Abstract
Immune evasion is a common reason causing the failure of anticancer immune therapy. Small interfering RNA (siRNA), which can activate the innate and adaptive immune system responses by silencing immune-relevant genes, have been demonstrated to be a powerful tool for preventing or reversing immune evasion. However, siRNAs show poor stability in biological fluids and cannot efficiently cross cell membranes. Nanotechnology has shown great potential for intracellular siRNA delivery in recent years. Nano-immunotherapy can efficiently penetrate the tumor microenvironment (TME) and deliver multiple immunomodulatory agents simultaneously, which appears to be a promising method for combination therapy. Therefore, it provides a new perspective for siRNA delivery in immunomodulation and cancer immunotherapy. The current advances and challenges in nanotechnology-based siRNA delivery strategies for overcoming immune evasion will be discussed in this review. In addition, we also offer insights into therapeutic options, which may expand its applications in clinical cancer treatment.
1. Introduction
Cancer ranks as a one of the leading causes of death, with a substantial barrier to extend life expectancy worldwide [1]. In the past 30 years, despite the significant progress has been made for the mainstream cancer treatment methods including surgery, chemotherapy, radiotherapy, or their combinations, increasingly empirical consensus has shown that the failure of these standard treatments is largely attributable to inborn or acquired therapeutic resistance [2]. Targeted therapy, gene therapy, and immunotherapy are newly established treatment techniques that have expanded cancer treatment options and enabled individualized medicine [3,4].
Unlike the targeted therapy or gene therapy, the effect of immunotherapy is achieved by strengthening an anticancer immune response [5]. Although cancer immunotherapies have demonstrated promise in several preclinical models, a limited proportion of patients react to therapy [6]. The growth and progression of cancer are governed by immune cells [7]. However, connections between immune system components and tumor cells in TME frequently result in immune evasion, which enables cancer cells to evade killing by immune cells [8]. Selecting tumor variants resistant to immune effectors and the continual formation of an immunologically suppressive milieu within the tumor contribute to immune evasion [9]. Indeed, it has been demonstrated that the recruitment and expansion of immunosuppressive cells, such as regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), are related to tumor aggressiveness and poor prognosis [10]. For effective tumor control, therapeutic treatments focus on modifying the TME and/or reducing immunosuppression, as well as counteracting insufficient tumor immunogenicity and antigenicity [11]. Immunomodulatory substances such as cytokines, adjuvants, and monoclonal antibodies are utilized to modify the TME and enhance antitumor immunity [12]. Despite the preliminary success of immunotherapeutic agents, some patients do not benefit from them. In addition, a substantial number of people have been reported to suffer serious side effects, such as gastrointestinal, hematologic, and endocrine illnesses, arthritis, dermatitis, neuropathy, and acute renal injury [13,14].
SiRNA is essential for efficiently suppressing multi-drug-resistance-related protein, inhibiting tumorigenesis protein/suppressor mutations, and activating tumor-associated immune cells [15,16]. Applying siRNA methodology in cancer treatment, particularly beyond liver targets, has been intensively researched. However, as a significant drawback, unmodified siRNA is unstable in the bloodstream and is prone to immunogenicity. Therefore, it cannot readily enter cells for crossing membranes [17]. The lack of unharmful and convenient transport systems is a fundamental obstacle to realize the vast potential of siRNA-based therapies [18]. As shown in Figure 1, chemical modifications and delivery systems have been established to safely transport siRNA to its location of action [19]. At present, the nanoparticles that are used as loading systems for antitumor drugs usually have a size of 1–100 nm, including nano-liposomes, nano-polymers, nano-gene carriers, nano-inorganic materials, and other drug carriers [20,21]. The active targeting can be achieved by the targeted transportation modes of antitumor drug nanocarriers, including passive transportation, active transportation, and physical and chemical transportation [22,23]. The tumor microenvironment (TME) comprises a range of stimuli, such as enzymes, mildly acidic pH, hypoxia, and GSH, which can help with the development of stimuli-responsive nanoscale drug delivery systems (SRNDs). Because these SRNDs preserve their stealth characteristics in the normal physiological environment, they are sensitive and release their contents when homing in on particular lesions [24,25]. Therefore, in comparison to conventional medications, nanotechnology-based siRNA delivery systems can alter the immunosuppressive environment by targeting primary components in the TME, hence enhancing the efficiency of cancer immunotherapy [15]. Furthermore, nano-siRNA may increase retention time and enable targeted delivery, thus minimizing toxicity. The application of nano-siRNA as a drug delivery strategy has been extensively explored [18]. In general, nanotechnology-based siRNA delivery systems have predominantly evolved as novel techniques for modifying the immune system in two ways [26]: initially, targeting and/or removing immunosuppressive cells or pathways that implicated in tumor immune evasion; secondly, activating cytotoxic T lymphocytes (CLTs) to generate immunogenic cell death (ICD).
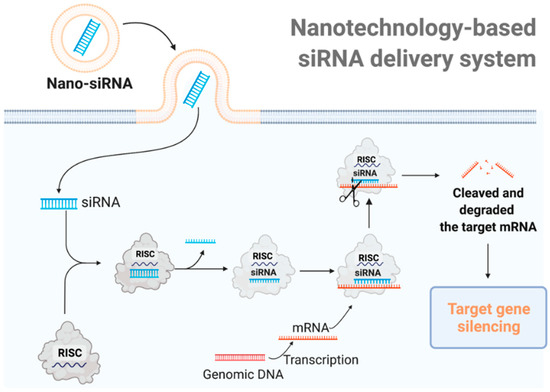
Figure 1.
The mechanism of nanotechnology-based siRNA delivery systems. Utilizing delivery materials, siRNA can be delivered directly into the cell. The siRNA is integrated into the RNA-induced silencing complex (RISC) and the sense (passenger) strand is degraded by the RISC protein Argo-2. The remaining antisense strand acts as a guide for recognizing the complementary messenger RNA. The activated RISC–siRNA complex binds to and degrades the target mRNA, leading to the silence of the target gene.
In this review, we summarize the immune evasion mechanisms that lead to the failure of cancer treatment modalities. In addition, we also highlight the current advances and challenges in nanotechnology-based siRNA delivery strategies for overcoming immune evasion.
2. Immune Evasion Mechanisms in Tumor
Cancer immune surveillance is a crucial process that enables the immune system to monitor, identify, and eliminate tumor cells during the early stages of carcinogenesis [27]. This process has three fundamental steps called elimination, balance, and escape [28]. Several investigations have revealed that the immune system has the potential to identify and eliminate tumor cells via the recruitment of innate and adaptive effectors [29]. In the context of antitumor responses, innate immunity promotes fast and non-specific responses, while adaptive immunity is more specific [30]. The first line of protection is the innate immune system, which detects microbes and endogenous danger signals by recognizing damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) through host pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nuclear receptors [31]. It has been reported that immune cells including NK cells, monocytes/macrophages, and neutrophils can contribute to the elimination of tumor cells through indirect or direct mechanisms, such as the generation of antitumor chemicals or antibody-dependent cellular cytotoxicity (ADCC) [32]. Professional antigen-presenting cells (APCs), such as dendritic cells (DCs), act as bridges between innate and adaptive immunity, which connect activating T cells (CTL or helper T cells) and B cells [33]. DCs move to neighboring lymph nodes (LN) during maturation, where they present tumor antigens and activate tumor-specific CD4+ and CD8+ T cells [34]. Then, these tumor-specific T lymphocytes will migrate to the tumor location and aid in its elimination.
