Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study
Abstract
1. Introduction
2. Material and Methods
2.1. Drug and Chemicals
2.2. Drug and Excipient Compatibility Studies
2.2.1. Thermal Analysis
2.2.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. Preparation of Solid Lipid Nanoparticles (SLNs)
2.4. Characterization of SLNs
2.4.1. Particle Size, Polydispersity Index and Zeta Potential Measurement
2.4.2. Percent Drug Loading
2.4.3. Entrapment Efficiency
2.5. Preparation of Lyophilized SLNs
2.6. Characterization of Lyophilized SLNs
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. X-ray Diffraction Analysis (XRD)
2.7. Formulation of the TH-Loaded SLNs Hydrogel
2.8. Characterization of the TH-Loaded SLN-Based Hydrogel
2.8.1. Measurement of Viscosity and pH
2.8.2. Extrudability and Spreadability
2.8.3. Determination of Gel Strength
2.9. Determination of Drug Content
2.10. In Vitro Drug Release
2.11. ExVivo Skin Permeation Study
2.12. Evaluation of Antifungal Efficacy of Formulation
2.12.1. In Vitro Antifungal Activity
2.12.2. In Vivo Antifungal Activity
2.13. Stability Study
2.14. Statistical Analysis
3. Results and Discussion
3.1. Drug and Excipient Compatibility Study
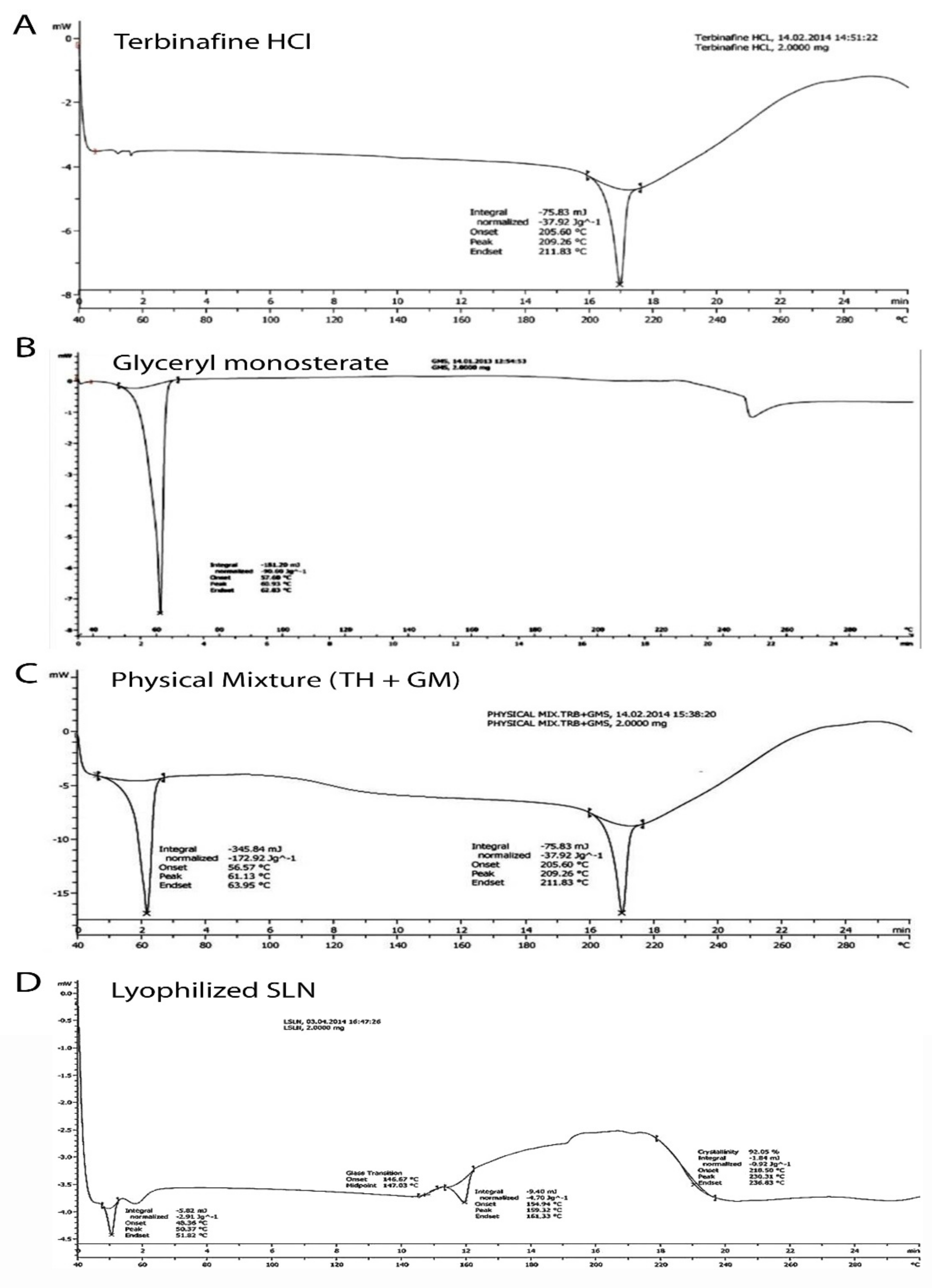
3.1.1. Thermal Analysis
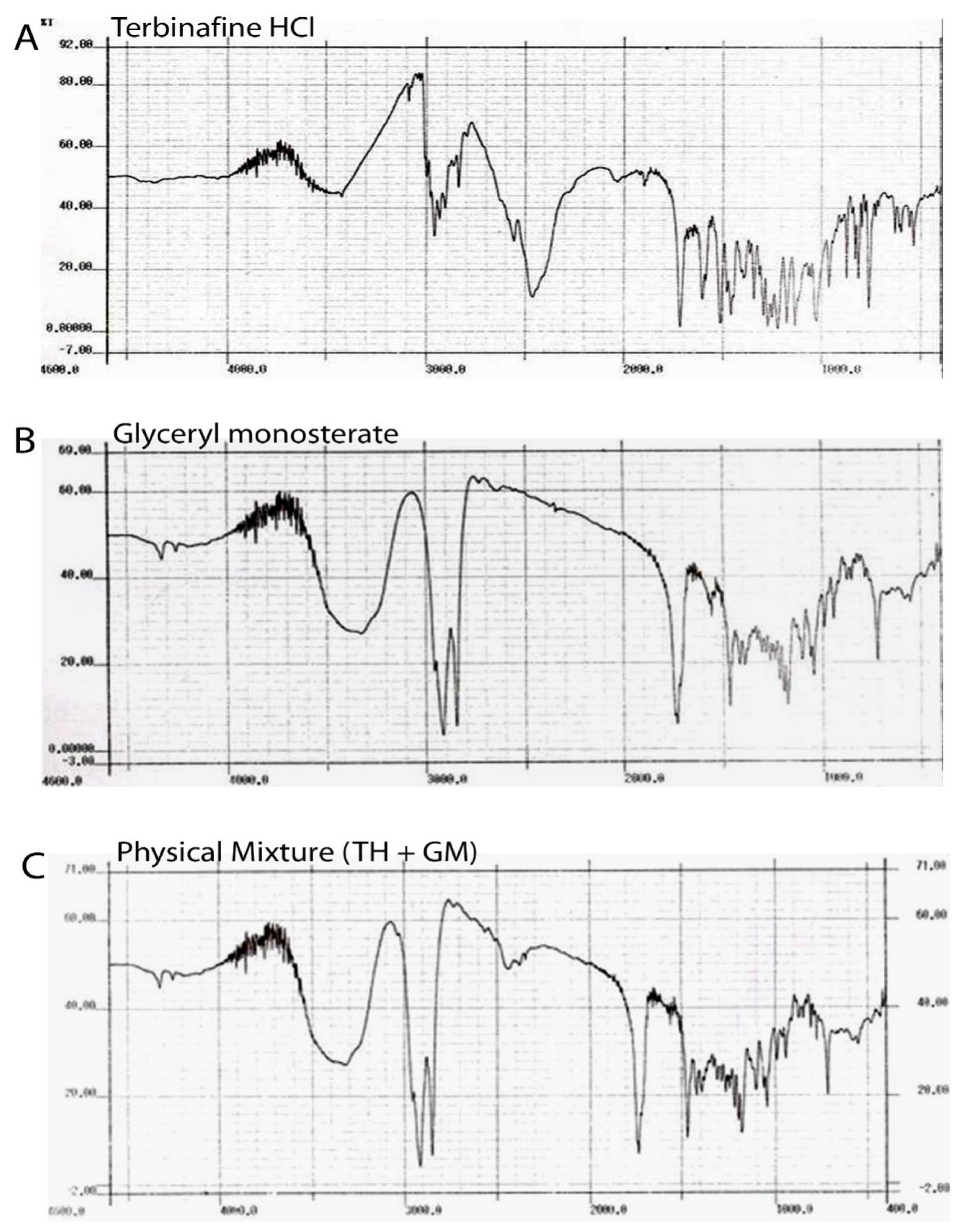
3.1.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.2. Characterization of SLNs
3.2.1. Particle Size, Polydispersity Index, and Zeta Potential
3.2.2. Percent Drug Loading and Encapsulation Efficiency
3.3. Characterization of Lyophilized SLNs
