“Targeting Design” of Nanoparticles in Tumor Therapy
Abstract
:1. Introduction
2. Effects of Physicochemical Properties of NPs on Targeting
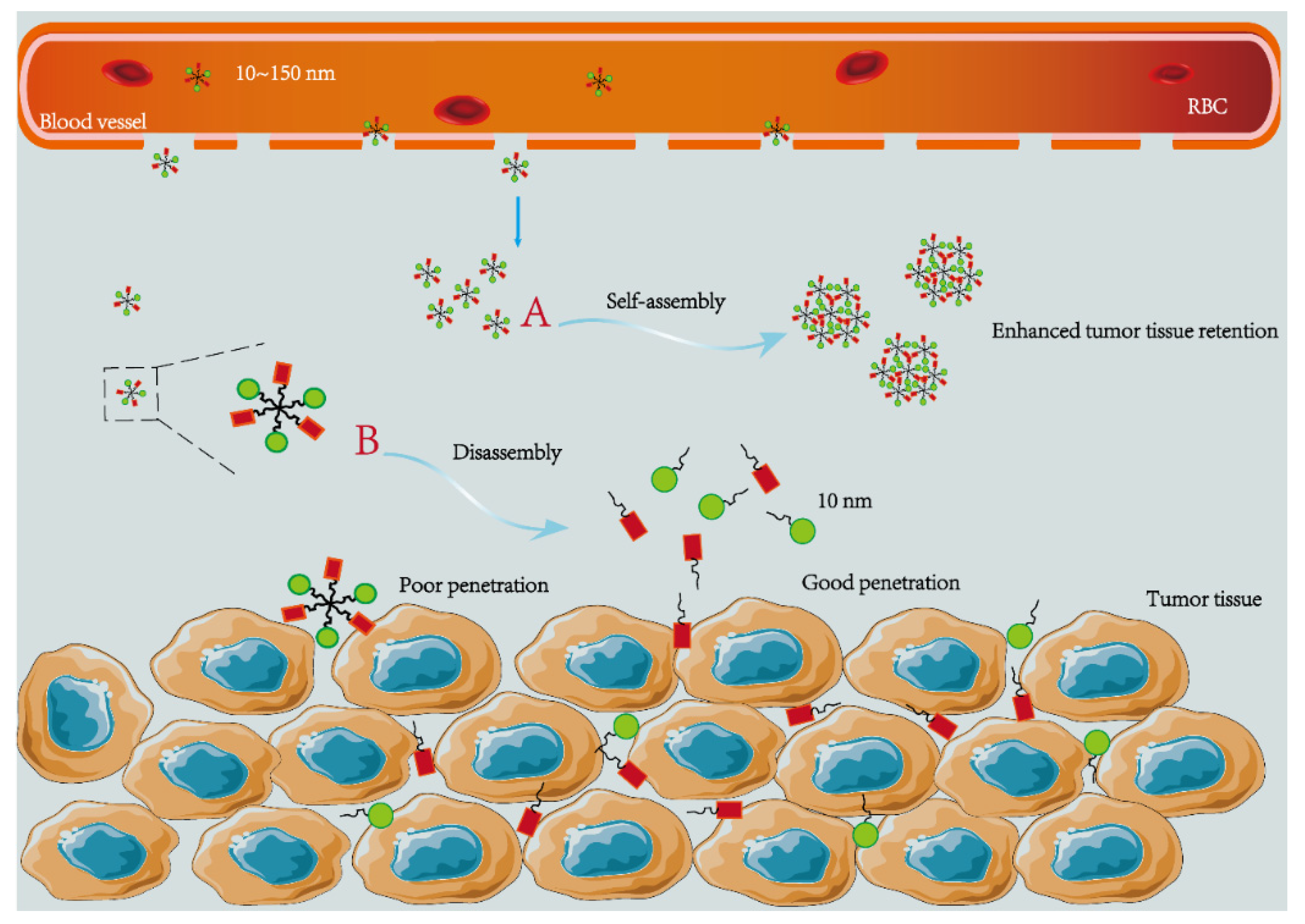
2.1. Size
2.2. Shape
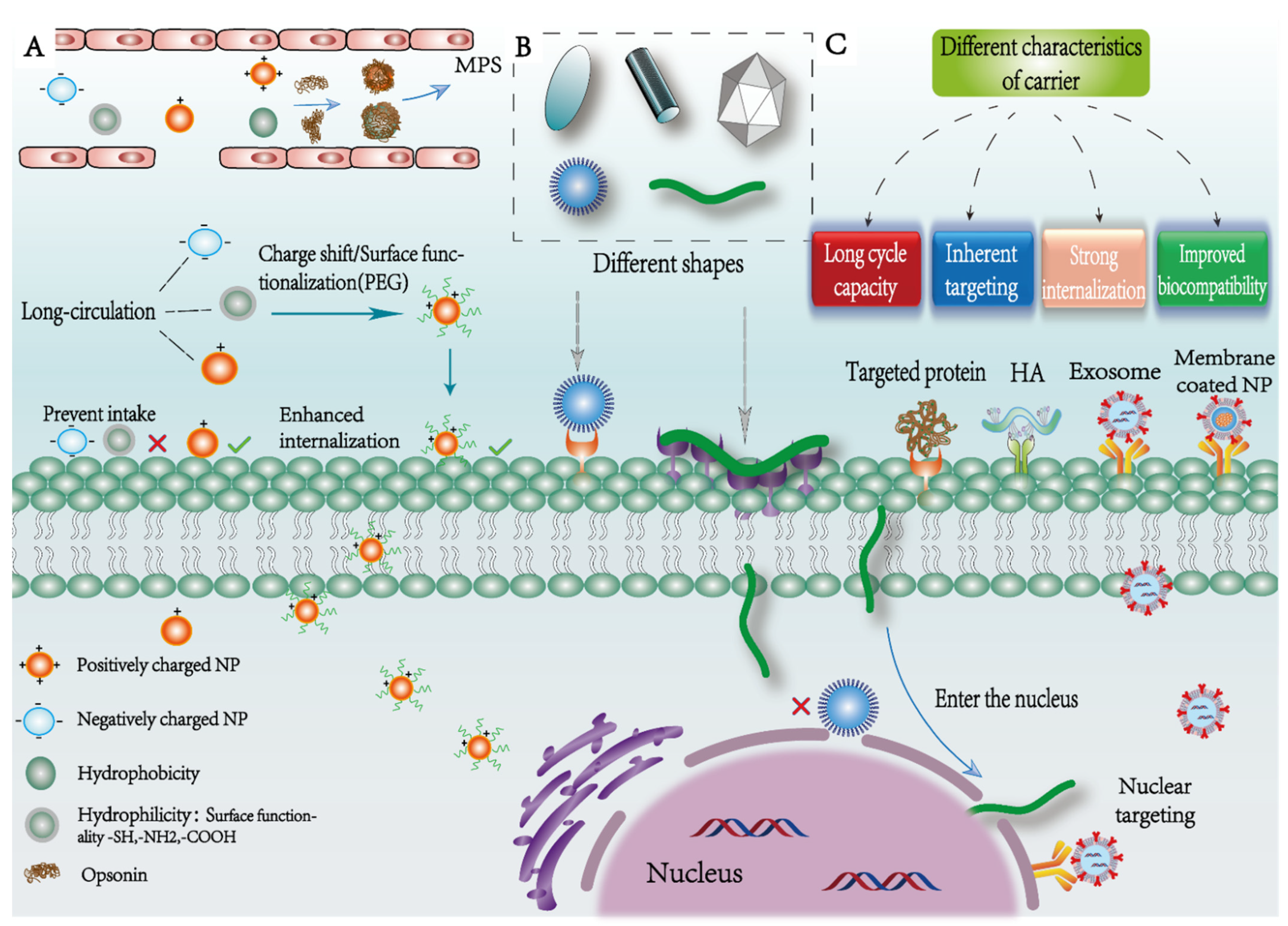
2.3. Surface Properties
2.4. Intrinsic Property of Particles
3. Improved Targeting Based on Ligand-Receptor Interactions
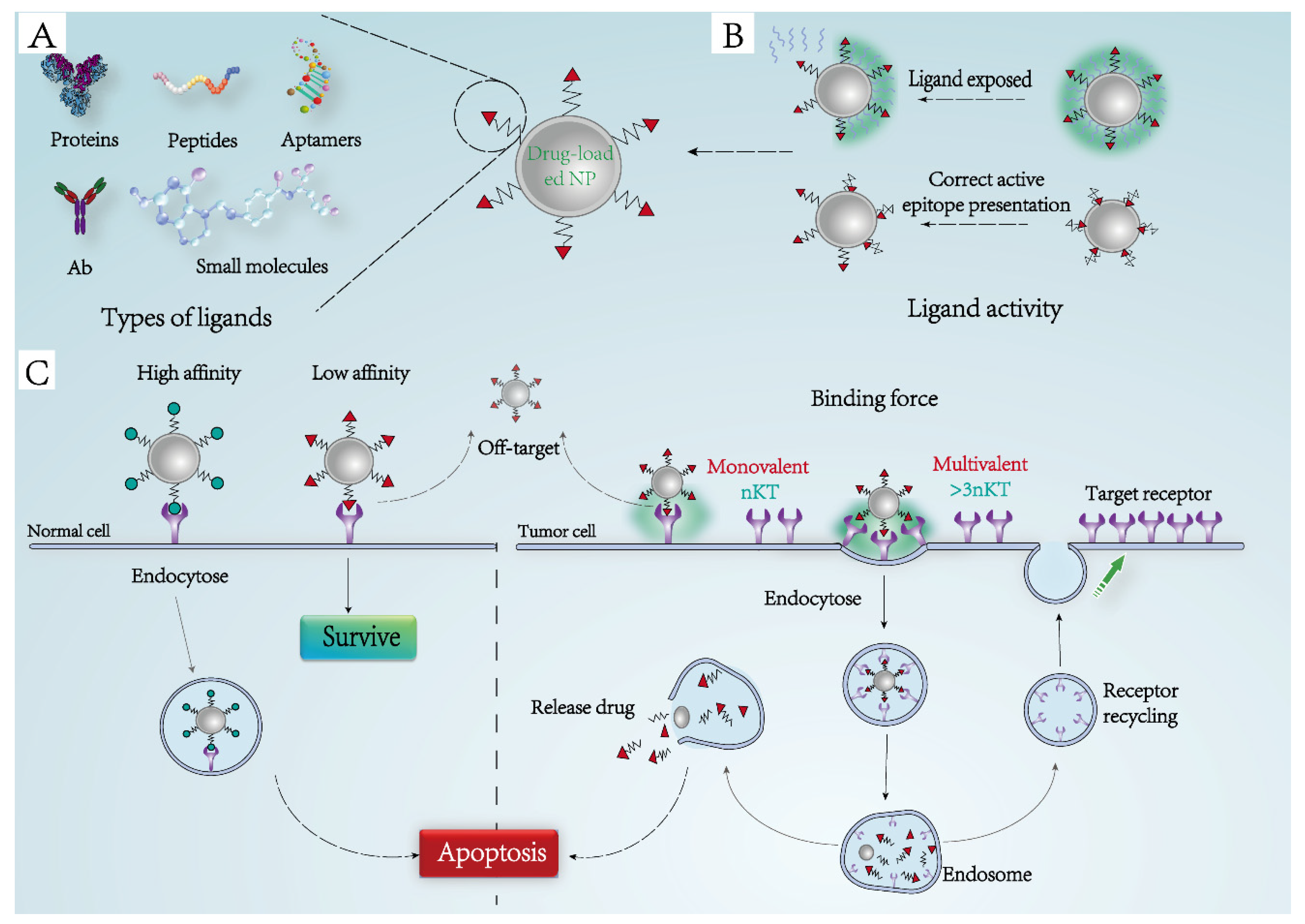
3.1. Ligand-Receptor Binding Force
3.2. Activity Maintenance of Targeted Ligands
3.3. Types of Bioactive Ligands
3.4. Receptor-Mediated Endocytosis
3.5. Distribution and Recycling of Receptors
3.6. Amplified Receptor Signal
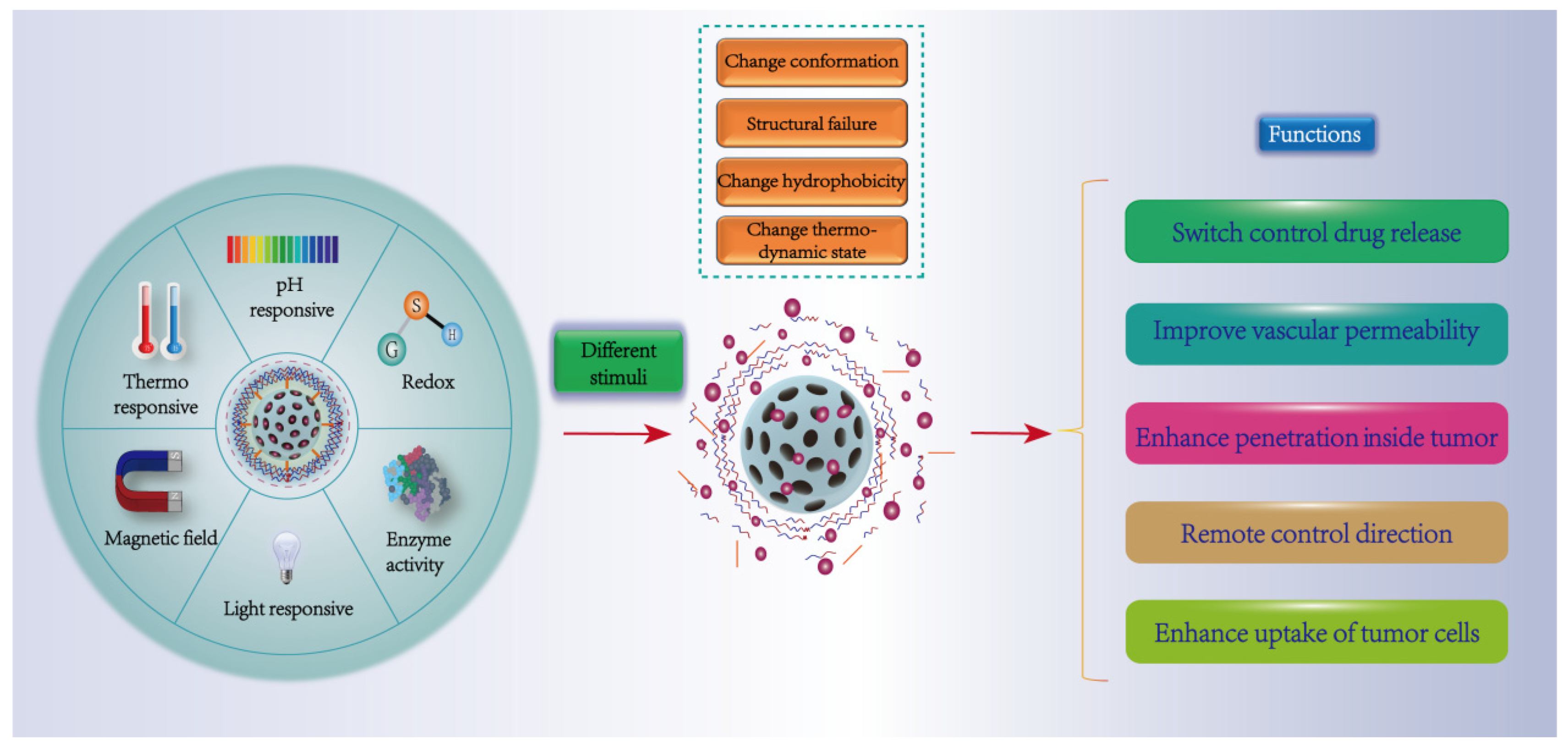
4. Stimulus-Responsive Targeting Strategies
4.1. Physical Response (Temperature, Magnetism, Light)
4.2. Chemical Response (pH, Reduction)
4.3. Biological Response (Enzyme)
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Torchilin, V. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef]
- Cheng, K.; Ding, Y.; Zhao, Y.; Ye, S.; Zhao, X.; Zhang, Y.; Ji, T.; Wu, H.; Wang, B.; Anderson, G.; et al. Sequentially Responsive Therapeutic Peptide Assembling Nanoparticles for Dual-Targeted Cancer Immunotherapy. Nano Lett. 2018, 18, 3250–3258. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.; Farbiak, L.; Siegwart, D. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Y.; Yang, Y.; Zhang, L.; Wang, H.; Shen, Y.; Lai, J.; Chen, J. Phospholipid-Decorated Glycogen Nanoparticles for Stimuli-Responsive Drug Release and Synergetic Chemophotothermal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 23311–23322. [Google Scholar] [CrossRef]
- Han, Q.; Lan, X.; Wen, Y.; Zhang, C.; Cleary, M.; Sayyed, Y.; Huang, G.; Tuo, X.; Yi, L.; Xi, Z.; et al. Matrix Metalloproteinase-9-Responsive Surface Charge-Reversible Nanocarrier to Enhance Endocytosis as Efficient Targeted Delivery System for Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2021, 10, e2002143. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.; Lin, Y.; Tang, M.; Zhai, C.; Xia, H.; Wang, J.; Zhang, Z.; Xie, Z.; Chen, G.; et al. Transformation of Viral Light Particles into Near-Infrared Fluorescence Quantum Dot-Labeled Active Tumor-Targeting Nanovectors for Drug Delivery. Nano Lett. 2019, 19, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, Q.; Li, Y.; Yuan, C.; Xing, R.; Yan, X. Smart Peptide-Based Supramolecular Photodynamic Metallo-Nanodrugs Designed by Multicomponent Coordination Self-Assembly. J. Am. Chem. Soc. 2018, 140, 10794–10802. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.J.; Saha, S. A Nanoparticle’s Journey to the Tumor: Strategies to Overcome First-Pass Metabolism and Their Limitations. Cancers 2022, 14, 1741. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front. Immunol. 2020, 11, 567365. [Google Scholar] [CrossRef]
- Seynhaeve, A.; Amin, M.; Haemmerich, D.; van Rhoon, G.; Ten Hagen, T. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163, 125–144. [Google Scholar] [CrossRef]
- Jang, S.; Wientjes, M.; Lu, D.; Au, J. Drug delivery and transport to solid tumors. Pharm. Res. 2003, 20, 1337–1350. [Google Scholar] [CrossRef]
- Steichen, S.; Caldorera-Moore, M.; Peppas, N. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef]
