Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis
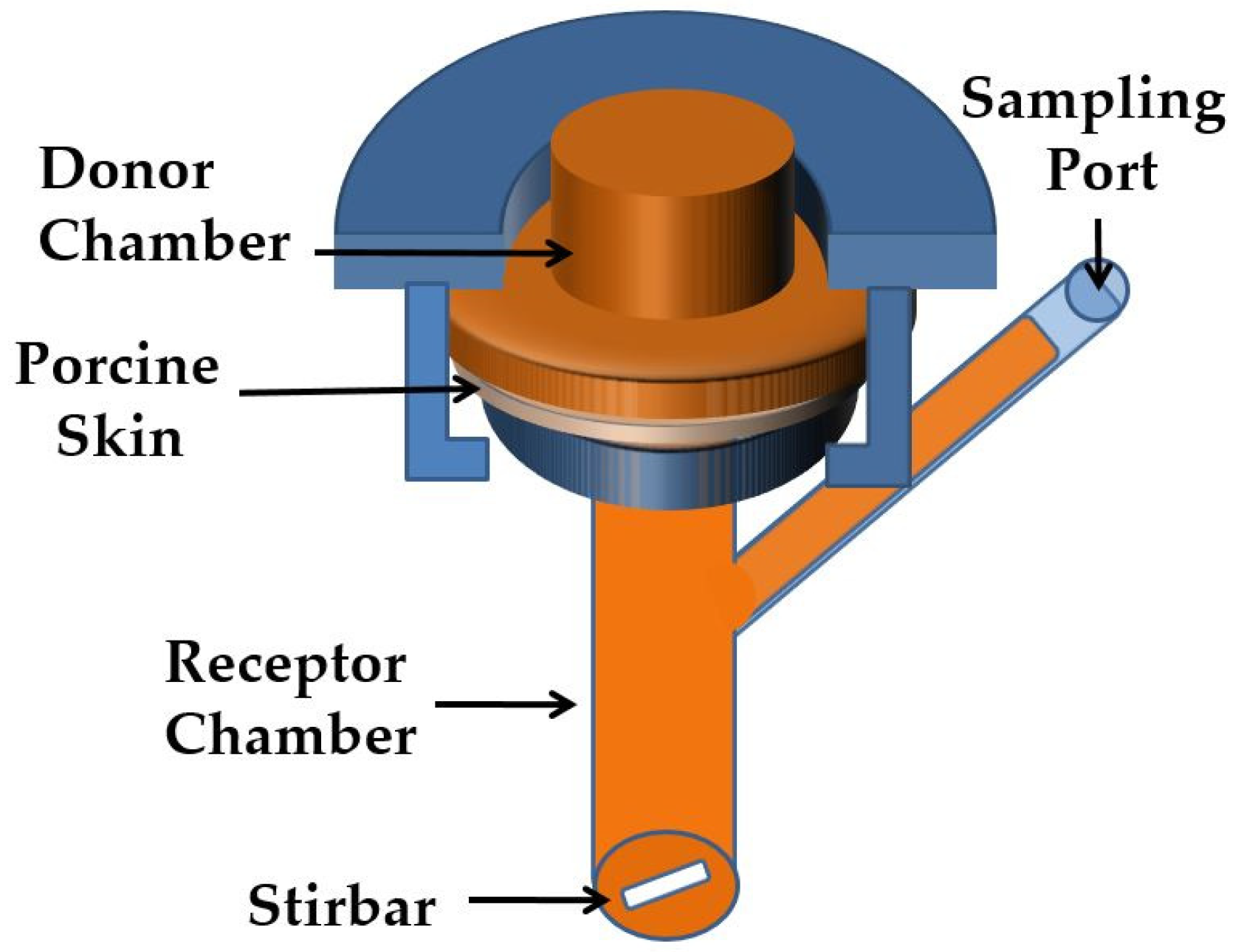
2.3. Release Studies by In Vitro Experiments
2.4. Tape Stripping In Vitro
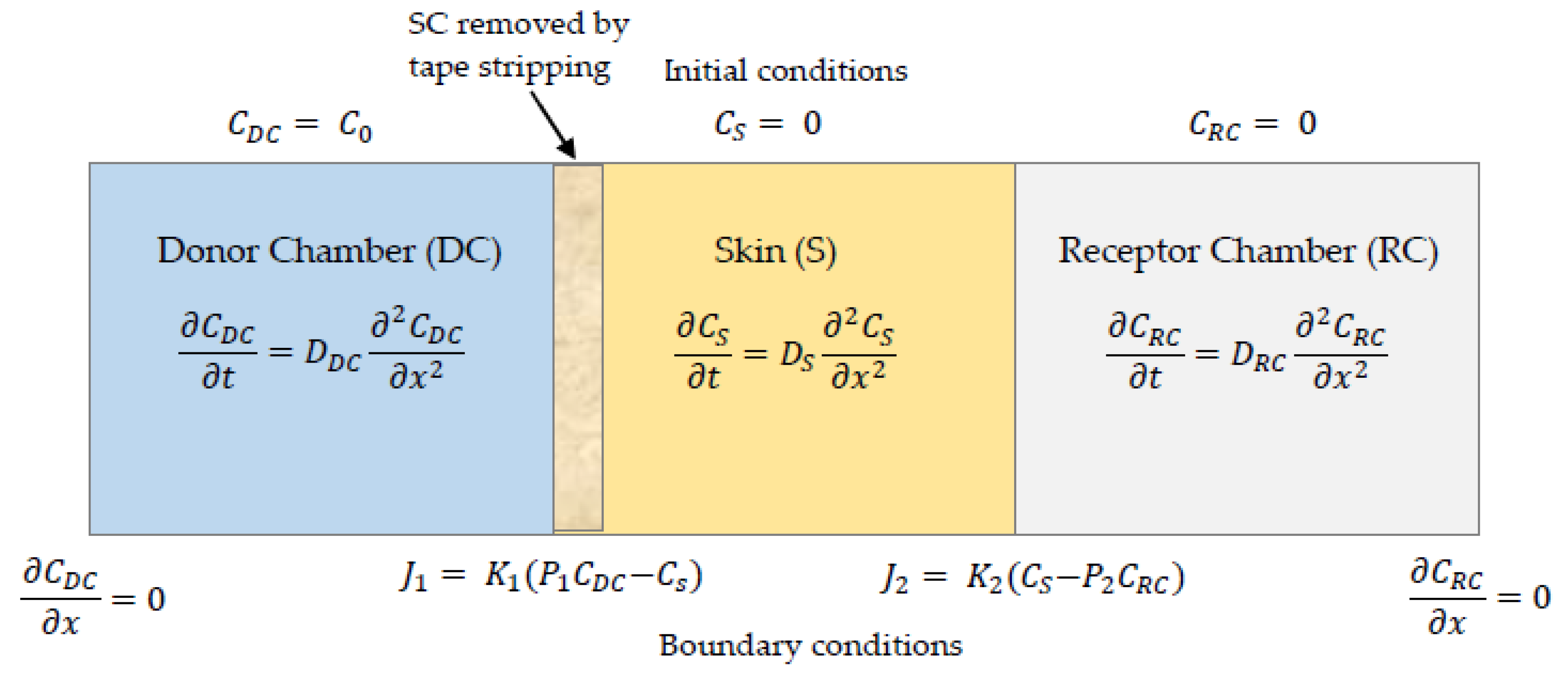
2.5. Numerical Simulations
3. Results
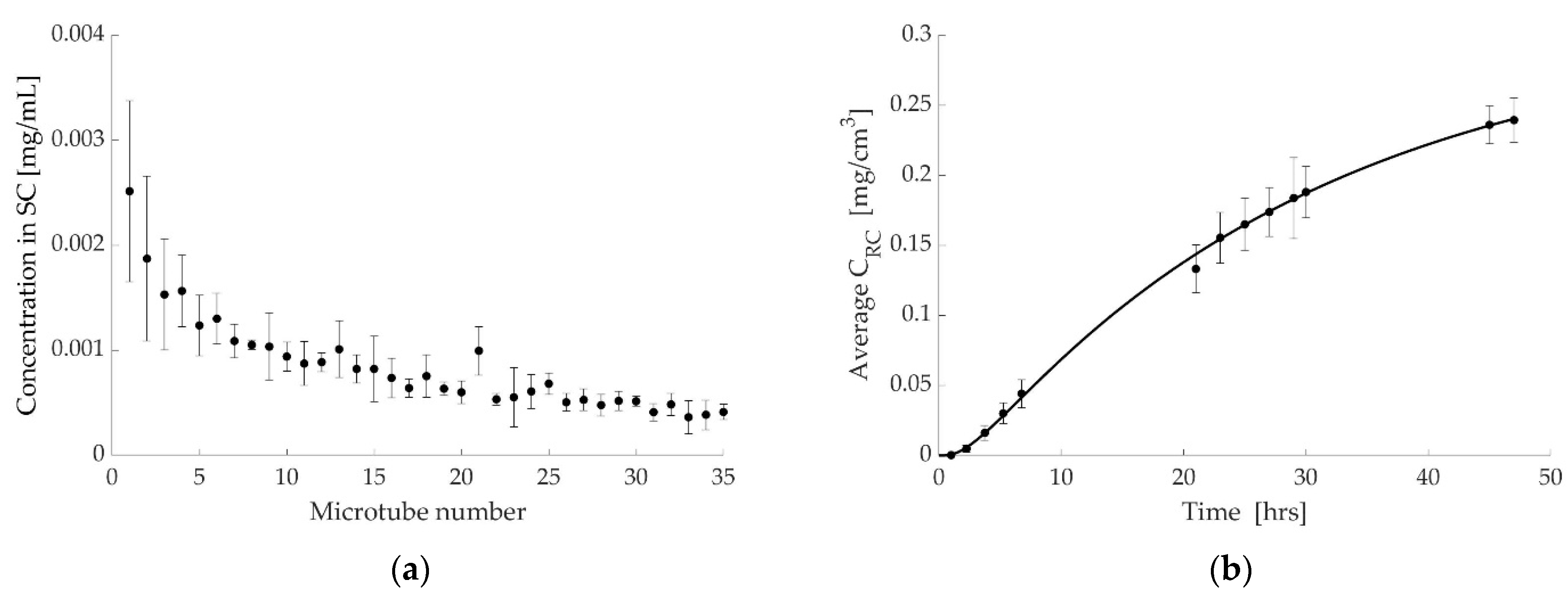
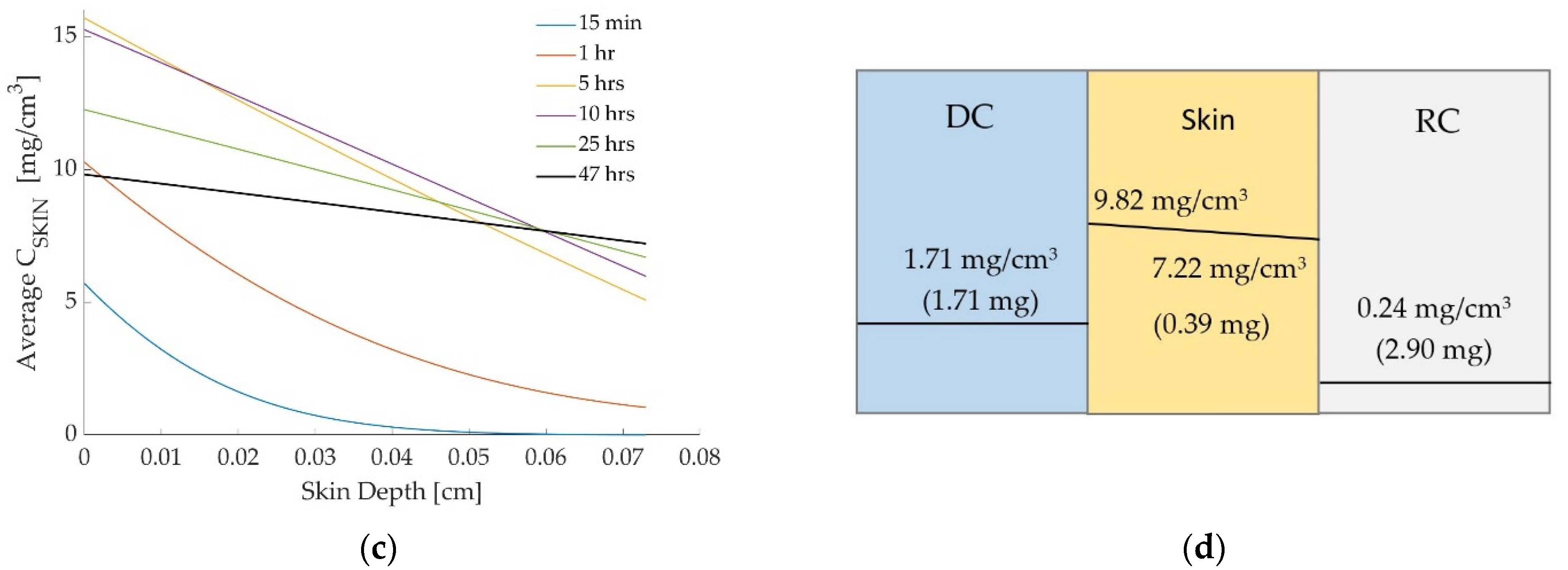
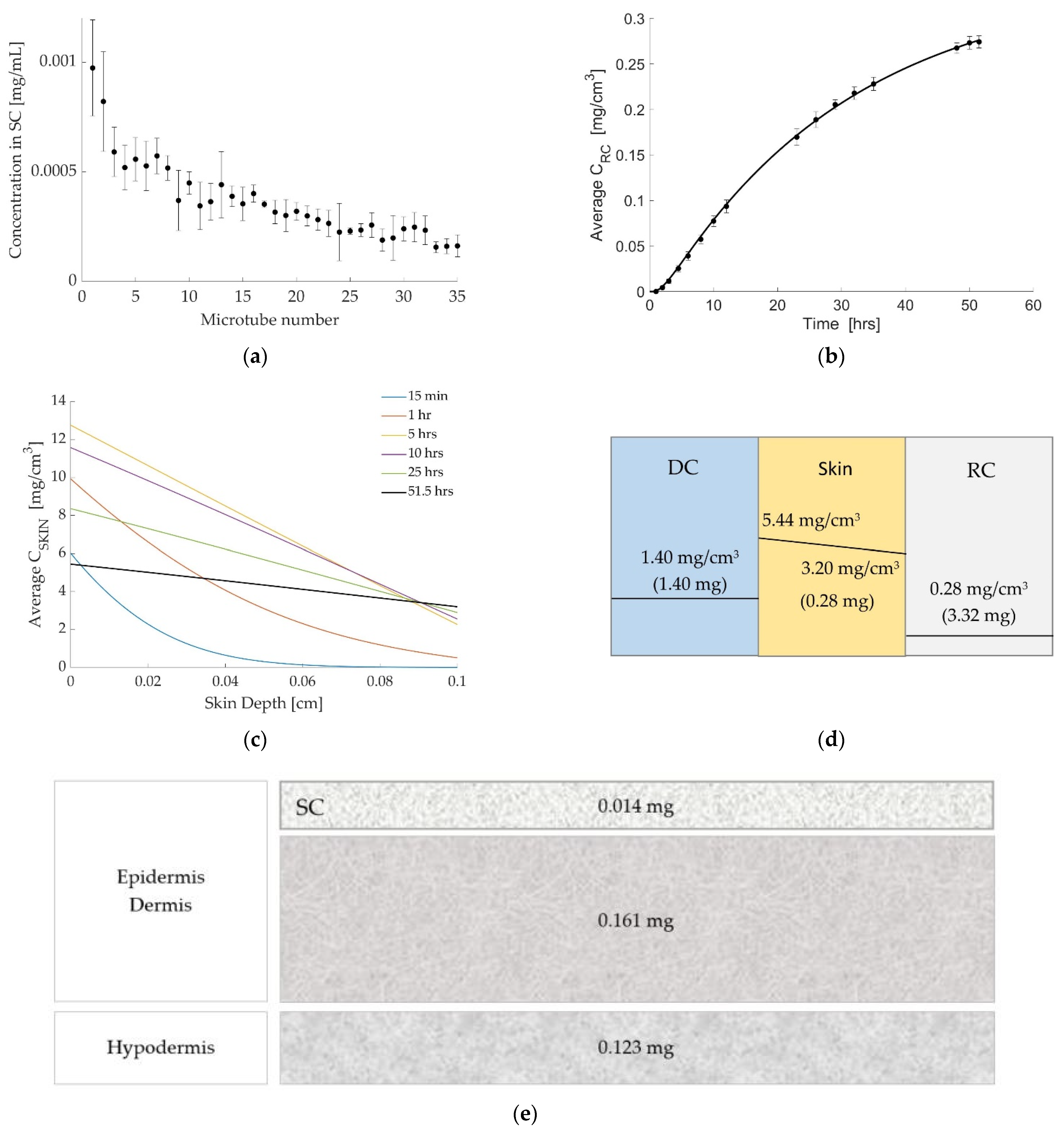
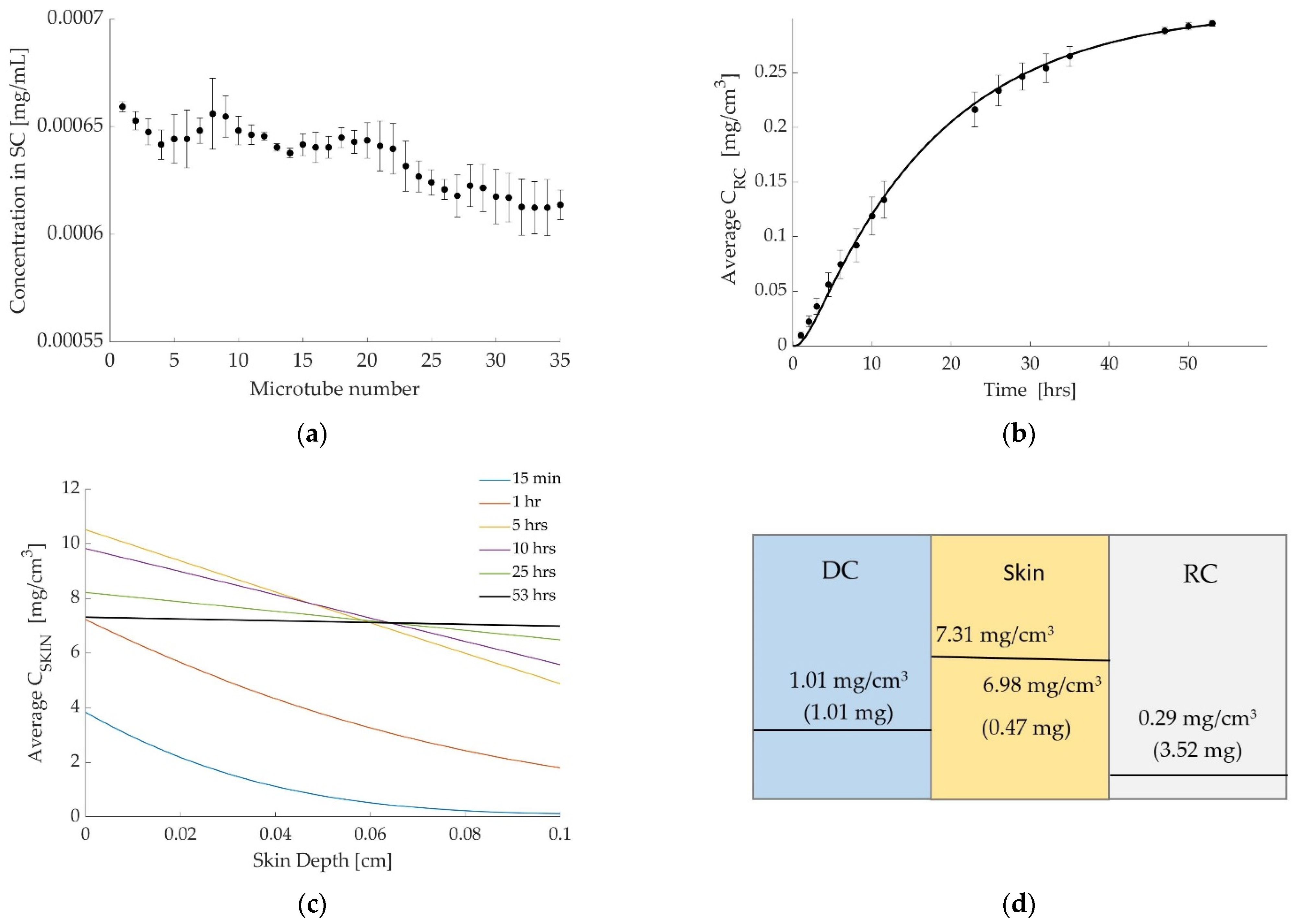
3.1. Case 1: Diclofenac Solution at Room Temperature
3.2. Case 2: Dicofenac Solution at 32 °C
3.3. Case 3: Caffeine at 32 °C
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, M.N.; Kalia, Y.; Horstmann, M.; Roberts, M. Transdermal patches: History, development and pharmacology. J. Cereb. Blood Flow Metab. 2015, 172, 2179–2209. [Google Scholar]
- Apfel, C.C.; Zhang, K.; George, E.; Shi, S.; Jalota, L.; Hornuss, C.; Fero, K.E.; Heidrich, F.; Pergolizzi, J.V.; Cakmakkaya, O.S.; et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: A systematic review and meta-analysis. Clin. Ther. 2010, 32, 1987–2002. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar]