3.3.1. Scanning Electron Microscopy
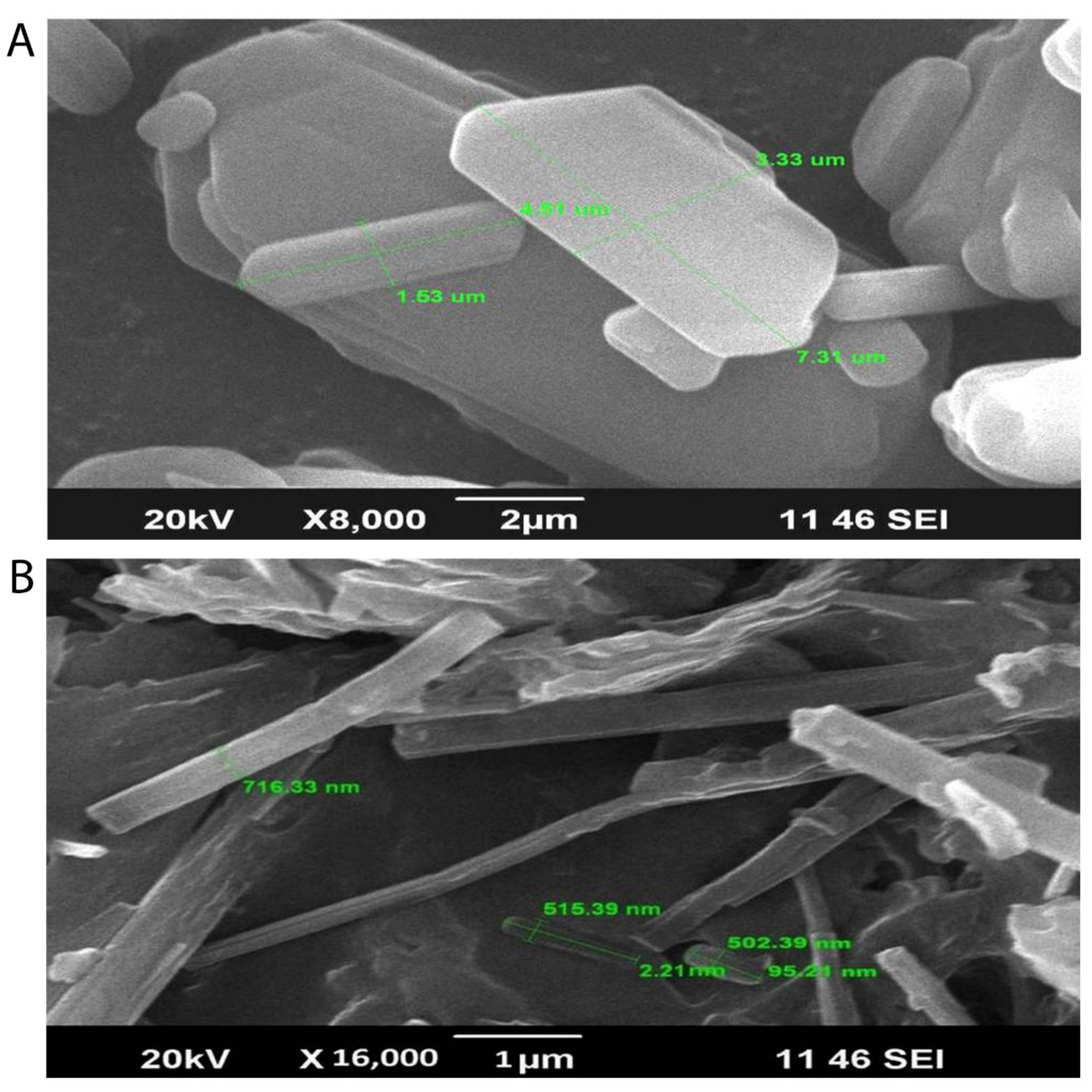
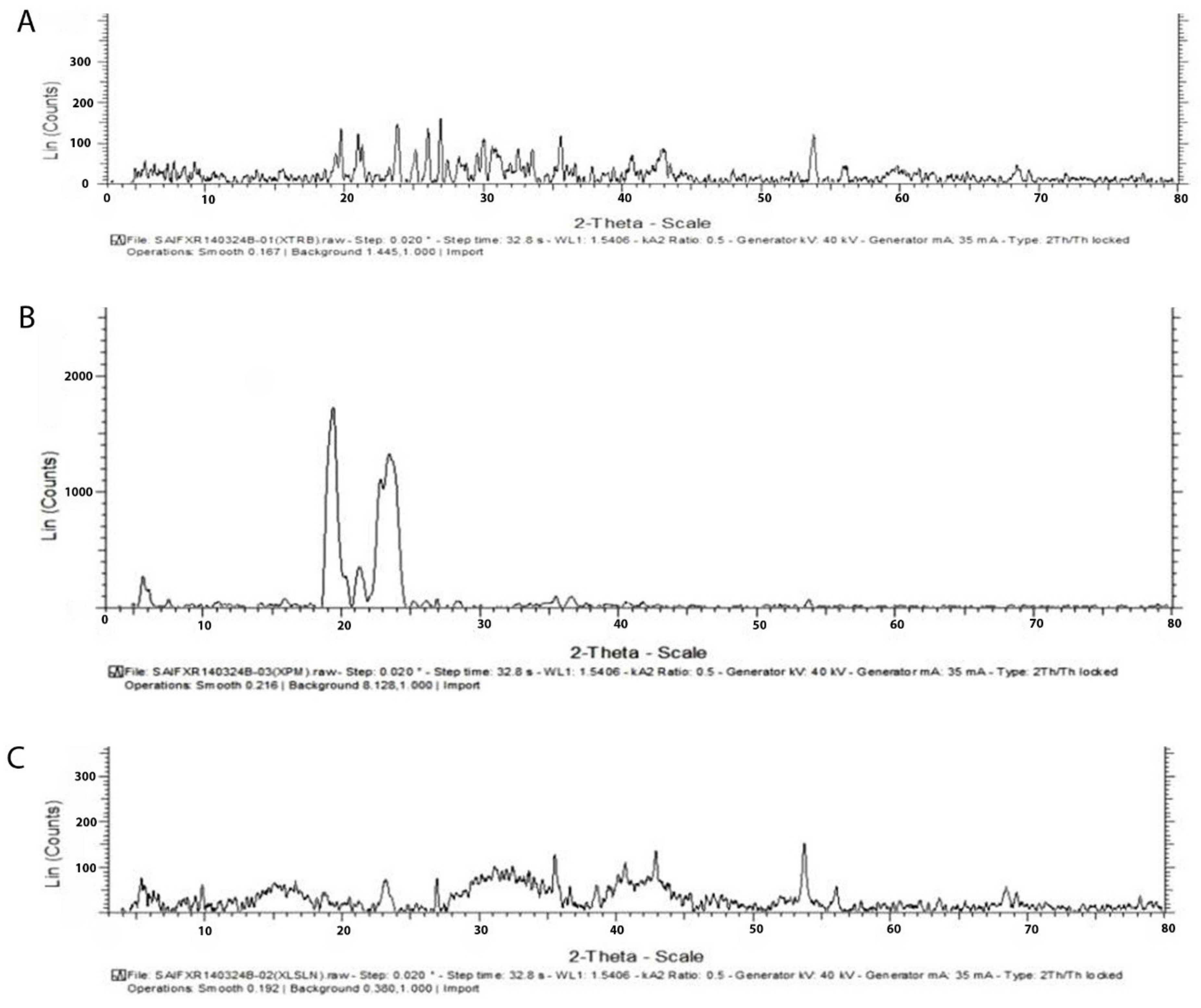
3.3.2. X-ray Diffraction Studies
3.4. Characterization of the TH-Loaded SLN-Based Hydrogel
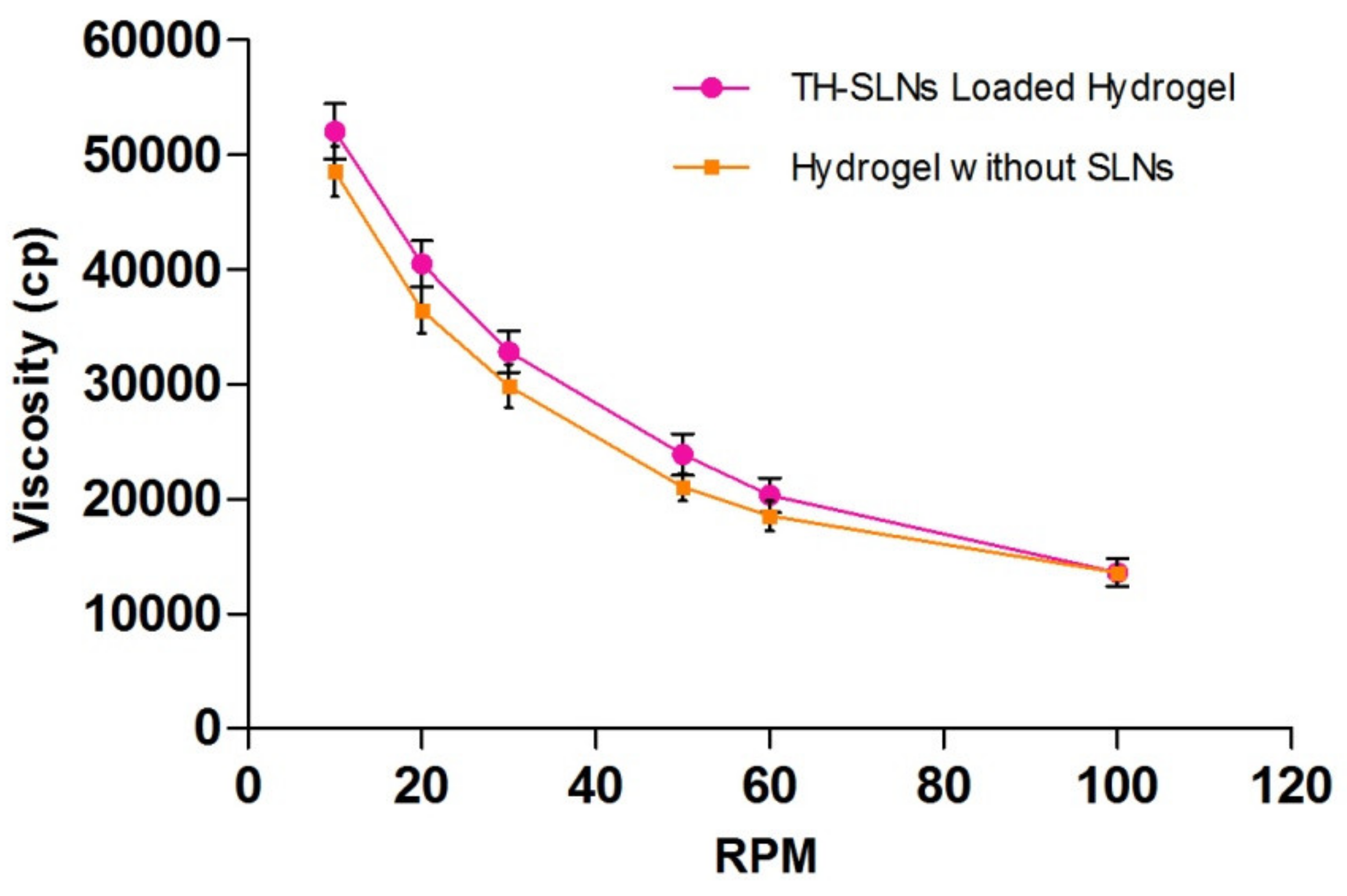
3.4.1. Rheological Behavior
3.4.2. Determination of Viscosity, pH, Gel Strength, Spreadability, Extrudability and Drug Content
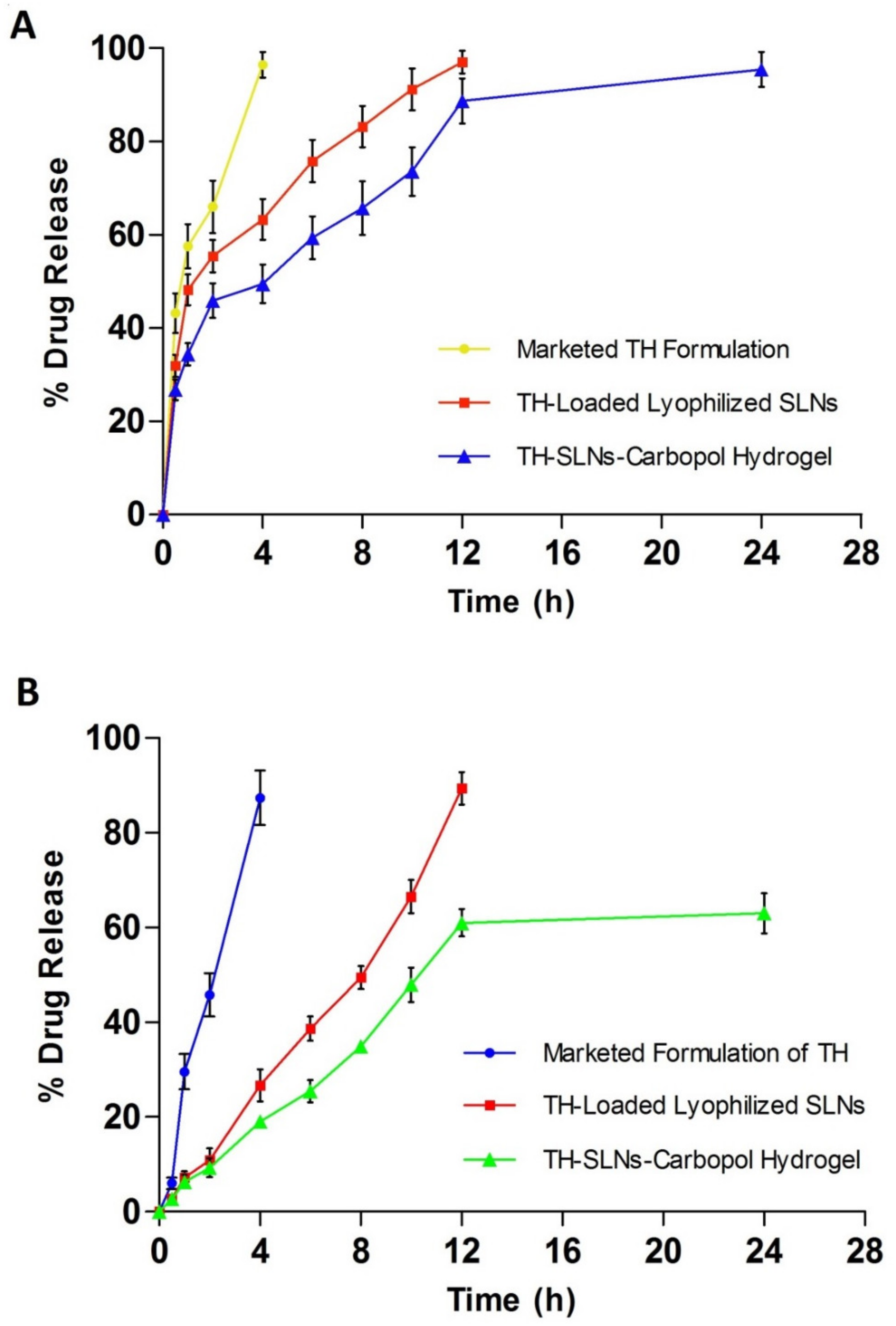
3.5. In Vitro Release Study
3.6. ExVivo Skin Permeation Studied
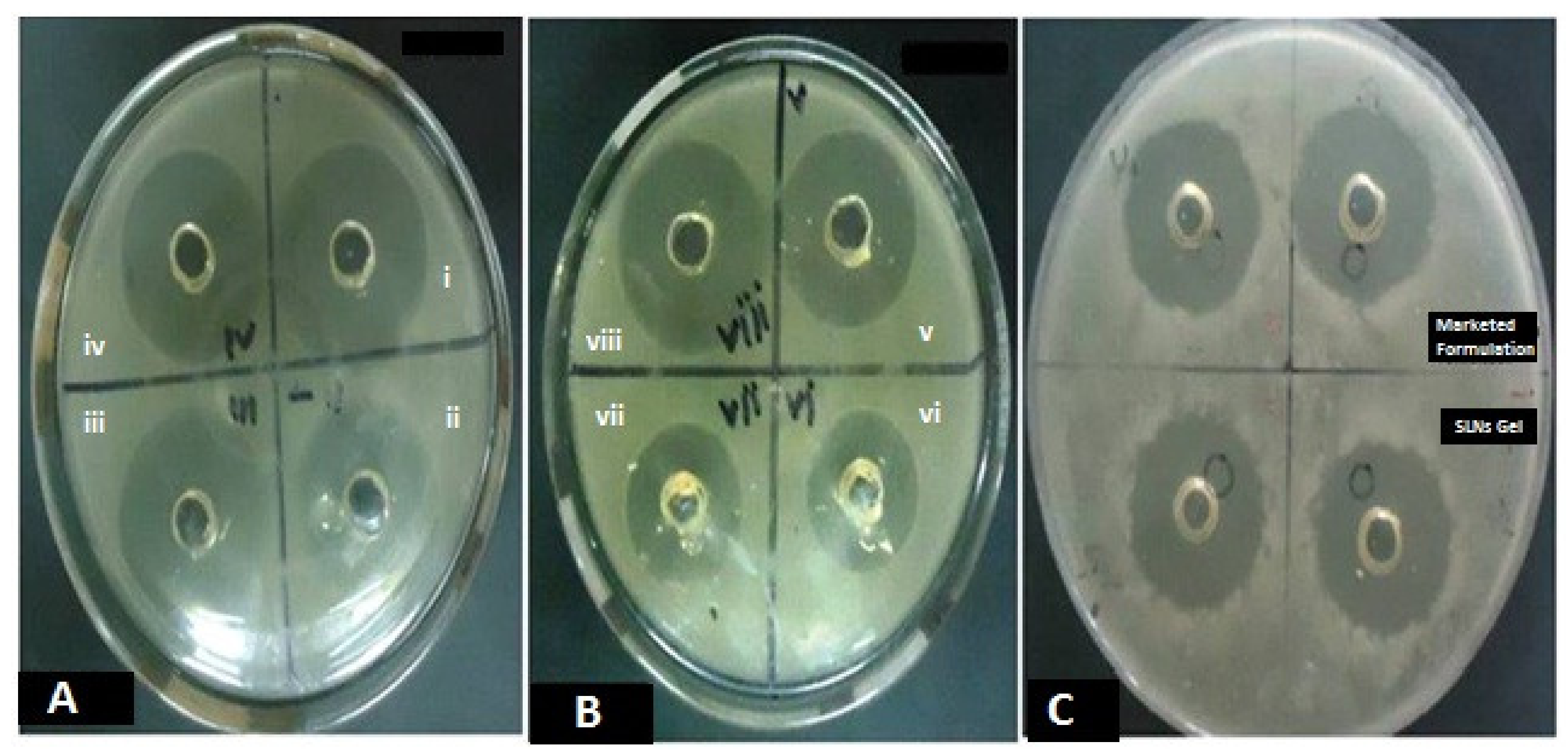
3.7. In Vitro Antifungal Activity
3.8. In Vivo Efficacy of the SLN Hydrogel against C. albicans-Induced Dermal Mycosis in Rats
3.9. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applica-tions. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Malec-Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula, E.S.A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Jain, S.K.; Chourasia, M.K.; Masuriha, R.; Soni, V.; Jain, A.; Jain, N.K.; Gupta, Y. Solid Lipid Nanoparticles Bearing Flurbiprofen for Transdermal Delivery. Drug Deliv. 2005, 12, 207–215. [Google Scholar] [CrossRef][Green Version]
- Maia, C.; Mehnert, W.; Schäfer-Korting, M. Solid lipid nanoparticles as drug carriers for topical glucocorticoids. Int. J. Pharm. 2000, 196, 165–167. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef]
- Mei, Z.; Wu, Q.; Hu, S.; Lib, X.; Yang, X. Triptolide Loaded Solid Lipid Nanoparticle Hydrogel for Topical Application. Drug Dev. Ind. Pharm. 2005, 31, 161–168. [Google Scholar] [CrossRef]