- Jain, R. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2012, 64, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release Off. J. Control. Release Soc. 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.; Ho, J.; Chan, M.; Lau, C.; Chang, T.; Leung, H.; Liu, L.; Wang, F.; Chan, L.; Tin, C.; et al. Penetrating the Blood-Brain Barrier by Self-Assembled 3D DNA Nanocages as Drug Delivery Vehicles for Brain Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 28928–28940. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, J.; Liu, S.; Lu, Q.; He, J.; Zhou, Z.; Hu, Y. Enzyme sensitive, surface engineered nanoparticles for enhanced delivery of camptothecin. J. Control. Release Off. J. Control. Release Soc. 2015, 216, 111–120. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2021, 69, 166–177. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.; Farokhzad, O. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Revi, N.; Murugappan, S.; Singh, S.; Rengan, A. Enhanced Permeability and Retention Effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, P.; Chen, X. Hierarchical Targeting Strategy for Enhanced Tumor Tissue Accumulation/Retention and Cellular Internalization. Adv. Mater. 2016, 28, 7340–7364. [Google Scholar] [CrossRef]
- Huang, P.; Gao, Y.; Lin, J.; Hu, H.; Liao, H.; Yan, X.; Tang, Y.; Jin, A.; Song, J.; Niu, G.; et al. Tumor-Specific Formation of Enzyme-Instructed Supramolecular Self-Assemblies as Cancer Theranostics. ACS Nano 2015, 9, 9517–9527. [Google Scholar] [CrossRef]
- Truong, N.; Whittaker, M.; Mak, C.; Davis, T. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Li, X.; Montague, E.; Pollinzi, A.; Lofts, A.; Hoare, T. Design of Smart Size-, Surface-, and Shape-Switching Nanoparticles to Improve Therapeutic Efficacy. Small 2022, 18, e2104632. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Irvine, D.; Discher, D.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nature reviews. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Youn, Y.; Oh, K.; Bae, Y. A virus-mimetic nanogel vehicle. Angew. Chem. 2008, 47, 2418–2421. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.; Chan, W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Xiong, Q.; Hornburg, D.; Tao, W.; Farokhzad, O. Nano-Bio Interactions in Cancer: From Therapeutics Delivery to Early Detection. Acc. Chem. Res. 2021, 54, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, H.; Palanikumar, L.; Go, E.; Jana, B.; Park, S.; Kim, H.; Kim, K.; Seo, J.; Kwak, S.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Scarso, A.; Hossain, K.; Florean, L.; Del Tedesco, A.; Cattaruzza, E.; Geppi, M.; Borsacchi, S.; Canton, P.; Benedetti, A.; Riello, P. Modification of Amorphous Mesoporous Zirconia Nanoparticles with Bisphosphonic Acids: A Straightforward Approach for Tailoring the Surface Properties of the Nanoparticles. Chemistry 2021, 27, 17941–17951. [Google Scholar]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Saha, S.; Yakati, V.; Shankar, G.; Jaggarapu, M.; Moku, G.; Madhusudana, K.; Banerjee, R.; Ramkrishna, S.; Srinivas, R.; Chaudhuri, A. Amphetamine decorated cationic lipid nanoparticles cross the blood-brain barrier: Therapeutic promise for combating glioblastoma. J. Mater. Chemistry. B 2020, 8, 4318–4330. [Google Scholar] [CrossRef]
- Saha, S.; Venu, Y.; Bhattacharya, D.; Kompella, S.; Madhusudana, K.; Chakravarty, S.; Ramakrishna, S.; Chaudhuri, A. Combating Established Mouse Glioblastoma through Nicotinylated-Liposomes-Mediated Targeted Chemotherapy in Combination with Dendritic-Cell-Based Genetic Immunization. Adv. Biosyst. 2017, 1, e1600009. [Google Scholar] [CrossRef]
- Ramalho, M.; Loureiro, J.; Coelho, M.; Pereira, M. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Villa, C.; Cines, D.; Siegel, D.; Muzykantov, V. Erythrocytes as Carriers for Drug Delivery in Blood Transfusion and Beyond. Transfus. Med. Rev. 2017, 31, 26–35. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Yang, Z.; Peng, H.; Wei, R.; Wang, C.; Feng, M. Tunneling Nanotubular Expressways for Ultrafast and Accurate M1 Macrophage Delivery of Anticancer Drugs to Metastatic Ovarian Carcinoma. ACS Nano 2019, 13, 1078–1096. [Google Scholar] [CrossRef] [PubMed]