- Wasley, M.A.; McNagny, S.E.; Phillips, V.; Ahluwalia, J.S. The Cost-Effectiveness of the Nicotine Transdermal Patch for Smoking Cessation. Prev. Med. 1997, 26, 264–270. [Google Scholar]
- Field, C.S.; Ory, S.J.; Wahner, H.W.; Herrmann, R.R.; Judd, H.L.; Riggs, B.L. Preventive effects of transdermal 17β-estradiol on osteoporotic changes after surgical menopause: A two-year placebo-controlled trial. Am. J. Obstet. Gynecol. 1993, 168, 114–121. [Google Scholar]
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2010, 28, 469–472. [Google Scholar]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar]
- Shahzad, Y.; Louw, R.; Gerber, M.; du Plessis, J. Breaching the skin barrier through temperature modulations. J. Control. Release 2015, 202, 1–13. [Google Scholar]
- Ali, S.; Shabbir, M.; Shahid, N. The Structure of Skin and Transdermal Drug Delivery System-A Review. Res. J. Pharm. Technol. 2015, 8, 103. [Google Scholar]
- Bardal, S.K.; Waechter, J.E.; Martin, D.S. Chapter 2-Pharmacokinetics. In Applied Pharmacology; Bardal, S.K., Waechter, J.E., Martin, D.S., Eds.; Content Repository Only: Philadelphia, PA, USA, 2011; pp. 17–34. [Google Scholar]