- Pople, P.V.; Singh, K.K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech 2006, 7, E63–E69. [Google Scholar] [CrossRef]
- Souto, E.; Anselmi, C.; Centini, M.; Müller, R. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN®). Int. J. Pharm. 2005, 295, 261–268. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. The use of SLN and NLC as topical particulate carriers for imidazole antifungal agents. Pharm.-Int. J. Pharm. Sci. 2006, 61, 431–437. [Google Scholar]
- Chen, H.; Chang, X.; Du, D.; Liu, W.; Liu, J.; Weng, T.; Yang, Y.; Xu, H.; Yang, X. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J. Control. Release 2006, 110, 296–306. [Google Scholar] [CrossRef]
- Liu, B.; Han, L.; Liu, J.; Han, S.; Chen, Z.; Jiang, L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int. J. Nanomed. 2017, 12, 955–968. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical de-livery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 4, 311–322. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Chen, Y.-C.; Liu, D.-Z.; Chang, T.-W.; Ho, H.-O. Development of terbinafine solid lipid nanoparticles as a topical delivery system. Int. J. Nanomed. 2012, 7, 4409–4418. [Google Scholar] [CrossRef]
- Khanna, D.; Bharti, S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Évid. 2014, 9, 113–124. [Google Scholar] [CrossRef]
- Sudaxshina, M. Drug delivery to the nail following topical application. Int. J. Pharm. 2004, 226, 1–26. [Google Scholar]
- Rarokar, N.R.; Khedekar, P.B.; Bharne, A.P.; Umekar, M.J. Development of self-assembled nanocarriers to enhance antitumor efficacy of docetaxel trihydrate in MDA-MB-231 cell line. Int. J. Biol. Macromol. 2019, 125, 1056–1068. [Google Scholar] [CrossRef]
