Prostatic Therapeutic Efficacy of LENILUTS®, a Novel Formulation with Multi-Active Principles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Composition
2.3. Methods
2.3.1. Cell Cultures
LNCaP Cell Culture
THP-1 Cell Culture
Caco-2 Cell Culture
2.3.2. Evaluation of LENILUTS® Antioxidant Activity
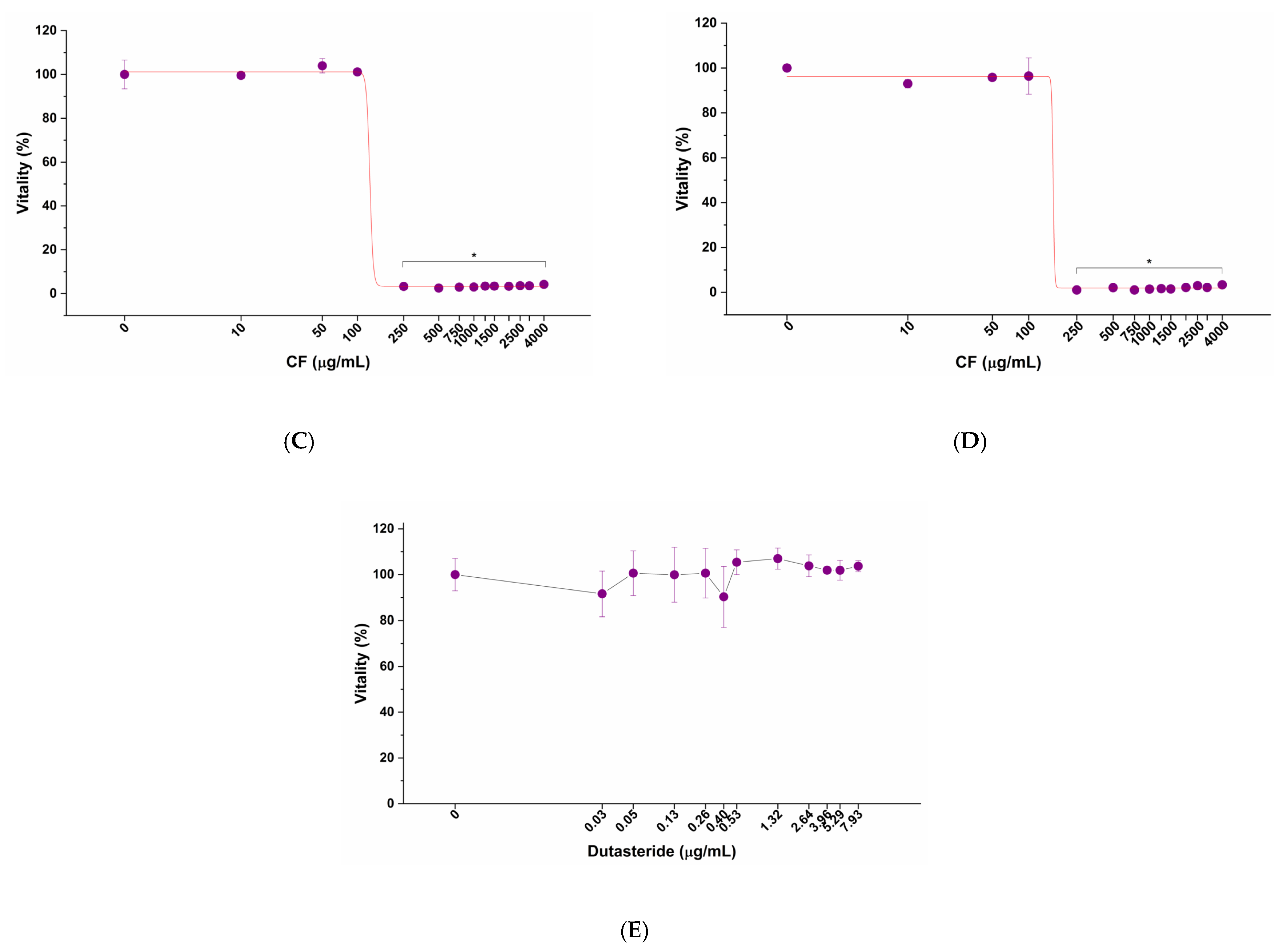
2.3.3. Evaluation of the Impact of Tested Formulations, Dutasteride and Diclofenac, on LNCaP Cells
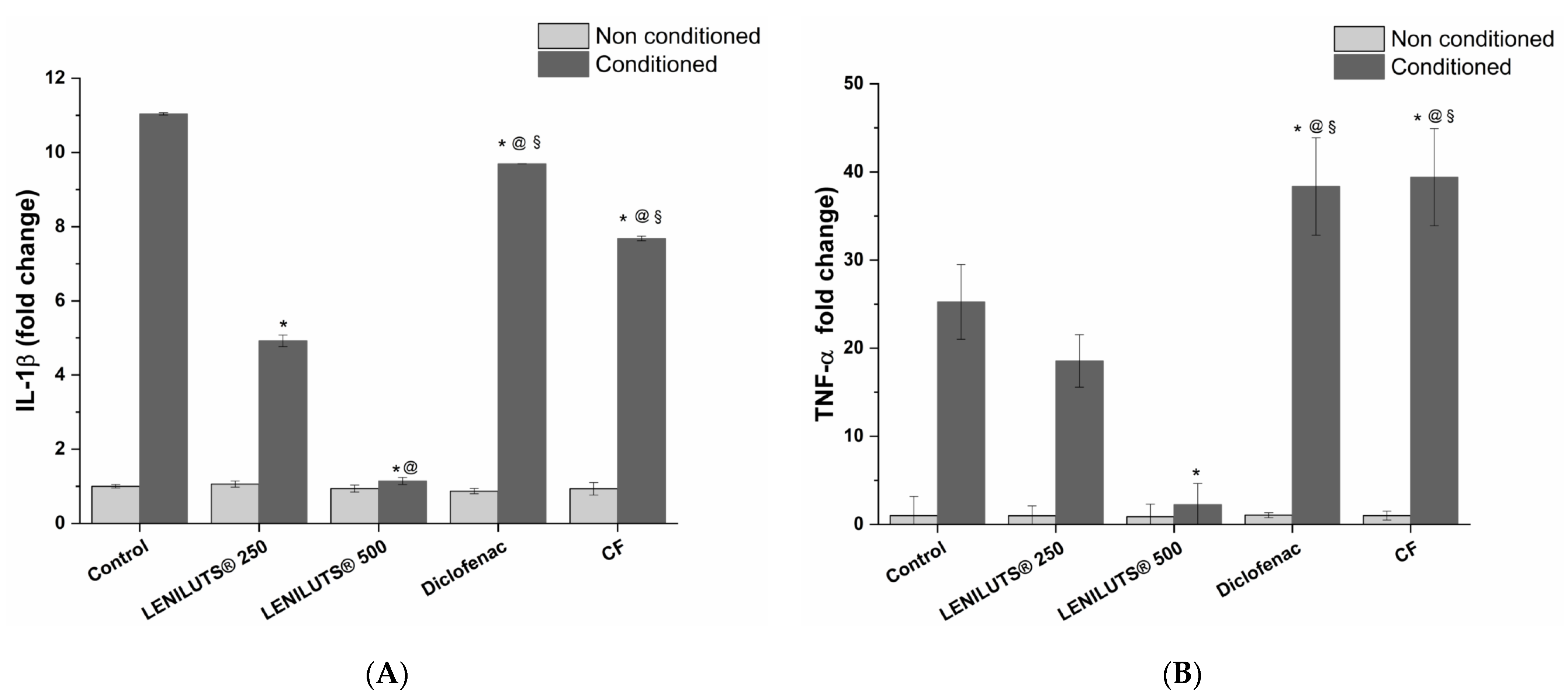
2.3.4. Prostate-Specific Anti-Inflammatory Activity
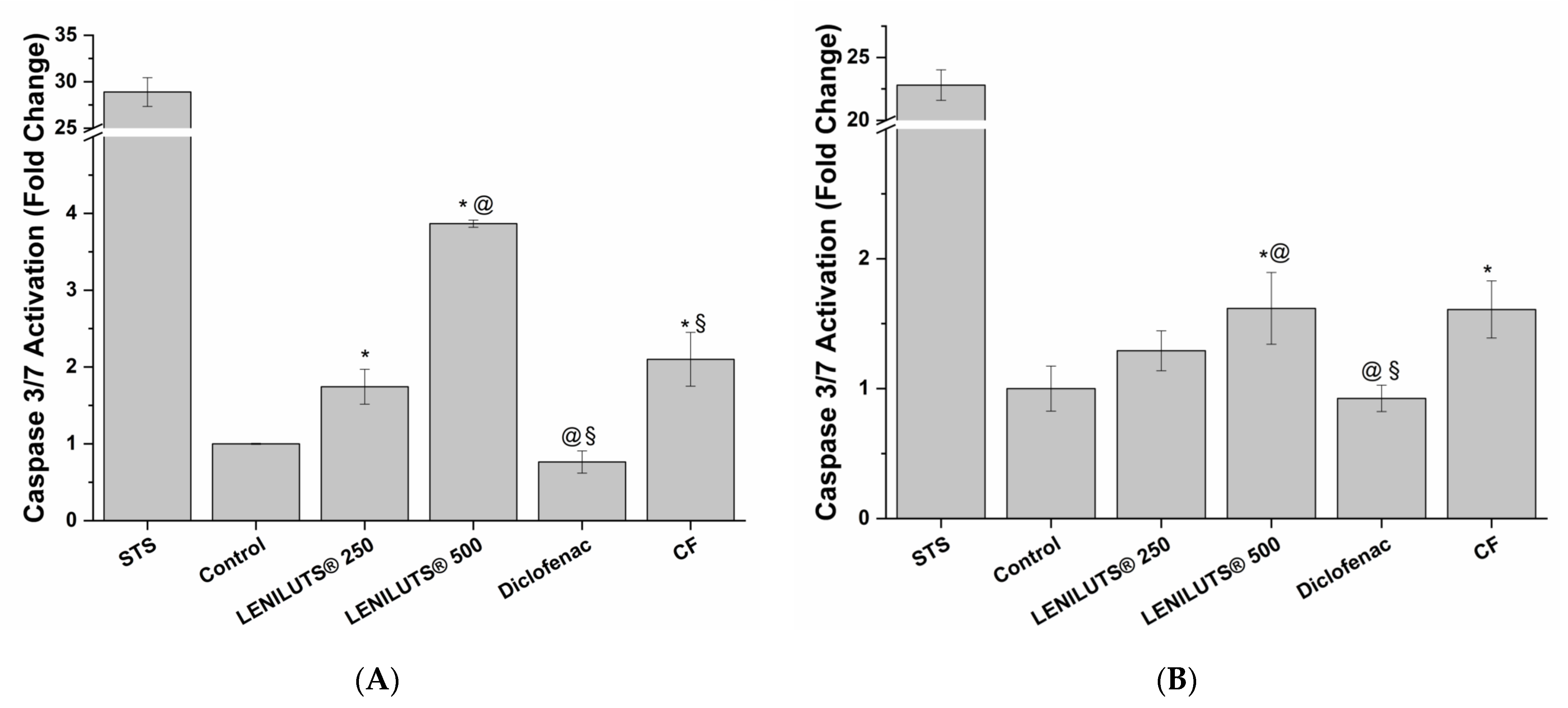
2.3.5. LENILUTS® Pro-Apoptotic Activity
2.3.6. 5α-R Activity
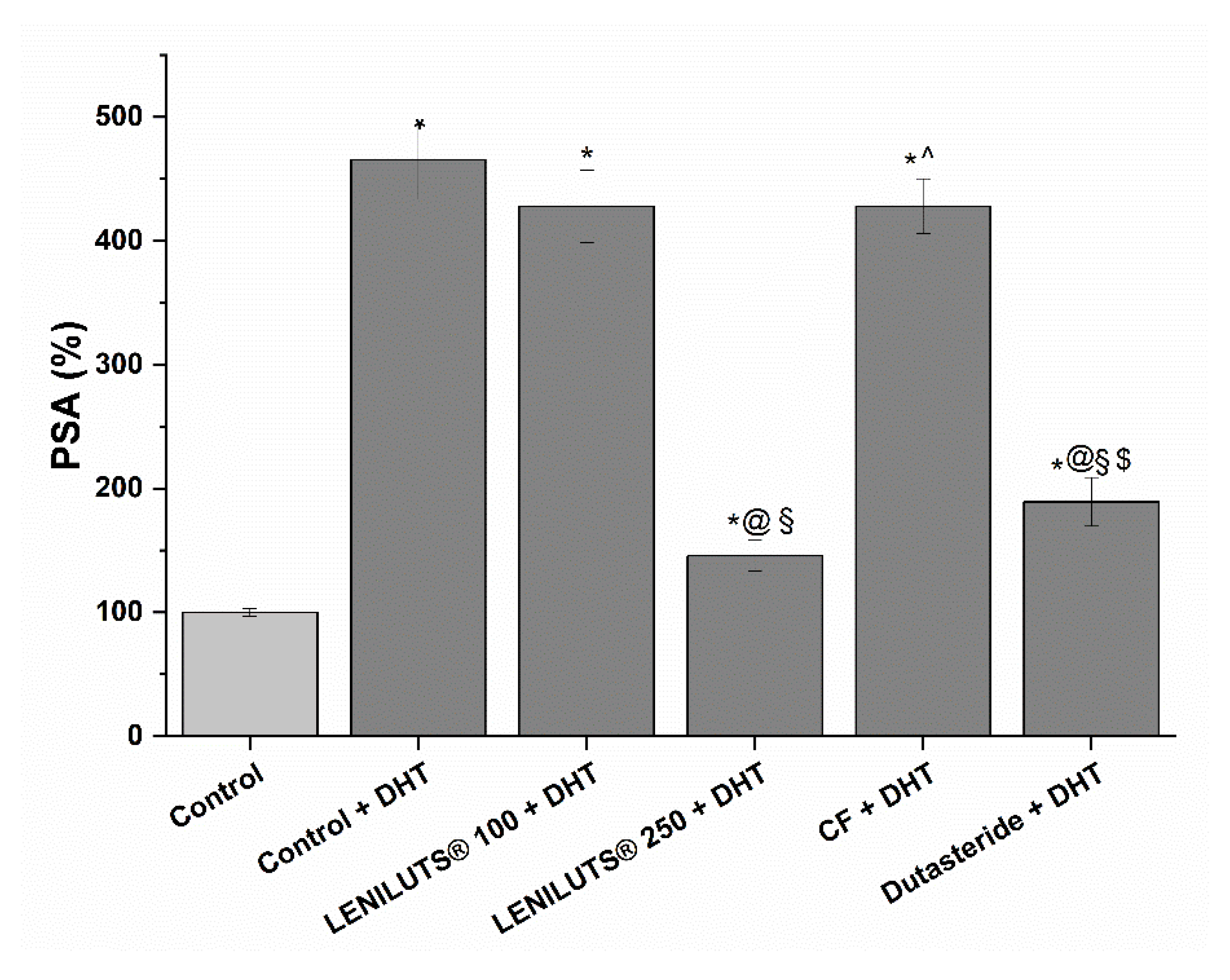
2.3.7. Measurement of PSA Secretion by LNCaP Prostatic Cells
2.3.8. In Vitro Digestion Process
2.3.9. Curcumin Determination
2.3.10. In Vitro Model of Human Intestinal Epithelium
2.3.11. Digested Formulations’ Impact on the Viability of the Intestinal Epithelium
2.3.12. Evaluation of the Bioavailability of Beta-Sitosterol, Curcumin and OPA
2.3.13. Evaluation of Post-Intestinal Absorption Caco-2 Monolayer Barrier Integrity and Viability
2.3.14. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Impact of LENILUTS®, CF, Dutasteride and Diclofenac on the In Vitro Prostate model
3.3. Effect of LENILUTS®, CF and Dutasteride Treatment on Pro-Inflammatory Cytokine Release from the In Vitro Prostate model
3.4. Pro-Apoptotic Activity of LENILUTS®, CF and Dutasteride
3.5. Impact of LENILUTS®, CF and Dutasteride Treatment on 5αR Activity in an In Vitro Prostate Model
