Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles
Abstract
1. Introduction
1.1. Current Treatment Protocols for H. pylori Infection and Emerging Antibiotic-Resistant Strains
1.2. Potential Use of AgNPs as a Complementary or Alternative Therapy for H. pylori in the Context of Antibiotic Resistance
2. Methods
2.1. Literature Search
2.2. Selection of Studies
3. The Pathology of Helicobacter pylori Infection: From Inflammation to Gastric Neoplasia

| Bacterial Element | Virulence Factors | Mechanism of Bacterial Virulence |
|---|---|---|
| Flagella | Flagellum | Bacterial motility |
| Leukocyte chemotaxis | ||
| Biofilm formation | ||
| Inflammation and immune response | ||
| Adhesins | Blood group antigen-binding adhesin (BabA) | Adherence to gastric epithelial cells |
| Toxins delivery | ||
| Increasing inflammatory responses | ||
| Sialic acid-binding adhesin (SabA) | Neutrophil activation | |
| Colonization | ||
| Oxidative stress | ||
| Adherence-associated lipoproteins A and B | Adherence to gastric epithelial cells | |
| Colonization | ||
| Biofilm formation | ||
| Release of proinflammatory factors | ||
| LacdiNAc-specific adhesin (LabA) | Adherence to gastric epithelial cells | |
| Enzymes | Urease | Protection from acidity |
| Colonization | ||
| Bacterial nutrition | ||
| Control of the host immune response | ||
| Platelet activation | ||
| Angiogenesis | ||
| Catalase | DNA damage and mutagenesis | |
| Induction of inflammation | ||
| Survival of phagocytosis | ||
| Superoxidase dismutase (SOD) | Gastric colonization | |
| Protection from reactive oxygen species | ||
| Inhibition of the synthesis of cytokines | ||
| Activation of macrophages | ||
| Arginase | Apoptosis | |
| Protection from acidity | ||
| Dysregulation of the immune response by inhibition of T and B cells production | ||
| Macrophage apoptosis | ||
| Phospholipases (PLAs) | Degradation of lipids | |
| Lysis of the mucous layer | ||
| Chronic inflammation induction | ||
| Gastric colonization | ||
| Cholesteryl ꭤ-glucosyltransferase | Protection from immune responses and phagocytosis | |
| Secretion of proinflammatory factors | ||
| Antibiotic resistance | ||
| Bacterial growth | ||
| ɤ-glutamyl-transpeptidase (GGT) | Stimulation of the release of SOR | |
| Apoptosis and necrosis | ||
| Induction of the release of proinflammatory factors | ||
| DNA damage | ||
| Decrease in cell viability | ||
| High-temperature-requirement serine protease A (HTRA) | Damage of the gastric epithelium | |
| Bacterial mobility | ||
| Proteins | Cytotoxin-associated gene A (CagA) | Induction of the inflammatory response |
| Bacterial motility | ||
| Activation of fibroblasts | ||
| Oncogenesis (by dysregulation of the RUNX3, ASPP2, CDX1, and AFADIN genes) | ||
| Decrease in microRNA-134, PDCD4, GSK-3 | ||
| Tumor progression by induction of cancer stem cell-like characteristics | ||
| Vacuolating cytotoxin A (VacA) | Induction of autophagy and formation of autophagosomes | |
| Induction of cellular apoptosis and necrosis | ||
| Dysregulation of the immune response by inhibition of T and B cells production | ||
| Outer inflammatory protein A (OipA) | Induction of apoptosis | |
| Induction of the release of proinflammatory factors | ||
| CagA delivery Stimulation of the microRNA-30b level | ||
| Outer membrane protein Q (HopQ) | Bacterial adherence to gastric epithelial cells | |
| Protection from gastric acidity | ||
| Induction of the release of proinflammatory factors | ||
| Inhibition of the immune response | ||
| Outer membrane protein Z | Adherence to gastric epithelial cells | |
| Increase in gastric secretion | ||
| Neutrophil-activating protein (NAP) | Stimulation of neutrophils adhesion to gastric epithelial cells | |
| Heat shock proteins (Hsps) | Maintenance of intact functional and structural characteristics of cellular proteins | |
| Chronic inflammation and angiogenesis | ||
| Adherence to gastric epithelial cells | ||
| Induction of apoptosis autophagy | ||
| Urease activation | ||
| Gastric tumor cells migrations | ||
| Toxins | Lipopolysaccharide (LPS) | Stimulation of the inflammatory response by neutrophil activation |
| Induction of bacterial protection | ||
| Impairment of the gastric mucosa mucus production | ||
| Others | Lewis antigens | Protection from host defense |
| Bacterial protection | ||