Growing tumors can defy immune responses by excluding or hiding from immune cells through intrinsic pathways [35] and extrinsic pathways [36], resulting in the formation of a favorable environment for tumor development.
2.1. Intrinsic Mechanisms Mediating Immune Evasion
Tumor cells have evolved a variety of mechanisms to evade immune responses. Indeed, these escape mechanisms are selected by the cancer cells after a period of engagement with the immune system [37]. Multiple tumor cells exhibit reduced levels of MHC class I, downmodulation of tumor antigens, and weak immunogenicity, which are highly associated with tumor immune evasion (Figure 2).
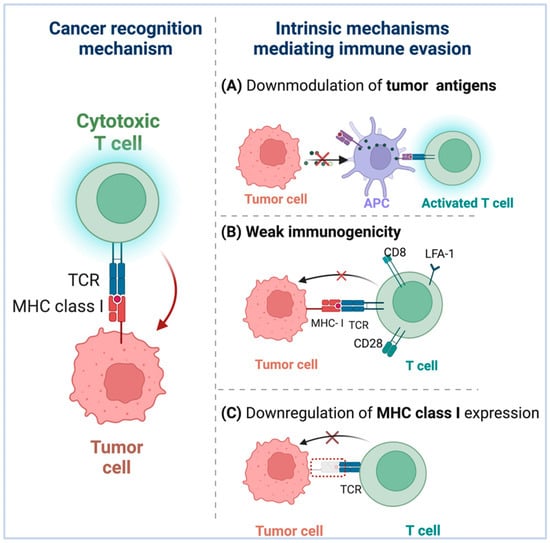
Figure 2.
Tumor cells have evolved various strategies for evading immune responses. (A) Downregulation of tumor antigens: Certain malignancies lack pre-existing tumor T cell infiltration, allowing them to escape immunosurveillance due to low tumor antigen expression levels, resulting in inadequate APC recruitment and activation. (B) Weak immunogenicity: Immune selection permits tumors with weak immunogenicity to avoid immune surveillance and grow preferentially, and the weak immunogenicity of tumor antigens may be owing to incorrect or non-expression of costimulatory molecules on tumor cells. (C) Downregulation of MHC-I expression: By evading immune identification by tumor cells, the ability of tumor-associated antigen (TAA)-specific CTLs to kill cancer cells is compromised.
To elicit an efficient antitumor response, antigen presentation requires two independent steps. First, cancer antigens must be picked up by DCs and cross-presented to CD8+ T cells for priming. Second, the tumor needs to directly deliver the antigens for detection and killing by primed CD8+ T cells. Tumors employ a variety of mechanisms to avoid detection by the immune system during both stages [38]. For example, tumor-specific CD8+ T cells recognize and activate tumor antigens, and the particular killing of tumor cells is dependent on the T cell receptors (TCR) specifically recognizing and attaching to MHC-I-peptide complexes [39]. To successfully evade immune identification, tumor cells can change in the interaction between MHC molecules and antigenic peptides, which have an effect on the TCR recognition of MHC–antigenic peptide complexes [40,41]. Additionally, certain tumors lack pre-existing tumor T cell infiltration, allowing them to elude immunosurveillance as a result of low tumor antigen expression levels [41,42] The low expression levels of tumor antigen result in poor APC recruitment and activation, hence impeding the beginning of an efficient immunological response [43].
Immune surveillance can detect and kill tumor cells at the early stages of cellular transformation [44]. However, since tumor cells vary in their immunogenicity, tumor cells with strong immunogenicity can induce an effective antitumor immune response and are easily eliminated, whereas tumor cells with weak immunogenicity can corrupt the host’s antitumor immune response and arise, survive, and grow [45]. Immune selection enables tumors cells with low immunogenicity to evade immune monitoring and proliferate preferentially [46]. Additionally, studies indicate that the lack of immunogenicity may be attributable to the improper expression or absence of costimulatory molecules on tumor cells [47].
Downregulation of MHC-I on tumor cells, a widely used tactic by tumor cells to evade specific immune responses, may be linked to coordinated silencing of antigen-presenting machinery genes [48,49,50]. Direct or indirect mutations in genes encoding antigen processing or presentation components, such as proteasome subunits and endoplasmic reticulum peptide transporters, can inhibit the expression of MHC-I in malignant cells [50,51]. Evading immune recognition by tumor cells through downregulating the expression of MHC-I compromises the ability of tumor associated antigen (TAA)-specific CTLs to kill cancer cells but boosts recognition and killing by NK cells when total MHC I levels fall below a threshold [52].
2.2. Extrinsic Mechanisms Mediating Immune Evasion
Immune cells, fibroblasts, endothelial cells, inflammatory cells, and lymphocytes make up the TME, along with extracellular matrix (ECM), vasculature, and chemokines [53,54]. Immune cells are involved in innate and adaptive immune responses. The adaptive immune system is capable of destroying tumor cells precisely and is perceived as the most efficient means of removing malignancies [55]. The TME is distinguished by an atypical tumor vasculature that appears and functions abnormally, resulting in a hypoxic condition [56]. In addition, hypoxia in the TME can alter abilities of the normal microenvironment, increase the growth of the tumor, and limit the therapeutic effects [57]. Thus, connections between tumor cells and components of the TME influence tumor growth, metastasis, and clinical outcome. TME has shown a significant effect on medication penetration and function, as well as being connected with drug resistance and low response rates [58]. Effective cancer remission is mediated by immunotherapy that induces sufficient immune responses. Unfortunately, the interaction between the immune system and tumor cells leads to increased immunosuppression in the TME and reduced immunogenicity of tumor cells, allowing tumor cells to resist immune elimination [59,60]. Malignant cells secrete a variety of chemokines and cytokines that encourage immune cells such as Tregs, TAMs, and MDSCs to infiltrate into the tumor [61]. Due to the regional production of immunosuppressive cytokines, chemokines, and growth factors, as well as their interaction with TME components, these immune cells are recognized as cancer immune suppressors (Figure 3) [62]. To this end, researchers interested in cancer immunotherapy have placed a premium on TME modulation.
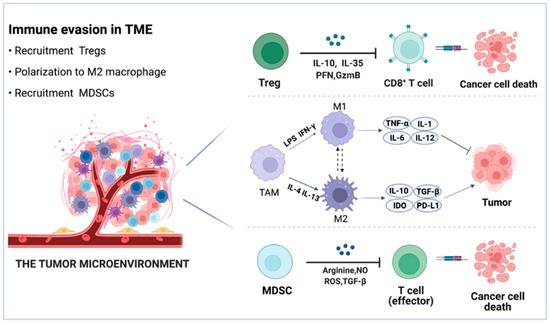
Figure 3.
Schematic view of the TME. Tregs exert immunosuppressive effects via the release of IL10, IL-35, PFN, and GzmB. The preponderance of M2-like TAMs in the TME promotes tumor immune evasion. TAMs suppress the immune system in a variety of ways, including the release of IL-10 and TGF-β, activation of the IDO, and overexpression of the PD-L1 checkpoints. MDSCs limit CD8+ T cell and natural killer cell responses via arginine, NO, ROS, and TGF-β.
