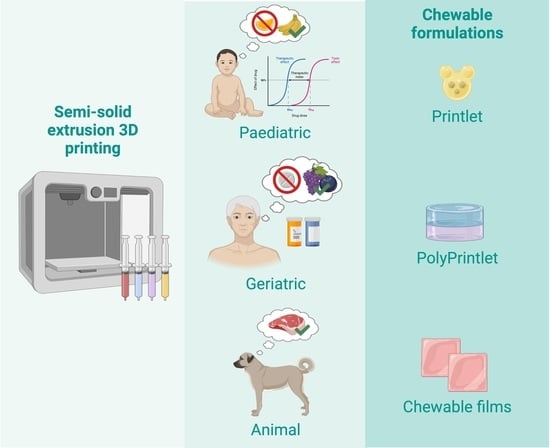
Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design
Abstract
:1. Introduction
2. Advantages and Disadvantages
3. Target Population for Chewable Tablets
3.1. Dysphagia
3.2. Geriatric Population
3.3. Paediatric Population
4. Types of Chewable Formulations and Conventional Manufacturing Methods
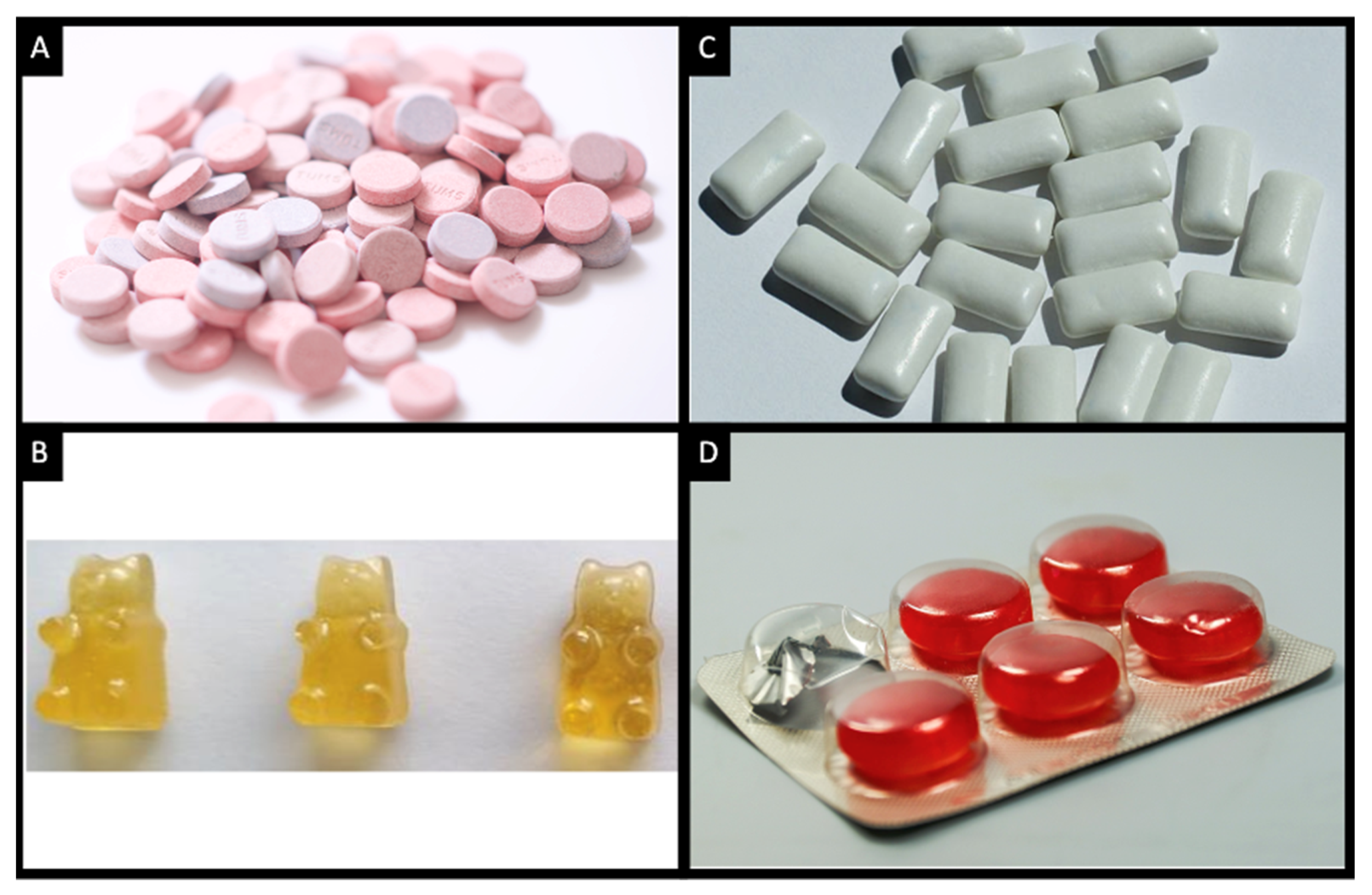
4.1. Chewable Tablets
4.2. Chewing Gums
4.2.1. Conventional or Fusion Method
4.2.2. Cooling, Grinding and Tableting
4.2.3. Direct Compression
4.3. Chewable Lozenges
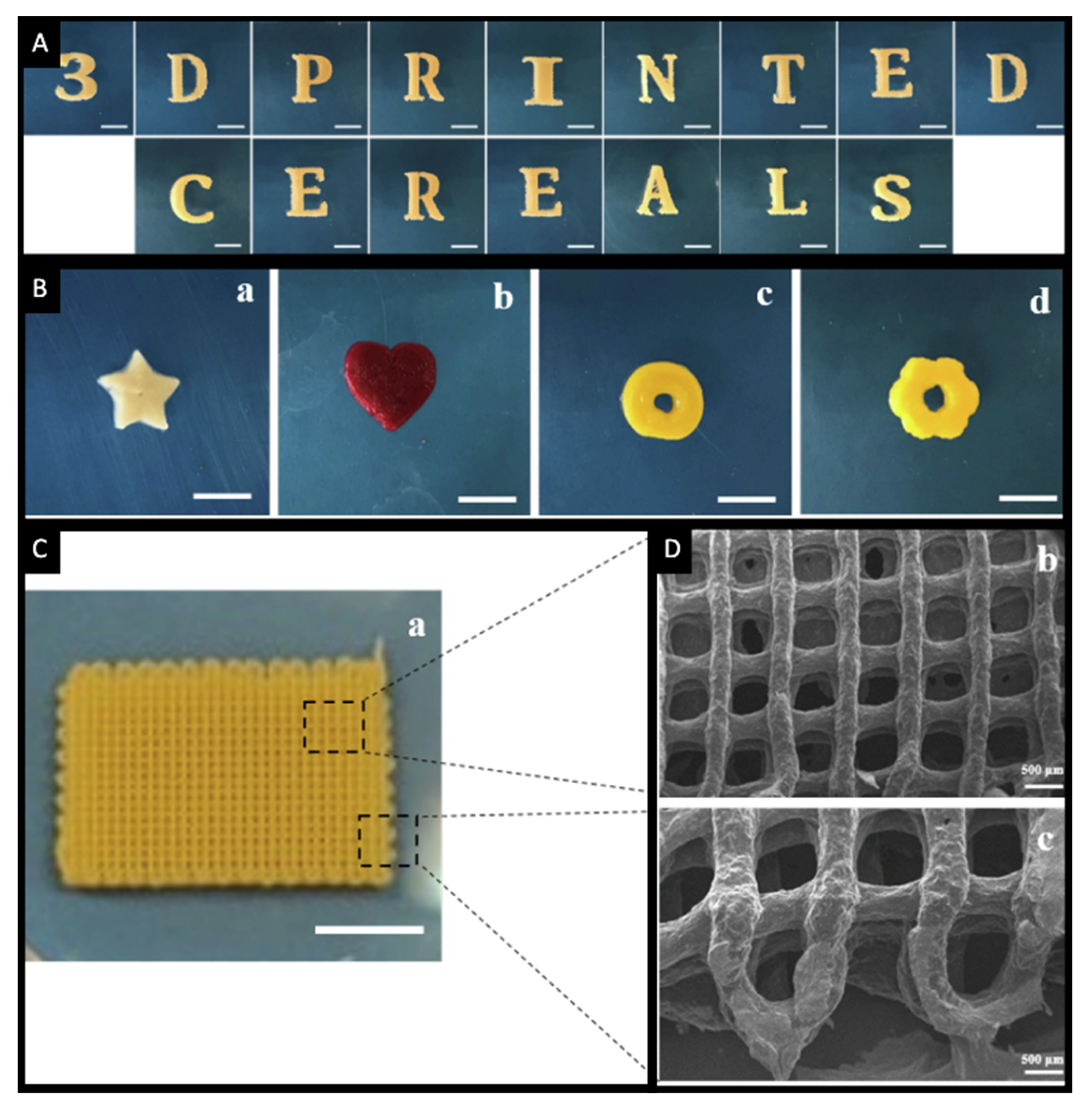
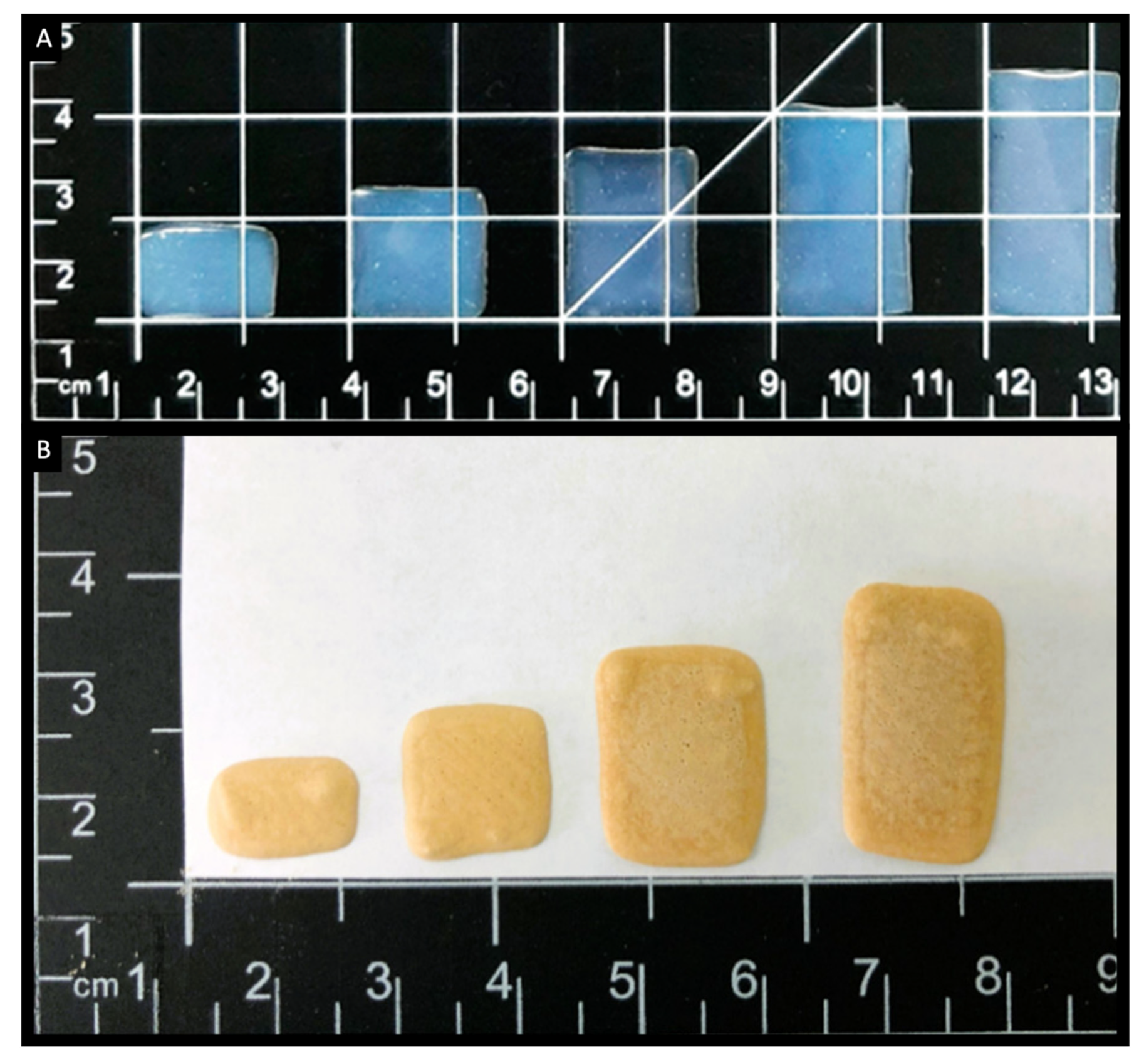
5. 3D Printing of Chewable Tablets: An Innovative Approach
6. Excipients for Chewable Medicines
7. Veterinary Applications
8. Considerations and Requirements of Chewable Tablets—A Regulatory Aspect
8.1. Mechanical Properties
8.2. Disintegration and Dissolution
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| API | Active pharmaceutical ingredient |
| ASTM | American Society for Testing and Materials |
| CAD | Computer-aided design |
| CDI | Chewing Difficulty Index |
| DLP | Digital light processing |
| EMA | European Medicines Agency |
| EP | European Pharmacopoeia |
| FDA | Food and Drug Administration |
| FDM | Fused deposition modelling |
| GI | Gastrointestinal |
| GMP | Good Manufacturing Practices |
| Kgf | kilogram-force |
| Kp | Kilopond |
| MSUD | Maple Syrup Urine Disease |
| N | Newton |
| PIP | Paediatric Investigation Plan |
| PolyPrintlets | Multi-drug dosage forms obtained by 3D printing |
| Printlets | 3D printed dosage forms |
| Scu | Strong–Cobb Units |
| SLS | Selective laser sintering |
| SSE | Semi-solid extrusion |
| USP | United States Pharmacopoeia |
References
- Awad, A.; Trenfield, S.J.; Basit, A.W. Chapter 19—Solid oral dosage forms. In Remington, 23rd ed.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 333–358. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Wening, K.; Breitkreutz, J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int. J. Pharm. 2011, 404, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sam, T.; Ernest, T.B.; Walsh, J.; Williams, J.L. A benefit/risk approach towards selecting appropriate pharmaceutical dosage forms—An application for paediatric dosage form selection. Int. J. Pharm. 2012, 435, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Madla, C.M.; Gavins, F.K.H.; Allahham, N.; Trenfield, S.J.; Basit, A.W. Chapter 20—Liquid dosage forms. In Remington, 23rd ed.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 359–379. [Google Scholar] [CrossRef]
- Schiele, J.T.; Quinzler, R.; Klimm, H.-D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalencse, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948. [Google Scholar] [CrossRef]
- Moore, K.T. Quite Simply a Pile of Tums; WikiMedia: St. Petersburg, FL, USA, 2016. [Google Scholar]
- Matulyte, I.; Mataraite, A.; Velziene, S.; Bernatoniene, J. The Effect of Myristica fragrans on Texture Properties and Shelf-Life of Innovative Chewable Gel Tablets. Pharmaceutics 2021, 13, 238. [Google Scholar] [CrossRef]
- Cancer Research UK. Can Nicotine Gum Cause Mouth Cancer? Available online: https://news.cancerresearchuk.org/2009/04/24/can-nicotine-gum-cause-mouth-cancer/ (accessed on 27 July 2022).
- Taken. Lozenge, Pixabay. 2014. Available online: https://pixabay.com/photos/lozenge-pill-medicine-drug-cure-462867/ (accessed on 27 July 2022).
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Xian Tan, H.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharm. 2020, 577, 119066. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Ong, J.J.; Basit, A.W.; Goyanes, A. To infinity and beyond: Strategies for fabricating medicines in outer space. Int. J. Pharm. X 2022, 4, 100121. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Isreb, M.; Forbes, R.T.; Oga, E.F.; Alhnan, M.A. Additive Manufacturing of a Point-of-Care “Polypill:” Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease. Adv. Healthc. Mater. 2020, 9, 2000236. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Yao, A.; Trenfield, J.S.; Goyanes, A.; Gaisford, S.; Basit, W.A. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Carnaby-Mann, G.; Crary, M. Pill Swallowing by Adults with Dysphagia. Arch. Otolaryngol.–Head Neck Surg. 2005, 131, 970–975. [Google Scholar] [CrossRef]
- Krekeler, B.N.; Vitale, K.; Yee, J.; Powell, R.; Rogus-Pulia, N. Adherence to Dysphagia Treatment Recommendations: A Conceptual Model. J. Speech Lang. Hear. Res. 2020, 63, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef]
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar] [CrossRef]
- Roden, D.; Altman, K. Causes of Dysphagia Among Different Age Groups A Systematic Review of the Literature. Otolaryngol. Clin. N. Am. 2013, 46, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Prasse, J.E.; Kikano, G.E. An Overview of Pediatric Dysphagia. Clin. Pediatr. 2008, 48, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; D’Cruz, G.; Wright, D. A Qualitative Study of the Problems Surrounding Medicine Administration to Patients with Dysphagia. Dysphagia 2008, 24, 49. [Google Scholar] [CrossRef]
- Wright, D. Medication administration in nursing homes. Nurs. Stand. (Through 2013) 2002, 16, 33–38. [Google Scholar] [CrossRef]
- Stubbs, J.; Haw, C.; Dickens, G. Dose form modification—A common but potentially hazardous practice. A literature review and study of medication administration to older psychiatric inpatients. Int. Psychogeriatr. 2008, 20, 616–627. [Google Scholar] [CrossRef]
