Scope and Limitations of Current Antibiotic Therapies against Helicobacter pylori: Reviewing Amoxicillin Gastroretentive Formulations
Abstract
1. Introduction
2. Materials and Methods
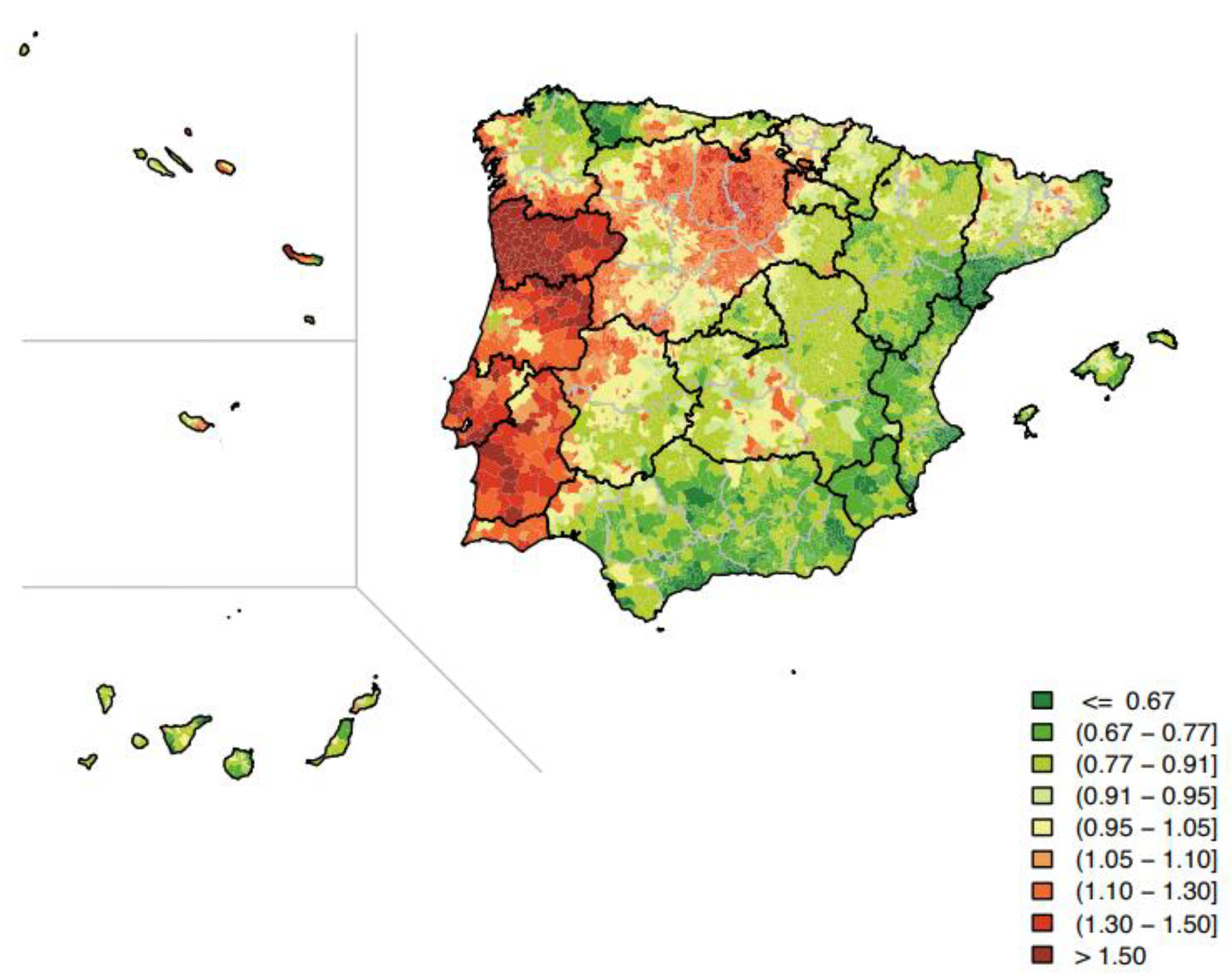
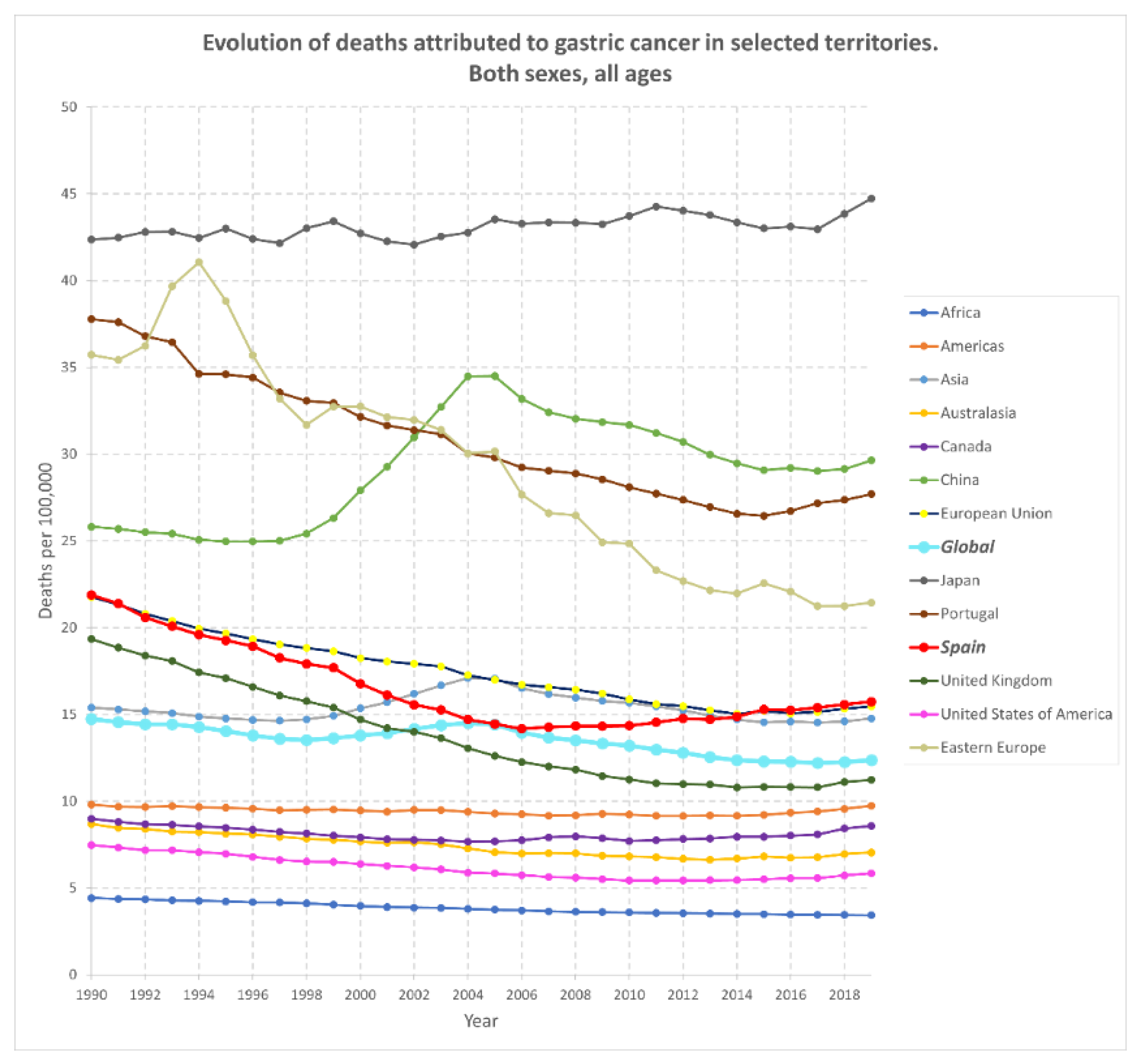
3. Gastric Cancer: Stats around the World
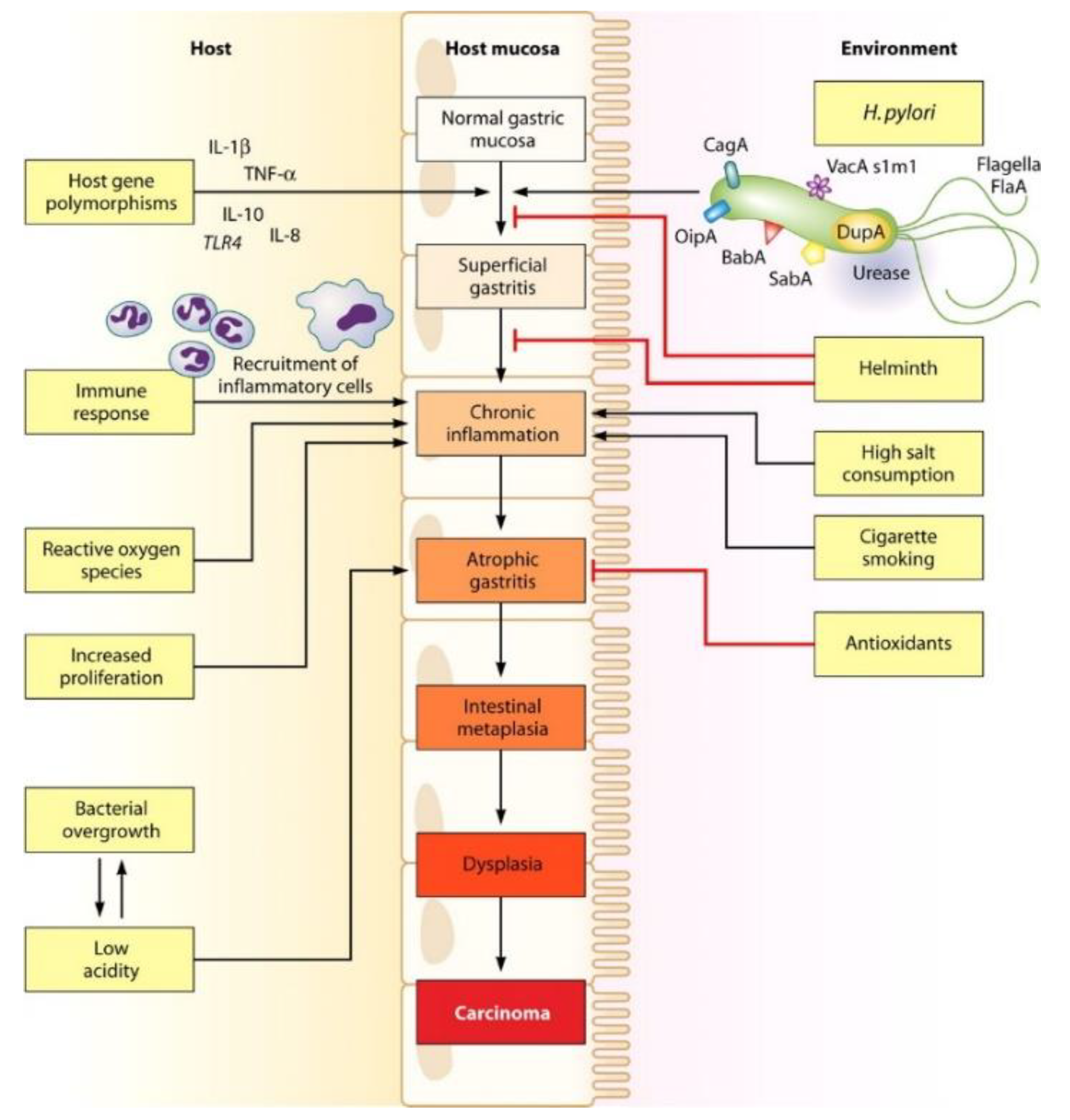
4. Clinical and Biological Characterization of Helicobacter pylori
5. Therapeutic Approaches against Helicobacter pylori: Precedents
| Region | Africa [40] | Australasia [41] | Americas [42] | Northern Europe (Norway) b [43] | Southern Europe b [43] | Asia | World [44] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vietnam [45] | Russia [46] | Iran [47] | China [48] | Continent [49] | |||||||||
| Antibiotic | 1986–2017 | pre-2000 | 2000 Onwards | 2007–2017 | 2013–2020 | 2000–2016 | 2011–2020 | 2010–2020 | 2013–2020 | 2006–2009 | 2015–2017 | ||
| Clarithromycin | 29.2 | 6.46 | 16.1 | 10 | 7.0 | 28.0 | 34.1 | 10.4 | 25.3 | 55.2 | 18.9 | 27.2 | |
| Metronidazole | 75.8 | 50.1 | 50.5 | 23 | 26.0 | 30.5 | 69.4 | 34.0 | 64.9 | 68.0 | 37.1 | 39.7 | |
| Levofloxacin | 17.4 a | N/A | 2.92 a | 15 | 2.5 | 23.5 | 27.9 | 20.0 | 21.9 | 49.7 | 11.6 | 22.5 | |
| Amoxicillin | 72.6 | 0.13 | 2.09 | 10 | 0 | 0.20 | 15.0 | 1.35 | 20.7 | 0.7 | 11.6 | 4.55 | |
6. Solution I: Amelioration of APIs Combinations
7. Solution II: Development of Smart, Gastroretentive DDSs
7.1. Gastroretentive Formulations for the Sustained Release of Amoxicillin in the Stomach in Helicobacter pylori Treatment
7.2. Floating Formulations
| Drug | Formulation | Matrix-Forming Polymers | Other Components | Floating Time (FT) Floating Lag Time (FLT) | DL (%) EE (%) | Sustained Release: Time (h), and Cumulative Drug Release (%) | Preparation Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| AMOX | Floating oral in situ gelling system | NaAlg HPMC K100 (thickening agent) | CaCl2 (Xrlinker), sodium citrate, NaHCO3 | >24 h FLT ≤ 30 s | 7.5% (w/v) … | pH 1.2: Burst release of drug >40% in 1 h 66–85% in 6 h | In situ gelation by Ca2+ ions | [78] |