3.6. Effect of LENILUTS®, CF and Dutasteride on the Release of PSA
3.7. Bioaccessibility of LENILUTS® Active Principles
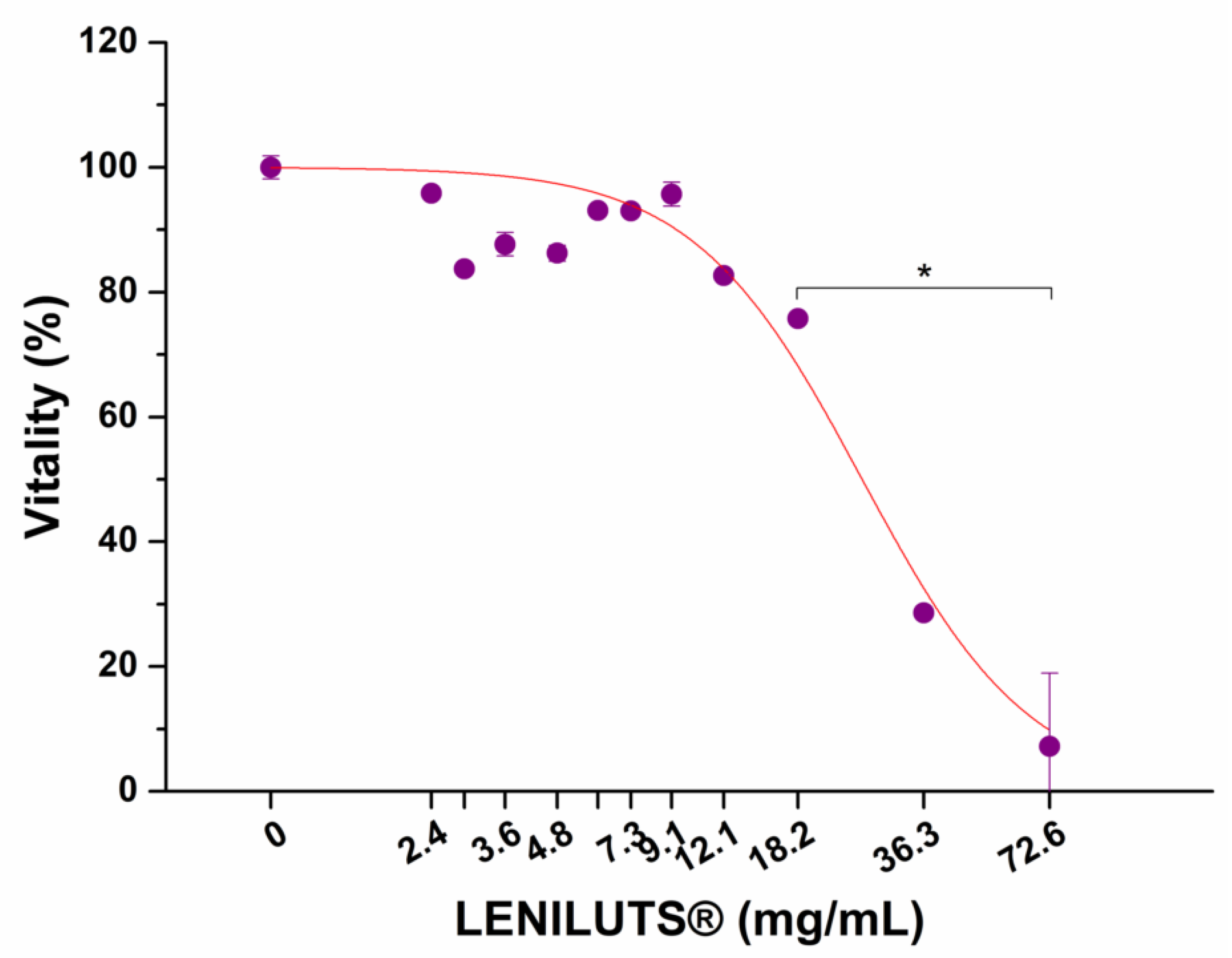
3.8. Impact of Digested LENILUTS® on Intestinal Epithelium Viability
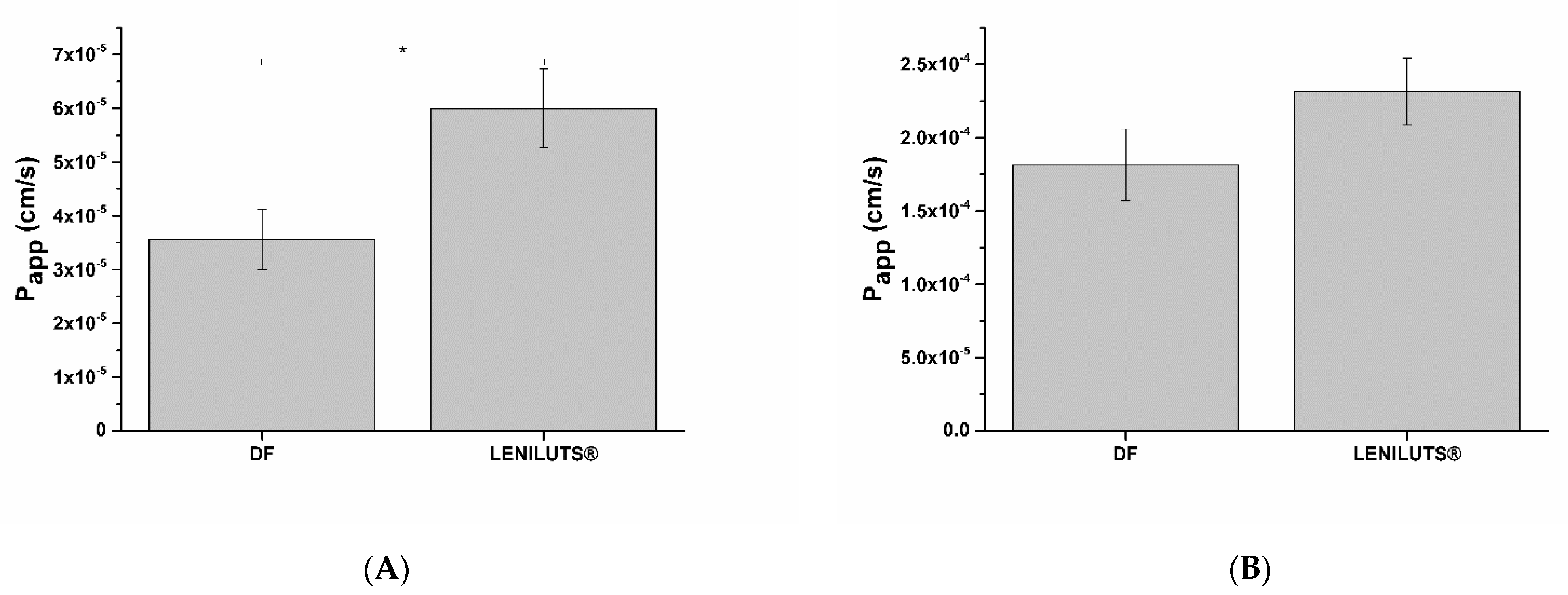
3.9. Active Principles Bioavailability
Curcumin
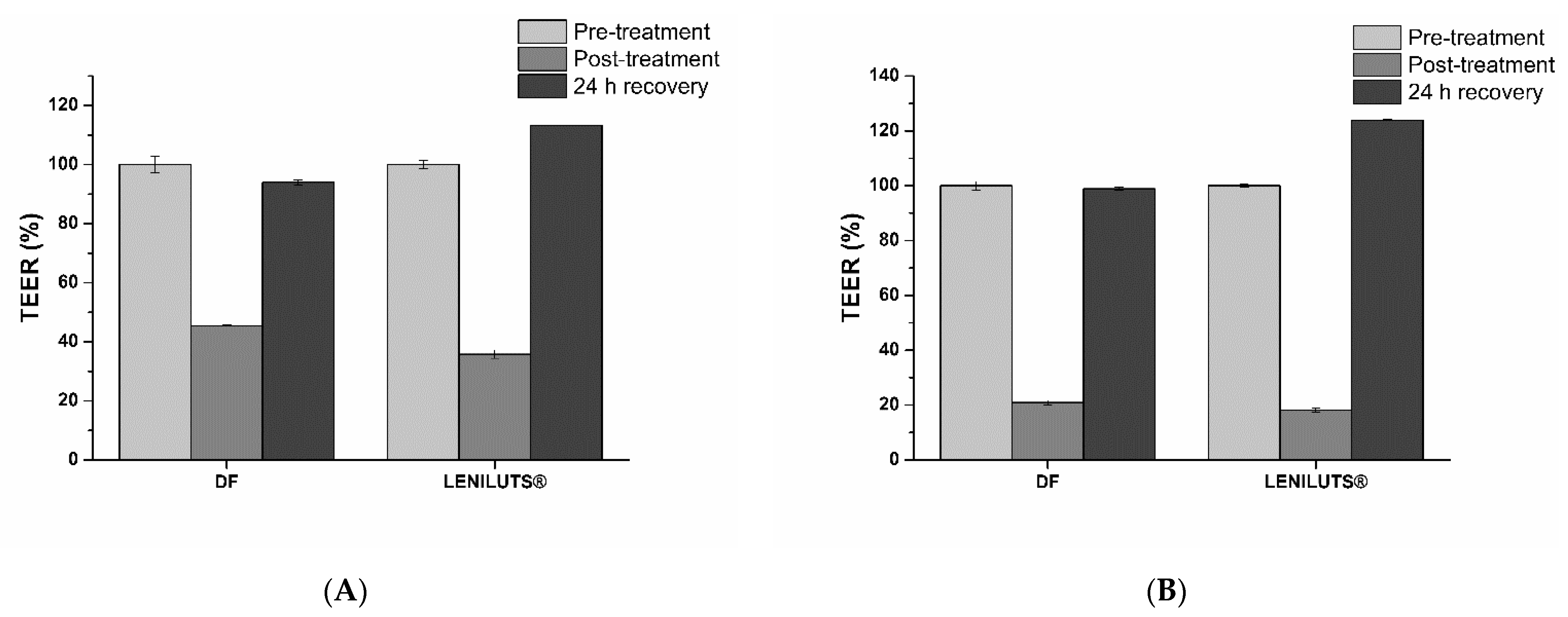
3.10. Impact of Digested Formulations on Intestinal Mucosa Viability and Integrity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ficarra, V.; Rossanese, M.; Zazzara, M.; Giannarini, G.; Abbinante, M.; Bartoletti, R.; Mirone, V.; Scaglione, F. The Role of Inflammation in Lower Urinary Tract Symptoms (LUTS) Due to Benign Prostatic Hyperplasia (BPH) and Its Potential Impact on Medical Therapy. Curr. Urol. Rep. 2014, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Mendes, P.; Silva, J.; Cruz, F. Pharmacology of the Lower Urinary Tract: Update on LUTS Treatment. Ther. Adv. Urol. 2020, 12, 175628722092242. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Freedland, S.J. Lifestyle and Lower Urinary Tract Symptoms: What Is the Correlation in Men? Curr. Opin. Urol. 2015, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lucia, M.S.; Lambert, J.R. Growth Factors in Benign Prostatic Hyperplasia: Basic Science Implications. Curr. Urol. Rep. 2008, 9, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kunit, T.; Ciotkowska, A.; Rutz, B.; Schreiber, A.; Strittmatter, F.; Waidelich, R.; Liu, C.; Stief, C.G.; Gratzke, C.; et al. Inhibition of Prostate Smooth Muscle Contraction and Prostate Stromal Cell Growth by the Inhibitors of Rac, NSC23766 and EHT1864. Br. J. Pharmacol. 2015, 172, 2905–2917. [Google Scholar] [CrossRef]