- Thomson, F.C.; Naysmith, M.R.; Lindsay, A. Managing drug therapy in patients receiving enteral and parenteral nutrition. Hosp. Pharm. 2000, 7, 155–164. [Google Scholar]
- Watson, C.J.; Whitledge, J.D.; Siani, A.M.; Burns, M.M. Pharmaceutical Compounding: A History, Regulatory Overview, and Systematic Review of Compounding Errors. J. Med. Toxicol. 2021, 17, 197–217. [Google Scholar] [CrossRef]
- Shirkey, H. Editorial Comment: Therapeutic Orphans. Pediatrics 1999, 104, 583. [Google Scholar] [CrossRef]
- Van der Veken, M.; Brouwers, J.; Budts, V.; Lauwerys, L.; Pathak, S.M.; Batchelor, H.; Augustijns, P. Practical and operational considerations related to paediatric oral drug formulation: An industry survey. Int. J. Pharm. 2022, 618, 121670. [Google Scholar] [CrossRef]
- Ernest, T.B.; Craig, J.; Nunn, A.; Salunke, S.; Tuleu, C.; Breitkreutz, J.; Alex, R.; Hempenstall, J. Preparation of medicines for children—A hierarchy of classification. Int. J. Pharm. 2012, 435, 124–130. [Google Scholar] [CrossRef]
- Litalien, C.; Bérubé, S.; Tuleu, C.; Gilpin, A.; Landry, É.K.; Valentin, M.; Strickley, R.; Turner, M.A. From paediatric formulations development to access: Advances made and remaining challenges. Br. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Pirmohamed, M.; Nunn, T. Off-label and unlicensed medicine use and adverse drug reactions in children: A narrative review of the literature. Eur. J. Clin. Pharmacol. 2012, 68, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug Formulations: Standards and Novel Strategies for Drug Administration in Pediatrics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Kirby, D.; Bryson, S.; Shah, M.; Rahman Mohammed, A. Paediatric specific dosage forms: Patient and formulation considerations. Int. J. Pharm. 2022, 616, 121501. [Google Scholar] [CrossRef]
- EMA. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: Amsterdam, The Netherlands, 2012; Volume 2, pp. 1–24. [Google Scholar]
- FDA. Quality Attribute Considerations for Chewable Tablets Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; Volume 83, pp. 1–13. [Google Scholar]
- Nyamweya, N.; Kimani, S. Chewable Tablets: A Review of Formulation Considerations. Pharm. Technol. N. Am. 2020, 44, 38–44. [Google Scholar]
- Gawade, N.L.; Shendge, R.S. A review on chewable tablet. J. Emerg. Technol. Innov. Res. 2020, 7, 342–353. [Google Scholar]
- Strickley, R.G.; Iwata, Q.; Wu, S.; Dahl, T.C. Pediatric drugs—A review of commercially available oral formulations. J. Pharm. Sci. 2008, 97, 1731–1774. [Google Scholar] [CrossRef]
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef]
- Renu, J.D.; Jalwal, P.; Singh, B. Chewable Tablets: A Comprehensive Review. Pharma Innov. J. 2015, 4, 100–105. [Google Scholar]
- Prajapati, S.T.; Mehta, A.P.; Modhia, I.P.; Patel, C.N. Formulation and optimisation of raft-forming chewable tablets containing H2 antagonist. Int. J. Pharm. Investig. 2012, 2, 176–182. [Google Scholar] [CrossRef]
- Nour, S.A.; Abdelmalak, N.S.; Naguib, M.J. Novel chewable colon targeted tablets of bumadizone calcium for treatment of ulcerative colitis: Formulation and optimization. J. Drug Deliv. Sci. Technol. 2016, 35, 172–183. [Google Scholar] [CrossRef]
- Sharma, K.S.C.; Kumar, Y.K.; Reddy, K.R. Effect of drug release on albendazole chewable tablets by using different formulation techniques. Int. J. Pharm. Sci. Res. 2014, 5, 4543–4547. [Google Scholar]
- Iqubal, M.K.; Singh, P.K.; Shuaib, M.; Iqubal, A.; Singh, M. Recent advances in direct compression technique for pharmaceutical tablet formulation. Int. J. Pharm. Res. Dev. 2014, 6, 49–57. [Google Scholar]
- Gohel, M.; Jogani, P.D. A review of co-processed directly compressive excipents. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar] [PubMed]
- Ahjel, S.; Lupuliasa, D. Directly compressible adjuvants—A pharmaceutical approach. Farmacia 2008, 56, 591–599. [Google Scholar]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Wang, Y. Using Multivariate Analysis for Pharmaceutical Drug Product Development. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2016. [Google Scholar]
- US Pharmacopeia. ⟨1151⟩ Pharmaceutical dosage forms. In United States Pharmacopeia; USP-NF, Ed.; The United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Committee For Medicinal Products For Human Use. CPMP List of Allowed Terms for the Pharmaceutical Dosage Form, Route of Administration, Container, Closure and Administration Devices; European Medicines Agency: Brussels, Belgium, 1991; Volume III. [Google Scholar]
- Krämer, J.; Gajendran, J.; Guillot, A.; Barakat, A. Chewable Oral Drug Products. In In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 27–53. [Google Scholar] [CrossRef]
- Banakar, M.; Moayedi, S.; Shamsoddin, E.; Vahedi, Z.; Banakar, M.H.; Mousavi, S.M.; Rokaya, D.; Bagheri Lankarani, K. Chewing Gums as a Drug Delivery Approach for Oral Health. Int. J. Dent. 2022, 2022, 9430988. [Google Scholar] [CrossRef]
- Jacobsen, J.; Christrup, L.L.; Jensen, N.-H. Medicated chewing gum. Am. J. Drug Deliv. 2004, 2, 75–88. [Google Scholar] [CrossRef]
- Rømer Rassing, M. Chewing gum as a drug delivery system. Adv. Drug Deliv. Rev. 1994, 13, 89–121. [Google Scholar] [CrossRef]
- Drugs.com. Aspergum Gum. Available online: https://www.drugs.com/cdi/aspergum-gum.html (accessed on 20 July 2021).