| AMOX | Bilayer floating tablets | Aloe vera gel powder HPMC K4M, HPMC K100M | NaHCO3, Citric acid | >8 h FLT: 24–36 s | pH 1.2: 97% in 8 h | Prepared by applying direct compression technique | [80] | |
| AMOX + CLA | Floating modular DDS (hollow tablet) | - For AMOX: HPMC K100M - For CLA: HPMC K15M, PVP K30, PEG 6000 | Talc, magnesium stearate | 5 h FLT: 0 s | AMOX: 65% CLA: 84% … | - AMOX at pH 1.2: ~55% in 3 h - CLA at pH 3.0: 75–90% in 3 h | * Direct compression for AMOX * Compression of CLA-loaded granules | [81] |
| AMOX | Floating 3D-printed capsular devices | PVA filaments | BaSO4 | * In vitro: FT = 14 h; FLT = 0 s * In vivo (rabbits): FT = 10 h | -- | pH 1.2 ca. 80–100% in 1.5–3 h | Fused deposition modeling (FDM) 3D print 3D printing and thermal crosslinking HME-3D | [82] |
| Mz | FRS | (1) NaAlg (2) G | COM, PRE, GMS Sodium citrate and CaCO3 | >24 h TLF: 1 min | pH 2: 75–90% in 4–6 h | Raft systems prepared by ionotropic gelation | [89] | |
| AMOX | FRS | GG | GMS (lipid phase) CaCO3 + Sodium citrate (Xrlinker, gas generating agent) | FLT: 1–5.5 min | … | pH 1.2: 80–97% in 24 h | Emulsion and ionic crosslinking method | [83] |
| Luteolin | Floating microsponge | Eudragit SR100, EC | Tween 80 emulsifier | >8 h FLT: 0 s | -- | pH 1.2: 20–50% in 12 h | Quasi-emulsion method | [91] |
| AMOX | Floating microballoons | CAP Eudragit S100 | PVA Mixture of CH2Cl2, EtOH, iPrOH | Buoyancy: 43–96% | … EE = 57–93% | pH 1.2 34–75% in 8 h | Emulsion-solvent diffusion method | [84] |
7.3. Mucoadhesive Formulations
7.4. Micro and Nanostructured Mucoadhesive GRDDSs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Institute for Health Metrics and Evaluation (IHME). GBD Compare; IHME, University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Goodwin, C.S.; Armstrong, J.A.; Chilvers, T.; Peters, M.; Collins, M.D.; Sly, L.; McConnell, W.; Harper, W.E.S. Transfer of Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov., respectively. Int. J. Syst. Bacteriol. 1989, 39, 397–405. [Google Scholar] [CrossRef]