- Hennenberg, M.; Miljak, M.; Herrmann, D.; Strittmatter, F.; Walther, S.; Rutz, B.; Hocaoglu, Y.; Kunit, T.; Schreiber, A.; Andersson, K.E.; et al. The Receptor Antagonist Picotamide Inhibits Adrenergic and Thromboxaneinduced Contraction of Hyperplastic Human Prostate Smooth Muscle. Am. J. Physiol. Ren. Physiol. 2013, 305, 1383–1391. [Google Scholar] [CrossRef]
- Torkko, K.C.; Wilson, R.S.; Smith, E.E.; Kusek, J.W.; Van Bokhoven, A.; Lucia, M.S. Prostate Biopsy Markers of Inflammation Are Associated with Risk of Clinical Progression of Benign Prostatic Hyperplasia: Findings from the MTOPS Study. J. Urol. 2015, 194, 454–461. [Google Scholar] [CrossRef]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef]
- Taoka, R.; Kakehi, Y. The Influence of Asymptomatic Inflammatory Prostatitis on the Onset and Progression of Lower Urinary Tract Symptoms in Men with Histologic Benign Prostatic Hyperplasia. Asian. J. Urol. 2017, 4, 158–163. [Google Scholar] [CrossRef]
- Neill, M.G.; Appu, S.; Zlotta, A.R. Strategies to Preserve Prostate Health. Drugs. Today 2009, 45, 63–80. [Google Scholar] [CrossRef]
- Comhaire, F.; Mahmoud, A. Preventing Diseases of the Prostate in the Elderly Using Hormones and Nutriceuticals. Aging. Male. 2004, 7, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Keehn, A.; Taylor, J.; Lowe, F.C. Phytotherapy for Benign Prostatic Hyperplasia. Benign Prostatic Hyperlasia 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Keehn, A.; Lowe, F.C. Complementary and Alternative Medications for Benign Prostatic Hyperplasia. Can. J. Urol. 2015, 22, 18–23. [Google Scholar] [PubMed]