- CIMA. Ficha Tecnica Nicotinell Cool Mint 2 mg Chicle Medicamentoso. Available online: https://cima.aemps.es/cima/dochtml/ft/65670/FT_65670.html (accessed on 20 July 2021).
- Jensen, E.J.; Schmidt, E.; Pedersen, B.; Dahl, R. Effect of nicotine, silver acetate, and ordinary chewing gum in combination with group counselling on smoking cessation. Thorax 1990, 45, 831–834. [Google Scholar] [CrossRef]
- Kralikova, E.; Kozak, J.T.; Rasmussen, T.; Gustavsson, G.; Le Houezec, J. Smoking cessation or reduction with nicotine replacement therapy: A placebo-controlled double blind trial with nicotine gum and inhaler. BMC Public Health 2009, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, T.; Stead, L.F. Silver acetate for smoking cessation. Cochrane Database Syst. Rev. 2012, 2012, CD000191. [Google Scholar] [CrossRef] [PubMed]
- Oliveby, A.; Ekstrand, J.; Lagerlöf, F. Effect of Salivary Flow Rate on Salivary Fluoride Clearance after Use of a Fluoride-Containing Chewing Gum. Caries Res. 1987, 21, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.A.; Milgrom, P.; Rothen, M. The Potential of Dental-Protective Chewing Gum in Oral Health Interventions. J. Am. Dent. Assoc. 2008, 139, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, J.A.N.; Birkhed, D.; Lindgren, L.-E.; Oliveby, A.; Edwardsson, S.; Frostell, G. Effect of repeated intake of a sugar free fluoride-containing chewing gum on acido genicity and microbial composition of dental plaque. Eur. J. Oral Sci. 1985, 93, 309–314. [Google Scholar] [CrossRef]
- Simons, D.; Brailsford, S.; Kidd, E.A.M.; Beighton, D. The effect of chlorhexidine acetate/xylitol chewing gum on the plaque and gingival indices of elderly occupants in residential homes. J. Clin. Periodontol. 2001, 28, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Moran, J.; Dangler, L.V.; Leight, R.S.; Addy, M. The efficacy of an anti-gingivitis chewing gum. J. Clin. Periodontol. 1996, 23, 19–21. [Google Scholar] [CrossRef]
- Burt, B.A. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J. Am. Dent. Assoc. 2006, 137, 190–196. [Google Scholar] [CrossRef]
- Söderling, E.; Mäkinen, K.K.; Chen, C.Y.; Pape, H.R., Jr.; Loesche, W.; Mäkinen, P.L. Effect of Sorbitol, Xylitol, and Xylitol/Sorbitol Chewing Gums on Dental Plaque. Caries Res. 1989, 23, 378–384. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Wiater, A.; Bachanek, T.; Szczodrak, J. Enzymes in therapy of biofilm-related oral diseases. Biotechnol. Appl. Biochem. 2017, 64, 337–346. [Google Scholar] [CrossRef]
- Anderson, G.B.; McLean, T.N.; Caffesse, R.G.; Smith, B.A. Effects of zirconium silicate chewing gum on plaque and gingivitis. Quintessence Int. 1990, 21, 479–489. [Google Scholar] [PubMed]
- Porciani, P.F.; Grandini, S. The effect of zinc acetate and magnolia bark extract added to chewing gum on volatile sulfur-containing compounds in the oral cavity. J. Clin. Dent. 2012, 23, 76–79. [Google Scholar] [PubMed]
- Sjögren, K.; Birkhed, D.; Persson, L.G.; Norén, J.G. Salivary fluoride clearance after a single intake of fluoride tablets and chewing gums in children, adults, and dry mouth patients. Eur. J. Oral Sci. 1993, 101, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, T. Chewing Gum—Facts and Fiction: A Review of Gum-Chewing and Oral Health. Crit. Rev. Oral Biol. Med. 1999, 10, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Söderling, E. Controversies around Xylitol. Eur. J. Dent. 2009, 3, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Rindum, J.L.; Holmstrup, P.; Pedersen, M.; Rassing, M.R.; Stoltze, K. Miconazole chewing gum for treatment of chronic oral candidosis. Eur. J. Oral Sci. 1993, 101, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Bastian, H.L.; Rindum, J.; Lindeberg, H. A double-dummy, double-blind, placebo-controlled phase III study comparing the efficacy and efficiency of miconazole chewing gum with a known drug (Brentan® gel) and a placebo in patients with oral candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 423–428. [Google Scholar] [CrossRef]
- Wertalik, F.; Bonorden, R. Salivary Levels of Antibiotics from Use of Neomycin-Gramicidin Chewing Troches. J. Pharm. Sci. 1968, 57, 530–531. [Google Scholar] [CrossRef]
- Samiei, N.; Olyaie, E.; Saberi, S.; Zolfaghari, M.E. Development of a gum base formulation for nystatin; a new drug delivery approach for treatment of oral candidiasis. J. Drug Deliv. Sci. Technol. 2018, 48, 59–65. [Google Scholar] [CrossRef]
- Kamimori, G.H.; Karyekar, C.S.; Otterstetter, R.; Cox, D.S.; Balkin, T.J.; Belenky, G.L.; Eddington, N.D. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int. J. Pharm. 2002, 234, 159–167. [Google Scholar] [CrossRef]
- Newman, R.A.; Kamimori, G.H.; Wesensten, N.J.; Picchioni, D.; Balkin, T.J. Caffeine Gum Minimizes Sleep Inertia. Percept. Mot. Ski. 2013, 116, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Effects of caffeine in chewing gum on mood and attention. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Seibel, K.; Schaffler, K.; Reitmeir, P.; Golly, I. A Randomised, Placebo-controlled Study Comparing Two Formulations of Dimenhydrinate with Respect to Efficacy in Motion Sickness and Sedation. Arzneimittelforschung 2002, 52, 529–536. [Google Scholar] [CrossRef] [PubMed]
- CIMA. Ficha Tecnica Biodramina 20 mg. Available online: https://cima.aemps.es/cima/dochtml/ft/57681/FT_57681.html (accessed on 20 January 2022).