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting therapeutic strategies for h. Pylori treatment in the context of antibiotic resistance: Focus on alternative and complementary therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.M.; Gerwig, G.J.; Pitman, R.S.; Potts, L.F.; Williams, N.A.; Greenman, J.; Weinzweig, I.P.; Hirst, T.R.; Millar, M.R. Biofilm formation by Helicobacter pylori. Lett. Appl. Microbiol. 1999, 28, 121–126. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Alam, J.; Dilnawaz, F.; Sahoo, S.; Singh, D.; Mukhopadhyay, A.; Hussain, T.; Pati, S. Curcumin Encapsulated into Biocompatible Co-Polymer PLGA Nanoparticle Enhanced Anti-Gastric Cancer and Anti-Helicobacter Pylori Effect. Asian Pac. J. Cancer Prev. 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Pasechnikov, V.; Chukov, S.; Fedorov, E.; Kikuste, I.; Leja, M. Gastric cancer: Prevention, screening and early diagnosis. World J. Gastroenterol. 2014, 20, 13842–13862. [Google Scholar] [CrossRef]
- Fernández-Navarro, P.; Roquette, R.; Nuñez, O.; de Sousa-Uva, M.; García-Pérez, J.; López-Abente, G.; Nunes, B.; González-Sánchez, M.; Dinis, J.; Carmona, R.; et al. Atlas of Cancer Mortality in Portugal and Spain (2003–2012); Instituto de Salud Carlos III: Madrid, Spain, 2021; p. 834210075. [Google Scholar]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Aspirin and Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study. JNCI J. Natl. Cancer Inst. 2018, 110, 743–749. [Google Scholar] [CrossRef]
- Hansson, L.-E.; Nyrén, O.; Hsing, A.W.; Bergström, R.; Josefsson, S.; Chow, W.-H.; Fraumeni, J.F.; Adami, H.-O. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 1996, 335, 242–249. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Dore, M.P.; Graham, D.Y. Diagnosis and Treatment of Helicobacter pylori Infection. Chin. J. Gastroenterol. 2022, 73, 183–195. [Google Scholar] [CrossRef]
- Programas de Cribado de Cáncer. Ministerio de Sanidad (Gobierno de España). Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/Cribado/cribadoCancer.htm (accessed on 2 November 2021).
