Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano–Bio Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Transmission Electron Microscopy (TEM) and Image Analysis
2.4. 3D Cell Explorer
2.5. MNP Uptake Assay
2.6. Protein Preparation and Extraction
2.7. Western Blot Analysis
2.8. Confocal Microscopy
2.9. Knockdown of 67LR Using esiRNA
2.10. Statistical Analysis
3. Results
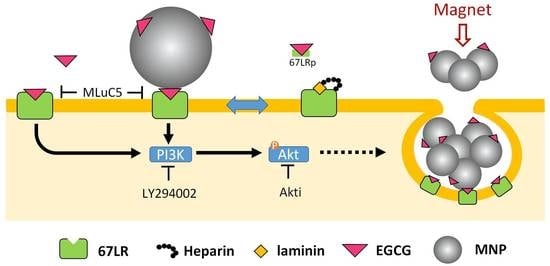
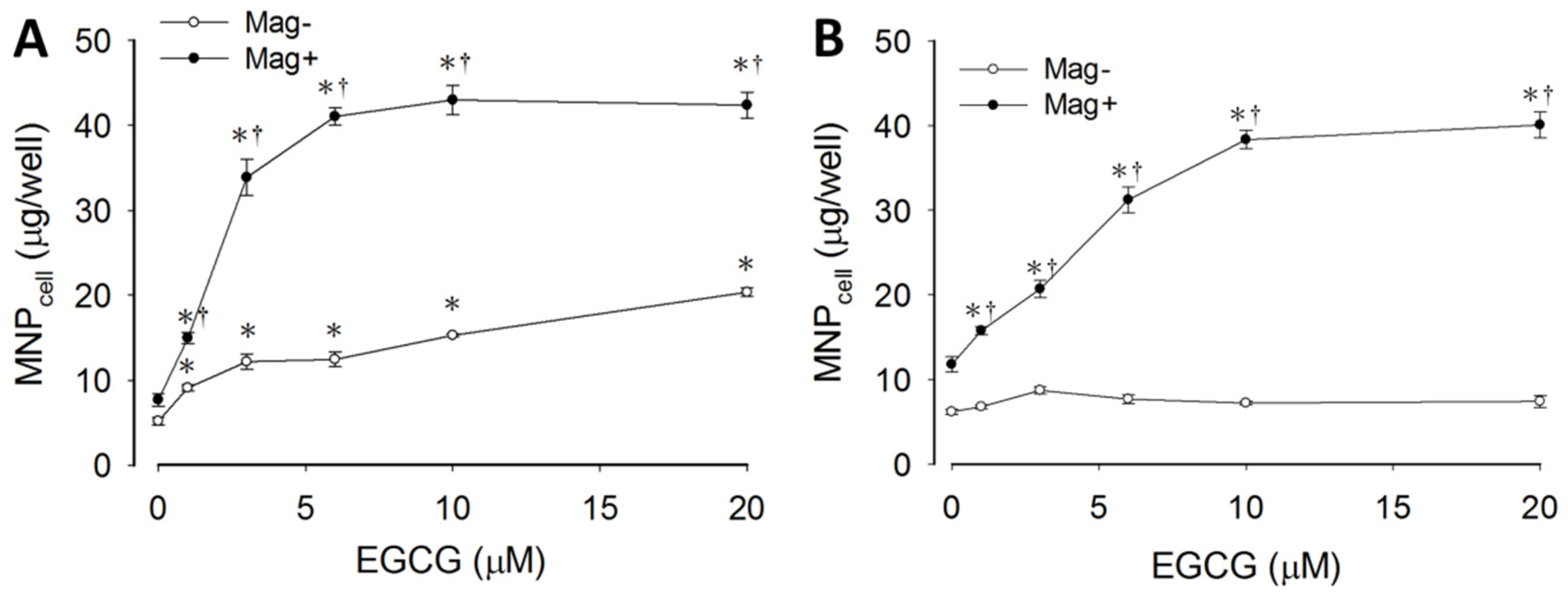
3.1. Synergetic Effects of EGCG and Magnetic Force on MNP Uptake
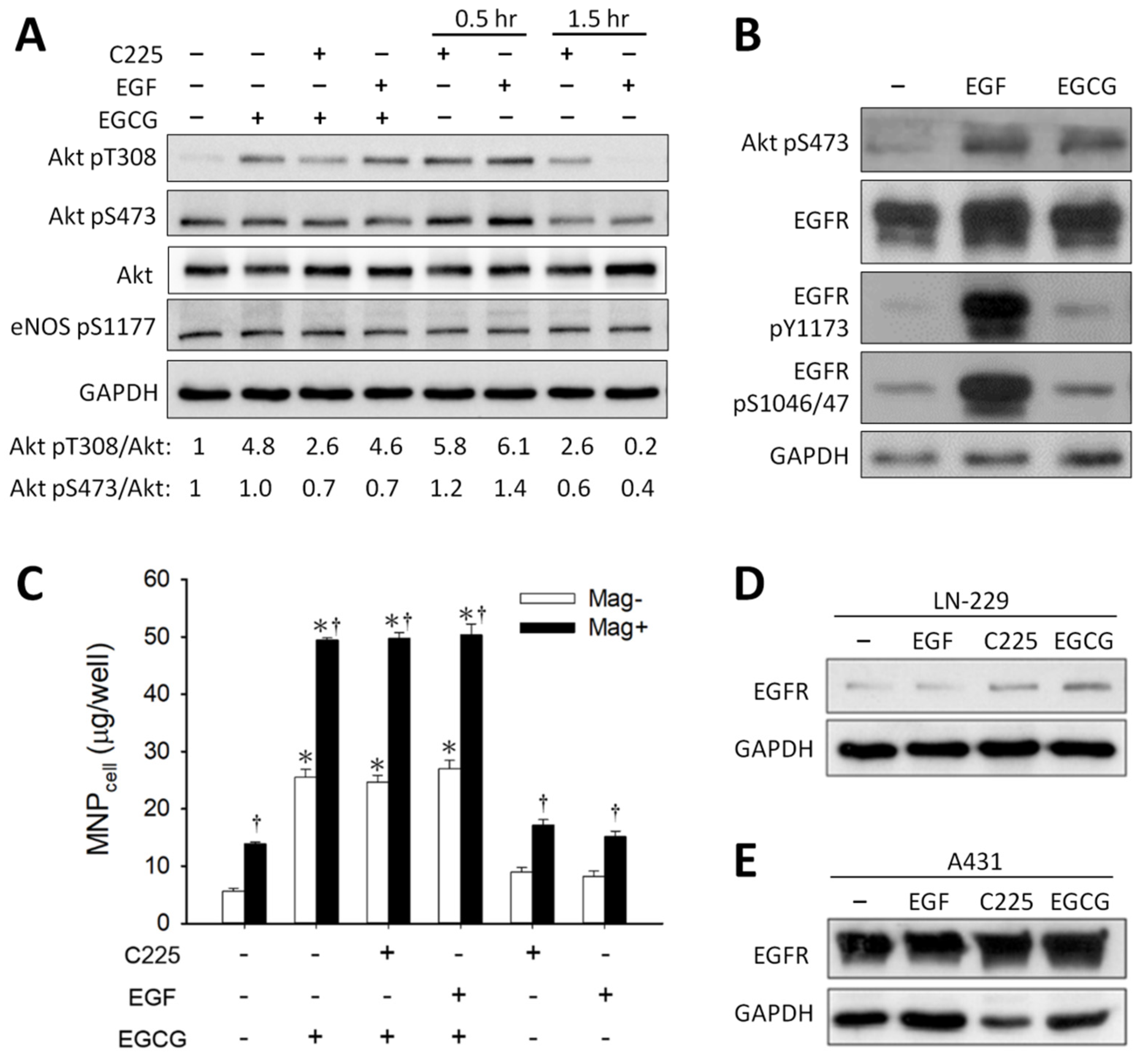
3.2. 67LR-Mediated MNP Uptake Induced by EGCG
3.3. Effect of Signaling Downstream of 67LR on EGCG-Induced MNP Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, E.H.; Harford, J.B.; Eaton, M.A.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future-a global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Nanoparticle therapeutics: Technologies and methods for overcoming cancer. Eur. J. Pharm Biopharm. 2015, 97, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K. Cellular interactions of therapeutically delivered nanoparticles. Expert Opin. Drug Deliv. 2011, 8, 141–151. [Google Scholar] [CrossRef]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar]
- Yu, M.; Yang, Y.; Zhu, C.; Guo, S.; Gan, Y. Advances in the transepithelial transport of nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Asp. Med. 2022, 83, 100993. [Google Scholar] [CrossRef]
- Ma, Y.C.; Li, C.; Gao, F.; Xu, Y.; Jiang, Z.B.; Liu, J.X.; Jin, L.Y. Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway. Oncol. Rep. 2014, 31, 1343–1349. [Google Scholar] [CrossRef]
- Yan, J.J.; Liao, J.Z.; Lin, J.S.; He, X.X. Active radar guides missile to its target: Receptor-based targeted treatment of hepatocellular carcinoma by nanoparticulate systems. Tumour Biol. 2015, 36, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Raucher, D.; Sheetz, M.P. Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 1999, 144, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Hsieh, H.Y.; Lu, K.Y.; Wang, S.Y.; Mi, F.L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine 2016, 11, 9–30. [Google Scholar] [CrossRef]
- Lu, Y.C.; Luo, P.C.; Huang, C.W.; Leu, Y.L.; Wang, T.H.; Wei, K.C.; Wang, H.E.; Ma, Y.H. Augmented cellular uptake of nanoparticles using tea catechins: Effect of surface modification on nanoparticle-cell interaction. Nanoscale 2014, 6, 10297–10306. [Google Scholar] [CrossRef]