| Adhesive proprieties | ||
| Duodenal ulcer promoting gene A (DupA) | Increase in the inflammatory response | |
| Induction of apoptosis (intrinsic pathway) | ||
| Bacterial resistance to the acidic microenvironment |
3.1. Gastric Inflammation Induced by H. pylori Infection
3.2. Gastric Neoplasia Induced by H. pylori Infection
3.3. Extragastric Pathology Induced by H. pylori Infection
4. Alternative and Complementary AgNPs-Based Approaches in the Treatment of Helicobacter pylori Infection
4.1. Therapies Using AgNPs per se in the Eradication of Helicobacter pylori Infections
4.2. Therapies Using AgNPs in Combination with Antibiotics or Embedded in Polymeric Nanocarriers in the Eradication of Helicobacter pylori Infections
5. The Antibacterial Mechanism of Silver Nanoparticles: An Extended Paradigm towards the Treatment of Helicobacter pylori
5.1. Oxidative Stress
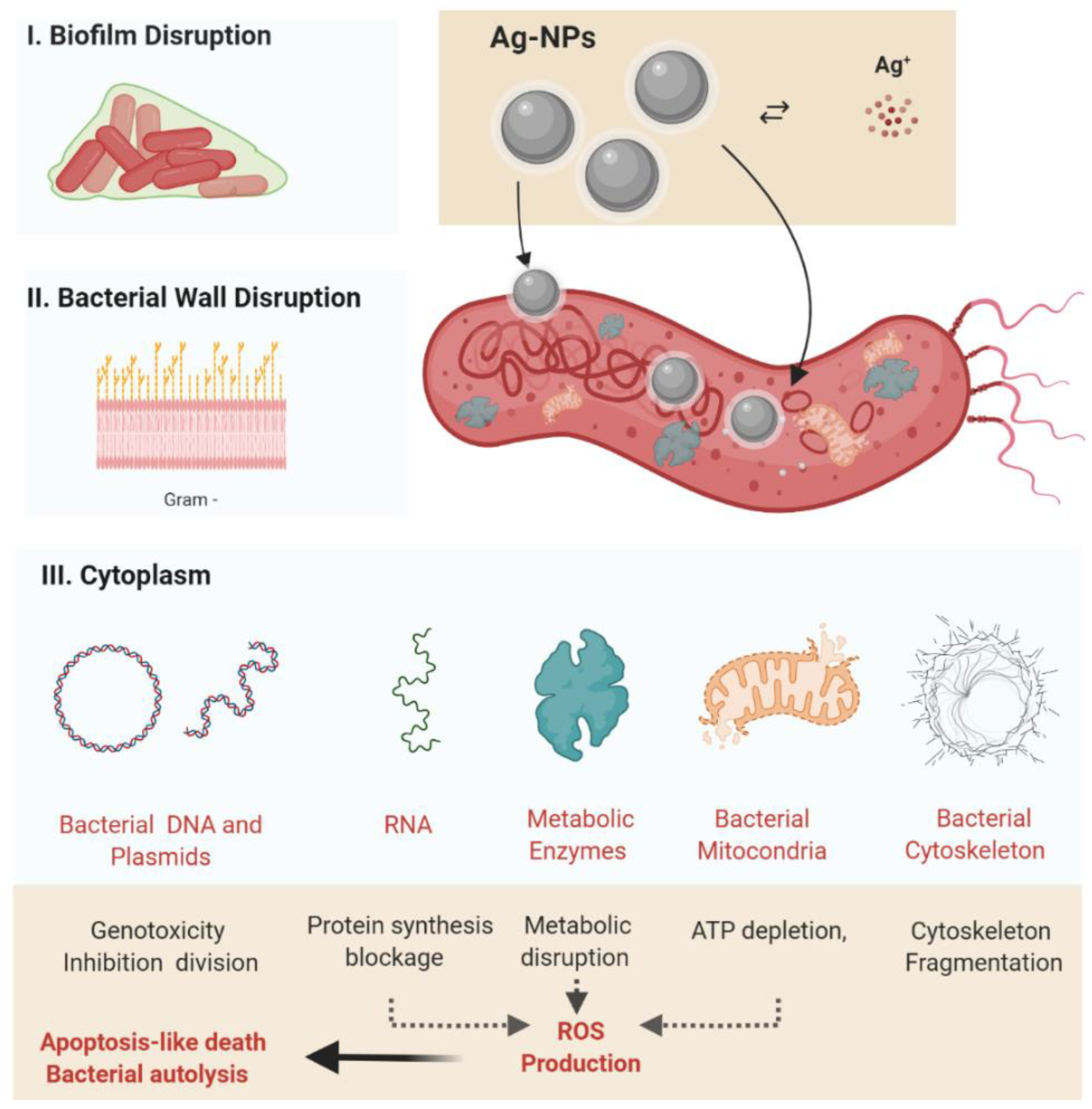
5.2. Release of Metal Ions
5.3. Stages of Antibacterial Activity of Silver Nanoparticles
5.3.1. The Interaction of Nanoparticles with the Bacterial Cell Wall
5.3.2. Inhibition of Bacterial Protein and DNA Synthesis
5.4. In Vitro Experiments Assessing the Anti-H. pylori Activity of Silver Nanoparticles
5.5. In Vivo Experiments Assessing the Anti-H. pylori Activity of Silver Nanoparticles
6. Other Metal-Based Nanoparticles Used in the Treatment of Helicobacter pylori Infection
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The puzzle of coccoid forms of Helicobacter pylori: Beyond basic science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Gao, W.; Obonyo, M.; Zhang, L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 17600–17605. [Google Scholar] [CrossRef]
- Wolle, K.; Malfertheiner, P. Treatment of Helicobacter pylori. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 315–324. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Cristofari, F.; Andriani, A.; De Francesco, V.; Ierardi, E.; Tomao, S.; Stolte, M.; Morini, S.; Vaira, D. Effects of Helicobacter pylori eradication on early stage gastric mucosa–associated lymphoid tissue lymphoma. Clin. Gastroenterol. Hepatol. 2010, 8, 105–110. [Google Scholar] [CrossRef]
- Franceschi, F.; Gasbarrini, A. Helicobacter pylori and extragastric diseases. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-S.; Yang, T.-Y.; Shen, W.-C.; Lin, C.-L.; Lin, M.-C.; Kao, C.-H. Association between Helicobacter pylori infection and dementia. J. Clin. Neurosci. 2014, 21, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, H.; Wu, Y.; Zhang, D.; Jiang, H. Meta-analysis: Association of Helicobacter pylori infection with Parkinson’s diseases. Helicobacter 2017, 22, e12398. [Google Scholar] [CrossRef]
- Álvarez-Arellano, L.; Maldonado-Bernal, C. Helicobacter pylori and neurological diseases: Married by the laws of inflammation. World J. Gastrointest. Pathophysiol. 2014, 5, 400. [Google Scholar] [CrossRef]
- Blecker, U.; Renders, F.; Lanciers, S.; Vandenplas, Y. Syncopes leading to the diagnosis of a Helicobacter pylori positive chronic active haemorrhagic gastritis. Eur. J. Pediatr. 1991, 150, 560–561. [Google Scholar] [CrossRef]
- Mendall, M.A.; Goggin, P.M.; Molineaux, N.; Levy, J.; Toosy, T.; Strachan, D.; Camm, A.J.; Northfield, T.C. Relation of Helicobacter pylori infection and coronary heart disease. Heart 1994, 71, 437–439. [Google Scholar] [CrossRef]
- Boutari, C.; Perakakis, N.; Mantzoros, C.S. Association of adipokines with development and progression of nonalcoholic fatty liver disease. Endocrinol. Metab. 2018, 33, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Sung, J.J. Helicobacter pylori and benign upper digestive disease. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Argent, R.H.; Atherton, J.C. The inflammatory and immune response to Helicobacter pylori infection. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.I.; Wu, D.-C.; Wu, J.-Y.; Graham, D.Y. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter 2011, 16, 146. [Google Scholar] [CrossRef]
- Zullo, A.; De Francesco, V.; Hassan, C.; Morini, S.; Vaira, D. The sequential therapy regimen for Helicobacter pylori eradication: A pooled-data analysis. Gut 2007, 56, 1353–1357. [Google Scholar] [CrossRef]
- O’Morain, C.; Smith, S. Helicobacter pylori treatment failure: The rationale for alternative antibiotics. Digestion 2016, 93, 309–310. [Google Scholar] [CrossRef]