- Silva, A.; Gonzalez, E.; García, M.L.; Egea, M.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the cur-rent-time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured Cubosomes in a Thermoresponsive Depot System: An Alternative Approach for the Controlled Delivery of Docetaxel. AAPS PharmSciTech 2015, 17, 436–445. [Google Scholar] [CrossRef]
- Novelli, F.; De Santis, S.; Diociaiuti, M.; Giordano, C.; Morosetti, S.; Punzi, P.; Sciubba, F.; Viali, V.; Masci, G.; Scipioni, A. Curcumin loaded nanocarriers obtained by self-assembly of a linear d,loctapeptide-poly(ethylene glycol) conjugate. Eur. Polym. J. 2018, 98, 28–38. [Google Scholar] [CrossRef]
- Saoji, S.D.; Raut, N.A.; Dhore, P.W.; Borkar, C.D.; Popielarczyk, M.; Dave, V.S. Preparation and Evaluation of Phospholipid-Based Complex of Standardized Centella Extract (SCE) for the Enhanced Deliv-ery of Phytoconstituents. AAPS J. 2016, 18, 102–114. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; González-Mira, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef]
- Benoit, S.; Afizah, M.N.; Ruttarattanamongkol, K.; Rizvi, S. Effect of pH and Temperature on the Viscosity of Texturized and Commercial Whey Protein Dispersions. Int. J. Food Prop. 2013, 16, 322–330. [Google Scholar] [CrossRef]