Tregs are a T cell subset with regulatory immunological capabilities that act as immunosuppressive factors in the TME and encourage tumor progression [63]. Chemokines in the TME have been demonstrated to attract naturally existing Tregs from the thymus, bone marrow, lymph nodes, and periphery via the Treg receptor CCR4, hence weakening immunity [64].
Tregs largely contribute to the immune evasion of tumor cells by inhibiting effector T cells and DCs via many mechanisms [65]. First, Tregs can secrete perforin (PFN) and granzyme B (GzmB), which directly act on effector cells to promote apoptosis [66]. In addition, Tregs could secrete immunosuppressive cytokines such as TGF-β, IL-10, and IL-35, which attach to immunological cells and inhibit the immune system [67,68]. Furthermore, numerous surface receptors, incorporating cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) [68], programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin, and mucin-containing molecule 3 (TIM-3) [69,70], as well as upregulation of indoleamine 2,3-dioxygenase 1 (IDO) and activation of CD28 family-induced costimulatory molecules (ICOS), contribute to the immunosuppressive function of Tregs which promotes the secretion of inhibitory cytokines [71]. As a corollary, it is envisaged that eliminating Tregs or inhibiting their immunosuppressive actions will restore the antitumor effects of immunotherapies [72].
TAMs, which are myeloid-derived and tissue-resident macrophages prominent in the microenvironment of solid tumors, become immunosuppressive when they interact with tumor cells [73]. They have been shown to be critical ingredients of the TME and to encourage tumor growth. TAMs seem to be synonymous with M2 macrophages due to that they share M2 phenotypic traits, including boosting angiogenesis, reducing inflammation, and matrix remolding [74].
TAMs are classified into two subtypes based on their cellular context: tumor-suppressive M1 macrophages and tumor-promoting M2 macrophages [75]. Preclinical and clinical research has revealed that TAMs are largely M2 phenotype, which participate in the tumorigenesis and cancer progression [76]. M1 macrophages have microbicidal and anticancer capabilities [77]. In comparison, anti-inflammatory cytokines IL-4, IL-10, and IL-13 are secreted by M2 macrophages, as well as a reduction in reactive oxygen species (ROS), NO, and TNF production, which are responsible for the anti-inflammatory effect [74,78].
The predominance of M2-like TAMs in the TME contributes to tumor immune evasion and chemoresistance. Through the secretion of soluble substances such as IL6 and PGE2, tumor cells directly stimulate the M2 polarization of macrophages [78,79]. M2-macrophages induce a Th2-type response, which inhibits proinflammatory stimuli. TAMs exert their immunosuppressive activity in a variety of ways, including the release of IL-10 and TGF-β, activation of IDO, generation of prostaglandins, and upregulation of the programmed death-ligand 1(PD-L1), programmed death-ligand 2 (PD-L2), and VISTA checkpoints [74].
MDSCs, as a heterogeneous population of immature myeloid cells, have a high level of anti-T cell activity [80]. MDSCs accumulate in different kinds of tumors tissue, where they enhance tumor invasion, angiogenesis, and metastasis while inhibiting antitumor immunity [81]. Thus, MDSCs provide a vital function in helping tumor cells to evade immune monitoring, thereby promoting tumor proliferation and progression [81]. The accumulation of MDSCs in TME is primarily determined by two modulation schemes. To begin, tumor cells induce MDSCs generation and recruitment via the secretion of stem cell factor (SCF), granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), and macrophage colony-stimulating factor (MCSF) [82,83,84]. The second class of signals consists of cytokines and chemokines such as IL-4, IL-6, IL-1, and CXCL1, that are mostly produced by the tumor stroma, which induce the suppressive activity of MDSCs via NF-κB, STAT1, and STAT6 [83,84,85]. Intra-tumoral MDSCs can impair antitumor responses by upregulating pathways involved in the production of arginine, ROS, and nitric oxide (NO), as well as by secreting TGF-β, which can dampen effector T cells directly or indirectly to promote immune evasion [86].
3. Targeting Immunosuppressive Cells in the TME with Nanotechnology-Based siRNA Delivery Systems
Strategies for TME regulation have received considerable attention in cancer immunotherapy. Unlike conventional treatment, nanocarriers with distinct physical properties and complex structures can successfully penetrate TME and distribute to specific components [87]. At the same time, nanoparticles can increase the retention duration and distribute drugs more precisely, thereby lowering toxicity [88]. Additionally, nanoparticles have the potential to alter the immunosuppressive milieu within TME by targeting the key components [89]. The deformed blood vessels and rapid development of tumor cause hypoxia, which result in immunosuppression in TME through the accumulating immunosuppressive cells such as Tregs and MDSCs and secreting immunosuppressive factors such as VEGF and TGF-β. This substitute impairs the functions of DCs, converting macrophages to the tumor-promoting M2 phenotype, and resulting in immune evasion [90]. Nanoparticles can target certain components in the TME and convert them to an immune supporting state, hence increasing the efficacy of cancer immunotherapy [91]. Nanotechnology-based siRNA delivery systems have been extensively investigated as potential novel therapy modalities for a variety of cancers over the last few decades [92]. When modified with various ligands, nanoparticles function as efficient drug delivery systems that can selectively target TME components such as Tregs, macrophages, MDSCs, fibroblasts, tumor vasculature, tdLNs, and the hypoxic state. By encapsulating siRNA in nanoparticles, we can preserve siRNA from degradation and efficiently distribute it to tumor cells [93]. Nanotechnology-based siRNA delivery systems have been utilized extensively for in vivo gene silencing and tumor therapy [94]. Here, we will discuss the specific siRNA delivery systems used to target important immunosuppressive cell populations or pathways.
3.1. Targeted Delivery of siRNA to Tregs
Depletion of Tregs or inhibiting their immunosuppressive actions could restore the antitumor activity of CTLs, hence preventing the development of tumors (Figure 4) [95]. Unfortunately, clinical implementation of Treg-depleting methods remains unsatisfied due to the paucity of Treg cell-specific markers and small molecules that specifically target Treg functions [96]. It has been reported that covalently linking siRNA to an aptamer (apt) that specifically binds cytotoxic T lymphocyte-associated antigen 4 CTLA4 (apt) enables gene silencing, as well as CTLA4-expressing malignant T cells. CTLA4 (apt) link to a STAT3-targeting siRNA (CTLA4 (apt)-STAT3 siRNA), resulting in STAT3 suppression and the activation of tumor antigen-specific T cells. Additionally, CTLA4(apt)-STAT3 siRNA demonstrated a substantial inhibiting impact on tumor development and metastasis in a variety of mouse tumor models [97]. Recent improvements in nanoparticles have facilitated the creation of novel Treg-depleting strategies. For example, Wang et al. constructed nanoparticles to deliver CTLA-4-siRNA (NPsiCTLA-4), which are used to transfer specific siRNAs to T cells, therefore promoting the activation and proliferation of T cells. They created siCTLA-4-encapsulated nanoparticles using a biocompatible and biodegradable poly (ethylene glycol)-block-poly (D, L-lactide) (PEG5 k-PLA11 k) copolymer and N-bis(2-hydroxyethyl)-N-methyl-N-(2-cholesteryloxycarbonyl aminoethyl) ammonium bromide (BHEM-Chol). The findings confirmed that the nanocarrier delivery system could deliver CTLA-4-siRNA to CD4+ and CD8+ T cell subsets at tumor sites, while decreasing the proportion of suppressor Tregs among tumor-infiltrating lymphocytes (TILs), thereby increasing the antitumor immune response of tumor-infiltrating T cells [98]. To investigate the antitumor effects of various combined strategies, Zhang et al. created several spherical nucleotide nanoparticles (SNPs) that were loaded with CTLA-4-siRNA aptamer (cSNPs), PD-1 siRNA (pSNPs) or both (hybrid SNPs, or hSNPs). The findings showed that hSNPs could promote considerably higher antitumor immune responses than a combination of pSNPs and cSNPs (pSNPs and cSNPs),through regulating the immune suppressive function of both Tregs and TIM3+ exhausted-like CD8 T cells [99]. Overall, utilizing nano-siRNA delivery systems to achieve Tregs depletion may be a realistic strategy.