- Suzuki, M.; Ito, Y.; Fujino, T.; Abe, M.; Umegaki, K.; Onoue, S.; Noguchi, H.; Yamada, S. Pharmacological Effects of Saw Palmetto Extract in the Lower Urinary Tract. Acta. Pharmacol. Sin. 2009, 30, 271–281. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C. Serenoa Repens for Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia: Current Evidence and Its Clinical Implications in Naturopathic Medicine. J. Altern. Complement. Med. 2017, 23, 599–606. [Google Scholar] [CrossRef]
- Latil, A.; Pétrissans, M.T.; Rouquet, J.; Robert, G.; De La Taille, A. Effects of Hexanic Extract of Serenoa Repens (Permixon® 160 mg) on Inflammation Biomarkers in the Treatment of Lower Urinary Tract Symptoms Related to Benign Prostatic Hyperplasia. Prostate 2015, 75, 1857–1867. [Google Scholar] [CrossRef]
- Russo, A.; Capogrosso, P.; La Croce, G.; Ventimiglia, E.; Boeri, L.; Briganti, A.; Damiano, R.; Montorsi, F.; Salonia, A. Serenoa Repens, Selenium and Lycopene to Manage Lower Urinary Tract Symptoms Suggestive for Benign Prostatic Hyperplasia. Expert. Opin. Drug. Saf. 2016, 15, 1661–1670. [Google Scholar] [CrossRef]
- Minutoli, L.; Bitto, A.; Squadrito, F.; Marini, H.; Irrera, N.; Morgia, G.; Passantino, A.; Altavilla, D. Serenoa Repens, Lycopene and Selenium: A Triple Therapeutic Approach to Manage Benign Prostatic Hyperplasia. Curr. Med. Chem. 2013, 20, 1306–1312. [Google Scholar] [CrossRef]
