Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study
Abstract
:Highlights
- 1)
- What are the main findings?
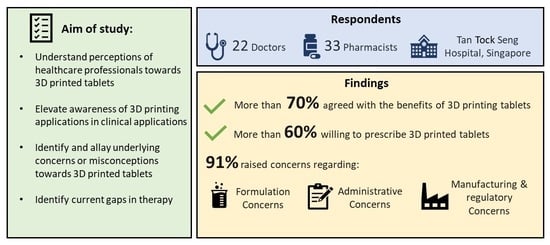
- More than 70% of the respondents agreed about the benefits of 3D printed tablets.
- More than 60% of the respondents were willing to prescribe 3D printed tablets.
- Many of the respondents were concerned about formulation considerations, manufacturing processes, and administrative issues.
- 2)
- What is the implication of the main finding?
- Healthcare professionals are receptive to adopting 3D printed tablets.
- Addressing formulation, manufacturing process and administrative concerns is important for technology adoption.
- 3D printing companies, healthcare professionals and regulators should closely collaborate to advance the benefits of 3D printed tablets.
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Data Collection and Analysis
3. Results and Discussion
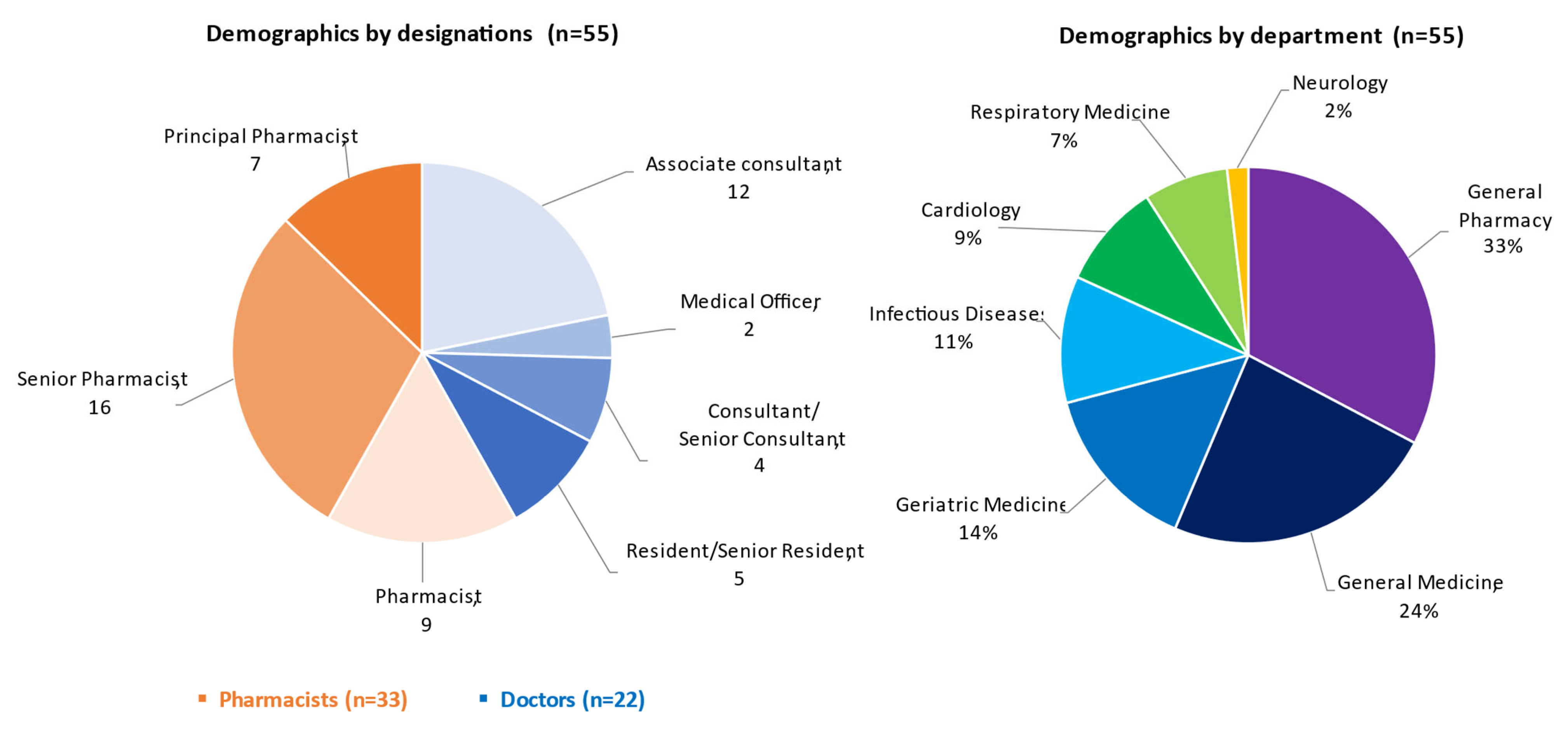
3.1. Demographics of Respondents
3.2. Survey Results
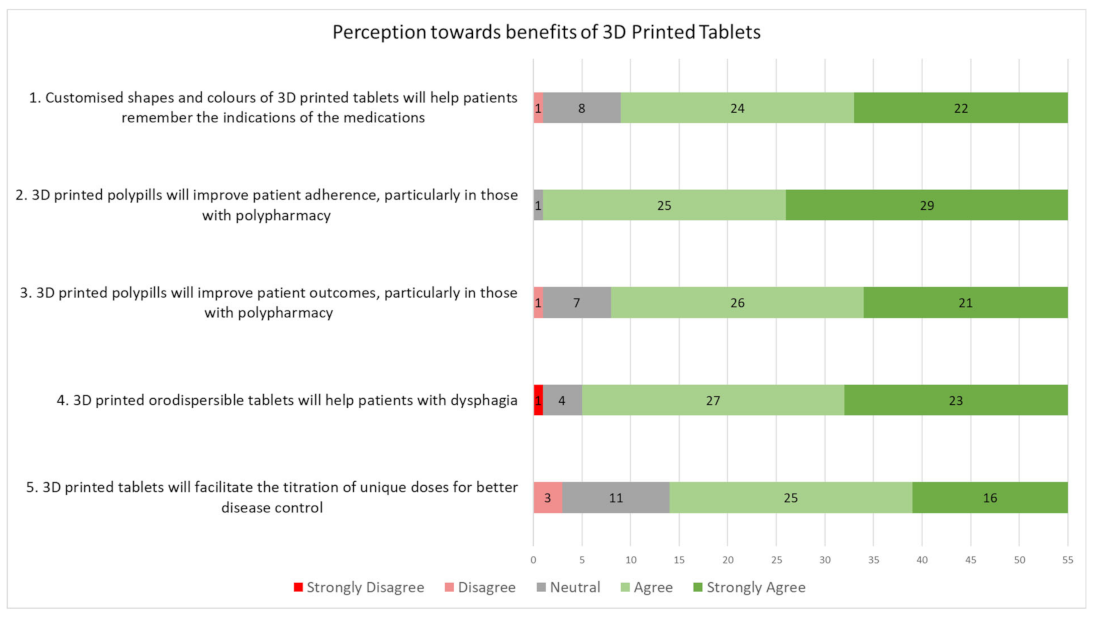
3.3. General Perceptions toward 3D Printed Tablets
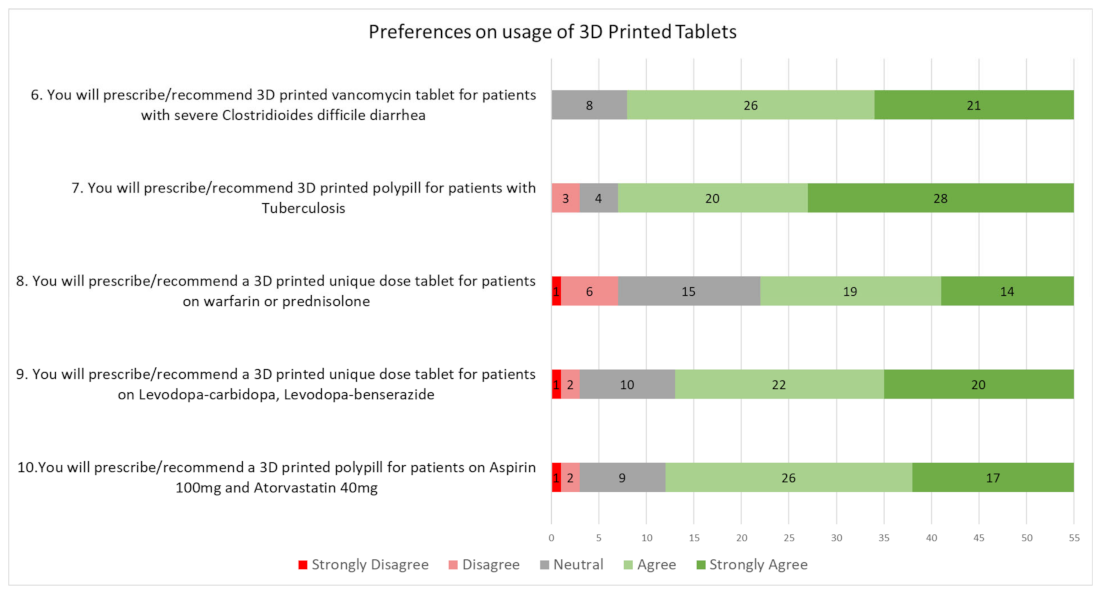
3.4. Preferences on Prescribing 3D Printed Tablets
3.5. Areas of Concern
3.5.1. Formulation Issues
3.5.2. Administrative Concerns
3.5.3. Manufacturing and Regulatory Concerns