- Cheng, M.C.; Lu, Y.C.; Wu, J.; Ma, Y.H. Gallate-induced nanoparticle uptake by tumor cells: Structure-activity relationships. Coll. Surf. B Biointerfaces 2019, 179, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Siow, W.X.; Chang, Y.T.; Babič, M.; Lu, Y.C.; Horák, D.; Ma, Y.H. Interaction of poly-l-lysine coating and heparan sulfate proteoglycan on magnetic nanoparticle uptake by tumor cells. Int. J. Nanomed. 2018, 13, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Stangl, V.; Dreger, H.; Stangl, K.; Lorenz, M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 2007, 73, 348–358. [Google Scholar] [CrossRef]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Dusterhoft, T.; Baumann, G.; Stangl, K.; Stangl, V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004, 279, 6190–6195. [Google Scholar] [CrossRef]
- Gundimeda, U.; McNeill, T.H.; Elhiani, A.A.; Schiffman, J.E.; Hinton, D.R.; Gopalakrishna, R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cε. J. Biol. Chem. 2012, 287, 34694–34708. [Google Scholar] [CrossRef]
- Kumazoe, M.; Fujimura, Y.; Tachibana, H. 67-kDa laminin receptor mediates the beneficial effects of green tea polyphenol EGCG. Curr. Pharmacol. Rep. 2020, 6, 1–6. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H. Cellular sensing system for green tea polyphenol epigallocatechin gallate. AGri-Biosci. Monogr. 2014, 4, 19–35. [Google Scholar] [CrossRef][Green Version]
- Nelson, J.; McFerran, N.V.; Pivato, G.; Chambers, E.; Doherty, C.; Steele, D.; Timson, D.J. The 67 kDa laminin receptor: Structure, function and role in disease. Biosci. Rep. 2008, 28, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Robey, F.A.; Graf, J.; Sasaki, M.; Kleinman, H.K.; Yamada, Y.; Martin, G.R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science 1987, 238, 1132–1134. [Google Scholar] [CrossRef]
- Yu, H.N.; Zhang, L.C.; Yang, J.G.; Das, U.N.; Shen, S.R. Effect of laminin tyrosine-isoleucine-glycine-serine-arginine peptide on the growth of human prostate cancer (PC-3) cells in vitro. Eur. J. Pharmacol. 2009, 616, 251–255. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- Castronovo, V.; Taraboletti, G.; Sobel, M.E. Functional domains of the 67-kDa laminin receptor precursor. J. Biol. Chem. 1991, 266, 20440–20446. [Google Scholar] [CrossRef]
- Butò, S.; Tagliabue, E.; Ardini, E.; Magnifico, A.; Ghirelli, C.; van den Brûle, F.; Castronovo, V.; Colnaghi, M.I.; Sobel, M.E.; Ménard, S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell. Biochem. 1998, 69, 244–251. [Google Scholar] [CrossRef]