- Paniagua-Pérez, R.; Flores-Mondragón, G.; Reyes-Legorreta, C.; Herrera-López, B.; Cervantes-Hernández, I.; Madrigal-Santillán, O.; Morales-González, J.A.; Álvarez-González, I.; Madrigal-Bujaidar, E. Evaluation of the Anti-Inflammatory Capacity of Beta-Sitosterol in Rodent Assays. African. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 123–130. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol Exhibits Anti-Inflammatory Activity in Human Aortic Endothelial Cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The Anti-Inflammatory Activity of Curcumin Is Mediated by Its Oxidative Metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-Inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-Related Diseases. Eur. Cardiol. Rev. 2019, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, R.; Yu, S.; Lu, G.; Yu, Y.; Jiang, C. Anti-Inflammatory Activity of Oligomeric Proanthocyanidins via Inhibition of NF-ΚB and MAPK in LPS-Stimulated MAC-T Cells. J. Microbiol. Biotechnol. 2020, 30, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.L.; Siddiqui, F.A. Beta-Sitosterol: As Immunostimulant, Antioxidant and Inhibitor of SARS-CoV-2 Spike Glycoprotein. Arch. Pharmacol. Ther. 2020, 2, 12–16. [Google Scholar] [CrossRef]
- Manisha, P.; Chandrashekhar, P.; Raghunath, M. Phytochemical Investigation and Validation of Antioxidant Potential of β-Sitosterol from Tubers of Eulophia Herbacea and Eulophia Ochreata. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 309–316. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant Effects of Curcumin in Models of Neurodegeneration, Aging, Oxidative and Nitrosative Stress: A Review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring Recent Developments to Improve Antioxidant, Anti-Inflammatory and Antimicrobial Efficacy of Curcumin: A Review of New Trends and Future Perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Lai, S.; Liu, C.; Huang, S.; Yang, H. Structure, Antioxidant and α-Amylase Inhibitory Activities of Longan Pericarp Proanthocyanidins. J. Funct. Foods 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Lu, M.C.; Yang, M.D.; Li, P.C.; Fang, H.Y.; Huang, H.Y.; Chan, Y.C.; Bau, D.T. Effect of Oligomeric Proanthocyanidin on the Antioxidant Status and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. In Vivo 2018, 32, 753–758. [Google Scholar] [CrossRef]
- Wong, C.P.; Bray, T.M.; Ho, E. Induction of Proinflammatory Response in Prostate Cancer Epithelial Cells by Activated Macrophages. Cancer. Lett. 2009, 276, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Assinder, S.J. Oxytocin Increases 5α-reductase Activity of Human Prostate Epithelial Cells, but Not Stromal Cells. Prostate 2008, 68, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Papakonstanti, E.A.; Hatzoglou, A.; Stathopoulos, E.N.; Stournaras, C.; Castanas, E. The Human Prostate Cancer Cell Line LNCaP Bears Functional Membrane Testosterone Receptors That Increase PSA Secretion and Modify Actin Cytoskeleton. FASEB J. 2002, 16, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Fokkink, R.; Peters, R.; Tromp, P.; Herrera