3.6. Limitations of Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Unique Features of 3D Printed Tablets | Impact/Advantages | Examples of Applications | 3DP Technology Used |
|---|---|---|---|
| Customised colors and shapes |
| Tablets with braille and moon patterns for visually impaired patients [12] | Selective laser sintering (SLS) |
| Chewable tablets [27]/gummies [28] shaped like candy for pediatric use | Fused deposition Modeling [27] Embedded 3D printing [28] | ||
| Polypills |
| Four-in-one polypill with multiple release profiles [29] | Semi-solid extrusion |
| Polypills with bespoke release patterns for multiple drugs [30] | Fused deposition Modeling | ||
| Multi-layered polypill containing six drugs [31] | Stereolithographic (SLA) | ||
| Oro-dispersible tablets |
| Spritam®, first commercialised 3D printed drug [32] | Binder jet 3D Printing |
| Oro-dispersible pills in cartoon shapes for pediatric applications [33] | Color jet 3D Printing | ||
| Customized doses |
| First clinical trial using chewable isoleucine tablets with varying personalised dosages and flavors [34] | Semi-solid extrusion |
| Indication | Drugs | Current State of Treatment | Significance of 3D Printed Tablets |
|---|---|---|---|
| Clostridioides difficile Diarrhea | Vancomycin (antibiotic) |