- Harish, N.M.; Prabhu, P.; Charyulu, R.N.; Gulzar, M.A.; Subrahmanyam, E.V. Formulation and Evaluation of in situ Gels Containing Clotrimazole for Oral Candidiasis. Indian J. Pharm. Sci. 2009, 71, 421–427. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.-Z.; Rhee, J.-D.; Kim, C.-K.; Lim, S.-J.; Kim, K.-M.; Oh, P.-S.; Choi, H.-G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Ankola, D.D.; Durbin, E.W.; Buxton, G.A.; Schäfer, J.; Bakowsky, U.; Kumar, M.R. Preparation, characterization and in silico mod-eling of biodegradable nanoparticles containing cyclosporine A and coenzyme Q10. Nanotechnology 2010, 21, 065104. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical de-livery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Vaishya, P.; Jain, S.; Pandey, V.; Bansal, D.; Dubey, N. Delivery of amphotericin B for effective treatment of Candida albicans induced dermal mycosis in rats via emulgel system: Formulation and evaluation. Indian J. Dermatol. 2014, 59, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Deshkar, S.S.; Bhalerao, S.G.; Jadhav, M.S.; Shirolkar, S.V. Formulation and Optimization of Topical Solid Lipid Nanoparticles based Gel of Dapsone Using Design of Experiment. Pharm. Nanotechnol. 2018, 6, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Dua, K.; Khude, P. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef]
- Freitas, C.; Muller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Oehlke, K.; Behsnilian, D.; Mayer-Miebach, E.; Weidler, P.G.; Greiner, R. Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS ONE 2017, 12, e0171662. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-loaded solid lipid nanoparticles: Preparation, characterisation, stability and transdermal behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Padhye, S.G.; Nagarsenker, M.S. Simvastatin Solid Lipid Nanoparticles for Oral Delivery: Formulation Development and In vivo Evaluation. Indian J. Pharm. Sci. 2013, 75, 591–598. [Google Scholar]
- Andreozzi, E.; Seo, J.W.; Ferrara, K.; Louie, A. Novel Method to Label Solid Lipid Nanoparticles with 64Cu for Positron Emission Tomography Imaging. Bioconjugate Chem. 2011, 22, 808–818. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Im-pact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Pathak, S.; Tak, J.W.; Lee, H.H.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Effects of Formulation Variables on the Particle Size and Drug Encapsulation of Imatinib-Loaded Solid Lipid Nanoparticles. AAPS PharmSciTech 2016, 17, 652–662. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Bendas and Mohamed A. El-Nabarawi. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: For-mulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef]
- Molan, P. The role of honey in the management of wounds. J. Wound Care 1999, 8, 415–418. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells NanomedBiotechnol. 2018, 46 (Suppl. 1), 1039–1050. [Google Scholar] [CrossRef]
- Jessup, C.J.; Ghannoum, M.A.; Ryder, N.S. An evaluation of the in vitro activity of terbinafine. Med. Mycology. 2000, 38, 155–159. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Khedekar, P.B. Investigation of effectiveness of some extensively used polymers on thermoreversible properties of Pluronic® tri-block copolymers. J. Drug Deliv. Sci. Technol. 2018, 44, 220–230. [Google Scholar] [CrossRef]