- Malekian, F.; Shamsian, A.; Kodam, S.; Ullah, M. Exosome engineering for efficient and targeted drug delivery: Current status and future perspective. J. Physiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, S.; Li, S.; Wang, H.; Xu, J.; Wang, Y.; Liang, G. Strategies for Engineering Exosomes and Their Applications in Drug Delivery. J. Biomed. Nanotechnol. 2021, 17, 2271–2297. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Dehari, D.; Shekher, A.; Gupta, S.C.; Majumdar, S.; Krishnamurthy, S.; Singh, S.; Kumar, D.; Agrawal, A.K. Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer. Life 2022, 12, 1143. [Google Scholar] [CrossRef]
- Kumar, D.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.; Agrawal, A. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.; Sugimoto, H.; Yang, S.; Ruivo, C.; Melo, S.; Lee, J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Liang, G.; Kan, S.; Zhu, Y.; Feng, S.; Feng, W.; Gao, S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int. J. Nanomed. 2018, 13, 585–599. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sinica. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Zhou, X.; Chen, D.; Li, Y.; Yang, J.; Zhu, X. Hyaluronic Acid-Methotrexate Conjugates Coated Magnetic Polydopamine Nanoparticles for Multimodal Imaging-Guided Multistage Targeted Chemo-Photothermal Therapy. Mol. Pharm. 2018, 15, 4049–4062. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, J.; Yuan, Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J. Mater. Chemistry. B 2020, 8, 3959–3971. [Google Scholar] [CrossRef]
- Woythe, L.; Tito, N.; Albertazzi, L. A quantitative view on multivalent nanomedicine targeting. Adv. Drug Deliv. Rev. 2021, 169, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Veracoechea, F.; Frenkel, D. Designing super selectivity in multivalent nano-particle binding. Proc. Natl. Acad. Sci. USA 2011, 108, 10963–10968. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.; Majoros, I.; Orr, B.; Baker, J.; Banaszak Holl, M. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem. Biol. 2007, 14, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Mowery, P.; Owen, R.; Dykhuizen, E.; Kiessling, L. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem. Biol. 2007, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hartel, N.; Ren, K.X.; Graham, N.A.; Malmstadt, N. Effect of protein corona on nanoparticle-plasma membrane and nanoparticle-biomimetic membrane interactions. Environ. Sci.-Nano 2020, 7, 963–974. [Google Scholar] [CrossRef]
- Ahsan, S.; Rao, C.; Ahmad, M. Nanoparticle-Protein Interaction: The Significance and Role of Protein Corona. Adv. Exp. Med. Biol. 2018, 1048, 175–198. [Google Scholar]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Lee, H. Molecular Modeling of Protein Corona Formation and Its Interactions with Nanoparticles and Cell Membranes for Nanomedicine Applications. Pharmaceutics 2021, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Pinals, R.; Chio, L.; Ledesma, F.; Landry, M. Engineering at the nano-bio interface: Harnessing the protein corona towards nanoparticle design and function. Anal. 2020, 145, 5090–5112. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Nuffer, J.; Muñiz-Papandrea, V.; Colón, W.; Siegel, R.; Dordick, J. Cytochrome C on silica nanoparticles: Influence of nanoparticle size on protein structure, stability, and activity. Small 2009, 5, 470–476. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Yan, B. Regulation of enzyme activity through interactions with nanoparticles. Int. J. Mol. Sci. 2009, 10, 4198–4209. [Google Scholar] [CrossRef] [PubMed]