- Pandya, H.B.; Agravat, H.H.; Patel, J.S.; Sodagar, N.R.K. Emerging antimicrobial resistance pattern of Helicobacter pylori in central Gujarat. Indian J. Med. Microbiol. 2014, 32, 408–413. [Google Scholar] [CrossRef]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, Z.F.; Wang, Y.H.; Wang, H.; Liu, X.-Q.; Xie, Y.; Zhou, X.-J. Standard triple therapy for Helicobacter pylori infection in China: A meta-analysis. World J. Gastroenterol. 2014, 20, 14973–14985. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; He, L.; Ding, Z.; Gu, Y.; Zhang, J.; Zhou, L. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter 2011, 16, 356–362. [Google Scholar] [CrossRef]
- Dang, B.N.; Graham, D.Y. Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H. Inadequate antimicrobial treatment: An important determinant of outcome for hospitalized patients. Clin. Infect.Dis. 2000, 31 (Suppl. S4), S131–S138. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Clinical impact and relevance of antibiotic resistance. Adv. Drug Deliv.Rev. 2005, 57, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Rodriguez, T.; Silva, F.M.; Barbuti, R.C.; Mattar, R.; Moraes-Filho, J.P.; de Oliveira, M.N.; Bogsan, C.S.; Chinzon, D.; Eisig, J.N. Association of a Probiotic to a Helicobacter pylori Eradication Regimen Does Not Increase efficacy or decreases the adverse effects of the treatment: A prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 56. [Google Scholar] [CrossRef]
- Akcam, M.; Koca, T.; Salman, H.; Karahan, N. The effects of probiotics on treatment of Helicobacter pylori eradication in children. Saudi Med. J. 2013, 36, 286. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.-H. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front. Cell. Infect. Microbiol. 2017, 7, 168. [Google Scholar] [CrossRef]
- Ko, S.W.; Kim, Y.; Chung, W.C.; Lee, S.J. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter 2019, 24, e12565. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, D.J.; Chung, J.-W. Antibiotic treatment for Helicobacter pylori: Is the end coming? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Iqbal, M.S.; Hughes, R.W.; Khan, S.A.; Reynolds, P.A.; Enne, V.I.; Sajjad-ur-Rahman Mirza, A.S. Mechanochemical synthesis and in vitro anti-Helicobacter pylori and uresase inhibitory activities of novel zinc (II)–famotidine complex. J. Enzyme Inhib. Med. Chem. 2010, 25, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bruggraber, S.F.; French, G.; Thompson, R.P.; Powell, J.J. Selective and effective bactericidal activity of the cobalt (II) cation against Helicobacter pylori. Helicobacter 2004, 9, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomedicine 2017, 12, 3941. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; MubarakAli, D.; Loke, M.F.; Thajuddin, N.; Alharbi, N.S.; Yadavalli, T.; Alagiri, M.; Vadivelu, J. Anti-Helicobacter pylori, cytotoxicity and catalytic activity of biosynthesized gold nanoparticles: Multifaceted application. Arab. J. Chem. 2019, 12, 33–40. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; You-Sheng, O.-Y.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Geng, W.; Jiang, N.; Qing, G.-Y.; Liu, X.; Wang, L.; Busscher, H.J.; Tian, G.; Sun, T.; Montelongo, Y.; Janiak, C.; et al. Click reaction for reversible encapsulation of single yeast cells. ACS Nano 2019, 13, 14459–14467. [Google Scholar] [CrossRef]
- Geng, W.; Wang, L.; Jiang, N.; Cao, J.; Xiao, Y.-X.; Wei, H.; Yetisen, A.K.; Yang, X.-Y.; Su, B.-L. Single cells in nanoshells for the functionalization of living cells. Nanoscale 2018, 10, 3112–3129. [Google Scholar] [CrossRef]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef]
- Bao, C.; Beziere, N.; del Pino, P.; Pelaz, B.; Estrada, G.; Tian, F.; Ntziachristos, V.; de la Fuente, J.M.; Cui, D. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small 2013, 9, 68–74. [Google Scholar] [CrossRef]
- Chen, N.-T.; Tang, K.-C.; Chung, M.-F.; Cheng, S.-H.; Huang, C.-M.; Chu, C.-H.; Chou, P.-T.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; et al. Enhanced plasmonic resonance energy transfer in mesoporous silica-encased gold nanorod for two-photon-activated photodynamic therapy. Theranostics 2014, 4, 798. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://prisma-statement.org (accessed on 27 June 2022).