- Ruela, A.L.M.; Perissinato, A.; Lino, M.E.D.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar]
- Jhawat, V.; Saini, V.; Kamboj, S.; Maggon, N. Transdermal Drug Delivery Systems: Approaches and Advancements in Drug Absorption through Skin. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 47–56. [Google Scholar]
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30+ years of war and still fighting. J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products. AAPS J. 2012, 15, 41–52. [Google Scholar] [CrossRef]
- Lee, C.K.; Uchida, T.; Kitagawa, K.; Yagi, A.; Kim, N.-S.; Goto, S. Skin Permeability of Various Drugs with Different Lipophilicity. J. Pharm. Sci. 1994, 83, 562–565. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- Obata, Y.; Takayama, K. Related Topic: Determination of Partition Coefficient from Vehicle to Skin. In Skin Permeation and Disposition of Therapeutic and Cosmeceutical Compounds; Springer: Tokyo, Japan, 2017; pp. 385–389. [Google Scholar]
- Herkenne, C.; Alberti, I.; Naik, A.; Kalia, Y.N.; Mathy, F.-X.; Préat, V.; Guy, R.H. In Vivo Methods for the Assessment of Topical Drug Bioavailability. Pharm. Res. 2007, 25, 87–103. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- McGinty, S.; Pontrelli, G. A general model of coupled drug release and tissue absorption for drug delivery devices. J. Control. Release 2015, 217, 327–336. [Google Scholar] [CrossRef]
- Pontrelli, G.; de Monte, F. A two-phase two-layer model for transdermal drug delivery and percutaneous absorption. Math. Biosci. 2014, 257, 96–103. [Google Scholar] [CrossRef]
- Rim, J.E.; Pinsky, P.M.; Van Osdol, W.W. Multiscale Modeling Framework of Transdermal Drug Delivery. Ann. Biomed. Eng. 2009, 37, 1217–1229. [Google Scholar] [CrossRef]
- Rim, J.E.; Pinsky, P.M.; Van Osdol, W.W. Finite Element Modeling of Coupled Diffusion with Partitioning in Transdermal Drug Delivery. Ann. Biomed. Eng. 2005, 33, 1422–1438. [Google Scholar] [CrossRef]