- American Association of Pharmaceutical Scientists. Novel Chewing Gum Formulation Helps Prevent Motion Sickness. Available online: https://www.sciencedaily.com/releases/2012/10/121017123908.htm (accessed on 15 February 2022).
- Kumar, K.; Sharma, A.; Teotia, D.; Kaur, G. A Comprehensive Review On Medicated Chewing Gums—A Novel Drug Delivery System. J. Pharma Res. 2020, 9, 117–120. [Google Scholar] [CrossRef]
- Gadhavi, A.G.; Patel, B.N.; Patel, D.M.; Patel, C.N. Medicated chewing gum-A 21st century drug delivery system. Int. J. Pharm. Sci. Res. 2011, 2, 1961–1974. [Google Scholar]
- International Chewing Gum Association. Manufacturing Process. Available online: http://www.gumassociation.org/index.cfm/science-technology/manufacturing-process/ (accessed on 26 July 2022).
- Kaushik, P.; Kaushik, D. Medicated Chewing Gums: Recent Patents and Patented Technology Platforms. Recent Pat. Drug Deliv. Formul. 2019, 13, 184–191. [Google Scholar] [CrossRef]
- Choursiya, S.; Andheriya, D. Review on Lozenges. J. Drug Deliv. Ther. 2018, 8, 124–128. [Google Scholar]
- Renuka Pothu, M.R.Y. Lozenges formulation and evaluation: A review. Int. J. Adv. Pharm. Res. 2014, 5, 290–298. [Google Scholar]
- Chandrawanshi, M.J.; Sakhare, R.S.; Nagoba, S.N.; Bhalekar, R.V. A review on medicated lozenges. World J. Pharm. Res. 2018, 8, 396–412. [Google Scholar]
- Eleftheriadis, G.K.; Kantarelis, E.; Monou, P.K.; Andriotis, E.G.; Bouropoulos, N.; Tzimtzimis, E.K.; Tzetzis, D.; Rantanen, J.; Fatouros, D.G. Automated digital design for 3D-printed individualized therapies. Int. J. Pharm. 2021, 599, 120437. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Lee, V.H. Personalised medicines: More tailored drugs, more tailored delivery. Int. J. Pharm. 2011, 415, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Rastogi, V.; Ginn, M. A review on 3D printing techniques for medical applications. Curr. Opin. Chem. Eng. 2020, 28, 152–157. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Poojary, R. A review on 3D printing: Advancement in healthcare technology. In Proceedings of the 2018 Advances in Science and Engineering Technology International Conferences (ASET), Sharjah, Dubai, 6 February–5 April 2018; pp. 1–5. [Google Scholar]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Aprecia. FDA Approves the First 3D Printed Drug Product. Available online: https://www.aprecia.com/news/fda-approves-the-first-3d-printed-drug-product (accessed on 30 July 2022).
- Rodríguez-Pombo, L.; Xu, X.; Seijo-Rabina, A.; Ong, J.J.; Alvarez-Lorenzo, C.; Rial, C.; García, D.N.; Gaisford, S.; Basit, A.W.; Goyanes, A. Volumetric 3D printing for rapid production of medicines. Addit. Manuf. 2022, 52, 102673. [Google Scholar] [CrossRef]
- Chen, G.; Yihua, X.; Kwok, P.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef]
- Ong, J.J.; Awad, A.; Martorana, A.; Gaisford, S.; Stoyanov, E.; Basit, A.W.; Goyanes, A. 3D printed opioid medicines with alcohol-resistant and abuse-deterrent properties. Int. J. Pharm. 2020, 579, 119169. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.-J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?—A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Mostafavi, A.; Quint, J.; Panayi, A.; Baldino, K.; Williams, T.; Daubendiek, J.; Sanchez, V.; Bonick, Z.; Trujillo-Miranda, M.; et al. In Situ Printing of Adhesive Hydrogel Scaffolds for the Treatment of Skeletal Muscle Injuries. ACS Appl. Bio Mater. 2020, 3, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Derossi, A.; Caporizzi, R.; Azzollini, D.; Severini, C. Application of 3D printing for customized food. A case on the development of a fruit-based snack for children. J. Food Eng. 2018, 220, 65–75. [Google Scholar] [CrossRef]
- Hao, L.; Mellor, S.; Seaman, O.; Henderson, J.; Sewell, N.; Sloan, M. Material characterisation and process development for chocolate additive layer manufacturing. Virtual Phys. Prototyp. 2010, 5, 57–64. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Dong, X.; Prakash, S. Feasibility study of hydrocolloid incorporated 3D printed pork as dysphagia food. Food Hydrocoll. 2020, 107, 105940. [Google Scholar] [CrossRef]
- International, A. Additive Manufacturing, Design, Requirements, Guidelines and Recommendations. Available online: https://www.iso.org/obp/ui#iso:std:iso-astm:52910:ed-1:v1:en (accessed on 27 October 2021).
- Awad, A.; Gaisford, S.; Basit, A.W. Fused Deposition Modelling: Advances in Engineering and Medicine. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–132. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T. Basics of Fused Deposition Modelling (FDM). In Fused Deposition Modeling; Springer: Cham, Germany, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Ong, J.J.; Luzardo-Álvarez, A.; González-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2020, 16, 110–119. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Gómez-Lado, N.; Lázare-Iglesias, H.; García-Otero, X.; Antúnez-López, J.R.; Ruibal, Á.; Varela-Correa, J.J.; Aguiar, P.; Basit, A.W.; Otero-Espinar, F.J.; et al. 3D Printed Tacrolimus Rectal Formulations Ameliorate Colitis in an Experimental Animal Model of Inflammatory Bowel Disease. Biomedicines 2020, 8, 563. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2018, 36, 4. [Google Scholar] [CrossRef]
- Firth, J.; Basit, A.W.; Gaisford, S. The Role of Semi-Solid Extrusion Printing in Clinical Practice. In 3D Printing of Pharmaceuticals; Springer: Cham, Germany, 2018; Volume 31, pp. 133–151. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ando, M.; Nagata, N.; Goto, E.; Yoshimura, N.; Takeuchi, T.; Noda, T.; Ozeki, T. Fabrication of Naftopidil-Loaded Tablets Using a Semisolid Extrusion-Type 3D Printer and the Characteristics of the Printed Hydrogel and Resulting Tablets. J. Pharm. Sci. 2019, 108, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchon, H.; Rodriguez-Gonzalez, D.; Alejandro Fernandez, M.; Sune-Pou, M.; Perez-Lozano, P.; Garcia-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Kimaro, E.; Tibalinda, P.; Shedafa, R.; Temu, M.; Kaale, E. Formulation development of chewable albendazole tablets with improved dissolution rate. Heliyon 2019, 5, e02911. [Google Scholar] [CrossRef]
- El-Gazayerly, O.N.; Rakkanka, V.; Ayres, J.W. Novel Chewable Sustained-Release Tablet Containing Verapamil Hydrochloride. Pharm. Dev. Technol. 2004, 9, 181–188. [Google Scholar] [CrossRef]
- Jagdale, S.; Gattani, M.; Bhavsar, D.; Kuchekar, B.; Chabukswar, A. Formulation and evaluation of chewable tablet of levamisole. Int. J. Res. Pharm. Sci. 2010, 1, 282–289. [Google Scholar]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Pineiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sanchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of developing hospital preparation by semisolid extrusion 3D printing: Personalized amlodipine besylate chewable tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Castro, B.M.; Gaisford, S.; Cabalar, P.; Basit, A.W.; Pérez, G.; Goyanes, A. Accelerating 3D printing of pharmaceutical products using machine learning. Int. J. Pharm. X 2022, 4, 100120. [Google Scholar] [CrossRef]
- Karavasili, C.; Zgouro, P.; Manousi, N.; Lazaridou, A.; Zacharis, C.K.; Bouropoulos, N.; Moschakis, T.; Fatouros, D.G. Cereal-Based 3D Printed Dosage Forms for Drug Administration During Breakfast in Pediatric Patients within a Hospital Setting. J. Pharm. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Muñiz Castro, B.; Gavins, F.K.H.; Jie Ong, J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, Á. M3DISEEN: A Novel Machine Learning Approach for Predicting the 3D Printability of Medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef]
- Muñiz Castro, B.; Elbadawi, M.; Ong, J.J.; Pollard, T.D.; Song, Z.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J. Control. Release 2021, 337, 530–545. [Google Scholar] [CrossRef]
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine learning directed drug formulation development. Adv. Drug Deliv. Rev. 2021, 175, 113806. [Google Scholar] [CrossRef]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical biosensors—Illuminating the path to personalized drug dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical biosensors: A nexus for precision medicine. Drug Discov. Today 2020, 26, 69–79. [Google Scholar] [CrossRef]
- Hann, S.Y.; Cui, H.; Nowicki, M.; Zhang, L.G. 4D printing soft robotics for biomedical applications. Addit. Manuf. 2020, 36, 101567. [Google Scholar] [CrossRef]
- Miyashita, S.; Guitron, S.; Yoshida, K.; Li, S.; Damian, D.D.; Rus, D. Ingestible, controllable, and degradable origami robot for patching stomach wounds. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 909–916. [Google Scholar]
- Trenfield, S.J.; Awad, A.; McCoubrey, L.E.; Elbadawi, M.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advancing pharmacy and healthcare with virtual digital technologies. Adv. Drug Deliv. Rev. 2022, 182, 114098. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Jie Ong, J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected Healthcare: Improving Patient Care using Digital Health Technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Seijo-Rabina, A.; Awad, A.; Rial, C.; Gaisford, S.; Basit, A.W.; Goyanes, A. Smartphone-enabled 3D printing of medicines. Int. J. Pharm. 2021, 609, 121199. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio Mater. 2020, 4, 325–369. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid Gel Particles: Formation, Characterization, and Application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery Gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef]
- EMC. Singulair Paediatric 4 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/6500/smpc (accessed on 15 November 2021).