- Blaser, M. The role of Helicobacter pylori in gastritis and its progression to peptic ulcer disease. Aliment. Pharmacol. Ther. 1995, 9, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Audibert, C.; Burucoa, C.; Janvier, B.; Fauchere, J. Implication of the structure of the Helicobacter pylori cag pathogenicity island in induction of interleukin-8 secretion. Infect. Immun. 2001, 69, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Sillén, A.; Eriksson, L.; Strand, M.-L.; Enroth, H.; Normark, S.; Falk, P.; Engstrand, L. Correlation between cag pathogenicity island composition and Helicobacter pylori-associated gastroduodenal disease. Infect. Immun. 2003, 71, 6573–6581. [Google Scholar] [CrossRef]
- Hacker, J.; Blum-Oehler, G.; Mühldorfer, I.; Tschäpe, H. Pathogenicity islands of virulent bacteria: Structure, function and impact on microbial evolution. Mol. Microbiol. 1997, 23, 1089–1097. [Google Scholar] [CrossRef]
- Queiroz, D.; Mendes, E.; Rocha, G.; Moura, S.; Resende, L.; Barbosa, A.; Coelho, L.; Passos, M.; Castro, L.; Oliveira, C. Effect of Helicobacter pylori eradication on antral gastrin-and somatostatin-immunoreactive cell density and gastrin and somatostatin concentrations. Scand. J. Gastroenterol. 1993, 28, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Sheu, B.-S.; Wu, J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Nomura, A.M.; Pérez-Pérez, G.I.; Lee, J.; Stemmermann, G.; Blaser, M.J. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am. J. Epidemiol. 2002, 155, 1054–1059. [Google Scholar] [CrossRef]
- Cover, T.L. The vacuolating cytotoxin of Helicobacter pylori. Mol. Microbiol. 1996, 20, 241–246. [Google Scholar] [CrossRef]
- Park, S.M.; Park, J.; Kim, J.G.; Yoo, B.C. Relevance of vacA genotypes of Helicobacter pylori to cagA status and its clinical outcome. Korean J. Intern. Med. 2001, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Miehlke, S.; Yu, J.; Schuppler, M.; Frings, C.; Kirsch, C.; Negraszus, N.; Morgner, A.; Stolte, M.; Ehninger, G.; Bayerdörffer, E. Helicobacter pylori vacA, iceA, and cagA status and pattern of gastritis in patients with malignant and benign gastroduodenal disease. Am. J. Gastroenterol. 2001, 96, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.M., Jr.; Thompson, S.A.; Donahue, J.P.; Tham, K.T.; Atherton, J.C.; Blaser, M.J.; Miller, G.G. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc. Assoc. Am. Physicians 1998, 110, 531–544. [Google Scholar]
- Ernst, P.B.; Gold, B.D. The disease spectrum of Helicobacter pylori: The immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 2000, 54, 615–640. [Google Scholar] [CrossRef]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.J.; Mobley, H.L. Pathogenesis of Helicobacter pylori infection. Curr. Opin. Gastroenterol. 2000, 16, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Radosz-Komoniewska, H.; Bek, T.; Jóźwiak, J.; Martirosian, G. Pathogenicity of Helicobacter pylori infection. Clin. Microbiol. Infect. 2005, 11, 602–610. [Google Scholar] [CrossRef]
- Chang, W.L.; Yeh, Y.C.; Sheu, B.S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 68. [Google Scholar] [CrossRef]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M. H. pylori virulence factors: Influence on immune system and pathology. Mediat. Inflamm. 2014, 2014, 426309. [Google Scholar] [CrossRef]
- Lee, S. Future candidates for indications of Helicobacter pylori eradication: Do the indications need to be revised? J. Gastroenterol. Hepatol. 2012, 27, 200–211. [Google Scholar] [CrossRef]
- Lee, S.Y. Endoscopic gastritis: What does it mean? Dig. Dis.Sci. 2011, 56, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Leib, M. Therapy of GI Diseases: What’s New with Antiemetics, Antacids, and Probiotics. Available online: http://www.dvm360storage.com/cvc/proceedings/dc/Gastrointestinal%20Medicine/Leib/Leib,%20Michael_Therapy_GI_diseases_STYLED.pdf (accessed on 31 May 2021).