- Gudnason, K.; Sigurdsson, S.; Snorradottir, B.S.; Masson, M.; Jonsdottir, F. A numerical framework for drug transport in a multi-layer system with discontinuous interlayer condition. Math. Biosci. 2018, 295, 11–23. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef]
- OECD. Skin Absorption: In Vivo Method. Test Guideline No. 427; Series on Testing and Assessment. No. 428; OECD: Paris, France, 2004. [Google Scholar]
- Council of Europe. European Pharmacopoeia; Council of Europe: European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010. [Google Scholar]
- Gudnason, K.; Solodova, S.; Vilardell, A.; Masson, M.; Sigurdsson, S.; Jónsdóttir, F. Numerical simulation of Franz diffusion experiment: Application to drug loaded soft contact lenses. J. Drug Deliv. Sci. Technol. 2017, 38, 18–27. [Google Scholar] [CrossRef]
- Sinko, P.J.; Martin, A.N. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; 795p. [Google Scholar]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic considerations in the dermatokinetics of topical formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25, reprinted in Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
| Settings | Mobile Phase | Flow Rate mL/min | UV Signal nm | Retention Time min |
|---|---|---|---|---|
| Diclofenac | Acetonitrile: 1% acetic acid (40:60) | 1.5 | 281 | 2.2 |
| Caffeine | Methanol: MilliQ water (40:60) | 0.8 | 272 | 4.1 |
| Starting Concentration C0 | 5.00 mg/mL |
|---|---|
| Experiment time | 47 h |
| Skin thickness | 0.07 cm |
| Diffusion coefficient | 1.2 × 10−3 cm2/h |
| Partition coefficient P1 | 8 |
| Partition coefficient P2 | 25 |
| Mass transfer coefficient K1 | 0.08 cm/h |
| Mass transfer coefficient K2 | 0.04 cm/h |
| Starting Concentration C0 | 5.00 mg/mL |
|---|---|
| Experiment time | 51.5 h |
| Skin thickness | 0.1 cm |
| Diffusion coefficient | 1.8 × 10−3 cm2/h |
| Partition coefficient P1 | 5 |
| Partition coefficient P2 | 10 |
| Mass transfer coefficient K1 | 0.12 cm/h |
| Mass transfer coefficient K2 | 0.10 cm/h |
| Starting Concentration C0 | 5.00 mg/mL |
|---|---|
| Experiment time | 53 h |
| Skin thickness | 0.1 cm |
| Diffusion coefficient | 5.0 × 10−3 cm2/h |
| Partition coefficient P1 | 8 |
| Partition coefficient P2 | 23 |
| Mass transfer coefficient K1 | 0.11 cm/h |
| Mass transfer coefficient K2 | 0.08 cm/h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonsdottir, F.; Snorradottir, B.S.; Gunnarsson, S.; Georgsdottir, E.; Sigurdsson, S. Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics 2022, 14, 1880. https://doi.org/10.3390/pharmaceutics14091880
Jonsdottir F, Snorradottir BS, Gunnarsson S, Georgsdottir E, Sigurdsson S. Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics. 2022; 14(9):1880. https://doi.org/10.3390/pharmaceutics14091880
Chicago/Turabian StyleJonsdottir, Fjola, Bergthora S. Snorradottir, Skuli Gunnarsson, Elina Georgsdottir, and Sven Sigurdsson. 2022. "Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling" Pharmaceutics 14, no. 9: 1880. https://doi.org/10.3390/pharmaceutics14091880
APA StyleJonsdottir, F., Snorradottir, B. S., Gunnarsson, S., Georgsdottir, E., & Sigurdsson, S. (2022). Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics, 14(9), 1880. https://doi.org/10.3390/pharmaceutics14091880