| 3D printing can change the dosage forms of conventional liquid medications to solid oral forms, ensuring accurate dosing and improving patients’ adherence. 3D printing is suited for small batch manufacturing, keeping costs low for “low-demand” or orphan drugs. |
| Tuberculosis | Combination of drugs must be taken. These include: Rifampicin, Isoniazid, Pyrazinamide, Ethambutol, and Pyridoxine [36,37] |
| 3D printing can produce polypills that combine various drugs into one tablet. This would greatly reduce the number of pills patients need to take daily and reduce the associated monitoring time by clinicians and caregivers. |
| Anticoagulation | Warfarin (anticoagulant) |
| 3D printing can print on-demand with the accurate dosing amount required in the pill. Patients would not need to “mix-and-match” from fixed dose tablets, thereby reducing the chances of inaccurate dosing and medication error. |
| Anti-inflammation | Prednisolone (corticosteroid) |
| |
| Parkinson’s disease | Levodopa-carbidopa/Levodopa-benserazide |
| 3D printing can print sustained-release tablets and personalize dosages for patients. This would reduce the frequency of pill-taking. |
| Cardiovascular | Aspirin and Atorvastatin |
| Drugs can be combined into a generic polypill for easy prescription; reducing the overall number of drugs. |
Appendix B. Survey Questions
- 1.