- Guilford, W.G.; Center, S.A.; Strombeck, D.R.; Williams, D.A.; Meyer, D.J. Strombeck’s Small Animal Gastroenterology; WB Saunders Co.: Philadelphia, PA, USA, 1990. [Google Scholar]
- Westblom, T.U.; Czinn, S.J.; Nedrud, J.G. Gastroduodenal Disease and Helicobacter pylori: Pathophysiology, Diagnosis and Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Blok, P.; Craanen, M.E.; Johan Offerhaus, G.; Dekker, W.; Kuipers, E.J.; Meuwissen, S.G.; Tytgat, G.N. Molecular alterations in early gastric carcinomas: No apparent correlation with Helicobacter pylori status. Am. J. Clin. Pathol. 1999, 111, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Villako, K.; Kekki, M.; Maaroos, H.-I.; Sipponen, P.; Uibo, R.; Tammur, R.; Tamm, A. Chronic gastritis: Progression of inflammation and atrophy in a six-year endoscopic follow-up of a random sample of 142 Estonian urban subjects. Scand. J. Gastroenterol. 1991, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Watanabe, N.; Hiraishi, H.; Terano, A. Redox regulation of interleukin-8 expression in MKN28 cells. Dig. Dis. Sci. 1999, 44, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Montalban, C.; Santon, A.; Redondo, C.; García-Cosio, M.; Boixeda, D.; Vazquez-Sequeiros, E.; Norman, F.; de Argila, C.; Alvarez, I.; Abraira, V. Long-term persistence of molecular disease after histological remission in low-grade gastric MALT lymphoma treated with H. pylori eradication. Lack of association with translocation t (11; 18): A 10-year updated follow-up of a prospective study. Ann. Oncol. 2005, 16, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Ruskoné-Fourmestraux, A. Les lymphomes gastriques du MALT. Rev. Médecine Interne 2004, 25, 573–581. [Google Scholar] [CrossRef]
- Hussell, T.; Isaacson, P.G.; Crabtree, J.; Dogan, A.; Spencer, J. Immunoglobulin specificity of low grade B cell gastrointestinal lymphoma of mucosa-associated lymphoid tissue (MALT) type. Am. J. Pathol. 1993, 142, 285. [Google Scholar]
- Wotherspoon, A. Gastric MALT lymphoma and Helicobacter pylori. Yale J. Biol. Med. 1996, 69, 61. [Google Scholar]
- Lai, C.-Y.; Yang, T.-Y.; Lin, C.-L.; Kao, C.-H. Helicobacter pylori infection and the risk of acute coronary syndrome: A nationwide retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 69–74. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Shi, S. Helicobacter pylori infection increase the risk of myocardial infarction: A meta-analysis of 26 studies involving more than 20,000 participants. Helicobacter 2015, 20, 176–183. [Google Scholar] [CrossRef]
- Lenzi, C.; Palazzuoli, A.; Giordano, N.; Alegente, G.; Gonnelli, C.; Campagna, M.S.; Santucci, A.; Sozzi, M.; Papakostas, P.; Rollo, F. H. pylori infection and systemic antibodies to CagA and heat shock protein 60 in patients with coronary heart disease. World J. Gastroenterol. WJG 2006, 12, 7815. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, Y.; Ariyama, I.; Hamada, M.; Otaguro, S.; Machi, T.; Taira, Y.; Hayashi, J. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT). Atherosclerosis 2005, 178, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Mizuno, M.; Take, S.; Suwaki, K.; Honda, T.; Kawai, K.; Fujita, M.; Tamura, T.; Yokota, K.; Oguma, K. Eradication of Helicobacter pylori increases platelet count in patients with idiopathic thrombocytopenic purpura in Japan. Eur. J. Clin. Investig. 2005, 35, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Tsuzuki, T.; Mizuno, T.; Minami, M.; Ina, K.; Kusugami, K.; Takamatsu, J.; Adachi, K.; El-Omar, E.; Ohta, M. Characteristics of Helicobacter pylori-induced gastritis and the effect of H. pylori eradication in patients with chronic idiopathic thrombocytopenic purpura. Helicobacter 2004, 9, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.; Kuwana, M.; Kurata, Y.; Imamura, M.; Harada, H.; Sakamaki, H.; Teramura, M.; Koda, K.; Nomura, S.; Sugihara, S. Is eradication therapy useful as the first line of treatment in Helicobacter pylori-positive idiopathic thrombocytopenic purpura? Analysis of 207 eradicated chronic ITP cases in Japan. Int. J. Hematol. 2005, 81, 162–168. [Google Scholar] [CrossRef]
- Hynes, S.; McGuire, J.; Wadstrom, T. The effect of bile on protein expression by intestinal Helicobacters, determined using ProteinChip (r) Technology. Gut 2001, 49, A67. [Google Scholar]
- Leong, R.; Sung, J. Helicobacter species and hepatobiliary diseases. Aliment. Pharmacol. Ther. 2002, 16, 1037–1045. [Google Scholar] [CrossRef]
- Harvey, P.; Upadhya, G.A.; Strasberg, S. Immunoglobulins as nucleating proteins in the gallbladder bile of patients with cholesterol gallstones. J. Biol. Chem. 1991, 266, 13996–14003. [Google Scholar] [CrossRef]
- Swidsinski, A.; Lee, S.P. The role of bacteria in gallstone pathogenesis. Front. Biosci. 2001, 6, 93–103. [Google Scholar] [CrossRef]
- Stewart, L.; Ponce, R.; Oesterk, A.L.; Griffiss, J.M.; Way, L.W. Pigment gallstone pathogenesis: Slime production by biliary bacteria is more important than beta-glucuronidase production. J. Gastrointest. Surg. 2000, 4, 547–553. [Google Scholar] [CrossRef]