- Aubin-Tam, M.; Hamad-Schifferli, K. Gold nanoparticle-cytochrome C complexes: The effect of nanoparticle ligand charge on protein structure. Langmuir ACS J. Surf. Colloids 2005, 21, 12080–12084. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Chatterjee, T.; Joshi, P.; Poddar, A.; Bhattacharyya, B.; Singh, S.; Gupta, V.; Chakrabarti, P. Structure and activity of lysozyme on binding to ZnO nanoparticles. Langmuir ACS J. Surf. Colloids 2010, 26, 3506–3513. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.; Li, W.; Zhang, Y. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnology 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.; Karp, J.; Langer, R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin. Drug Deliv. 2006, 3, 311–324. [Google Scholar] [CrossRef]
- Eck, W.; Craig, G.; Sigdel, A.; Ritter, G.; Old, L.; Tang, L.; Brennan, M.; Allen, P.; Mason, M. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano 2008, 2, 2263–2272. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Lin, Y. Electroactive silica nanoparticles for biological labeling. Small 2006, 2, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Mahon, E.; Salvati, A.; Baldelli Bombelli, F.; Lynch, I.; Dawson, K. Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J. Control. Release: Off. J. Control. Release Soc. 2012, 161, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.; Bhatnagar, S.; Prabha, S. Synthetic Receptor-Based Targeting Strategies to Improve Tumor Drug Delivery. AAPS PharmSciTech 2021, 22, 93. [Google Scholar] [CrossRef]
- Tietjen, G.; Bracaglia, L.; Saltzman, W.; Pober, J. Focus on Fundamentals: Achieving Effective Nanoparticle Targeting. Trends Mol. Med. 2018, 24, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Aubin-Tam, M.; Hwang, W.; Hamad-Schifferli, K. Site-directed nanoparticle labeling of cytochrome c. Proc. Natl. Acad. Sci. USA 2009, 106, 4095–4100. [Google Scholar] [CrossRef]
- Caracciolo, G.; Cardarelli, F.; Pozzi, D.; Salomone, F.; Maccari, G.; Bardi, G.; Capriotti, A.; Cavaliere, C.; Papi, M.; Laganà, A. Selective targeting capability acquired with a protein corona adsorbed on the surface of 1,2-dioleoyl-3-trimethylammonium propane/DNA nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 13171–13179. [Google Scholar] [CrossRef]
- Koneru, T.; McCord, E.; Pawar, S.; Tatiparti, K.; Sau, S.; Iyer, A. Transferrin: Biology and Use in Receptor-Targeted Nanotherapy of Gliomas. ACS Omega 2021, 6, 8727–8733. [Google Scholar] [CrossRef]
- Lam, F.; Morton, S.; Wyckoff, J.; Vu Han, T.; Hwang, M.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.; Floyd, S.; Hammond, P. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Liszbinski, R.; Romagnoli, G.; Gorgulho, C.; Basso, C.; Pedrosa, V.; Kaneno, R. Anti-EGFR-Coated Gold Nanoparticles In Vitro Carry 5-Fluorouracil to Colorectal Cancer Cells. Materials 2020, 13, 375. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Zhang, P.; Yi, X.; Xiao, C.; Chen, X. Polypeptides-Drug Conjugates for Anticancer Therapy. Adv. Healthc. Mater. 2021, 10, e2001974. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liang, R.P.; Ding, D.B.; Zheng, X.M.; Zhu, X.D.; Hu, S.X.; Wei, H.B.; Wei, B. A Smart Multifunctional Nanoparticle for Enhanced Near-Infrared Image-Guided Photothermal Therapy Against Gastric Cancer. Int. J. Nanomed. 2021, 16, 2897–2915. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368. [Google Scholar] [CrossRef] [PubMed]
- Vachutinsky, Y.; Oba, M.; Miyata, K.; Hiki, S.; Kano, M.; Nishiyama, N.; Koyama, H.; Miyazono, K.; Kataoka, K. Antiangiogenic gene therapy of experimental pancreatic tumor by sFlt-1 plasmid DNA carried by RGD-modified crosslinked polyplex micelles. J. Control. Release Off. J. Control. Release Soc. 2011, 149, 51–57. [Google Scholar] [CrossRef]