| Formulation Code | Particle Size (nm) | Zeta Potential (mV) | Polydispersity Index | % Drug Loading | % Encapsulation Efficiency |
|---|---|---|---|---|---|
| P F 1 | 241.3 | −15.2 | 0.415 | 6.3602 | 98.36 |
| P F 2 | 248.7 | −18.1 | 0.47 | 4.2192 | 97.84 |
| P F 3 | 274.7 | −19.4 | 0.542 | 3.1652 | 97.49 |
| P F 4 | 302.4 | −20.2 | 0.577 | 2.5322 | 96.89 |
| P F 5 | 321.8 | −24.8 | 0.543 | 2.12 | 95.39 |
| Viscosity(Cp) | RPM | Viscosity(Cp) | RPM |
|---|---|---|---|
| 52,000 | 10 | 13,570 | 100 |
| 40,460 | 20 | 18,500 | 60 |
| 32,800 | 30 | 21,000 | 50 |
| 23,890 | 50 | 29,800 | 30 |
| 20,320 | 60 | 36,400 | 20 |
| 13,570 | 100 | 48,500 | 10 |
| Treatment | No. of Animals with Positive Culture/Total No. of Animals | Log CFU/Infected Sites |
|---|---|---|
| Control (Base formulation) | 7/7 | 5.69 ± 0.45 |
| TH solution in ethanol | 6/7 | 4.01 ± 0.33 |
| TH-loaded SLN-based hydrogel | 2/7 | 2.23 ± 0.19 *,# |
| Conventional marketed formulation of TH | 1/7 | 1.46 ± 0.15 ** |
| Drug Content | Particle Size | ||
|---|---|---|---|
| Room Temperature | Refrigerator Temperature | Room Temperature | |
| Initial | 100.0 | 100.0 | 241 nm |
| After 1 month | 99.7 ± 0.6 | 99.8 ± 0.2 | 248 nm |
| After 2 months | 99.4 ± 0.4 | 99.7 ± 0.5 | 250 nm |
| After 3 months | 99.1 ± 0.2 | 99.5 ± 0.6 | 269 nm |
| Drug content | pH | ||
|---|---|---|---|
| Room Temperature | Refrigerator Temperature | Room Temperature | |
| Initial | 100.0 | 100.0 | 6.5 |