Rivera, Z.E.; Rietjens, I.M.C.M.; Hendriksen, P.J.M.; Bouwmeester, H. Behaviour of Silver Nanoparticles and Silver Ions in an in Vitro Human Gastrointestinal Digestion Model. Nanotoxicology 2013, 7, 1198–1210. [Google Scholar] [CrossRef]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of Simulated Intestinal Fluid for Caco-2 Permeability Assay of Lipophilic Drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Cosentino, V.; Fratter, A.; Cosentino, M. Anti-Inflammatory Effects Exerted by Killox®, an Innovative Formulation of Food Supplement with Curcumin, in Urology. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1390–1398. [Google Scholar]
- Kumar, R.; Gupta, Y.K.; Singh, S. Anti-Inflammatory and Anti-Granuloma Activity of Berberis Aristata DC. in Experimental Models of Inflammation. Indian J. Pharmacol. 2016, 48, 155–161. [Google Scholar]
- Kim, E.H.; Brockman, J.A.; Andriole, G.L. The Use of 5-Alpha Reductase Inhibitors in the Treatment of Benign Prostatic Hyperplasia. Asian J. Urol. 2018, 5, 28–32. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of Permeability-Related Hurdles in Oral Delivery of Curcumin Using the Caco-2 Cell Model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]


| Product | Hydrophilic ORAC (µmol TE/g ± SD) | Lipophilic ORAC (µmol TE/g ± SD) | Total ORAC (µmol TE/g ± SD) |
|---|---|---|---|
| LENILUTS® | 32.5 ± 2.2 | 506.5 ± 61.2 | 539.0 ± 63.4 |
| Active Principle | Recovery (%) | Supernatant (%) |
|---|---|---|
| CURCUMIN | 67.0 ± 5.4 | 5.1 ± 0.1 |