- Ki, M.-R.; Goo, M.-J.; Park, J.-K.; Hong, I.-H.; Ji, A.-R.; Han, S.-Y.; You, S.-Y.; Lee, E.-M.; Kim, A.-Y.; Park, S.-J. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β 1-induced inflammatory signaling. Lab. Investig. 2010, 90, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Franceschi, F.; Nishizawa, T.; Gasbarrini, A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 2011, 16, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, D.; Cannan, R.; Stubbs, R. Common presence of Helicobacter DNA in the gallbladder of patients with gallstone diseases and controls. Dig. Liver Dis. 2003, 35, 237–243. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Carloni, E.; Gasbarrini, G.; Ménard, A. Helicobacter pylori and extragastric diseases–other Helicobacters. Helicobacter 2003, 8, 68–76. [Google Scholar] [CrossRef][Green Version]
- dela Pena-Ponce, M.G.; Jimenez, M.T.; Hansen, L.M.; Solnick, J.V.; Miller, L.A. The Helicobacter pylori type IV secretion system promotes IL-8 synthesis in a model of pediatric airway epithelium via p38 MAP kinase. PLoS ONE 2017, 12, e0183324. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, T.; Shang, S.; Chen, H.; Kao, C.; Wu, C.; Yang, T. Helicobacter pylori infection increases the risk of adult-onset asthma: A nationwide cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, J.-J.; Kwon, Y.; Kim, J.-H.; Sohn, J.-H. Clinical implications of associations between headache and gastrointestinal disorders: A study using the hallym smart clinical data warehouse. Front. Neurol. 2017, 8, 526. [Google Scholar] [CrossRef]
- Yang, G.-T.; Zhao, H.-Y.; Kong, Y.; Sun, N.-N.; Dong, A.-Q. Correlation between serum vitamin B12 level and peripheral neuropathy in atrophic gastritis. World J. Gastroenterol. 2018, 24, 1343. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, Y.; Li, X.; Liuyang, Z.; Shentu, Y.; Jing, X.; Liang, J.; Zhou, X.; Wang, X.; Wang, J. Long-term Helicobacter pylori infection does not induce tauopathy and memory impairment in SD rats. Curr. Med. Sci. 2017, 37, 823–827. [Google Scholar] [CrossRef]
- Lewinska, A.; Wnuk, M. Helicobacter pylori-induced premature senescence of extragastric cells may contribute to chronic skin diseases. Biogerontology 2017, 18, 293–299. [Google Scholar] [CrossRef]
- Bakry, O.A.; Basha, M.; El Hefnawy, S.; Mekkawy, S. Relationship between disease activity and Helicobacter pylori infection in patients with vitiligo. Indian Dermatol. Online J. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Ražuka-Ebela, D.; Giupponi, B.; Franceschi, F. Helicobacter pylori and extragastric diseases. Helicobacter 2018, 23, e12520. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, W.; Zhang, J.; Xia, X. Eradication of Helicobacter pylori: The power of nanosized formulations. Nanomedicine 2020, 15, 527–542. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, B.A.F.; Silva, D.E.S.; da Silva, A.N.; Campos, D.L.; Ribeiro, T.R.M.; Mieli, M.J.; Zanatta, M.B.T.; da Silva, P.B.; Pavan, F.R.; Moreira, C.G.; et al. New silver (I) coordination compound loaded into polymeric nanoparticles as a strategy to improve in vitro anti-Helicobacter pylori activity. Mol. Pharm. 2020, 17, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Saffari, M.; Moniri, R.; Sedaghat, H.; Moosavi, G.A.; Molaghanbari, M.; Razavizade, M. Investigation of the Effect of Silver Nanoparticles alone and Their Combination with Clarithromycin on H. pylori Isolates. 2022. Available online: https://assets.researchsquare.com/files/rs-1631922/v1/6ec0820c-530c-443f-adb4-097c17d48bf8.pdf?c=1653660296 (accessed on 27 June 2022).
- Walker, B.; Barrett, S.; Polasky, S.; Galaz, V.; Folke, C.; Engström, G.; Ackerman, F.; Arrow, K.; Carpenter, S.; Chopra, K. Looming global-scale failures and missing institutions. Science 2009, 325, 1345–1346. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Gaynes, R.; National Nosocomial Infections Surveillance System. The impact of antimicrobial-resistant, health care–associated infections on mortality in the United States. Clin. Infect. Dis. 2008, 47, 927–930. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.-T.; Sun, H. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Yang, X.; Xu, X.; Hao, Q.; Yan, A.; Hu, M.; Lobinski, R.; Li, H.; Sun, H. Antimicrobial silver targets glyceraldehyde-3-phosphate dehydrogenase in glycolysis of E. coli. Chem. Sci. 2019, 10, 7193–7199. [Google Scholar] [CrossRef]
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Review of materials and fabrication methods for flexible nano and micro-scale physical and chemical property sensors. Appl. Sci. 2021, 11, 8563. [Google Scholar] [CrossRef]
- Cassir, N.; Rolain, J.-M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Pal, T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J. Nanosci. Nanotechnol. 2007, 7, 2151–2156. [Google Scholar] [CrossRef]
- Awad, M.A.; Eisa, N.E.; Virk, P.; Hendi, A.A.; Ortashi, K.M.O.O.; Mahgoub, A.S.A.; Elobeid, M.A.; Eissa, F.Z. Green synthesis of gold nanoparticles: Preparation, characterization, cytotoxicity, and anti-bacterial activities. Mater. Lett. 2019, 256, 126608. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227. [Google Scholar] [CrossRef]