- What is 3D Printing?
- 2.
- Has 3D Printing been used in pharmaceuticals?
- 3.
- What are the advantages of 3D printing in pharmaceuticals?
- Combination of various medicines or supplements into a single polypill
- Reformulation of various medicines into a new controlled release profile (immediate/sustained/enteric/delayed/ orodispersible controlled release forms)
- Rapid small batch manufacturing of new formulations
- 3D printing of new shapes, geometries or colours for easier recognition
- 4.
- Patient centric pharmaceutical dosage forms
- (1)
- Medicines which are unavailable in a suitable tablet form can be 3D printed quickly. Oro-dispersible tablets can also be created for patients with dysphagia.
- (2)
- Patients on unique doses who require breaking the usual mass-produced dosage forms (e.g., breaking a 2mg table to achieve a daily dose of 3.5 mg) can now obtain a 3D printed tablet with a personalized dose.
- (3)
- Embossing designs have the potential to include Braille and can help visually impaired patients to identify the correct medications to take.
- (4)
- Polypills can be designed where up to 5 different active pharmaceutical ingredients with immediate release and sustained release profiles all in one.

| 1. What is your profession? | ||||
| (a) Doctor | (b) Pharmacist | |||
| 2. What is your current designation? | |||||
| (a) Associate consultant and above | (b) Resident/Senior resident | (c) Medical Officer | (d) Principal pharmacist | (e) Senior pharmacist | (f) Pharmacist |
| 3. What is your specialty? | ||||
| (a) General Medicine | (b) Cardiology | (c) Infectious diseases | (d) Neurology | (e) Geriatric Medicine |
| (f) Respiratory medicine | (g) Pharmacy | |||
| 4. Customized shapes and colours of 3D printed tablets will help patients remember the indications of the medicines. For instance, different colours and patterns may be used in the identification and differentiation of tablets that look alike. Different shapes may also be 3D printed to appeal to certain demographics such as paediatric patients. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 5. 3D printed polypills will improve patient adherence particularly in those with polypharmacy. For instance, multiple medicines may be combined into a single tablet. In such cases, the patient may be able to reduce the number of pills to be taken daily. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 6. 3D printed polypills will improve patient outcomes particularly in those with polypharmacy. This may be associated with the reduction in the number of pills to be taken daily, allowing easier persuasion of the patient to take their medicines and to reduce episodes of forgetfulness. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 7. 3D printed orodispersible tablets will help patients with dysphagia (difficulty in swallowing). Orodispersible tablets can dissolve quickly with limited water, or in the patient’s mouth, reducing the need to swallow. Tablets may be 3D printed as an orodispersible formulation depending on the medication required. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 8. 3D printed tablets will facilitate the titration of unique doses for better disease control. For instance, half doses of medicines may be 3D printed instead of the current practice of splitting the tablet or taking the tablet on alternate days. Also, the dosing of medicine may be personalized to the individual depending on the requirements for the condition, under the prescribing doctor’s supervision. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 9. You will prescribe/recommend 3D printed vancomycin tablet for patients with severe Clostridiodes difficile diarrhea. Oral vancomycin tablet/capsule is currently not available in Singapore. The required dose of vancomycin may be 3D printed into an oral tablet for the individual patient. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 10. You will prescribe/recommend a 3D printed polypill for patients with Tuberculosis. The typical regimen for Tuberculosis treatment involves 4 different medications to be taken daily, and medication adherence is important in controlling the condition and preventing the risk of spreading and development of resistance. 3D printing allows all the medications to be formulated in a single tablet. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 11. You will prescribe/recommend a 3D printed unique dose tablet for patients on warfarin or prednisolone. Warfarin and prednisolone dosing typically require dose titration depending on INR testing or the disease condition. 3D printed tablets allow for the exact dose required to be produced for the individual patient. | ||||