- EMC. Lamictal Chewable/Dispersible Tablets. Available online: https://www.medicines.org.uk/emc/product/1286/smpc (accessed on 15 November 2021).
- EMC. Calcichew-D3 1000 mg/800 IU Once Daily Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/12843/smpc (accessed on 15 November 2021).
- EMC. Remegel. Available online: https://www.medicines.org.uk/emc/product/1308/smpc (accessed on 15 November 2021).
- Drugs.com. Childrens Chewable Antacid. Available online: https://www.drugs.com/otc/126203/childrens-chewable-antacid.html (accessed on 9 April 2021).
- EMC. Gaviscon Advance Mint Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/73/smpc (accessed on 17 November 2021).
- EMC. Bisodol Original Indigestion Relief Tablets. Available online: https://www.medicines.org.uk/emc/product/9014/smpc (accessed on 15 November 2021).
- EMC. Fosrenol 1000mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/7494/smpc (accessed on 15 November 2021).
- EMC. Neomag. Available online: https://www.medicines.org.uk/emc/product/2678/smpc (accessed on 17 November 2021).
- EMC. Epanutin Infatabs 50 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/2259/smpc (accessed on 17 November 2021).
- Drugs.com. Childrens Chewable Acetaminophen. Available online: https://www.drugs.com/otc/1438384/childrens-chewable-acetaminophen.html# (accessed on 9 April 2021).
- Drugs.com. Chewable Low Dose Aspirin. Available online: https://www.drugs.com/otc/109731/chewable-low-dose-aspirin.html (accessed on 9 April 2021).
- Bayer. Bayer® Chewable Aspirin. Available online: https://www.bayeraspirin.com/products/bayer-chewable-aspirin (accessed on 9 April 2021).
- FDA. Isentress® (Raltegravir) Label; FDA: Silver Spring, MD, USA, 2013; pp. 1–37. [Google Scholar]
- EMC. Isentress® 25 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/3026/smpc (accessed on 17 November 2021).
- DailyMed. Children’s Loratadine Chewable Tablets USP, 5 mg. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=8ec562d3-9d4c-4e28-bc8e-f725079f603a&type=display (accessed on 27 April 2021).
- EMC. Lipitor 20 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/5241/smpc#gref (accessed on 26 May 2021).
- CIMA. Ficha Tecnica Aeroflat 5 mg/77.5 mg Comprimidos Masticables. Available online: https://cima.aemps.es/cima/dochtml/ft/53610/FT_53610.html#6-datos-farmac-uticos (accessed on 27 October 2021).
- EMC. Nipatra 25 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/11299/smpc (accessed on 17 November 2021).
- EMC. Velphoro 500 mg Chewable Tablets. Available online: https://www.medicines.org.uk/emc/product/3532/smpc (accessed on 17 November 2021).
- Fahmy, R.; Danielson, D.; Martinez, M. Formulation and Design of Veterinary Tablets. In Pharmaceutical Dosage Forms-Tablets; CRC Press: Boca Raton, FL, USA, 2008; pp. 399–448. [Google Scholar] [CrossRef]
- Ahmed, I.; Kasraian, K. Pharmaceutical challenges in veterinary product development. Adv. Drug Deliv. Rev. 2002, 54, 871–882. [Google Scholar] [CrossRef]
- Chappell, K.; Paarlberg, T.; Seewald, W.; Karadzovska, D.; Nanchen, S. A randomized, controlled field study to assess the efficacy and safety of lotilaner flavored chewable tablets (CredelioTM CAT) in eliminating fleas in client-owned cats in the USA. Parasites Vectors 2021, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Walsh, K.; King, V.; Sture, G.; Caneva, L. Acceptance of oclacitinib maleate (Apoquel®) chewable tablets in client-owned dogs with allergic and atopic dermatitis. BMC Vet. Res. 2022, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Directorate, V.M. Aderexa Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1575787.PDF (accessed on 13 January 2022).
- FDA. InterceptorTM Plus Milbemycin Oxime/Praziquantel Chewable Tablets. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/1504 (accessed on 21 January 2022).
- Directorate, V.M. Amodip 1.25 mg Chewable Tablets for Cats. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_753508.PDF (accessed on 13 January 2022).
- CIMA. Amodip 1.25 mg Comprimidos Masticables Para Gatos. Available online: https://cimavet.aemps.es/cimavet/pdfs/es/ft/3183+ESP/FT_3183+ESP.pdf (accessed on 21 January 2022).
- Directorate, V.M. Apoquel 16 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_2222828.PDF (accessed on 13 January 2022).
- EMA. Bravecto Chewable Tablets for Dogs. Available online: https://www.ema.europa.eu/en/documents/product-information/bravecto-epar-product-information_en.pdf (accessed on 13 January 2022).
- FDA. Bravecto Chewable Tablets. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/1502 (accessed on 21 January 2022).
- EMA. Cardalis 2.5 mg/20 mg Chewable Tablets for Dogs. Available online: https://www.ema.europa.eu/en/documents/product-information/cardalis-epar-product-information_en.pdf (accessed on 14 January 2022).
- Directorate, V.M. Carprodyl Quadri 120 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_262886.PDF (accessed on 14 January 2022).
- FDA. Carprofen Chewable Tablets. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/1251 (accessed on 21 January 2022).
- EMA. Cimalgex 80 mg Chewable Tablets for Dogs. Available online: https://www.ema.europa.eu/en/documents/product-information/cimalgex-epar-product-information_en.pdf (accessed on 14 January 2022).
- Directorate, V.M. Cladaxxa 200 mg/50 mg Chewable Tablets for Cats and Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_2124104.PDF (accessed on 14 January 2022).
- Directorate, V.M. Clindabactin 55 mg Chewable Tablets for Dogs and Cats. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1588067.PDF (accessed on 14 January 2022).
- Directorate, V.M. Zodon 150 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_618381.PDF (accessed on 15 January 2022).
- EMA. Comfortis 140 mg Chewable Tablets for Dogs and Cats. Available online: https://www.ema.europa.eu/en/documents/product-information/comfortis-epar-product-information_en.pdf (accessed on 14 January 2022).
- EMA. Credelio 56 mg Chewable Tablets for Dogs (1.3–2.5 kg). Available online: https://www.ema.europa.eu/en/documents/product-information/credelio-epar-product-information_en.pdf (accessed on 14 January 2022).
- EMA. Credelio Plus 56.25 mg/2.11 mg Chewable Tablets for Dogs (1.4–2.8 kg). Available online: https://www.ema.europa.eu/en/documents/product-information/credelio-plus-epar-product-information_en.pdf (accessed on 14 January 2022).
- Directorate, V.M. Dexacortone 0.5 mg Chewable Tablets for Dogs and Cats. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1316256.PDF (accessed on 14 January 2022).
- Directorate, V.M. Efex 10 mg Chewable Tablets for Cats and Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_492508.PDF (accessed on 14 January 2022).
- Directorate, V.M. Equimax Tabs 150 mg/20 mg Chewable Tablet for Horses. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_225500.PDF (accessed on 14 January 2022).