- Goral, V. Etiopathogenesis of Gastric Cancer. Scand. J. Surg. 2016, 17, 2745–2750. [Google Scholar]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lu, C.W.; Lin, C.J. Treatment of Helicobacter pylori infection: Current status and future concepts. World J. Gastroenterol. 2014, 20, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869. [Google Scholar] [CrossRef]
- Sankararaman, S.; Moosavi, L. Urea Breath Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542286/ (accessed on 22 May 2022).
- Sgouras, D.; Tegtmeyer, N.; Wessler, S. Activity and Functional Importance of Helicobacter pylori Virulence Factors. Adv. Exp. Med. Biol. 2019, 1149, 35–56. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 7343–7350. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Shichijo, S.; Hirata, Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J. Gastroenterol. 2018, 24, 2163–2172. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Terebiznik, M.R.; Raju, D.; Vázquez, C.L.; Torbricki, K.; Kulkarni, R.; Blanke, S.R.; Yoshimori, T.; Colombo, M.I.; Jones, N.L. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy 2009, 5, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Bismuth therapy in gastrointestinal diseases. Gastroenterology 1990, 99, 863–875. [Google Scholar] [CrossRef]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. Fluoroquinolones. Consumer Notice. 2020. Available online: https://www.consumernotice.org/drugs-and-devices/fluoroquinolones/ (accessed on 30 April 2022).
- Kiyotoki, S.; Nishikawa, J.; Sakaida, I. Efficacy of vonoprazan for helicobacter pylori eradication. Intern. Med. 2020, 59, 153–161. [Google Scholar] [CrossRef]
- Scott, D.R.; Sachs, G.; Marcus, E.A. The role of acid inhibition in Helicobacter pylori eradication [version 1; referees: 3 approved]. F1000Research 2016, 5, 1747. [Google Scholar] [CrossRef]
- Furuta, T.; Graham, D.Y. Pharmacologic Aspects of Eradication Therapy for Helicobacter pylori Infection. Gastroenterol. Clin. N. Am. 2012, 39, 465–480. [Google Scholar] [CrossRef]
- Reed, M.D. Optimal antibiotic dosing. The pharmacokinetic-pharmacodynamic interface. Postgrad. Med. 2000, 108, 17–24. [Google Scholar] [CrossRef]
- Gisbert, J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820968736. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–238. [Google Scholar] [CrossRef]
- Guo, B.; Cao, N.W.; Zhou, H.Y.; Chu, X.J.; Li, B.Z. Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line Helicobacter pylori eradication: A systematic review and meta-analysis. Microb. Pathog. 2021, 152, 104661. [Google Scholar] [CrossRef]
- Li, B.Z.; Threapleton, D.E.; Wang, J.Y.; Xu, J.M.; Yuan, J.Q.; Zhang, C.; Li, P.; Ye, Q.L.; Guo, B.; Mao, C.; et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: Systematic review and network meta-analysis. BMJ 2015, 351, h4052. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.D.; Rodrigues, D.A.; Figueiras, A.; Zapata-Cachafeiro, M.; Roque, F.; Herdeiro, M.T. Antibiotic dispensation without a prescription worldwide: A systematic review. Antibiotics 2020, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007, 12, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Jaka, H.; Rhee, J.A.; Östlundh, L.; Smart, L.; Peck, R.; Mueller, A.; Kasang, C.; Mshana, S.E. The magnitude of antibiotic resistance to Helicobacter pylori in Africa and identified mutations which confer resistance to antibiotics: Systematic review and meta-analysis. BMC Infect. Dis. 2018, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.P.; Gehlert, J.; Rayner, C.K.; Roberts-Thomson, I.C.; Costello, S.; Mangoni, A.A.; Bryant, R.V. Antibiotic resistance of Helicobacter pylori in Australia and New Zealand: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1450–1456. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lerang, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Gasbarrini, A.; et al. Antibiotic resistance prevalence and trends in patients infected with helicobacter pylori in the period 2013–2020: Results of the european registry on h. pylori management (hp-eureg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef]