- Zhao, L.; Ashraf, M. Influence of silver-hydroxyapatite nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med. J. 2015, 64, 506. [Google Scholar]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Tăbăran, A.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver nanoparticles for the therapy of tuberculosis. Int. J. Nanomed. 2020, 15, 2231. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Li, Y.; Wang, G.; Yang, L.; Jin, J.; Chen, Q.; Huang, M. Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial. Biomed. Mater. 2014, 10, 015001. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Geilich, B.M.; Webster, T.J. Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomed. 2014, 9, 3801. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Chen, Y.; Li, M.; Liu, K. Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 2015, 5, 15824. [Google Scholar] [CrossRef]
- Grande, R.; Sisto, F.; Puca, V.; Carradori, S.; Ronci, M.; Aceto, A.; Muraro, R.; Mincione, G.; Scotti, L. Antimicrobial and antibiofilm activities of new synthesized Silver Ultra-NanoClusters (SUNCs) against Helicobacter pylori. Front. Microbiol. 2020, 11, 1705. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci. Rep. 2019, 9, 5787. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A. In Vitro Study Of Antimicrobial Activity Of Silver Nanoparticles Usage (Curcuma Longa L.) Rhizomes Against Helicobacter pylori. Plant Arch. 2019, 19, 1102–1106. [Google Scholar]
- Prasad, A.; Baker, S.; Prasad, M.N.; Devi, A.T.; Satish, S.; Zameer, F.; Shivamallu, C. Phytogenic synthesis of silver nanobactericides for anti-biofilm activity against human pathogen H. pylori. SN Appl. Sci. 2019, 1, 341. [Google Scholar] [CrossRef]
- Sampath, G.; Shyu, D.J.; Rameshkumar, N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Synthesis and Characterization of Pyrogallol Capped Silver Nanoparticles and Evaluation of Their In Vitro Anti-Bacterial, Anti-cancer Profile against AGS Cells. J. Clust. Sci. 2020, 32, 549–557. [Google Scholar] [CrossRef]
- Amin, M.; Hameed, S.; Ali, A.; Anwar, F.; Shahid, S.A.; Shakir, I.; Yaqoob, A.; Hasan, S.; Khan, S.A. Green synthesis of silver nanoparticles: Structural features and in vivo and in vitro therapeutic effects against Helicobacter pylori induced gastritis. Bioinorg. Chem. Appl. 2014, 2014, 135824. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Masanam, E.; Ramkumar, V.S.; Baskaraligam, V.; Selvaraj, G. Influence of N-acylhomoserine lac-tonase silver nanoparticles on the quorum sensing system of Helicobacter pylori: A potential strategy to combat biofilm formation. J. BasicMicrobiol. 2020, 60, 207–215. [Google Scholar]
- Jang, Y.; Zhang, X.; Zhu, R.; Li, S.; Sun, S.; Li, W.; Liu, H. Viola betonicifolia-Mediated Biosynthesis of Silver Nanoparticles for Improved Biomedical Applications. Front.Microbiol. 2022, 13, 891144. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Lu, C.-Y.; Yang, Y.-C.; Chin, C.; Weng, B.-C.; Liu, C.-J.; Chen, Y.-H.; Chang, L.-L.; Kuo, F.-C.; Wu, D.-C. Does Long-Term Use of Silver Nanoparticles Have Persistent Inhibitory Effect on H. pylori Based on Mongolian Gerbil’s Model? BioMed. Res. Int. 2014, 2014, 461034. [Google Scholar] [CrossRef] [PubMed]
- Borase, H.P.; Salunkhe, R.B.; Patil, C.D.; Suryawanshi, R.K.; Salunke, B.K.; Wagh, N.D.; Patil, S.V. Innovative approach for urease inhibition by Ficus carica extract–fabricated silver nanoparticles: An in vitro study. Biotechnol. Appl. Biochem. 2015, 62, 780–784. [Google Scholar] [CrossRef]
- Al-Bahrani, R.M.; Radif, H.M.; Albaayit, S.F.A. Evaluation of potent silver nanoparticles production from Agaricus bisporus against Helicobacter pylori. Pak. J. Agri. Sci. 2020, 57, 1197–1201. [Google Scholar]
- Sampath, G.; Govarthanan, M.; Sridharan, K.; Prabhusaran, N.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K. Isolation and identification of metronidazole resistance Helicobacter pylori from gastric patients in the southeastern region of India and its advanced antibacterial treatment using biological silver oxide nanoparticles. Biochem. Eng. J. 2022, 108445. [Google Scholar] [CrossRef]
- Lopes, D.; Nunes, C.; Martins MC, L.; Sarmento, B.; Reis, S. Eradication of Helicobacter pylori: Past, present and future. J. Control Release 2014, 189, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of bismuth in the eradication of Helicobacter pylori. Am. J.Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; So, M.H.; Yang, J.; Deng, F.; Che, C.M.; Sun, H. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem.Commun. 2006, 21, 2265–2267. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bhattacharya, S.; Chowdhury, R.; Chakrabarti, P. The molecular basis of inactivation of metronidazole-resistant Helicobacter pylori using polyethyleneimine functionalized zinc oxide nanoparticles. PLoS ONE 2013, 8, e70776. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Gong, M.; Lin, Y.; Xu, Y.; Ye, L.; Yu, X.; Liu, J.; Liu, J.; He, B.; et al. Synergistic effects of nanoparticle heating and amoxicillin on H. pylori inhibition. J. Magn. Magn. Mater. 2019, 485, 95–104. [Google Scholar] [CrossRef]
- Umamaheshwari, R.; Jain, N. Receptor mediated targeting of lectin conjugated gliadin nanoparticles in the treatment of Helicobacter pylori. J. Drug Target. 2003, 11, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Huang, W.-Y.; Lai, C.-H.; Hsu, Y.-M.; Yao, Y.-H.; Chen, T.-Y.; Wu, J.-Y.; Peng, S.-F.; Lin, Y.-H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef] [PubMed]