- Kinoshita, K.; Kaneda, Y.; Sato, M.; Saeki, Y.; Wataya-Kaneda, M.; Hoffmann, A. LBP-p40 binds DNA tightly through associations with histones H2A, H2B, and H4. Biochem. Biophys. Res. Commun. 1998, 253, 277–282. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin-gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Yamada, K.; Tachibana, H. A lipid raft-associated 67kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FcepsilonRI expression. Biochem. Biophys. Res. Commun. 2005, 336, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Involvement of 67-kDa laminin receptor-mediated myosin phosphatase activation in antiproliferative effect of epigallocatechin-3-O-gallate at a physiological concentration on caco-2 colon cancer cells. Biochem. Biophys. Res. Commun. 2008, 371, 172–176. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin gallate-gold nanoparticles exhibit superior antitumor activity compared to conventional gold nanoparticles: Potential synergistic interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]
- Mu, M.; Chen, H.; Fan, R.; Wang, Y.; Tang, X.; Mei, L.; Zhao, N.; Zou, B.; Tong, A.; Xu, J.; et al. A tumor-specific ferric-coordinated epigallocatechin-3-gallate cascade nanoreactor for glioblastoma therapy. J. Adv. Res. 2021, 34, 29–41. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef]
- Gan, N.; Wakayama, C.; Inubushi, S.; Kunihisa, T.; Mizumoto, S.; Baba, M.; Tanino, H.; Ooya, T. Size dependency of selective cellular uptake of epigallocatechin gallate-modified gold nanoparticles for effective radiosensitization. ACS Appl. Bio. Mater. 2022, 5, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Weinstein, I.B. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat. Res. 2005, 591, 147–160. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Adachi, S.; Nagao, T.; To, S.; Joe, A.K.; Shimizu, M.; Matsushima-Nishiwaki, R.; Kozawa, O.; Moriwaki, H.; Maxfield, F.R.; Weinstein, I.B. (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis 2008, 29, 1986–1993. [Google Scholar] [CrossRef]
- Zhu, W.; Li, M.C.; Wang, F.R.; Mackenzie, G.G.; Oteiza, P.I. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem. Pharmacol. 2020, 175, 113923. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Shimizu, M.; Shirakami, Y.; Yamauchi, J.; Natsume, H.; Matsushima-Nishiwaki, R.; To, S.; Weinstein, I.B.; Moriwaki, H.; Kozawa, O. (−)-Epigallocatechin gallate downregulates egf receptor via phosphorylation at ser1046/1047 by p38 mapk in colon cancer cells. Carcinogenesis 2009, 30, 1544–1552. [Google Scholar] [CrossRef]
- Lowenstein, C.J. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc. Res. 2007, 75, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Moniri, N.H.; Ozawa, K.; Stamler, J.S.; Daaka, Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. USA 2006, 103, 1295–1300. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver nanoparticle-mediated cellular responses in various cell lines: An in vitro model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef]