- Kasahun, G.G.; Demoz, G.T.; Desta, D.M. Primary Resistance Pattern of Helicobacter pylori to Antibiotics in Adult Population: A Systematic Review. Infect. Drug Resist. 2020, 13, 1567–1573. [Google Scholar] [CrossRef]
- Van Khien, V.; Thang, D.M.; Hai, T.M.; Duat, N.Q.; Khanh, P.H.; Ha, D.T.; Binh, T.T.; Dung, H.D.Q.; Trang, T.T.H.; Yamaoka, Y. Management of antibiotic-resistant helicobacter pylori infection: Perspectives from Vietnam. Gut Liver 2019, 13, 483–497. [Google Scholar] [CrossRef]
- Andreev, D.N.; Maev, I.V.; Kucheryavyy, Y.A. Helicobacter pylori resistance in the Russian Federation: A meta-analysis of studies over the past 10 years. Ter. Arkhiv 2020, 92, 24–30. [Google Scholar]
- Khademi, F.; Sahebkar, A. An Updated Systematic Review and Meta-Analysis on the Helicobacter pylori Antibiotic Resistance in Iran (2010–2020). Microb. Drug Resist. 2020, 26, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Li, J.; Dong, X.H.; Teng, G.G.; Zhang, W.; Cheng, H.; Gao, W.; Dai, Y.; Zhang, X.H.; Wang, W.H. The effect of previous eradication failure on antibiotic resistance of Helicobacter pylori: A retrospective study over 8 years in Beijing. Helicobacter 2021, 26, e12804. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointest. Liver Dis. 2010, 19, 409–414. [Google Scholar]
- Hafeez, M.; Qureshi, Z.A.; Khattak, A.L.; Saeed, F.; Asghar, A.; Azam, K.; Khan, M.A. Helicobacter Pylori Eradication Therapy: Still a Challenge. Cureus 2021, 13, 14872. [Google Scholar] [CrossRef]
- Wolle, K.; Malfertheiner, P. Treatment of Helicobacter pylori. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Phiphatpatthamaamphan, K.; Vilaichone, R.K.; Siramolpiwat, S.; Tangaroonsanti, A.; Chonprasertsuk, S.; Bhanthumkomol, P.; Pornthisarn, B.; Mahachai, V. Effect of IL-1 polymorphisms, CYP2C19 genotype and antibiotic resistance on Helicobacter pylori eradication comparing between 10-day sequential therapy and 14-day standard triple therapy with four-times-daily-dosing of amoxicillin in Thailand. Asian Pac. J. Cancer Prev. 2016, 17, 1903–1907. [Google Scholar] [CrossRef]
- Hong, J.; Shu, X.; Liu, D.; Zhu, Y.; Xie, C.; Xie, Y.; Zhang, K.; Wang, A.; Xiong, H.; Zeng, H.; et al. Antibiotic resistance and CYP2C19 polymorphisms affect the efficacy of concomitant therapies for Helicobacter pylori infection: An open-label, randomized, single-centre clinical trial. J. Antimicrob. Chemother. 2016, 71, 2280–2285. [Google Scholar] [CrossRef][Green Version]
- Vítor, J.M.B.; Vale, F.F. Alternative therapies for Helicobacter pylori: Probiotics and phytomedicine. FEMS Immunol. Med. Microbiol. 2011, 63, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Astruc, B.; Jenkins, H.; Jenkins, R. Effect of Therapeutic and Supratherapeutic Doses of Vonoprazan on the QT/QTc Interval in a Phase I Randomized Study in Healthy Subjects. Clin. Transl. Sci. 2017, 10, 208–216. [Google Scholar] [CrossRef]
- Otake, K.; Sakurai, Y.; Nishida, H.; Fukui, H.; Tagawa, Y.; Yamasaki, H.; Karashima, M.; Otsuka, K.; Inatomi, N. Characteristics of the Novel Potassium-Competitive Acid Blocker Vonoprazan Fumarate (TAK-438). Adv. Ther. 2016, 33, 1140–1157. [Google Scholar] [CrossRef] [PubMed]
- Mulford, D.J.; Leifke, E.; Hibberd, M.; Howden, C.W. The Effect of Food on the Pharmacokinetics of the Potassium-Competitive Acid Blocker Vonoprazan. Clin. Pharmacol. Drug Dev. 2022, 11, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.; Sakurai, Y.; Nishimura, A.; Okamoto, H.; Hibberd, M.; Jenkins, R.; Yoneyama, T.; Ashida, K.; Ogama, Y.; Warrington, S. Randomised clinical trial: Safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment. Pharmacol. Ther. 2015, 41, 636–648. [Google Scholar] [CrossRef]
- Sugano, K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: Safety and clinical evidence to date. Ther. Adv. Vaccines 2018, 11, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Echizen, H. The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations. Clin. Pharmacokinet. 2016, 55, 409–418. [Google Scholar] [CrossRef]
- Takeda Pharmaceutical Company Limited TAKECAB® Now Available for the Treatment of Acid-Related Diseases in Japan. Available online: https://www.takeda.com/newsroom/newsreleases/2015/takecab-now-available-for-the-treatment-of-acid-related-diseases-in-japan/ (accessed on 30 April 2022).