| Helicobacter Species/Strain | Experimental Model | Nanoformulation | Tested Doses | NP Shape and Size Distribution | Effect on Bacteria | References | |
|---|---|---|---|---|---|---|---|
| 1. | H. pylori (NCTC 11638 strains) | Bacterial culture | AgNPs (extract of Solanum xanthocarpum) | 2 μg/mL−1 MIC | Spherical 4–18 nm | Inactivation of H. pylori urease | [32] |
| 2. | H. pylori (clinical isolates from humans) | Bacterial culture | AgNPs (extract of Solanum xanthocarpum) | 2–8 μg/mL−1 MIC | Spherical 4–18 nm | Inactivation of H. pylori urease | [126] |
| 3. | H. pylori I (NCTC 11637 strains) | Bacterial culture | AgNPs (extract of Solanum xanthocarpum) | 4 μg/mL−1 MIC | Spherical 4–18 nm | Inactivation of H. pylori urease | [126] |
| 4. | H. pylori (NCTC 11637 NCTC 11638) | Bacterial culture | AgNPs (P. harmala seeds) | 4.0 μg/mL (NCTC 11637) 8.0 μg/mL (NCTC 11638) MIC | Spherical | Inhibition of growth | [131] |
| 5. | H. pylori (GS-13 Strain) | Bacterial culture | AgNPs (leaf extract of A. priceps) | 5.0 μg/mL MIC | Spherical 5 ± 30 nm (average, 20 nm) | Inhibition of growth Loss of viability ROS production DNA fragmentation | [125] |
| 6. | Helicobacter felis (GS-14 strain) | Bacterial culture | AgNPs (leaf extract of A. priceps) | 5.5 μg/mL MIC | Spherical 5 ± 30 nm (average, 20 nm) | Inhibition of growth Loss of viability ROS production DNA fragmentation | [125] |
| 7. | H. pylori (UM066), H. pylori J99, and H. pylori NCTC 11637 | Bacterial clinical isolates | AgNPs (soil-derived Pseudomonas putida MVP2) | 24 μg/mL | Spherical 6–16 nm | Inhibition of growth | [132] |
| 8. | H. pylori (MH179988) | Bacterial culture | Tv-AgNPs (extract of Toxicodendron vernicifluum) | 18.14 μg/mL−1 MIC | Spherical 2–40 nm | Inhibition of growth Damage to the bacterial membrane ROS production | [127] |
| 9. | H. pylori (BHI) | Bacterial culture | AgNPs (extract of Curcuma longa) | N/A dosage data | Spherical No size data | Inhibition of growth | [128] |
| 10. | H. pylori biofilm | Bacterial culture | AgNPs (extract of Acorus calamus L.) | 350 μg/mL (highest activity) | Spherical and near-spherical 5 to 60 nm | Inhibition of growth | [129] |
| 11. | H. pylori (ATCC 26695). | Bacterial culture | py-AgNPs (Acacia nilotica leaf extract-mediated compound pyrogallol) | 10–40 μg/mL | Spherical 22.68–55.16 nm | Antioxidant activity | [130] |
| 12. | H. pylori (ATCC 43504) | Bacterial culture | Silver ultrananoclusters | 0.16 to 0.33 mg/L | Non-spherical shape 1.83 ± 1.57 nm | Inhibition of growth | [124] |
| 13. | H. pylori ATCC 43504 | Bacterial culture | AiiA-AgNPs | 1–5 μM | No shape and size data | Inhibition of growth | [133] |
| 14. | Urease (from jack beans) | Bacterial culture | AgNPs (leaf extract of Ficus carica) | N/A dosage data | Spherical 21 nm (average size) | Urease inhibition | [137] |
| 15. | H. pylori strain 43504 | Bacterial culture | Ag (PhTSC∙HCl)2 (BPN: base polymeric nanoparticle) | 3.90 μg/mL MIC | Spherical shape No size data | Inhibition of growth | [97] |
| 16. | Helicobacter pylori (ATCC 43504TM) | Bacterial culture | LEVB-AgNAPs (leaf extract of Viola betonicifolia) | 120 μg/mL | 6–11 nm No shape data | Inhibition of growth | [134] |
| Helicobacter Species/Strain | Experimental Model | Nanoformulation | Tested Doses | NP Shape and Size Distribution | Effect on Bacteria | References | |
|---|---|---|---|---|---|---|---|
| 1 | H. pylori (NCTC 11637) | Male albino Wistar rats (72–112 days and weight of 295 ± 4.1 g) | AgNPs (P. harmala seeds) | 1–32 μg/mL MIC | Spherical 15–18 nm | Inhibition of growth | [131] |
| 2 | H. pylori (metronidazole-resistanr strain) | 50 biopsy samples | Ag2ONPs (Digiria muricata) | 25–100 μg/mL | 11–35.6 nm | Inhibition of growth | [139] |
| 3 | H. pylori (no strain data) | Eight-week-old gerbils with a bodyweight of 30–40 gm | AgNPs (lucentite SWN clay slurry) | 0.1% weight | No shape and size data | Inhibition of growth | [136] |
| 4. | H. pylori (no strain data) | 50 biopsies from patients with duodenal ulcer | AB-AgNPs (extract of A. bisporus) | 25–100 mg/mL | No shape and size data | Inhibition of growth | [138] |
| 5. | H. pylori (no strain data) | 40 gastric biopsies | AgNPs | 31.25–250 μg/mL MIC | Sspherical 5–8 nm | Inhibition of growth | [99] |
| 6. | H. pylori (no strain data) | 40 gastric biopsies | AgNPs in combination with clarithromycin | 31.25–125 µg/m MIC | Spherical 5–8 nm | Inhibition of growth | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, R.; Tăbăran, A.-F.; Ungur, A.P.; Negoescu, A.; Cătoi, C. Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles. Pharmaceutics 2022, 14, 1463. https://doi.org/10.3390/pharmaceutics14071463
Pop R, Tăbăran A-F, Ungur AP, Negoescu A, Cătoi C. Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles. Pharmaceutics. 2022; 14(7):1463. https://doi.org/10.3390/pharmaceutics14071463
Chicago/Turabian StylePop, Romelia, Alexandru-Flaviu Tăbăran, Andrei Paul Ungur, Andrada Negoescu, and Cornel Cătoi. 2022. "Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles" Pharmaceutics 14, no. 7: 1463. https://doi.org/10.3390/pharmaceutics14071463
APA StylePop, R., Tăbăran, A.-F., Ungur, A. P., Negoescu, A., & Cătoi, C. (2022). Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles. Pharmaceutics, 14(7), 1463. https://doi.org/10.3390/pharmaceutics14071463