- CIMA. Cardotek 30 plus (136 mcg Ivermectina/326 mg Pamoato de Pirantel). Available online: https://cimavet.aemps.es/cimavet/pdfs/es/ft/1082+ESP/FT_1082+ESP.pdf (accessed on 21 January 2022).
- Directorate, V.M. Eraquell Tabs, 20 mg Chewable Tablets for Horses. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_248256.PDF (accessed on 14 January 2022).
- EMA. Equioxx 57 mg Chewable Tablets for Horses. Available online: https://www.ema.europa.eu/en/documents/product-information/equioxx-epar-product-information_en.pdf (accessed on 14 January 2022).
- Directorate, V.M. Firodyl 250 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1741614.PDF (accessed on 14 January 2022).
- EMA. Frontpro 11 mg Chewable Tablets for Dogs 2–4 kg. Available online: https://www.ema.europa.eu/en/documents/product-information/frontpro-epar-product-information_en.pdf (accessed on 14 January 2022).
- FDA. NexGard® Chewable Tablet. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/3811 (accessed on 21 January 2022).
- EMA. Inflacam 1 mg Chewable Tablets for Dogs. Available online: https://www.ema.europa.eu/en/documents/product-information/inflacam-epar-product-information_en.pdf (accessed on 14 January 2022).
- EMA. Isemid 1 mg Chewable Tablets for Dogs (2.5–11.5 kg). Available online: https://www.ema.europa.eu/en/documents/product-information/isemid-epar-product-information_en.pdf (accessed on 14 January 2022).
- Directorate, V.M. Libeo 40 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_538426.PDF (accessed on 14 January 2022).
- EMA. MiPet Easecto 5 mg Chewable Tablets for Dogs 1.3–2.5 kg. Available online: https://www.ema.europa.eu/en/documents/product-information/mipet-easecto-epar-product-information_en.pdf (accessed on 15 January 2022).
- EMA. Nexgard Spectra 9 mg/2 mg Chewable Tablets for Dogs 2–3.5 kg. Available online: https://www.ema.europa.eu/en/documents/product-information/nexgard-spectra-epar-product-information_en.pdf (accessed on 15 January 2022).
- Directorate, V.M. Pimotab 1.25 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1787062.PDF (accessed on 15 January 2022).
- Directorate, V.M. Proin 15 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1350857.PDF (accessed on 15 January 2022).
- EMA. Simparica Trio Chewable Tablets for Dogs 1.25–2.5 kg. Available online: https://www.ema.europa.eu/en/documents/product-information/simparica-trio-epar-product-information_en.pdf (accessed on 15 January 2022).
- CIMA. Ficha técnica Simparica Trio Comprimidos Masticables Para Perros. Available online: https://cimavet.aemps.es/cimavet/pdfs/es/ft/EU%402%4019%40243%40010/FT_EU-2-19-243-010.pdf (accessed on 23 September 2021).
- FDA. Simparica Trio® (Sarolaner, Moxidectin, and Pyrantel Chewable Tablets). Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/11809 (accessed on 21 January 2022).
- Directorate, V.M. Spizobactin 1,500,000 IU/250 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1247508.PDF (accessed on 15 January 2022).
- Directorate, V.M. Tralieve 20 mg Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_1411433.PDF (accessed on 15 January 2022).
- EMA. Trocoxil 6 mg Chewable Tablets for Dogs. Available online: https://www.ema.europa.eu/en/documents/product-information/trocoxil-epar-product-information_en.pdf (accessed on 15 January 2022).
- Directorate, V.M. Veloxa Chewable Tablets for Dogs. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase/files/SPC_Documents/SPC_404527.PDF (accessed on 15 January 2022).
- Bray, J.; Kersley, A.; Downing, W.; Crosse, K.; Worth, A.; House, A.; Yates, G.; Coomer, A.; Brown, I. Clinical outcomes of patient-specific porous titanium endoprostheses in dogs with tumors of the mandible, radius, or tibia: 12 cases (2013–2016). J. Am. Vet. Med. Assoc. 2017, 251, 566–579. [Google Scholar] [CrossRef]
- Oxley, B.; Behr, S. Stabilisation of a cranial cervical vertebral fracture using a 3D-printed patient-specific drill guide. J. Small Anim. Pract. 2016, 57, 277. [Google Scholar] [CrossRef] [PubMed]
- Galicia, C.; Hernandez Urraca, V.; del Castillo, L.; Samour, J. Design and Use of a 3D Prosthetic Leg in a Red-lored Amazon Parrot (Amazona autumnalis). J. Avian Med. Surg. 2018, 32, 133–137. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Rajan Prakash, D.; Lindfors, L.; Wang, Q.; Wang, X.; Ojala, S.; Sandler, N. 3D-Printed Veterinary Dosage Forms—A Comparative Study of Three Semi-Solid Extrusion 3D Printers. Pharmaceutics 2020, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Mathiyalagan, R.; Wang, X.; Sandler, N. Compounding Tailored Veterinary Chewable Tablets Close to the Point-of-Care by Means of 3D Printing. Pharmaceutics 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Mathiyalagan, R.; Lindfors, L.; Wang, X.; Ojala, S.; Sandler, N. Semi-solid extrusion 3D printing of tailored ChewTs for veterinary use—A focus on spectrophotometric quantification of gabapentin. Eur. J. Pharm. Sci. 2022, 174, 106190. [Google Scholar] [CrossRef]
- Blake, C.; Birch, S.; Brandão, J. Medical Three-Dimensional Printing in Zoological Medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 331–348. [Google Scholar] [CrossRef]
- Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. [Google Scholar] [CrossRef] [PubMed]
- US Pharmacopeia. ⟨1217⟩ Tablet Breaking Force. In United States Pharmacopeia; USP-NF, Ed.; The United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- Jarosz, P.J.; Parrott, E.L. Tensile Strengths and Hardness of Tablets. J. Pharm. Sci. 1982, 71, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F. Methods for the practical determination of the mechanical strength of tablets—From empiricism to science. Int. J. Pharm. 2012, 436, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Sinka, I.C.; Jayaraman, B.; Pan, J. Break force and tensile strength relationships for curved faced tablets subject to diametrical compression. Int. J. Pharm. 2013, 442, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ambros, M.C.; Podczeck, F.; Podczeck, H.; Newton, J.M. The Characterization of the Mechanical Strength of Chewable Tablets. Pharm. Dev. Technol. 1998, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- David, S.T.; Augsburger, L.L. Flexure Test for Determination of Tablet Tensile Strength. J. Pharm. Sci. 1974, 63, 933–936. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Quality Attribute Considerations for Chewable Tablets Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Quality-Attribute-Considerations-for-Chewable-Tablets-Guidance-for-Industry.pdf (accessed on 26 July 2022).