| After 1 month | 99.7 ± 0.5 | 99.8 ± 0.1 | 6.6 ± 0.1 |
| After 2 months | 98.6 ± 0.7 | 98.9 ± 0.8 | 6.7 ± 0.0 |
| After 3 months | 97.1 ± 0.3 | 98.6 ± 0.4 | 6.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rarokar, N.R.; Menghani, S.S.; Kerzare, D.R.; Khedekar, P.B.; Bharne, A.P.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Sreeharsha, N.; Asdaq, S.M.B. Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study. Pharmaceutics 2022, 14, 1393. https://doi.org/10.3390/pharmaceutics14071393
Rarokar NR, Menghani SS, Kerzare DR, Khedekar PB, Bharne AP, Alamri AS, Alsanie WF, Alhomrani M, Sreeharsha N, Asdaq SMB. Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study. Pharmaceutics. 2022; 14(7):1393. https://doi.org/10.3390/pharmaceutics14071393
Chicago/Turabian StyleRarokar, Nilesh R., Sunil S. Menghani, Deweshri R. Kerzare, Pramod B. Khedekar, Ashish P. Bharne, Abdulhakeem S. Alamri, Walaa F. Alsanie, Majid Alhomrani, Nagaraja Sreeharsha, and Syed Mohammed Basheeruddin Asdaq. 2022. "Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study" Pharmaceutics 14, no. 7: 1393. https://doi.org/10.3390/pharmaceutics14071393
APA StyleRarokar, N. R., Menghani, S. S., Kerzare, D. R., Khedekar, P. B., Bharne, A. P., Alamri, A. S., Alsanie, W. F., Alhomrani, M., Sreeharsha, N., & Asdaq, S. M. B. (2022). Preparation of Terbinafin-Encapsulated Solid Lipid Nanoparticles Containing Antifungal Carbopol® Hydrogel with Improved Efficacy: In Vitro, Ex Vivo and In Vivo Study. Pharmaceutics, 14(7), 1393. https://doi.org/10.3390/pharmaceutics14071393