- Gu, W.; Meng, F.; Haag, R.; Zhong, Z. Actively targeted nanomedicines for precision cancer therapy: Concept, construction, challenges and clinical translation. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 676–695. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.; Langer, R.; Farokhzad, O.; Lippard, S. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. United States Am. 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jelonek, K.; Musiał-Kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single-versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef]
- Liang, X.; Xie, Y.; Wu, J.; Wang, J.; Petković, M.; Stepić, M.; Zhao, J.; Ma, J.; Mi, L. Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2021, 215, 112122. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, W.; Yu, L.; Yu, Y.; Liu, H.; Zhang, X. Transferrin-Decorated Protein-Lipid Hybrid Nanoparticle Efficiently Delivers Cisplatin and Docetaxel for Targeted Lung Cancer Treatment. Drug Des. Dev. Ther. 2021, 15, 3475–3486. [Google Scholar] [CrossRef]
- Acharya, S.; Dilnawaz, F.; Sahoo, S. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30, 5737–5750. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Q.; Liu, Y.; Zhang, X.; Shan, W.; Ye, S.; Zhou, X.; Ge, Y.; Wang, X.; Ren, L. Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine 2020, 15, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Feng, L.; Liu, F.; Zhang, L.; Zhang, N. Polymeric complex micelles with double drug-loading strategies for folate-mediated paclitaxel delivery. Colloids Surfaces. B Biointerfaces 2015, 131, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Muley, H.; Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020, 177, 113959. [Google Scholar] [CrossRef]
- Francia, V.; Montizaan, D.; Salvati, A. Interactions at the cell membrane and pathways of internalization of nano-sized materials for nanomedicine. Beilstein J. Nanotechnol. 2020, 11, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, A.G.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Nanopartiele-plasma Membrane interactions: Thermodynamics, Toxicity and Cellular Response. Curr. Med. Chem. 2020, 27, 3330–3345. [Google Scholar] [CrossRef]
- Zhao, J.C.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Varma, S.; Dey, S.; Palanisamy, D. Cellular Uptake Pathways of Nanoparticles: Process of Endocytosis and Factors Affecting their Fate. Curr. Pharm. Biotechnol. 2022, 23, 679–706. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Zhu, H.; Gong, Q.; Luo, K. Endocytosis of Nanoscale Systems for Cancer Treatments. Curr. Med. Chem. 2018, 25, 3017–3035. [Google Scholar] [CrossRef]
- Johnsen, K.; Burkhart, A.; Thomsen, L.; Andresen, T.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Qin, Y.; Liu, H.; Cheng, Z. Tumor-Targeting Peptides: Ligands for Molecular Imaging and Therapy. Anti-Cancer Agents Med. Chem. 2018, 18, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, L.; Song, F. Interplay of Nanoparticle Properties during Endocytosis. Crystals 2021, 11. [Google Scholar] [CrossRef]
- Saikawa, Y.; Knight, C.; Saikawa, T.; Page, S.; Chabner, B.; Elwood, P. Decreased expression of the human folate receptor mediates transport-defective methotrexate resistance in KB cells. J. Biol. Chem. 1993, 268, 5293–5301. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, Y.; Wang, M.; Zhang, C.; Zhang, T.; Zhou, H.; Zhao, W.; Zhao, W.; Xia, G.; Shao, R. Endocytosis and Organelle Targeting of Nanomedicines in Cancer Therapy. Int. J. Nanomed. 2020, 15, 9447–9467. [Google Scholar] [CrossRef]