- Gupta, A.; Chidambaram, N.; Khan, M.A. An index for evaluating difficulty of Chewing Index for chewable tablets. Drug Dev. Ind. Pharm. 2015, 41, 239–243. [Google Scholar] [CrossRef]
- Nyamweya, N.N.; Kimani, S.N.; Abuga, K.O. Chewable Antacid Tablets: Are Disintegration Tests Relevant? AAPS PharmSciTech 2020, 21, 139. [Google Scholar] [CrossRef]
- US Pharmacopeia. ⟨701⟩ Disintegration. In United States Pharmacopeia; USP-NF, Ed.; The United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- US Pharmacopeia. ⟨711⟩ Dissolution. In United States Pharmacopeia; USP-NF, Ed.; The United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]






| Excipient | Function(s) | Example(s) |
|---|---|---|
| Sweeteners | Mask unpleasant taste, microbial stability | Polyols (mannitol, sorbitol, xylitol), sugars (dextrose, lactose, sucrose, saccharine, sucralose), aspartame |
| Flavouring agents | Mask unpleasant taste | Fruit-based agents (mint, strawberry) |
| Colourants | Enhance aesthetic appeal, identification of the product, mask non-uniform colour, match the flavour | Powder-based colourants |
| Diluents | Increase the bulk volume | Polyols |
| pH regulators | Acidity modifiers | Citric acid, malic acid |
| Gelling agents | Initiate gelation | Gelatine, cellulose derivatives, starch, pectin, carrageenan, alginate, chitosan, hyaluronic acid, collagen and gellan gum |
| Active Pharmaceutical Ingredient(s) | Commercial Name | Dose(s) | Excipients | Indication(s) | Target Population(s) | Ref. |
|---|---|---|---|---|---|---|
| Montelukast sodium | Singulair paediatric chewable tablets | 4 and 5 mg | Mannitol, microcrystalline cellulose, sodium croscarmellose, aspartame, magnesium stearate, hyprolose, cherry flavour | Chronic asthma, prevention of exercise-induced bronchoconstriction | Children (2–5 years old) | [153] |
| Lamotrigine | Lamictal | 2, 5, 25 and 100 mg | Calcium carbonate, hydroxypropyl cellulose, sodium starch glycolate (Type A), povidone K30, saccharin sodium, magnesium stearate | Epilepsy, bipolar disorder | Adults, adolescents and children | [154] |
| Calcium carbonate/cholecalciferol | Calcichew-D3 chewable tablets | 1 g/800 IU, 500 mg/200 IU, and 500 mg/400 IU | Hydrated lactose, aspartame, sodium croscarmellose, maltodextrin | Vitamin D and calcium deficiency | Adults | [155] |
| Calcium carbonate | Remegel | 800 mg | Glucose syrup, sucrose, glycerol, hydrolysed milk protein, gelatine, sorbitol | Relief of acid indigestion and heartburn and associated stomach upsets (dyspepsia) | Adults and over 12 years old | [156] |
| Calcium carbonate | Children chewable antacid | 400 mg | Sucrose, mannitol | Antacid | Children | [157] |
| Sodium alginate/potassium bicarbonate | Gaviscon advance mint chewable tablets | 500/100 mg | Sodium, potassium, mannitol, macrogol 20,000, magnesium stearate, aspartame, acesulfame potassium, copovidone | Treatment of symptoms resulting from acid, bile and pepsin reflux into the oesophagus | Adults and children | [158] |
| Calcium carbonate/sodium bicarbonate/light magnesium carbonate | Bisodol original indigestion relief tablets | 522/64/68 mg | Saccharin soluble, maize, starch, sugar, calcium stearate | Relief of the symptoms of gastric hyperacidity, including indigestion, heartburn, dyspepsia and flatulence | Adults and children over 12 years old | [159] |
| Lanthanum carbonate hydrate | Fosrenol chewable tablets | 1000, 500 and 750 mg | Dextrates, anhydrous colloidal silica, magnesium stearate | Hyperphosphatemia in chronic renal failure | Adults | [160] |
| Magnesium glycerophosphate | Neomag | 97 mg | Maize starch, microcrystalline cellulose, talc, aspartame, magnesium stearate, anhydrous colloidal silica, povidone | Magnesium supplements for the treatment of patients with chronic magnesium loss or hypomagnesaemia and hypomagnesaemia due to the concomitant administration of loop and thiazide diuretics or other drugs | Adults and children over 4 years old | [161] |
| Phenytoin | Epanutin Infatabs | 50 mg | Confectioner′s sugar, saccharin sodium, spearmint flavour, magnesium stearate, talc, quinoline yellow (E104), sunset yellow FCF (E110) | Control of seizures and prevention and treatment of seizures occurring during or following neurosurgery and/or severe head injury | Adults and children | [162] |
| Acetaminophen | Children chewable acetaminophen | 160 mg | Mannitol | Analgesic, antipyretic | Children | [163] |
| Aspirin | Bayer chewable aspirin | 81 mg | Sucralose, maltodextrin, dextrose monohydrate, starch | Analgesic | Children | [164,165] |
| Raltegravir | Isentress | 25 and 100 mg | Hydroxypropyl cellulose, sucralose, fructose, aspartame, sucrose, sorbitol, magnesium stearate | Treatment of HIV-1 infection | Adults and children | [166,167] |
| Loratadine | Children′s loratadine chewable tablets USP | 5 mg | Aspartame, colloidal silicon dioxide, magnesium stearate, mannitol, microcrystalline cellulose, sodium starch glycolate | Relieve symptoms related to hay fever or other upper respiratory allergies | Children | [168] |
| Atorvastatin calcium trihydrate | Lipitor chewable tablets | 10 and 20 mg | Aspartame | Hypercholesterolemia and prevention of cardiovascular disease | Adults, adolescents, children aged 10 years or older | [169] |
| Metoclopramide/dimethicone | Aeroflat chewable tablet | 5/77.5 mg | Silicated microcrystalline cellulose, acesulfame K | Prevention and treatment of nausea and vomiting Symptomatic relief of aerophagia and meteorism | Adults | [170] |
| Sildenafil citrate | Nipatra chewable tablets | 25, 50 and 100 mg | Polacrilin potassium, anhydrous colloidal silica, lactose monohydrate, povidone K-30, aspartame, sodium croscarmellose, magnesium stearate, sodium hydroxide or hydrochloric acid | Erectile dysfunction | Adults | [171] |
| Sucroferric oxyhydroxide | Velphoro chewable tablets | 500 mg | Neohesperidin-dihydrochalcone, magnesium stearate, anhydrous colloidal silica | Control of serum phosphorus levels in chronic kidney disease | Adults and children | [172] |
| Active Pharmaceutical Ingredient(s) | Commercial Name(s) | Dose(s) | Excipients | Indication(s) | Animal(s) | Ref |
|---|---|---|---|---|---|---|
| Milbemycin oxime/Praziquantel | Aderexa and Interceptor plus | 12.5/125 and 2.5/25 mg | Microcrystalline cellulose, lactose monohydrate, povidone, sodium croscarmellose, anhydrous colloidal silica, meat flavour, yeast powder, magnesium stearate | Treatment of mixed infections by adult cestodes and nematodes | Dogs | [177,178] |
| Amlodipine | Amlodip | 1.25 mg | Artificial chicken flavour, malted yeast, microcrystalline cellulose, mannitol sodium croscarmellose, magnesium stearate, anhydrous colloidal silica | Treatment of systemic hypertension | Cats | [179,180] |
| Oclacitinib maleate | Apoquel | 3.6, 5.4 and 16 mg | Pork liver powder, crospovidone (Type A), sodium starch glycolate (Type A), glycerol monostearate 40–55 (Type II), macrogol 3350, glycerol sodium chloride, xanthan gum, brewer’s dried yeast, anhydrous colloidal silica, magnesium stearate | Treatment clinical manifestations of allergic and atopic dermatitis | Dogs | [181] |
| Fluralaner | Bravecto | 112.5, 250, 500, 1000 and 1400 mg | Pork liver flavour, sucrose, maize starch, sodium lauryl sulphate, disodium embonate monohydrate, magnesium stearate, aspartame 6, glycerol, soya-bean oil, macrogol 3350 | Treatment of tick and flea infestations | Dogs | [182,183] |
| Benazepril Hydrochloride/spironolactone | Cardalis | 2.5/20, 5/40 and 10/80 mg | Lactose monohydrate, microcrystalline cellulose, povidone K30 8, artificial beef flavour, compressible sugar, crospovidone, magnesium stearate | Treatment of congestive heart failure caused by chronic degenerative valvular disease | Dogs | [184] |
| Carprofen | Carprodyl Quadri | 120 mg | Pig liver flavour, yeast, sodium croscarmellose, copovidone, magnesium stearate, anhydrous colloidal silica, microcrystalline cellulose, lactose monohydrate | Anti-inflammatory and analgesic in musculoskeletal disorders and degenerative joint disease | Dogs | [185,186] |
| Cimicoxib | Cimalgex | 8, 30 and 80 mg | Lactose monohydrate, povidone K25, crospovidone, s odium lauryl sulphate, macrogol 400, sodium stearyl fumarate, pork liver powder | Analgesic and anti-inflammatory in osteoarthritis, perioperative pain due to orthopaedic or soft tissue surgery | Dogs | [187] |
| Amoxicilin/Clavulanic acid | Cladaxxa | 40/10, 200/50 and 400/100 mg | Microcrystalline cellulose, magnesium stearate, anhydrous colloidal silica, sodium starch glycollate (type A), dried autolysed yeast, erythrosine aluminium lake E127 | Treatment of infections caused by susceptible bacteria in skin, soft tissue, dental tissue, urine tract, respiratory tract and gut | Cats and dogs | [188] |
| Clindamycin hydrochloride | Zodon (dogs only) | 55, 88, 150, 220, 264 and 440 mg | Zodon: chicken flavour, yeast extract, sodium croscarmellose, copovidone, magnesium stearate, anhydrous colloidal silica, microcrystalline cellulose, lactose monohydrate | Cats: treatment of infected wounds and abscesses and oral cavity infections, including periodontal disease, caused by susceptible bacteria | Cats (only 55 mg) and dogs | [189,190] |
| Clindabactin (cats and dogs) | 55, 220 and 440 mg | Clindabactin: croscarmellose sodium, pregelatinised maize starch, microcrystalline cellulose, hydrated colloidal silica, yeast (dried), chicken flavour, magnesium stearate | Dogs: treatment of infected wounds and abscesses, oral cavity infections (including periodontal disease), superficial pyoderma and osteomyelitis caused by susceptible bacteria | |||