| BETA-SITOSTEROL | 4.6 ± 0.1 | 17.1 ± 1.0 |
| OPCs | 3.5 ± 0.3 | 26.6 ± 7.6 |
| 1 h | 3 h | |||
|---|---|---|---|---|
| Curcumin | Absorption (%) | Concentration (ng/mL) | Absorption (%) | Concentration (ng/mL) |
| Serosal | n.d. | n.d. | 1.7 ± 0.1 | 2.8 ± 0.3 |
| Intracellular | 9.8 ± 3.8 | 1.9 ± 0.7 | 32.2 ± 4.0 | 35.0 ± 3.4 |
| Absorbed | 1.9 | 37.8 | ||
| 1 h | 3 h | |||
|---|---|---|---|---|
| Beta-Sitosterol | Absorption (%) | Concentration (ng/mL) | Absorption (%) | Concentration (ng/mL) |
| Serosal | n.d. | n.d. | 62.0 ± 3.1 | 440.8 ± 66.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, E.; Benetti, F.; Castelli, S.; Fratter, A. Prostatic Therapeutic Efficacy of LENILUTS®, a Novel Formulation with Multi-Active Principles. Pharmaceutics 2022, 14, 1866. https://doi.org/10.3390/pharmaceutics14091866
Tedesco E, Benetti F, Castelli S, Fratter A. Prostatic Therapeutic Efficacy of LENILUTS®, a Novel Formulation with Multi-Active Principles. Pharmaceutics. 2022; 14(9):1866. https://doi.org/10.3390/pharmaceutics14091866
Chicago/Turabian StyleTedesco, Erik, Federico Benetti, Simone Castelli, and Andrea Fratter. 2022. "Prostatic Therapeutic Efficacy of LENILUTS®, a Novel Formulation with Multi-Active Principles" Pharmaceutics 14, no. 9: 1866. https://doi.org/10.3390/pharmaceutics14091866
APA StyleTedesco, E., Benetti, F., Castelli, S., & Fratter, A. (2022). Prostatic Therapeutic Efficacy of LENILUTS®, a Novel Formulation with Multi-Active Principles. Pharmaceutics, 14(9), 1866. https://doi.org/10.3390/pharmaceutics14091866