- Paulos, C.; Reddy, J.; Leamon, C.; Turk, M.; Low, P. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004, 66, 1406–1414. [Google Scholar] [CrossRef]
- Dalton, S.; Wiegert, R.; Casey, C. Receptor-mediated endocytosis by the asialoglycoprotein receptor: Effect of ethanol administration on endosomal distribution of receptor and ligand. Liver Int. Off. J. Int. Assoc. Study Liver 2003, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- von Maltzahn, G.; Park, J.; Lin, K.; Singh, N.; Schwöppe, C.; Mesters, R.; Berdel, W.; Ruoslahti, E.; Sailor, M.; Bhatia, S. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011, 10, 545–552. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- Bi, H.; Xue, J.; Jiang, H.; Gao, S.; Yang, D.; Fang, Y.; Shi, K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. Sci. 2019, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, C.; Fang, E.; Lu, X.; Wang, G.; Tong, Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS ONE 2014, 9, e92924. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Sharma, A.; Mahant, S.; Kapoor, D. Recent advancements in brain tumor targeting using magnetic nanoparticles. Ther. Deliv. 2020, 11, 97–112. [Google Scholar] [CrossRef]
- Price, P.; Mahmoud, W.; Al-Ghamdi, A.; Bronstein, L. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Singh, A.; Mohanty, C.; Sahoo, S. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Tan, X.; Li, J.; Wei, Z.; Zhang, D.; Zheng, Y.; Zheng, A.; Zhao, B.; Zeng, Y.; et al. Photoresponsive Nanovehicle for Two Independent Wavelength Light-Triggered Sequential Release of P-gp shRNA and Doxorubicin to Optimize and Enhance Synergistic Therapy of Multidrug-Resistant Cancer. ACS Appl. Mater. Interfaces 2018, 10, 19416–19427. [Google Scholar] [CrossRef] [PubMed]
- Schoppa, T.; Jung, D.; Rust, T.; Mulac, D.; Kuckling, D.; Langer, K. Light-responsive polymeric nanoparticles based on a novel nitropiperonal based polyester as drug delivery systems for photosensitizers in PDT. Int. J. Pharm. 2021, 597, 120326. [Google Scholar] [CrossRef]
- Pan, H.; Li, W.J.; Wu, L.T.; Huang, W.L.; Zhang, F. β-Cyclodextrin-Modified Mesoporous Silica Nanoparticles with Photo-Responsive Gatekeepers for Controlled Release of Hexaconazole. Coatings 2021, 11, 1489. [Google Scholar] [CrossRef]
- Choi, W.; Battistella, C.; Gianneschi, N. High efficiency loading of micellar nanoparticles with a light switch for enzyme-induced rapid release of cargo. Biomater. Sci. 2021, 9, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Li, C.; Shen, X. Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine. J. Control. Release Off. J. Control. Release Soc. 2020, 319, 46–62. [Google Scholar] [CrossRef]
- Piao, J.G.; Gao, F.; Li, Y.N.; Yu, L.; Liu, D.; Tan, Z.B.; Xiong, Y.J.; Yang, L.H.; You, Y.Z. pH-sensitive zwitterionic coating of gold nanocages improves tumor targeting and photothermal treatment efficacy. Nano Res. 2018, 11, 3193–3204. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Shen, J.; Zhuang, R.; Zhang, S.; Zhu, Z.; Ma, J. pH-Sensitive nanoparticles as smart carriers for selective intracellular drug delivery to tumor. Int. J. Pharm. 2018, 545, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. 2017, 56, 14025–14030. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Qi, J. Nano-Drug Design Based on the Physiological Properties of Glutathione. Molecules 2021, 26, 5567. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.; Zhang, Y.; Zhu, Q.; Waddad, A.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Cong, Y.; Zhou, D.; Li, J.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. A dextran-platinum(iv) conjugate as a reduction-responsive carrier for triggered drug release. J. Mater. Chemistry. B 2015, 3, 8203–8211. [Google Scholar] [CrossRef]