- Bunchorntavakul, C.; Buranathawornsom, A. Randomized clinical trial: 7-day vonoprazan-based versus 14-day omeprazole-based triple therapy for Helicobacter pylori. J. Gastroenterol. Hepatol. 2021, 36, 3308–3313. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef]
- Sue, S.; Shibata, W.; Sasaki, T.; Kaneko, H.; Irie, K.; Kondo, M.; Maeda, S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J. Gastroenterol. Hepatol. 2019, 34, 686–692. [Google Scholar] [CrossRef]
- Scarpignato, C.; Leifke, E.; Smith, N.; Mulford, D.J.; Lahu, G.; Facius, A.; Howden, C.W. A Population Pharmacokinetic Model of Vonoprazan: Evaluating the Effects of Race, Disease Status, and Other Covariates on Exposure. J. Clin. Pharmacol. 2022, 62, 801–811. [Google Scholar] [CrossRef]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the US and Europe: Randomized Clinical Trial. Gastroenterology 2022, 20, 109–112. [Google Scholar] [CrossRef]
- Jenkins, H.; Jenkins, R.; Patat, A. Effect of Multiple Oral Doses of the Potent CYP3A4 Inhibitor Clarithromycin on the Pharmacokinetics of a Single Oral Dose of Vonoprazan: A Phase I, Open-Label, Sequential Design Study. Clin. Drug Investig. 2017, 37, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Arrua, E.C.; Sanchez, S.V.; Trincado, V.; Hidalgo, A.; Quest, A.F.G.; Morales, J.O. Experimental design and optimization of a novel dual-release drug delivery system with therapeutic potential against infection with Helicobacter pylori. Colloids Surf. B Biointerfaces 2022, 213, 112403. [Google Scholar] [CrossRef] [PubMed]
- Gottesmann, M.; Goycoolea, F.M.; Steinbacher, T.; Menogni, T.; Hensel, A. Smart drug delivery against Helicobacter pylori: Pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 2020, 104, 5943–5957. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.K.; Singh, S.; Nath, G.; Dubey, R.K. Evaluation of pH Triggers in situ Porous Controlled Release Micro Balloon Delivery of Amoxicillin for Eradication of Helicobacter pylori. Curr. Drug Deliv. 2011, 8, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; De, P.K.; De, A.; Ojha, S.; De, R.; Mukhopadhyay, A.K.; Samanta, A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016, 89, 622–631. [Google Scholar] [CrossRef]
- Moogooee, M.; Ramezanzadeh, H.; Jasoori, S.; Omidi, Y.; Davaran, S. Synthesis and in vitro studies of cross-linked hydrogel nanoparticles containing amoxicillin. J. Pharm. Sci. 2011, 100, 1057–1066. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Romero-Azogil, L.; Benito, E.; Lucas, R.; García-Martín, M.G.; De-Paz, M.-V. In-Depth Study into Polymeric Materials in Low-Density Gastroretentive Formulations. Pharmaceutics 2020, 12, 636. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Balasubramaniam, J.; Mishra, B. Development and evaluation of a novel floating in situ gelling system of amoxicillin for eradication of Helicobacter pylori. Int. J. Pharm. 2007, 335, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Patel, D.K.; Patel, C.N. Formulation and Evaluation of Floating Oral In Situ Gelling System of Amoxicillin. ISRN Pharm. 2011, 2011, 276250. [Google Scholar] [CrossRef] [PubMed]
- Rajinikanth, P.S.; Mishra, B. Stomach-site specific drug delivery system of clarithromycin for eradication of Helicobacter pylori. Chem. Pharm. Bull. 2009, 57, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Ranade, A.N.; Wankhede, S.S.; Ranpise, N.S.; Mundada, M.S. Development of bilayer floating tablet of amoxicillin and aloe vera gel powder for treatment of gastric ulcers. AAPS PharmSciTech 2012, 13, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Conti, C.; Colombo, G.; Castrati, L.; Scarpignato, C.; Barata, P.; Sandri, G.; Caramella, C.; Bettini, R.; Buttini, F.; et al. Floating modular drug delivery systems with buoyancy independent of release mechanisms to sustain amoxicillin and clarithromycin intra-gastric concentrations. Drug Dev. Ind. Pharm. 2016, 42, 332–339. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Fabrication of floating capsule-in-3D-printed devices as gastro-retentive delivery systems of amoxicillin. J. Drug Deliv. Sci. Technol. 2020, 55, 101393. [Google Scholar] [CrossRef]
- Kamsali, A.; Eranti, B.; Ch, M.; Manne, R.; Barghav, G.C.; Reddy, P.S. Development and Optimization of Amoxicillin Floating Raft System to effectively treat Helicobacter pylori infection. Ars Pharm. 2020, 61, 163–168. [Google Scholar]
- Awasthi, R.; Kulkarni, G.T.; Pawar, V.K.; Garg, G. Optimization studies on gastroretentive floating system using response surface methodology. AAPS PharmSciTech 2012, 13, 85–93. [Google Scholar] [CrossRef][Green Version]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef]
- Tadros, M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010, 74, 332–339. [Google Scholar] [CrossRef]
- Badhan, A.C.; Mashru, R.C.; Shah, P.P.; Thakkar, A.R.; Dobaria, N.B. Development and evaluation of sustained release gastroretentive minimatrices for effective treatment of H. pylori Infection. AAPS PharmSciTech 2009, 10, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Thombre, N.A.; Gide, P.S. Floating-bioadhesive gastroretentive Caesalpinia pulcherrima-based beads of amoxicillin trihydrate for Helicobacter pylori eradication. Drug Deliv. 2016, 23, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Abou Youssef, N.A.H.; Kassem, A.A.; El-Massik, M.A.E.; Boraie, N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015, 486, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.K.; Singh, S.; Nath, G. Formulation and In-vitro evaluation of pH-sensitive oil entrapped polymeric blend amoxicillin beads for the eradication of Helicobacter pylori. Iran. J. Pharm. Res. 2012, 11, 447–455. [Google Scholar] [PubMed]