| (a) Strongly disagree | (b) Disagree | (c) Neutral | (d) Agree | (e) Strongly agree |
| 12. You will prescribe/recommend a 3D printed unique dose tablet for patients on Levodopa-carbidopa, Levodopa-benserazide. The current dose and dosing frequencies of these medicines may be complex (e.g., requires taking half tablets or up to five times a day.) This medicine schedule could be personalized using 3D printing. This may be done through the control of different drug release within the same tablet, such as immediate, sustained or delayed release formulations within one tablet. | ||||
| (f) Strongly disagree | (g) Disagree | (h) Neutral | (i) Agree | (j) Strongly agree |
| 13. You will prescribe/recommend a 3D printed polypill for patients on Aspirin 100mg and Atorvastatin 40mg. These medications may be 3D printed into a single, fixed dose combination tablet. | ||||
| (k) Strongly disagree | (l) Disagree | (m) Neutral | (n) Agree | (o) Strongly agree |
| 14. List any concerns that you have on 3D printing of tablets |
| 15. List other medical conditions that you think 3D printing tablets can be helpful |
| 16. List any concerns that you have on 3D printing of tablets |
References
- Brown, M.; Bussell, J. Medication Adherence: WHO Cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimmy, B.; Jose, J. Patient Medication Adherence: Measures in Daily Practice. Oman Med. J. 2011, 26, 155–159. [Google Scholar] [CrossRef]
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Warsi, M.H.; Yusuf, M.; Al Robaian, M.; Khan, M.; Muheem, A.; Khan, S. 3D Printing Methods for Pharmaceutical Manufacturing: Opportunity and Challenges. Curr. Pharm. Des. 2019, 24, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fastø, M.M.; Genina, N.; Kaae, S.; Sporrong, S.K. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: A pilot study. Int. J. Clin. Pharm. 2019, 41, 1290–1298. [Google Scholar] [CrossRef]
- Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algahtani, M. Assessment of Pharmacist’s Knowledge and Perception toward 3D Printing Technology as a Dispensing Method for Personalized Medicine and the Readiness for Implementation. Pharmacy 2021, 9, 68. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 1–16. [Google Scholar] [CrossRef]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; DiMatteo, M.R. The challenge of patient adherence. Ther. Clin. Risk Manag. 2005, 1, 189. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1661624/ (accessed on 24 March 2022).
- Lauffenburger, J.C.; Landon, J.E.; Fischer, M.A. Effect of Combination Therapy on Adherence Among US Patients Initiating Therapy for Hypertension: A Cohort Study. J. Gen. Intern. Med. 2017, 32, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Bangalore, S.; Kamalakkannan, G.; Parkar, S.; Messerli, F.H. Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis. Am. J. Med. 2007, 120, 713–719. [Google Scholar] [CrossRef]
- Collier, R. Reducing the “pill burden”. Can. Med Assoc. J. 2012, 184, E117–E118. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, A.R.; Borrelli, E.P. The art and science of drug titration. Ther. Adv. Drug Saf. 2020, 11, 204209862095891. [Google Scholar] [CrossRef]
- Panraksa, P.; Zhang, B.; Rachtanapun, P.; Jantanasakulwong, K.; Qi, S.; Jantrawut, P. ‘Tablet-in-Syringe’: A Novel Dosing Mechanism for Dysphagic Patients Containing Fast-Disintegrating Tablets Fabricated Using Semisolid Extrusion 3D Printing. Pharmaceutics 2022, 14, 443. [Google Scholar] [CrossRef]
- Annual Report & Other Reports: Hyphens Pharma International Limited, (n.d.). Available online: https://www.hyphensgroup.com/investor-relations/annual-report-other-reports/ (accessed on 14 July 2022).