| Spinosad A/D 85:15 | Comfortis | 90, 140, 180, 270, 425, 665, 1040 and 1620 mg | Microcrystalline cellulose, artificial beef flavour, hydroxypropyl cellulose, colloidal silicon, anhydrous, croscarmellose sodium, magnesium stearate | Treatment and prevention of flea infestations | Cats (except 665, 1040 and 1620 mg) and dogs | [191] |
| Lotinaler | Credelio | 12, 48, 56, 112, 225, 450 and 900 mg | Cellulose powdered, lactose monohydrate, Silicified microcrystalline cellulose, dry meat flavour (not in cats), crospovidone, povidone K30, sodium lauryl sulphate, anhydrous colloidal silica, magnesium stearate | Treatment of flea and tick infestations | Cats (only 12 and 48 mg) and Dogs (only doses ≥ 56 mg) | [192] |
| Lotilaner/Milbemycin Oxime (A3 and A4) | Credelio Plus | 56.25/2.11, 112.5/4.22, 225/8.44, 450/16.88 and 900/33.75 mg | Cellulose powdered, lactose monohydrate, silicified microcrystalline cellulose, dry meat flavour, crospovidone, povidone K30, sodium lauryl sulphate, silica colloidal anhydrous, magnesium stearate | Treatment of mixed infestations/infections of ticks, fleas, gastrointestinal nematodes, heartworm and/or lungworm | Dogs | [193] |
| Dexamethasone | Dexacortone | 0.5 and 2 mg | Lactose monohydrate, potato starch, povidone K30, magnesium stearate, chicken flavour, yeast (dried) | Symptomatic treatment or as adjunct treatment of inflammatory and allergic conditions | Cats and Dogs | [194] |
| Marbofloxacin | Efex | 10, 40 and 100 mg | Lactose monohydrate, copovidone, silica colloidal anhydrous, croscarmellose sodium, hydrogenated castor oil, pig liver powder, malted yeast, microcrystalline cellulose | Cats: skin and soft tissue infections (wounds, abscesses, phlegmons) and upper respiratory tract infections caused by susceptible strains Dogs: skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis), UTI associated or not with prostatitis or epididymitis and respiratory tract infections caused by susceptible strains | Cats and Dogs | [195] |
| Ivermectin/Praziquantel | Equimax | 150/20 mg | Povidone, crospovidone, microcrystalline cellulose, cider applemarc (pressed apple pulp), glucose, pregelatinized liquid starch, compressible sugar, magnesium stearate | Treatment of mixed cestode, nematode and arthropod infestations | Horses | [196] |
| Ivermectin/Pyrantel pamoate | Cardotek 30 plus | 68 µg/163 mg, 136 µg/326 mg and 272 µg/652 mg | Polyoxyl 40, hydrogenated castor oil, distilled monoglycerides, ground corn cob, formulated antioxidant, tallow, lean beef, refined soy protein, purified water, dextrose, propylene glycol, sodium chloride, ethoxyquin, potassium sorbate, delta gluconolactone | Prevention of canine heartworm and treatment of infestations of nematodes (ascarids and hookworms) | Dogs | [197] |
| Ivermectin | Eraquell | 20 mg | Povidone, crospovidone, microcrystalline cellulose, cider applemarc (pressed apple pulp), glucose, pregelatinised liquid starch, compressible sugar, magnesium stearate | Treatment of nematode and arthropod infestations | Horses | [198] |
| Firocoxib | Equioxx (horses) and Firodyl | 57, 227 and 250 mg | Equioxx: lactose monohydrate, microcrystalline cellulose, chartor hickory smoke flavour, hydroxypropyl cellulose, sodium croscarmellose, magnesium stearate, caramel (E150d), anhydrous colloidal silica, yellow iron oxide (E172), red iron oxide (E172) Firodyl: hydroxypropyl cellulose, sodium croscarmellose, microcrystalline cellulose, anhydrous colloidal silica, lactose monohydrate, magnesium stearate, yeast, chicken flavour | Horses: Alleviation of pain and inflammation associated with osteoarthritis and reduction of associated lameness Dogs: Relief of pain and inflammation associated with osteoarthritis or For post-operative pain and inflammation associated with soft-tissue, orthopaedic and dental surgery | Horses (only 57 mg) and Dogs | [199,200] |
| Afoxolaner | Frontpro and NexGard | 11, 28, 68 and 136 mg | Maize starch, soy protein fines, braised beef flavouring, povidone (E1201), macrogol 400, macrogol 4000, macrogol 15 hydroxystearate, glycerol (E422), medium-chain triglycerides. | Treatment of flea and tick infestations, demodicosis and sarcoptic mange | Dogs | [201,202] |
| Meloxicam | Inflacam | 1 and 2.5 mg | Lactose monohydrate, silicified microcrystalline cellulose, sodium acid citrate, crospovidone, talc, pork flavour, magnesium stearate | Alleviation of inflammation and pain in chronic musculoskeletal disorders | Dogs | [203] |
| Torasemide | Isemid | 1, 2 and 4 mg | Lactose monohydrate, microcrystalline cellulose, povidone (K30), pork liver powder flavour, compressible sugar, crospovidone (type B), magnesium stearate | Treatment of clinical signs related to congestive heart failure in dogs, including pulmonary oedema | Dogs | [204] |
| Furosemide | Libeo | 10 and 40 mg | Chicken flavour, yeast extract, maltodextrin, magnesium stearate, anhydrous colloidal silica, microcrystalline cellulose, sodium croscarmellose, lactose monohydrate | Treatment of ascites and oedema, particularly associated with cardiac insufficiency | Dogs | [205] |
| Sarolaner | MiPet Easecto | 5, 10, 20, 40, 80 and 120 mg | Hypromellose acetate succinate (medium grade), lactose monohydrate, sodium starch glycolate, anhydrous colloidal silica, magnesium stearate, maize starch, confectioner’s sugar, glucose liquid (81.5% solids), spray-dried pork liver powder, hydrolysed vegetable protein, gelatine type A, wheat germ, calcium hydrogen phosphate anhydrous | Treatment of tick, flea, sarcoptic mange and ear mite infestations | Dogs | [206] |
| Afoxolaner/Milbemycin Oxime (A3 and A4) | Nexgard Spectra | 9/2, 19/4, 38/8, 75/15 and 150/30 mg | Maize starch, soy protein fines, braised beef flavouring, povidone (E1201), macrogol 400, macrogol 4000, macrogol 15 hydroxystearate, glycerol (E422), triglycerides medium-chain, citric acid monohydrate (E330), butylhydroxytoluene (E321) | Treatment of flea and tick infestations, concurrent prevention of heartworm disease, angiostrongylosis, thelaziosis and/or treatment of GI nematode infestations. Treatment of demodicosis and sarcoptic mange. Prevention of heartworm disease and angiostrongylosis | Dogs | [207] |
| Pimobendan | Pimotab | 1.25, 5, 10 and 15 mg | Citric acid anhydrous, povidone K25, lactose monohydrate, microcrystalline cellulose, sodium croscarmellose, chicken flavour, yeast, hydrated colloidal silica, magnesium stearate | Treatment of congestive heart failure originating from dilated cardiomyopathy or valvular insufficiency | Dogs | [208] |
| Phenylpropanolamine hydrochloride | Proin | 15 and 50 mg | Calcium hydrogen phosphate dehydrate, anhydrous colloidal silica, sorbitol, stearic acid, whey, powdered soy protein concentrate, chicken liver powder, dry liver flavour, dry garlic flavour, garlic powder, brewer’s yeast, dark brown lake LB506 | Management of urinary incontinence associated with urethral sphincter incompetence in the bitch, particularly that associated with ovariohysterectomy | Dogs | [209] |
| Moxidectin/Pyrantel embonate/Sarolaner | Simparica Trio | 0.06/12.5/3, 0.12/25/6, 0.24/50/12, 0.48/100/24, 0.96/200/48 and 1.44/300/72 mg | Hypromellose, lactose monohydrate, sodium starch glycolate (type A), meglumine, butylhydroxytoluene (E321), pigment blend 018 (E110, E129, E132), hydroxypropyl cellulose, anhydrous colloidal silica, magnesium stearate, maize starch, confectioner’s sugar, glucose liquid, pork liver powder, hydrolysed vegetable protein, gelatine, wheat germ, calcium hydrogen phosphate anhydrous | Treatment of mixed external and internal parasitic infestations (fleas, ticks and nematodes infestations) | Dogs | [210,211,212] |
| Spiramycin/Metronidazole | Spizobactin | 750,000 IU/125 mg, 1,500,000 IU/250 mg and 3,000,000 IU/500 mg | Pregelatinised starch, microcrystalline cellulose, lactose monohydrate, hydroxypropyl cellulose, yeast, chicken flavour, anhydrous colloidal silica, magnesium stearate | Adjunct treatment of mechanical or surgical periodontal therapy in the treatment of multi-bacterial infections of periodontal and related (peri)oral conditions | Dogs | [213] |
| Tramadol hydrochloride | Tralieve | 20 and 80 mg | Microcrystalline cellulose, lactose monohydrate, sodium starch glycolate (type A), magnesium stearate, hydrated colloidal silica, chicken flavour, yeast | Reduction of acute and chronic mild soft tissue and musculoskeletal pain | Dogs | [214] |
| Mavacoxib | Trocoxil | 6, 20, 30, 75 and 95 mg | Sucrose, silicified microcrystalline cellulose, artificial powdered beef flavour, sodium croscarmellose, sodium lauryl sulphate, magnesium stearate | Treatment of pain and inflammation associated with degenerative joint disease when continuous treatment exceeds one month | Dogs | [215] |
| Febantel/Praziquantel/Pyrantel | Veloxa and Veloxa XL | 150/50/50 and 525/175/175 mg | Cetyl palmitate, pregelatinised starch, sodium starch glycolate (type A), anhydrous colloidal silica, magnesium stearate, artificial beef flavour | Anthelmintic for treatment of mixed infections by roundworms and tapeworms in dogs and puppies | Dogs (Veloxa XL over 17.5 kg) | [216] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. https://doi.org/10.3390/pharmaceutics14081732
Rodríguez-Pombo L, Awad A, Basit AW, Alvarez-Lorenzo C, Goyanes A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics. 2022; 14(8):1732. https://doi.org/10.3390/pharmaceutics14081732
Chicago/Turabian StyleRodríguez-Pombo, Lucía, Atheer Awad, Abdul W. Basit, Carmen Alvarez-Lorenzo, and Alvaro Goyanes. 2022. "Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design" Pharmaceutics 14, no. 8: 1732. https://doi.org/10.3390/pharmaceutics14081732
APA StyleRodríguez-Pombo, L., Awad, A., Basit, A. W., Alvarez-Lorenzo, C., & Goyanes, A. (2022). Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics, 14(8), 1732. https://doi.org/10.3390/pharmaceutics14081732