- Lou, X.; Du, Y.; Xu, X. Endogenous Enzyme-responsive Nanoplatforms for Anti-tumor Therapy. Curr. Drug Targets 2021, 22, 845–855. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Qin, S.; Feng, J.; Rong, L.; Jia, H.; Chen, S.; Liu, X.; Luo, G.; Zhuo, R.; Zhang, X. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small 2014, 10, 599–608. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S.; et al. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, e2105254. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Syu, W.; Nishiyama, N.; Kataoka, K.; Lai, P. Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo. J. Control. Release Off. J. Control. Release Soc. 2011, 155, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Ng, K.; Zhao, Y. Integrated hollow mesoporous silica nanoparticles for target drug/siRNA co-delivery. Chemistry 2013, 19, 15593–15603. [Google Scholar]
- Zhang, Y.; Xiao, C.; Li, M.; Chen, J.; Ding, J.; He, C.; Zhuang, X.; Chen, X. Co-delivery of 10-hydroxycamptothecin with doxorubicin conjugated prodrugs for enhanced anticancer efficacy. Macromol. Biosci. 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, H.; Li, J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials 2014, 35, 1050–1062. [Google Scholar]
- Nam, K.; Nam, H.; Kim, P.; Kim, S. Paclitaxel-conjugated PEG and arginine-grafted bioreducible poly (disulfide amine) micelles for co-delivery of drug and gene. Biomaterials 2012, 33, 8122–8130. [Google Scholar] [CrossRef]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Kheiri, S.; Aldeghi, M.; Aspuru-Guzik, A.; Kumacheva, E. Self-Driving Platform for Metal Nanoparticle Synthesis: Combining Microfluidics and Machine Learning. Adv. Funct. Mater. 2021, 31, 2106725. [Google Scholar] [CrossRef]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W.K.; Zheng, F.; Xie, J.; Tian, I.P.S.; Jayavelu, S.; Mahfoud, Z.; Bash, D.; et al. Two-step machine learning enables optimized nanoparticle synthesis. Npj Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.; Cheng, Y.; He, C.; Monteiro-Riviere, N.; Riviere, J. Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar]
| Types of Ligands | Samples | Aim | Result | Ref. |
|---|---|---|---|---|
| Proteins | Tf | Cisplatin and docetaxel were loaded into lipid hybrid NPs and modified with Tf to prepare a targeted delivery vehicle | The Tf-modified group showed stronger targeting and cytotoxicity | [100] |
| EGFR antibodies | Combining EGFR antibodies with polymerized poly(lactide-coglycolide) NPs loaded with rapamycin for selective targeting of the extracellular ligand-binding domain of EGFR | MCF-7 breast cancer cells significantly augmented uptake of EGFR-coupled NPs and induced cell cycle arrest and apoptosis compared with free rapamycin and non-targeted NPs | [101] | |
| Polysaccharides | Angiopep-2 | Angiopep-2 is a complementary ligand for low-density lipoprotein-receptor-associated proteins. Coupling Angiopep-2 with paclitaxel-loaded poly(ethylene glycol)-copolymer (epsilon-caprolactone) NPs to cross the BBB and target delivery of the drug to glioma | The specific accumulation of Angiopep-2 conjugated particle complexes in the brain facilitates the nanocarrier crossing the blood-brain barrier | [102] |
| Artificial peptides | TGN and RGD peptides | Using virus-like particles as carriers, and selecting RGD peptides that can target tumor blood vessels and TGN, a brain-targeting peptide that can penetrate the BBB, as targeting ligands, a dual-targeted drug delivery system was developed to deliver PTX and siRNA | More effective and accurate delivery of small molecule chemotherapy drugs to the tumor site, to achieve a good anti-tumor effect | [103] |