- McGuire, M.; Iuga, A. Adherence and health care costs. Risk Manag. Healthc. Policy 2014, 7, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G. Mass Customisation: Singapore Start-Up Craft Health Develops Technique to 3D Print Nutraceuticals and Pharmaceuticals. 2021. Available online: https://www.nutraingredients-asia.com/Article/2021/12/08/Mass-customisation-Singapore-start-up-Craft-Health-develops-technique-to-3D-print-nutraceuticals-and-pharmaceuticals (accessed on 8 April 2022).
- FabRx Releases Pharmaceutical 3D Printer M3DIMAKER. 2020. Available online: https://manufacturingchemist.com/news/article_page/FabRx_releases_pharmaceutical_3D_printer_M3DIMAKER/164316 (accessed on 25 March 2022).
- Zidan, A. Promise and Potential of 3D Printed Pharmaceuticals. U.S. Food and Drug Administration. 2017. Available online: https://www.fda.gov/drugs/news-events-human-drugs/cder-researchers-explore-promise-and-potential-3d-printed-pharmaceuticals (accessed on 8 April 2022).
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; More, A.T. Some aesthetic considerations for over the-counter (OTC) pharmaceutical products. Int. J. Biotechnol. 2010, 11, 267. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. Int. J. Pharm. 2021, 598, 120360. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Isreb, M.; Forbes, R.T.; Oga, E.F.; Alhnan, M.A. Additive Manufacturing of a Point-of-Care “Polypill”: Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease. Adv. Healthc. Mater. 2020, 9, e2000236. [Google Scholar] [CrossRef] [PubMed]
- What Is SPRITAM?—SPRITAM (Levetiracetam). 2021. Available online: https://spritam.com/what-is-spritam/ (accessed on 24 March 2022).
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Han, X.; Chen, R.; Li, J.; Gao, J.; Zhang, H.; Liu, N.; Gao, X.; Zheng, A. Innovative color jet 3D printing of levetiracetam personalized paediatric preparations. Asian J. Pharm. Sci. 2021, 16, 374–386. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Bunnell, K.L.; Danziger, L.H.; Johnson, S. Economic barriers in the treatment of Clostridium difficile infection with oral vancomycin. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2017; Volume 4. [Google Scholar] [CrossRef] [Green Version]
- Treatment for TB Disease. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm (accessed on 24 March 2022).
- TB Drugs. TBFacts. Available online: https://tbfacts.org/tb-drugs/ (accessed on 24 March 2022).
- Solomon, S.; Tan, N.; Teo, S.; Lee, H. Tuberculosis Treatment in Singapore: Directly Observed Therapy (DOT)—HealthXchange. Available online: https://www.healthxchange.sg/heart-lungs/lung-conditions/tuberculosis-treatment-singapore-directly-observed-therapy (accessed on 24 March 2022).
- Warfarin. 2017. Available online: https://www.healthhub.sg/a-z/medications/116/warfarin (accessed on 24 March 2022).
- Ministry of Health. MOH Clinical Pharmacy Guidelines: Anticoagulation—Warfarin; Ministry of Health: Singapore, 2006; Volume 17, p. 6.