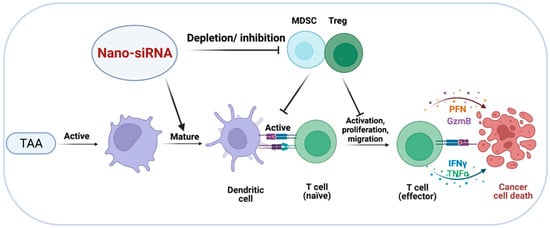
Figure 4.
Targeted delivery of siRNA to MDSCs and Tregs. Depletion of Tregs and MDSCs or suppression of their immunosuppressive effects may restore the antitumor activity of effector T cells, resulting in cancer cells’ death.
3.2. Targeted Delivery of siRNA to TAMs
Repolarizing or eradicating regulatory TAMs is a strategy that may be plausible for TAMs-targeted cancer immunotherapy (Figure 5) [100]. TAMs are an attractive population cell for such endeavors due to their plasticity, which allows them to transition into M1-like phenotype from M2-like phenotype. Additionally, macrophages readily internalize particles due to their natural phagocyte status, significantly increasing uptake efficiency [73]. Therefore, modifying the polarization of TAMs may impact their function, which has garnered much attention [101]. Nanoparticles are capable of delivering medications specifically to TAMs and modulating their polarized states, providing a promising strategy for cancer immunotherapy [102]. Thus, inhibiting macrophage infiltration, preventing the formation of M2-like TAMs, repolarizing majority M2-like TAMs to M1-like TAMs, or epigenetically suppressing the release of M2-like TAM-induced factors in the TME may all be plausible for TAMs-targeted cancer immunotherapy [103]. Toll-like receptor (TLR) agonists, such as CpG oligodeoxynucleotides (CpG ODNs), can activate antitumor macrophages, but their in vivo efficacy is limited due to the lack of effective delivery methods [104]. Naked CpG ODNs cannot permeate cell membranes and are rapidly removed by nucleases, posing a risk of an inflammatory reaction in the serum when given systemically [105,106]. It has been demonstrated that nanoparticles can deliver TLR agonists to TAMs, selectively concentrating in tumors and macrophages, eventually triggering TLR signaling and M1 polarization [107]. Therefore, a self-assembled nucleic acid system was used to construct an efficient siRNA and CpG ODNs delivery system (CpG-siRNA-tFNA). The combination of CpG-siRNA-tFNA efficiently polarized TAMs toward the M1 phenotype, which resulted in an enhanced production of proinflammatory cytokine and activation of the NF-κB signaling pathway, therefore eliciting robust anticancer immune responses. Furthermore, the combination of CpG-siRNA-tFNA increased antitumor activity without causing systemic toxicity in a mouse model of breast cancer xenograft [108].
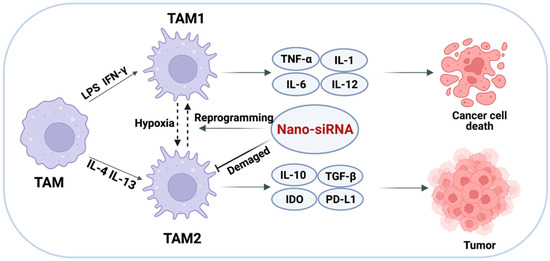
Figure 5.
Targeted delivery of siRNA to TAMs. Altering the polarization of TAMs or impairing the survival and function of TAMs to achieve the elimination of immune evasion.
Apart from altering the polarization of TAMs, another tactic is to impair their survival and function [109]. A recent study [110] used dual-targeting nanoparticles to deliver siRNA to M2-like TAMs to develop a molecular-targeted cancer immunotherapy strategy. A biocompatible fusion peptide-functionalized lipid nanoparticle with a dual-targeting entity for specific M2-like TAM binding, a sub-30 nm size for efficient solid tumor penetration [111], and stable loaded siRNA for systemic transport is the main component of this method [112] Qian et al. developed a new M2-like TAM-targeting core–shell fluorescent lipid nanoparticle (M2 NP), whose structure and function were controlled by α-peptide (a scavenger receptor B type 1 (SR-B1) targeting peptide) linked with M2 pep (an M2 macrophage binding peptide) [110]. This nanocomplex, which is encoded with anti-colony-stimulating factor-1 receptor siRNA on the M2 NP, is more selective and has a higher affinity for TAMs than other macrophages. Compared to control groups, M2 NP-based siRNA administration significantly reduced M2-like TAMs (52%), limited tumor size, and prolonged survival. Additionally, this molecularly targeted method decreased the production of immunosuppressive IL-10 and TGF-β, and enhanced the expression immunostimulatory cytokines and infiltration of CD8+ T cells in the TEM. Furthermore, the siRNA-carrying M2 NPs decreased the expression of PD-1 and Tim-3 on invading CD8+ T cells and increased the production of IFN-γ, showing that T cell immunological activity has been restored [110]. Thus, impairing the survival of TAMs with delivery of siRNA provides a potential strategy for clinical application of molecular-targeted cancer immunotherapy.
3.3. Targeted Delivery of siRNA to MDSCs
MDSCs are the primary facilitators of tumor survival and antitumor immune suppression, as evidenced by the secretion of Th2-type cytokines that lead to the development of an immunosuppressive TME [113]. MDSCs are involved in tumor immune evasion [114]. Hence, the concept of reprogramming or reducing MDSCs as a therapeutic strategy is evolving as a unique method for the continual improvement of existing cancer immunotherapies (Figure 5) [115]. Granulocytic MDSCs develop in patients with prostate cancer as the disease progresses. Hossain et al. [116] established the feasibility of targeting MDSCs using STAT3 siRNA to ameliorate arginase-dependent inhibition of T cells. The team developed an innovative technique for precisely silencing genes in Toll-like Receptor-9 (TLR9) positive myeloid cells utilizing CpG-siRNA conjugates. Human granulocytic MDSCs express TLR9 and rapidly internalize naked CpG-STAT3 siRNA, effectively suppressing the expression of STAT3. The inhibition of STAT3 abolishes the immunosuppressive effects of MDSCs generated from patients’ effector CD8+ T cells. These results demonstrate the viability of employing TLR9-targeted STAT3 siRNA delivery to reduce MDSC-mediated immunosuppression [116].