- Jafar, M.; Salahuddin, M.; Khan, M.S.A.; Alshehry, Y.; Alrwaili, N.R.; Alzahrani, Y.A.; Imam, S.S.; Alshehri, S. Preparation and in vitro-in vivo evaluation of luteolin loaded gastroretentive microsponge for the eradication of helicobacter pylori infections. Pharmaceutics 2021, 13, 2094. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef]
- Nitave, S.A.; Patil, V.A.; Kagalkar, A.A. Review on gastro retentive drug delivery system (GRDDS). Int. J. Pharm. Sci. Rev. Res. 2014, 27, 90–95. [Google Scholar]
- Amin, M.L.; Ahmed, T.; Mannan, M.A. Development of floating-mucoadhesive microsphere for site specific release of metronidazole. Adv. Pharm. Bull. 2016, 6, 195–200. [Google Scholar] [CrossRef]
- Grosso, R.; De-Paz, M.-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics 2021, 13, 854. [Google Scholar] [CrossRef]
- Zhao, S.; Lv, Y.; Zhang, J.B.; Wang, B.; Lv, G.J.; Ma, X.J. Gastroretentive drug delivery systems for the treatment of Helicobacter pylori. World J. Gastroenterol. 2014, 20, 9321–9329. [Google Scholar] [CrossRef]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, II, 575–584. [Google Scholar] [CrossRef]
- Onuigbo, E.; Onugwu, A.; Nwocha, M.; Odiase, A.; Attama, A. Preparation and in vitro evaluation of amoxicillin encapsulated in alginate-coated chitosan microparticles. Trop. J. Pharm. Res. 2016, 15, 2303–2309. [Google Scholar] [CrossRef][Green Version]
- Raval, J.; Patel, J.; Patel, M. Formulation and in vitro characterization of spray dried microspheres of amoxicillin. Acta Pharm. 2010, 60, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Huang, C.-H.; Yang, J.-C.; Wang, C.-H.; Shieh, M.-J. Residence Time-Extended Nanoparticles by Magnetic Field Improve the Eradication Efficiency of Helicobacter pylori. ACS Appl. Mater. Interfaces 2020, 12, 54316–54327. [Google Scholar] [CrossRef]
- Stephin, J.; Marina, K. Preparation and Investigation of Gastro-Retentive Mucoadhesive Microspheres of Clarithromycin-Resin Complex. Int. J. Pharm. Investig. 2020, 10, 445–451. [Google Scholar] [CrossRef]
- Hardenia, A.; Gupta, A.K. Development and optimization of gastroretentive mucoadhesive microspheres using 33 factorial design. Int. J. Pharm. Sci. Res. 2016, 7, 2020–2030. [Google Scholar] [CrossRef]
- Venkateswaramurthy, N.; Sambathkumar, R.; Perumal, P. Design and evaluation of controlled release mucoadhesive microspheres of amoxicillin for anti Helicobacter pylori therapy. Asian J. Pharm. 2011, 5, 238–245. [Google Scholar] [CrossRef]
- Angadi, S.C.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel composite blend microbeads of sodium alginate coated with chitosan for controlled release of amoxicillin. Int. J. Biol. Macromol. 2012, 51, 45–55. [Google Scholar] [CrossRef]
- Villegas, I.; Rosillo, M.Á.; Alarcón-de-la-Lastra, C.; Vázquez-Román, V.; Llorente, M.; Sánchez, S.; Gil, A.G.; Alcalde, P.; González, E.; Rosell, E.; et al. Amoxicillin and Clarithromycin Mucoadhesive Delivery System for Helicobacter pylori Infection in a Mouse Model: Characterization, Pharmacokinetics, and Efficacy. Pharmaceutics 2021, 13, 153. [Google Scholar] [CrossRef]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of Chitosan Determines Its Interactions with Mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef]
- Shastri, D.H.; Dodiya, H.D.; Shelat, P.; Bhanupriy, A.K. Formulation development and evaluation of a gastroretentive in situ oral gel of Cefuroxime Axetil. J. Young Pharm. 2016, 8, 324–329. [Google Scholar] [CrossRef]
- Earle, R.R.; Bharathi, V.V.; Lakshmi Usha, A.; Ksheera Bhavani, A.V.S. Cross-Linked Chitosan Based Stomach Specific Mucoadhesive Microspheres Loaded with Amoxicillin: Preparation and ex vivo Characterization. Int. J. Pharm. Investig. 2020, 10, 59–63. [Google Scholar] [CrossRef]
- Hadke, J.; Khan, S. Preparation of sterculia foetida-pullulan-based semi-interpenetrating polymer network gastroretentive microspheres of amoxicillin trihydrate and optimization by response surface methodology. Turk. J. Pharm. Sci. 2021, 18, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Sharaf, M.; Samreen; Dong, Q.; Wang, L.; Chi, Z.; Liu, C.-G. Bacteria-targeting chitosan/carbon dots nanocomposite with membrane disruptive properties improve eradication rate of Helicobacter pylori. J. Biomater. Sci. Polym. Ed. 2021, 32, 2423–2447. [Google Scholar] [CrossRef]
- Srivastava, A.; Verma, A.; Saraf, S.; Jain, A.; Tiwari, A.; Panda, P.K.; Jain, S.K. Mucoadhesive gastroretentive microparticulate system for programmed delivery of famotidine and clarithromycin. J. Microencapsul. 2021, 38, 151–163. [Google Scholar] [CrossRef]
- Abdelghany, A.; El-Desouky, M.A.; Shemis, M. Synthesis and characterization of amoxicillin-loaded polymeric nanocapsules as a drug delivery system targeting Helicobacter pylori. Arab J. Gastroenterol. 2021, 22, 278–284. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
| Type of Mucoadhesive Polymer | Polymer | Ref. |
|---|---|---|
| Cationic polymers | CTS | [72,98,99] |
| (Semi)synthetic anionic polymers | PAA | [73,100] |
| Carbopol® | [90,101,102,103] | |
| Polycarbophil® | [101] | |
| NaCMC | [104] | |
| Anionic polysaccharides and gums | NaAlg | [98,105] |
| Pectin | [106,107] | |
| Gellan gum | [90] | |
| Xanthan gum | [105,108] | |
