022. "SmartFilm Tablets for Improved Oral Delivery of Poorly Soluble Drugs" Pharmaceutics 14, no. 9: 1918. https://doi.org/10.3390/pharmaceutics14091918
APA StyleAbdelkader, A., Preis, E., & Keck, C. M. (2022). SmartFilm Tablets for Improved Oral Delivery of Poorly Soluble Drugs. Pharmaceutics, 14(9), 1918. https://doi.org/10.3390/pharmaceutics14091918