With the goal of achieving potent antitumor immunity, an immunochemotherapy regimen based on a redox-responsive nano-assembly (R-mPDV/PDV/DOX/siL) was designed. R-mPDV/PDV/DOX/siL was self-assembled by three synthesized amphiphilic polymers, including mPEG-DA-PVL (mPDV), RGD-mal-PEG-DA-PVL (R-mPDV), and PAMAM-DA-PVL (PDV), along with DOX encapsulated in core and LDHA siRNA (siL) compressed by PAMAM. These redox-responsive carrier materials were synthesized by conjugating hydrophobic polyester material PVL with hydrophilic mPEG or G2 PAMAM using 3, 3′-dithiodipropionic acid (DA) as the GSH-responsive linkage. This redox-responsive nano-assembly combined the strategies of inhibiting cytokine-mediated MDSC recruitment via LDHA silencing and enhancing tumor immunogenicity via anthracycline (DOX)-induced ICD effects. After egressing from endosomes/lysosomes, R-mPDV/PDV/DOX/siL is disintegrated by GSH-induced DA cleavage, enabling rapid drug release and very effective LDHA silencing. Reduced LDHA expression inhibits the production of G-CSF and GM-CSF cytokines, leading to inhibited MDSC recruitment and strengthened antitumor immunity [117].
Overall, these investigations suggest that targeted MDSCs reduction with siRNA delivery systems may be used as monotherapies or in conjunction with T cell-boosting drugs to enhance therapeutic efficacy.
4. Targeted Delivery of siRNA to Checkpoint Inhibitors
Immune checkpoint blockade therapies have been developed as effective treatment methods for a range of tumor types in recent years [118]. Checkpoint inhibitors inhibit the expression of specialized proteins on the surfaces of tumor cells and immune cells that are involved in immune evasion via the activation of certain signaling pathways [119]. PD-L1 is a type of cell membrane protein that is associated with tumor growth. By inhibiting the ligand–receptor interaction of PD-1/PD-L1, it is possible to prevent the activation of the PD-1/PD-L1 signaling pathway, reversing T cell fatigue and preventing immunological responses in the tumor microenvironment (Figure 6) [120]. Therefore, monoclonal antibodies against PD-1/PD-L1 have been the subject of numerous preclinical and clinical studies [121,122].
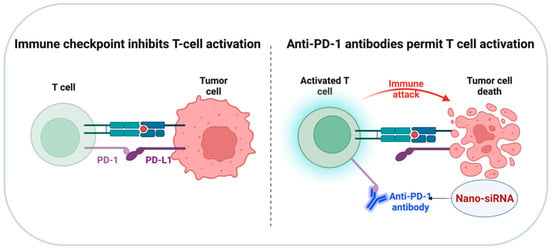
Figure 6.
Diagrammatic illustration of immune checkpoint blockade therapies. Inhibiting the ligand–receptor interaction of PD-1/PD-L1 to prevent the activation of PD-1/PD-L1 signaling pathway, leading to T cell activation and reduction of tumor cell proliferation.
Since overexpression of PD-L1 on the surface of tumor cells contributes to immune evasion, limiting PD-L1 expression has been proven to be an efficient method for facilitating immune system activation and inhibiting tumor development. Immune checkpoint inhibition has been accomplished in this manner by preloading nanocarriers with siRNA directed against specific checkpoint inhibitory pathways. Li et al. proposed using hybrid nanoparticles composed of polyethylene glycol-polylactic acid (PEG-PLA) and the cationic lipid BHEM-Chol to deliver anti-CTLA-4 siRNA in a recent study [98]. Anti-CTLA-4 siRNA-loaded nanoparticles were efficiently internalized by TILs (4–6%) after systemic distribution, resulting in tumor growth reduction and longer survival in B16 F10 melanoma-bearing mice. Teo and colleagues constructed folic acid (FA)-functionalized polyethyleneimine (PEI)-based nanoparticles to facilitate CTL-mediated killing of cancer epithelial cells (SKOV-3) through the release of siRNA that targets the PD1/PD-L1 pathway [123]. These findings reveal that siRNA-targeted silencing of PD-L1 can sensitize cancer cells to T cell-mediated death. Likewise, Roeven et al. used a lipoplex vector made up of SAINT-RED:DOPE liposomes precomplexed with anti-PD-L1 siRNA to achieve efficient and long-term PD-L1 silencing without compromising DC maturation or viability [124]. Together with other previously reported strategies for checkpoint inhibition, the PD-L1-siRNA loaded lipoplexes represent an innovative approach for improving the efficacy of antigen-specific immunotherapy, such as vaccines and ICD-inducing therapies, without risking treatment safety and tolerability. A recent work referred to as NPs@apt developed a tailored siRNA delivery system for transfecting PD-L1 siRNA into A549 cells to inhibit tumor immune evasion. The result revealed that PD-L1 siRNA was administered precisely to A549 cells, resulting in PD-L1 gene silencing, T cell activation, and reduction of tumor cell proliferation [125]. Additionally, this work developed an innovative method for targeted siRNA transfection to enhance antitumor immunity. These studies have shown that encapsulating siRNA in a nanoparticle can improve its stability and targetability, potentially addressing the issue of undesirable toxic effects associated with checkpoint blockade therapy.
5. Targeting Tumor Cells with Nano-siRNA: ICD-Inducing Strategies
Recent research suggests that traditional cancer therapies can be curative not only through directly killing malignant cells, but also through the induction of innate and adaptive antitumor responses, leading to the establishment of a new class of antitumor treatments known as ICD-inducing strategies [126]. ICD-inducing therapies are characterized by their capacity to enhance APC activation, priming of tumor-specific CD8+ T cells, and recruiting immune cells via the production of tumor antigens PAMPs and/or DAMPs, including calreticulin (CRT), heat shock proteins (HSPs) 70 and 90, and high-mobility group box 1 (HMGB1) (Figure 7) [127,128]. ICD has recently received considerable interest since it can be triggered by a variety of stimuli and anticancer treatment techniques, such as chemotherapy, radiotherapy, UVC irradiation, oncolytic viruses, and photodynamic therapy (PDT) [127,129,130,131]. ICD might differ in terms of DAMP profile in response to diverse stimulus and has also been associated with various cell death modalities, including apoptosis, necroptosis [132,133], and ferroptosis [134].
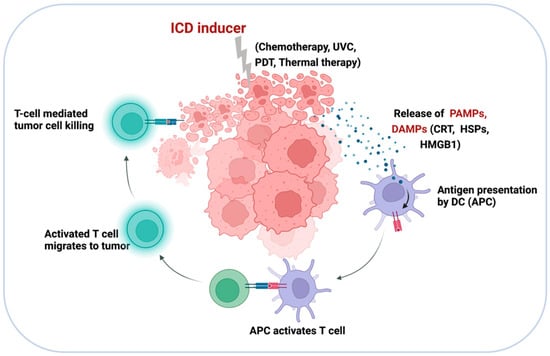
Figure 7.