- Bernstein, S. Prednisone Taper and Withdrawal Symptoms. WebMD. 2020. Available online: https://www.webmd.com/drug-medication/prednisone-taper (accessed on 24 March 2022).
- Madopar 50 mg/12.5 mg Dispersible Tablets—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/1111/smpc#gref (accessed on 24 March 2022).
- Hennekens, C.H.; Hollar, D.; Agatston, A.S. Aspirin and Statins to Decrease Risks of Cardiovascular Disease—The Need for Wider Utilization. US Cardiol. Rev. 2004, 1, 43–44. [Google Scholar] [CrossRef]
| Subcategory | Respondents’ Comments. (Comments Have Been Edited Slightly for Grammatical Clarity.) |
|---|---|
| Stability and bioequivalence | “Stability of tablets—How is the expiry date determined? What if the patient does not keep the tablet in the recommended conditions?” |
| “How is bioequivalence ensured?” | |
| “How would changes in release profile affect the pharmacokinetics & pharmacodynamics of the drug” | |
| Size of tablets | “What would the size of the tablet be like if the patient requires many medications?” |
| “Would the size of tablets be suitable and crushable for patients with dysphagia?” | |
| Drug interactions | “Would there be drug interaction issues in the polypills created?” |
| “It may be hard to know which component is causing side effects or allergic reactions, especially in polypills.” |
| Subcategory | Respondents’ Comments. (Comments Have Been Edited Slightly for Grammatical Clarity.) |
|---|---|
| Medication reconciliation | “How would healthcare providers identify the drug content or communicate across healthcare institutions to confirm the drugs the patients are on if each 3D pill is unique to the patient’s requirement?” |
| “Patients need to be able to distinguish between different polypills collected at different time points. For example: when there are dosage changes but the polypills collected have the same appearance” | |
| Reduced health literacy of patients | “There may be reduced patient awareness of the medications that patients are on. There needs to be good documentation of what each customised tab contains.” |
| “3D printed polypills may potentially reduce patient’s health literacy and incentive to participate in their medical care due to the reliance on prepacked medicine” |
| Subcategory | Respondents’ Comments. (Comments have Been Edited Slightly for Grammatical Clarity.) |
|---|---|
| Quality control and assurance | “There should be batch standardisation and confidence that for Narrow Therapeutic Index drugs, each product is within reasonable margin of error for product variance” |
| “Quality assurance of in-house 3D Printing should be ascertained” | |
| “How can cross contamination in the production process be prevented and consistency of dosages and active ingredients be ensured?” | |
| Manufacturing time and cost for patients | “Would the cost and time required to obtain the 3D printed pill be practical for patients requiring frequent titration of medication?” |
| “How fast is the turnaround time, to prevent interruption of therapy?” | |
| “Would it be time consuming to 3D print large quantities of medicines?” | |
| Regulatory challenges | “A long lead time and extensive testing for regulatory submission is needed. Would a change in formulation require application with HSA under the New Drug Application route?” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, O.; Goh, W.J.; Lim, S.H.; Hoo, G.S.; Liew, R.; Ng, T.M. Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study. Pharmaceutics 2022, 14, 1521. https://doi.org/10.3390/pharmaceutics14071521
Goh O, Goh WJ, Lim SH, Hoo GS, Liew R, Ng TM. Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study. Pharmaceutics. 2022; 14(7):1521. https://doi.org/10.3390/pharmaceutics14071521
Chicago/Turabian StyleGoh, Odelia, Wei Jiang Goh, Seng Han Lim, Grace S. Hoo, Raymond Liew, and Tat Ming Ng. 2022. "Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study" Pharmaceutics 14, no. 7: 1521. https://doi.org/10.3390/pharmaceutics14071521
APA StyleGoh, O., Goh, W. J., Lim, S. H., Hoo, G. S., Liew, R., & Ng, T. M. (2022). Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study. Pharmaceutics, 14(7), 1521. https://doi.org/10.3390/pharmaceutics14071521