- Cao, S.; Yao, J.; McCabe, T.J.; Yao, Q.; Katusic, Z.S.; Sessa, W.C.; Shah, V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J. Biol. Chem. 2001, 276, 14249–14256. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.R.; Chen, P.H.; Srinivasan, S.; Aguet, F.; Mettlen, M.; Schmid, S.L. Crosstalk between Akt/GSK3β signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 2015, 34, 2132–2146. [Google Scholar] [CrossRef]
- Fayol, D.; Luciani, N.; Lartigue, L.; Gazeau, F.; Wilhelm, C. Managing magnetic nanoparticle aggregation and cellular uptake: A precondition for efficient stem-cell differentiation and MRI tracking. Adv. Healthc. Mater. 2013, 2, 313–325. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chang, F.Y.; Tu, S.J.; Chen, J.P.; Ma, Y.H. Cellular uptake of magnetite nanoparticles enhanced by NdFeB magnets in staggered arrangement. J. Magn. Magn. Mater. 2017, 427, 71–80. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lin-shiau, S.Y.; Chen, C.F.; Lin, J.K. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell. Biochem. 1997, 67, 55–65. [Google Scholar] [CrossRef]
- Abeyrathna, P.; Su, Y. The critical role of Akt in cardiovascular function. Vascul. Pharmacol. 2015, 74, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.A.; Suarez, N.G.; Cappadocia, L.; Annabi, B. Functional targeting of the TGF-betaR1 kinase domain and downstream signaling: A role for the galloyl moiety of green tea-derived catechins in ES-2 ovarian clear cell carcinoma. J. Nutr. Biochem. 2021, 87, 108518. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Choi, B.Y.; Choi, H.S.; Kang, B.S.; Bode, A.M.; Dong, Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005, 280, 16882–16890. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-C.; Wu, N.-P.; Lu, Y.-C.; Ma, Y.-H. Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano–Bio Interface. Pharmaceutics 2022, 14, 1523. https://doi.org/10.3390/pharmaceutics14081523
Hsu S-C, Wu N-P, Lu Y-C, Ma Y-H. Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano–Bio Interface. Pharmaceutics. 2022; 14(8):1523. https://doi.org/10.3390/pharmaceutics14081523
Chicago/Turabian StyleHsu, Sheng-Chieh, Nian-Ping Wu, Yi-Ching Lu, and Yunn-Hwa Ma. 2022. "Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano–Bio Interface" Pharmaceutics 14, no. 8: 1523. https://doi.org/10.3390/pharmaceutics14081523
APA StyleHsu, S.-C., Wu, N.-P., Lu, Y.-C., & Ma, Y.-H. (2022). Laminin Receptor-Mediated Nanoparticle Uptake by Tumor Cells: Interplay of Epigallocatechin Gallate and Magnetic Force at Nano–Bio Interface. Pharmaceutics, 14(8), 1523. https://doi.org/10.3390/pharmaceutics14081523