Diagrammatic illustration of the ICD mechanism. ICD-inducing strategies promote APC activation and priming of tumor-specific CD8+ T cells through the release of tumor antigens, PAMPs, and DAMPs, such as CRT, HSP70, HSP90, and HMG1.
Using siRNA-based nanocarriers to activate ICD is appearing as an attractive fresh approach for producing a strong antigen-specific immune response, with potential to improve the efficiency and reliability of traditional ICD treatments, such as chemotherapies and PDT [135]. Several studies have demonstrated that the immunogenicity of these ICD-inducing monotherapies can be further enhanced by combining ICD-inducing monotherapies with other TME-targeting immunotherapies, [136]. For instance, an exosome-based dual-delivery biosystem has been demonstrated to enhance pancreatic ductal adenocarcinoma (PDAC) immunotherapy [136]. The delivery system is composed of exosomes derived from bone marrow mesenchymal stem cells, electroporation-loaded galectin-9 siRNA, and OXA prodrug used as an ICD inducer. Bone marrow mesenchymal stem cells (BM-MSCs) exosomes dramatically enhance tumor targeting efficacy, resulting in increased drug accumulation at the tumor location. The combination therapy (iEXO-OXA) induces antitumor immunity via tumor-suppressive macrophage polarization, recruitment of cytotoxic T cells, and downregulation of Tregs, and achieves considerable therapeutic efficacy in PDAC treatment [137].
PDT is a new non-invasive light-triggered therapeutic approach that has been clinically authorized and utilized to treat a range of malignancies [138]. PDT has the ability to increase the immunogenicity of tumors by stimulating the production of tumor antigens and triggering the release of a variety of DAMPs [139]. Nevertheless, due to tumor hypoxia and immune evasion, PDT is inefficient, thus reducing their photosensitizing efficiency and, consequently, the therapeutic effect [140]. Nanoparticles have successfully circumvented these constraints by delivering PDT in combination with immunotherapy [141]. Indocyanine green (ICG), which has notable near-infrared (NIR) optical properties within the optimal biological window for biomedical applications, has been extensively studied for NIR-fluorescence-guided imaging [142], as well as its great potential in PDT and photothermal therapy (PTT) due to deep permeation into tissues [143]. MnO2- and CaCO3-based nanomaterials have received a lot of attention in recent years for their ability to function as carriers for targeted medication delivery to regulate the TME inside solid tumors [144,145]. Herein, a nanoplatform of Mn@CaCO3/ICG@siRNA was designed and fabricated. The walnut-shaped MnO2 nanoparticles were generated by reducing potassium permanganate with polycyclic-aromatic hydrocarbons (PAH), and then the acquired MnO2 were modified with a pH-responsive CaCO3 cover layer while ICG was simultaneously entrapped. PD-L1-targeting siRNA was loaded via electrostatic contact onto the positively charged Mn@CaCO3/ICG to generate the nanoplatform (Mn@CaCO3/ICG). In vivo studies have proven that the nanoplatform is capable of delivering the medicine to tumor tissues and improving tumor hypoxia, hence enhancing the therapeutic efficiency of photodynamic therapy. Additionally, the combinatorial effects of silencing the checkpoint gene PD-L1, which mediates immune evasion, lead to a startling therapeutic impact on rousing the immune system [146]. In summary, recent studies support the hypothesis that PDT is a highly effective method for producing ICD in cancer immunotherapy. Nevertheless, while the majority of research has used mouse models, clinical validation of this method is important. PDT and ICD constitute an exciting field of research with numerous potential applications in cancer treatment.
6. Conclusions and Future Perspectives
In recent years, immunotherapy has made great advancements in the treatment of various solid tumor types. However, for the majority of patients, a positive initial reaction to treatment diminishes over time, resulting in relapse and recurrence of cancer, limiting its clinical use. A critical component that contributes to the limited response to immunotherapies is the occurrence of numerous pathways regulating tumor immune suppression. Understanding the molecular mechanisms underpinning immune evasion could identify novel therapeutic targets for improving immunotherapy efficacy. Therefore, we reviewed the immune evasion mechanisms that contribute to the failure of cancer immunotherapy in this review.
The rapid growth of nanomedicine in recent years has provided fresh insights into cancer immunotherapy. Due to the development of effective delivery mechanism, the engineering of siRNA carriers has generated considerable interest, being capable of delivering siRNA into tumor tissues and tumor cell cytoplasm. siRNA has shown the ability to target any cancer-related genes, therefore establishing a new class of cancer therapies. The ideal nanocarrier device would shield the RNAi therapeutic drug from the vascular environment and deliver it to tumor cells efficiently. We believe that nano-siRNA medications have significant therapeutic potential in cancer treatment, and the significant progress in siRNA-based formulation will continue to expand our understanding of their therapeutic potential. However, various obstacles must be overcome before they can become trustworthy delivery systems. In part, the shortcomings of nano-siRNA in cancer immunotherapy are partially due to our limited insufficient understanding of the immune network during carcinogenesis. In light of the fact that innate and adaptive immunity comprise a complex network, the effect of depleting or suppressing a particular component on the network as a whole is still unknown. In the context of targeted drugs, suppression of one or more components may be compensated by the overexpression of other processes. Second, it is important to enhance modification of siRNA by nanocarriers to protect it from nuclease-based degradation. Additionally, nanoparticle toxicity assays are underdeveloped at the moment. Given the potential of nanoparticles’ physicochemical qualities to alter when they combine with various biological molecules in the body, their final forms should be carefully assessed. As a result, significant work is still required to improve the size, shape, ligands, and other features of nanoparticles, as well as to identify possible dangers, before they can be transferred into clinical practice. Endosomal escape strategies, cell and tissue targeting, and the creation of novel biomaterials are critical for the translation of siRNA from laboratory to clinical.