| Aptamers | PSMA aptamers | The A10 RNA aptamer binds to the surface of polylactic acid NPs and encapsulates docetaxel to specifically recognize PSMA on the surface of prostate cancer cells | Significantly reduced tumor size in xenograft nude mice models of prostate cancer | [79] |
| Small molecules | FA | Combining PTX with pluronic123 polymer and attaching FA to the surface of the micelle by chemical coupling to obtain complex micelles | The FA-coupled polymer micelle system significantly enhanced cell uptake and anti-tumor activity, and exhibited higher anti-tumor effects and safety in animals | [104] |
| Stimulus Types | Carrier | Aim | Result | Ref. |
|---|---|---|---|---|
| Physical response (temperature, magnetism, light,) | Magnetic cationic liposomes | DPPC, DC-CHOL, DOAB, cholesterol-modified magnetic iron oxide co-delivery of DOX and SATB1-shRNA | In gastric cancer model, co-delivery of DOX and SATB1shRNA enhanced inhibition of cell growth compared with treatment alone. | [122] |
| NPs | an aqueous-based formulation of glycerol-monooleate-coated magnetic NPs (GMO-MNPs) co-delivery of paclitaxel and rapamycin | High encapsulation efficiency (~95%) and drug release synergistically enhance the anti-tumor effect | [125] | |
| Poly-ion complex micelles (PICs) | Dendrimer phthalocyanine-encapsulated polymeric micelle (DP c/m)-mediated PCI, combined with DOX | NP-mediated double PDT/PCI effect, DOX released from endo-lysosome to the nucleus after light irradiation, improved the efficacy of PDT and PDT in the tumor | [142] | |
| Chemical response (pH, reduction) | Hollow mesoporous silica NPs (HMSNP) | Folate-coated MSNPs bound to PEI carry DOX and siRNA | Controlled drug release and reduced the off-target action, greatly inhibiting the expression of Bcl-2 (anti-apoptotic) protein | [143] |
| Amphiphilic linear-dendritic prodrugs | The amphiphilic linear dendritic vector (MPEG-B-PAMAM) was synthesized and DOX was encapsulated | The release of the drug is pH-dependent, increases cell uptake, and effectively inhibits the growth of cancer cells | [144] | |
| Polyplex | Star-shaped cationic polymer was prepared by γ-cyclodextrin (γ-CD) and multiple oligo-ethylenimine (OEI) arms and combined with folic acid to carry PTX | Gene transfection was enhanced and apoptosis was significant | [145] | |
| Polymeric micelle | ABP-PEG3.5k-PTX (APP) for the co-delivery of genes and drugs. | Improved cell uptake efficiency, low cytotoxicity | [146] | |
| Biological response (enzyme) | Graphene oxide (GO) | Graphene oxide delivers DOX and DNA via MMP-2 cleavable PLGLAG peptide bond linked to PEI-PEG | MMP2 reacts with peptide cleavage to control drug release and enhance drug efficacy in vitro. The efficient transfection can be comparable to PEI25k | [140] |
| Polymeric micelles | MMP2 sensitive copolymer (PEG-PP-PEI-PE) co-delivers siRNA and drugs | The antitumor activity of PTX and siRNA was improved | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. https://doi.org/10.3390/pharmaceutics14091919
Yang T, Zhai J, Hu D, Yang R, Wang G, Li Y, Liang G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics. 2022; 14(9):1919. https://doi.org/10.3390/pharmaceutics14091919
Chicago/Turabian StyleYang, Tingting, Jingming Zhai, Dong Hu, Ruyue Yang, Guidan Wang, Yuanpei Li, and Gaofeng Liang. 2022. "“Targeting Design” of Nanoparticles in Tumor Therapy" Pharmaceutics 14, no. 9: 1919. https://doi.org/10.3390/pharmaceutics14091919
APA StyleYang, T., Zhai, J., Hu, D., Yang, R., Wang, G., Li, Y., & Liang, G. (2022). “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics, 14(9), 1919. https://doi.org/10.3390/pharmaceutics14091919