Author Contributions
Conceptualization, Y.Z.; writing: original draft preparation, K.D.; writing: review and editing, K.D. and D.Y.; supervision, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. Q22 H031268.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [Green Version]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Tuccitto, A.; Shahaj, E.; Vergani, E.; Ferro, S.; Huber, V.; Rodolfo, M.; Castelli, C.; Rivoltini, L.; Vallacchi, V. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch. 2019, 474, 407–420. [Google Scholar] [CrossRef]
- Allegrezza, M.J.; Conejo-Garcia, J.R. Targeted Therapy and Immunosuppression in the Tumor Microenvironment. Trends Cancer 2017, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Ahlquist, P. RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 2002, 296, 1270–1273. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef]
- Aghamiri, S.; Jafarpour, A.; Malekshahi, Z.V.; Mahmoudi Gomari, M.; Negahdari, B. Targeting siRNA in colorectal cancer therapy: Nanotechnology comes into view. J. Cell. Physiol. 2019, 234, 14818–14827. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thambi, T.; Park, J.H.; Lee, D.S.J.C.C. Hypoxia-responsive nanocarriers for cancer imaging and therapy: Recent approaches and future perspectives. Chem. Commun. 2016, 52, 8492–8500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gallis, B.; Taya, M.; Wang, S.; Ho, R.J.Y.; Sasaki, T. pH-responsive artemisinin derivatives and lipid nanoparticle formulations inhibit growth of breast cancer cells in vitro and induce down-regulation of HER family members. PLoS ONE 2013, 8, e59086. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv. Sci. 2022, 9, e2103444. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Nano-immunotherapy: Overcoming tumour immune evasion. Semin. Cancer Biol. 2021, 69, 238–248. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Pandya, P.H.; Murray, M.E.; Pollok, K.E.; Renbarger, J.L. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J. Immunol. Res. 2016, 2016, 4273943. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploquin, A.; Zhou, Y.; Sullivan, N.J. Ebola Immunity: Gaining a Winning Position in Lightning Chess. J. Immunol. 2018, 201, 833–842. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.-Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef] [Green Version]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018, 18, 139–147. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Immune responses to malignancies. J. Allergy Clin. Immunol. 2010, 125, S272–S283. [Google Scholar] [CrossRef] [Green Version]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Jewell, C.M. Induction of anti-cancer T cell immunity by in situ vaccination using systemically administered nanomedicines. Cancer Lett. 2019, 459, 192–203. [Google Scholar] [CrossRef]
- Piepenbrink, K.H.; Borbulevych, O.Y.; Sommese, R.F.; Clemens, J.; Armstrong, K.M.; Desmond, C.; Do, P.; Baker, B.M. Fluorine substitutions in an antigenic peptide selectively modulate T-cell receptor binding in a minimally perturbing manner. Biochem. J. 2009, 423, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006, 90, 51–81. [Google Scholar] [PubMed]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Tricks tumors use to escape from immune control. Oral Oncol. 2009, 45, e119–e123. [Google Scholar] [CrossRef]
- Shaaban, M.; Othman, H.; Ibrahim, T.; Ali, M.; Abdelmoaty, M.; Abdel-Kawi, A.-R.; Mostafa, A.; El Nakeeb, A.; Emam, H.; Refaat, A. Immune Checkpoint Regulators: A New Era Toward Promising Cancer Therapy. Curr. Cancer Drug Targets 2020, 20, 429–460. [Google Scholar] [CrossRef]
- Abken, H.; Hombach, A.; Heuser, C.; Kronfeld, K.; Seliger, B. Tuning tumor-specific T-cell activation: A matter of costimulation? Trends Immunol. 2002, 23, 240–245. [Google Scholar] [CrossRef]
- Algarra, I.; García-Lora, A.; Cabrera, T.; Ruiz-Cabello, F.; Garrido, F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: Implications for tumor immune escape. Cancer Immunol. Immunother. 2004, 53, 904–910. [Google Scholar] [CrossRef]
- Meissner, M.; Reichert, T.E.; Kunkel, M.; Gooding, W.; Whiteside, T.L.; Ferrone, S.; Seliger, B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: Association with clinical outcome. Clin. Cancer Res. 2005, 11, 2552–2560. [Google Scholar] [CrossRef] [Green Version]
- Ogino, T.; Shigyo, H.; Ishii, H.; Katayama, A.; Miyokawa, N.; Harabuchi, Y.; Ferrone, S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006, 66, 9281–9289. [Google Scholar] [CrossRef] [Green Version]
- Jongsma, M.L.M.; Guarda, G.; Spaapen, R.M. The regulatory network behind MHC class I expression. Mol. Immunol. 2019, 113, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.E.; Roberts, E.W.; Fearon, D.T. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1060, 99–114. [Google Scholar] [PubMed]
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Sun, X.; Xing, L.; Deng, X.; Hsiao, H.T.; Manami, A.; Koutcher, J.A.; Clifton Ling, C.; Li, G.C. Hypoxia targeted bifunctional suicide gene expression enhances radiotherapy in vitro and in vivo. Radiother. Oncol. 2012, 105, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Rajendrakumar, S.K.; Uthaman, S.; Cho, C.-S.; Park, I.-K. Nanoparticle-Based Phototriggered Cancer Immunotherapy and Its Domino Effect in the Tumor Microenvironment. Biomacromolecules 2018, 19, 1869–1887. [Google Scholar] [CrossRef]
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-W.; Choi, H.-J.; Ha, S.-J.; Lee, K.-T.; Kwon, Y.-G. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta 2013, 1835, 170–179. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012, 22, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasievich, E.A.; Huang, L. The suppressive tumor microenvironment: A challenge in cancer immunotherapy. Mol. Pharm. 2011, 8, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, N.; Kim, E.; Sprang, B.; Leukel, P.; Khafaji, F.; Ringel, F.; Sommer, C.; Tuettenberg, J.; Tuettenberg, A. GARP as an Immune Regulatory Molecule in the Tumor Microenvironment of Glioblastoma Multiforme. Int. J. Mol. Sci. 2019, 20, 3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Li, J.; Mo, H.-Y.; Qiu, F.; Zheng, L.-M.; Qian, C.-N.; Zeng, Y.-X. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol. Cancer 2010, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.S.K. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013, 45, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.-L.; Puccetti, P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef]
- Wang, H.; Franco, F.; Ho, P.-C. Metabolic Regulation of Tregs in Cancer: Opportunities for Immunotherapy. Trends Cancer 2017, 3, 583–592. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y.; Zhu, G.; Augustine, M.; Zheng, L.; Goode, D.J.; Broadwater, M.; Ruff, W.; Flies, S.; Xu, H.; et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity 2011, 34, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terme, M.; Colussi, O.; Marcheteau, E.; Tanchot, C.; Tartour, E.; Taieb, J. Modulation of immunity by antiangiogenic molecules in cancer. Clin. Dev. Immunol. 2012, 2012, 492920. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Gorain, B.; Alhakamy, N.A.; Md, S. Role of therapeutic agents on repolarisation of tumour-associated macrophage to halt lung cancer progression. J. Drug Target. 2020, 28, 166–175. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Pyzer, A.R.; Cole, L.; Rosenblatt, J.; Avigan, D.E. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int. J. Cancer 2016, 139, 1915–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.-I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Cassim, S.; Pouyssegur, J. Tumor Microenvironment: A Metabolic Player that Shapes the Immune Response. Int. J. Mol. Sci. 2019, 21, 157. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the Tumor Microenvironment with Emerging Nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Phuengkham, H.; Ren, L.; Shin, I.W.; Lim, Y.T. Nanoengineered Immune Niches for Reprogramming the Immunosuppressive Tumor Microenvironment and Enhancing Cancer Immunotherapy. Adv. Mater. 2019, 31, e1803322. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Su, Y.; Zhou, J.; Wang, W. Nanoparticles designed to regulate tumor microenvironment for cancer therapy. Life Sci. 2018, 201, 37–44. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Pi, F.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; Leggas, M.; Evers, B.M.; Guo, P. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 74–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy. J. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, U.B.; Gunnarsson, L.; Géraudie, S.; Scheffler, U.; Griep, R.A.; Reiersen, H.; Duncan, A.R.; Kiprijanov, S.M. Fully human antagonistic antibodies against CCR4 potently inhibit cell signaling and chemotaxis. PLoS ONE 2014, 9, e103776. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L.; Mandapathil, M.; Schuler, P. The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg). Curr. Med.Chem. 2011, 18, 5217–5223. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, A.; Priceman, S.J.; Swiderski, P.; Kujawski, M.; Xin, H.; Cherryholmes, G.A.; Zhang, W.; Zhang, C.; Lahtz, C.; Kowolik, C.; et al. CTLA4 aptamer delivers STAT3 siRNA to tumor-associated and malignant T cells. J. Clin. Investig. 2014, 124, 2977–2987. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-Y.; Liu, Y.; Xu, C.-F.; Shen, S.; Sun, R.; Du, X.-J.; Xia, J.-X.; Zhu, Y.-H.; Wang, J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 2016, 231, 17–28. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Liu, J.; Han, Y.; Xu, H.; Leng, X.; Kong, D.; Liu, L. Hybrid spherical nucleotide nanoparticles can enhance the synergistic anti-tumor effect of CTLA-4 and PD-1 blockades. Biomater. Sci. 2020, 8, 4757–4766. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Su, X.; Pan, Y.; Han, X.; Shao, C.; Shi, Y. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer 2019, 18, 177. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, J.; Huang, L. Tackling TAMs for Cancer Immunotherapy: It’s Nano Time. Trends Pharmacol. Sci. 2020, 41, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Zeng, Q.; Jewell, C.M. Directing toll-like receptor signaling in macrophages to enhance tumor immunotherapy. Curr. Opin. Biotechnol. 2019, 60, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef]
- Gao, M.; Ha, T.; Zhang, X.; Wang, X.; Liu, L.; Kalbfleisch, J.; Singh, K.; Williams, D.; Li, C. The Toll-like receptor 9 ligand, CpG oligodeoxynucleotide, attenuates cardiac dysfunction in polymicrobial sepsis, involving activation of both phosphoinositide 3 kinase/Akt and extracellular-signal-related kinase signaling. J. Infect. Dis. 2013, 207, 1471–1479. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale 2020, 12, 22268–22280. [Google Scholar] [CrossRef]
- Qian, H.; Zhou, T.; Fu, Y.; Guo, M.; Yang, W.; Zhang, D.; Fang, W.; Yao, M.; Shi, H.; Chai, C.; et al. Self-assembled tetrahedral framework nucleic acid mediates tumor-associated macrophage reprogramming and restores antitumor immunity. Mol. Ther. Nucleic Acids 2022, 27, 763–773. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Qian, Y.; Qiao, S.; Dai, Y.; Xu, G.; Dai, B.; Lu, L.; Yu, X.; Luo, Q.; Zhang, Z. Molecular-Targeted Immunotherapeutic Strategy for Melanoma via Dual-Targeting Nanoparticles Delivering Small Interfering RNA to Tumor-Associated Macrophages. ACS Nano 2017, 11, 9536–9549. [Google Scholar] [CrossRef]
- Huang, C.; Jin, H.; Qian, Y.; Qi, S.; Luo, H.; Luo, Q.; Zhang, Z. Hybrid melittin cytolytic Peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano 2013, 7, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Pluen, A.; Boucher, Y.; Ramanujan, S.; McKee, T.D.; Gohongi, T.; di Tomaso, E.; Brown, E.B.; Izumi, Y.; Campbell, R.B.; Berk, D.A.; et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 4628–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Messmer, M.N.; Netherby, C.S.; Banik, D.; Abrams, S.I. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol. Immunother. 2015, 64, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Amoozgar, Z.; Goldberg, M.S. Targeting myeloid cells using nanoparticles to improve cancer immunotherapy. Adv. Drug Deliv. Rev. 2015, 91, 38–51. [Google Scholar] [CrossRef]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin.Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Li, M.; Ran, G.; Wang, X.; Lu, Z.; Li, T.; Tang, X.; Zhang, Z.; He, Q. Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase A silencing for enhanced cancer immunochemotherapy. J. Control. Release 2021, 335, 557–574. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.-M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed Death-1 Blockade with Pembrolizumab in Patients with Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.Y.; Yang, C.; Whilding, L.M.; Parente-Pereira, A.C.; Maher, J.; George, A.J.T.; Hedrick, J.L.; Yang, Y.Y.; Ghaem-Maghami, S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: Strategies to enhance T cell killing. Adv. Healthc. Mater. 2015, 4, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Roeven, M.W.H.; Hobo, W.; van der Voort, R.; Fredrix, H.; Norde, W.J.; Teijgeler, K.; Ruiters, M.H.J.; Schaap, N.; Dolstra, H. Efficient nontoxic delivery of PD-L1 and PD-L2 siRNA into dendritic cell vaccines using the cationic lipid SAINT-18. J. Immunother. 2015, 38, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, T.; Ma, F.; Zhang, K.; Gao, T.; Pei, R.; Zhang, Y. Aptamer-functionalized targeted siRNA delivery system for tumor immunotherapy. Biomed. Mater. 2022, 17, 14. [Google Scholar] [CrossRef]
- Adkins, I.; Fucikova, J.; Garg, A.D.; Agostinis, P.; Špíšek, R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2014, 3, e968434. [Google Scholar] [CrossRef]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Tani, K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014, 21, 39–49. [Google Scholar] [CrossRef] [Green Version]
- De Munck, J.; Binks, A.; McNeish, I.A.; Aerts, J.L. Oncolytic virus-induced cell death and immunity: A match made in heaven? J. Leukoc. Biol. 2017, 102, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaes, T.L.; Kaczmarek, A.; Delvaeye, T.; De Craene, B.; De Koker, S.; Heyndrickx, L.; Delrue, I.; Taminau, J.; Wiernicki, B.; De Groote, P.; et al. Vaccination with Necroptotic Cancer Cells Induces Efficient Anti-tumor Immunity. Cell Rep. 2016, 15, 274–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysko, O.; Aaes, T.L.; Kagan, V.E.; D’Herde, K.; Bachert, C.; Leybaert, L.; Vandenabeele, P.; Krysko, D.V. Necroptotic cell death in anti-cancer therapy. Immunol. Rev. 2017, 280, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. 2019, 58, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, W.; Chen, X.; Wang, Q.; Li, C.; Chen, Q.; Zhang, Y.; Lu, Y.; Ding, X.; Jiang, C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm. Sin. B 2020, 10, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, X.; Liao, Y.-P.; Salazar, F.; Sun, B.; Jiang, W.; Chang, C.H.; Jiang, J.; Wang, X.; Wu, A.M.; et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 2017, 8, 1811. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; de Azevedo, R.B. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014, 5, 24381. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Hu, D.; Zheng, M.; Zhao, P.; Liu, H.; Gao, D.; Gong, P.; Gao, G.; Zhang, P.; Ma, Y.; et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano 2014, 8, 12310–12322. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Guo, X.; Wang, Z.; Kong, F.; Luo, L.; Li, Q.; Zhu, C.; Yang, J.; Lou, Y.; et al. Gold Nanospheres-Stabilized Indocyanine Green as a Synchronous Photodynamic-Photothermal Therapy Platform That Inhibits Tumor Growth and Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 3354–3367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, H.; Zhou, G.; Bai, H.; Liang, H.; Wang, R.; Zhang, X.; Tan, W. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Chem. Soc. 2014, 136, 11220–11223. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y.J. Multifunctional albumin–MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Cao, W.; Xia, F.; Liu, B.; Niu, J.; Alfranca, G.; Sun, X.; Ma, L.; de la Fuente, J.M.; et al. A tumor microenvironment responsive biodegradable CaCO/MnO-based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics 2019, 9, 6867–6884. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).