| Other polysaccharides and gums | Guar gum | [94,108] |
| Gum ghatti | [108] | |
| Sterculia foetida | [109] | |
| Pullulan | [109] | |
| Thiolated polymers | Thiolated CTS | [110] |
| Thiolated PAA | [111] |
| Drug | Formulations | Matrix-Forming Polymers | Other Components | Mucoadhesive Performance | DL EE | Sustained Release: Time (h), and Cumulative Drug Release (%) | Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| AMOX | Semi-IPN microspheres | Sterculia foetida Pullulan | - LLP (lipid phase), - Span 80 (surfactant) - Glutaraladehyde (Xrlinker) | Mucoadh. (goat intestinal mucosa): 81.7% at 12 h | - … - EE: 60–88% | pH 1.2: 60–89% in 12 h | Water-in-oil emulsification-crosslinking method | [109] |
| AMOX | Mucoadhesive microparticles | NaAlg CTS as coating agent | CaCl2 (XrL NaAlg) Na2SO4 (XrL CTS) | Mucoadh. (pig’s ileum): 76%, 100 mL SIF | - -- - EE: 96–97% | - SGF: 28–45% in 24 h - SIF: 21–39% in 24 h | Ionic gelation method | [98] |
| AMOX | Mucoadhesive microspheres | Carbopol® 934P EC | LP (lipid phase) Span 80 (surfactant) | Mucoadh. (rat stomach mucosa): 48–68% for 10 h | - … - EE: 66% | - pH 1.2 and - pH 7.8: ca. 90% in 10 h | Emulsion solvent evaporation method | [102] |
| AMOX | Mucoadhesive microspheres | Carbopol® 974P, HPMC K4M, Eudragit RS 100 | LP (lipid phase) | Mucoadh. (rat stomach mucosa): 56–89% at 6 h | - DL: 5% - EE: 57–88% | pH 1.2: Initial burst effect 75–100% in 7 h | Microspheres were prepared by solvent evaporation technique | [103] |
| AMOX | Mucoadhesive microspheres | CTS | Glutaraladehyde (Xrlinker) | Mucoadh. (rat stomach mucosa): 38–62% after 5 h | - DL: 25–50% - EE: 77–92% | pH: 1.2 It was measured the time required for 80% release: 3–10 h | Spray-drying method followed by chemical XrL (glutaraldehyde) | [99] |
| AMOX | Nanocomposites of CDs and CTS-based NP | Thiolated- ureido-CTS (mucoadh) Poly(malic acid) | CDs | Mucoadh. Measured as aggregation rate with mucin (fluorescence intensity after 3 h): 19–46% | - DL: 24–28% - … | Changing pH along the experiment (final time 12 h) - pH 1.2 (2 h): 35% - pH 6.0 (2 h): 60% - pH 7.0, 8 h: 80–95% | - CTS-based NP: ionic gelation method - CD-NP Composite: chemical reaction (Amide formation) between CDs and NP | [110] |
| AMOX | Composite blend microbeads | NaAlg NaCMC | - MAS particles - Chitosan (enteric coated) - CaCl2 | … | - … - EE: 52–92% | - pH 1.2 20–40% in 8 h (depending on matrix swelling). | Ionic gelation method | [104] |
| AMOX | Magnetic NP | CTS, PAA | SPIO | Mucoadhesion determined by changes in the surface charge potential of mucin particles upon absorption of mucoadhesive polymer | - DL: 0.1% (w/v) - EE: 77.8% | Changing pH along the experiment (final time 24 h) - pH 2.5 (2 h): ca. 20% - pH 6.5 (2 h): 30–40% in 4 h - pH 7.4 (20 h): 70% | Ionic gelation method | [100] |
| AMOX | Uncoated or pectin-coated liposomes (UCL, CL) | Pectin as coating agent | Lecithin, cholesterol, DDAB | Mucoadh. Measured by microviscometry (MX of UCL and CL with pig gastric mucin type III). | - … - EE: - UCL: 66% - CL: 83% | pH not stated - UCL: 85% (= 6.1 μM) in 1 h - CL: 75% (=62.3 μM) in 1 h | Thin-film hydration method | [106] |
| AMOX | Xrlinked hydrogel NPs | Monomers used: NIPAM (thermoresponsive) + AA (bioadhesive) + HEMA | BPO (radical initiatior) XrLinker: TEGDMA Poloxamer 188 (surfactant for emulsion-evaporation method) | … | - DL: 1–2% - EE: 70.2–91.4% | - pH 1.0: 88.5% in 4 h - pH 7.4: 45% in 4 h | - Synthesis of polymer: Radical polymerization - NP formation: Emulsion-evaporation technique | [73] |
| AMOX CLA | Liquid hydrogel | MUCOLAST®: NaAlg, NaCMC, XG | CaSO4 (XrLinker) Glycerol/GMS as drug solvents | Mucoadh. Time needed to detach formulation from simulated gastric mucosa: 4.0–6.7 h | - DL: - AMOX: 89.4–8.94 mg/100 μL - CLA: 44.7–4.47 mg/100 μL | pH: 5.0 AMOX: 50–60% CLA: 35–40% | Ionic gelation method | [105] |
| Drug | Formulation | Matrix-Forming Polymers | Other Components | Floating Time (FT) Floating Lag Time (FLT) Mucoadhesive Performance | DL EE | Sustained Release: Time (h), and Cumulative Drug Release (%) | Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| AMOX | Coated oil-entrapped beads | NaAlg HPMC | - CTS (coating and mucoadh.) - Sunflower oil -CaCl2 | FT > 24 h - FLT:43–50 s - Mucoad. (Sheep stomach mucosa): 75–85% | … 55.2–90.9% | pH 1.0: 59–78% in 7 h | Ionotropic gelation method | [72] |
| AMOX | Microspheres | CPG NaAlg | - CTS (coating and mucoadh.) - CaCO3 - CaCl2 | - Float. Capac.: 72–87% - FLT: 4–10 min - Mucoadh (in vivo, Wistar rats): 85%, 7 h | -- 65–89% | pH 1.2: 79–92% in 8 h | Ionotropic gelation method | [88] |
| Mz | Floating and mucoadh. microspheres | NaAlg, GG Carbopol® | CaCl2, NaHCO3, Eudragit® L100 | - FT > 8 h … - Mucoadh. (rat stomach mucosa): 61% | … 40–76% | pH 1.2: 29–73% in 8 h | Ionotropic gelation method | [94] |
| AMOX | Oil-entrapped buoyant beads | G HPMC or Carbopol® 934 | - Light mineral oil - CaCO3, CaCl2 - EC (coating) | Float. Capac.: 60–85% … … | 48–82% 73–96% | pH 1.2: 76% in 8 h pH 3.4: 62% in 8 h | Ionotropic gelation method | [90] |
| AMOX | Minimatrices | XG HPMC K100M CR/PEO | - NaHCO3 - Citric acid - Carbopol® 974P (lubricant and mucoadhesive) PVP K30 | - FT > 12 h - FLT: 7–32 min - Bioadhesive strength (goat stomach tissue): 5.6–18 dyn/cm2 | pH 1.2: 31.9–53.3% in 1 h 95% in 2.6–9.4 h | Non aqueous granulation method | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosso, R.; de-Paz, M.-V. Scope and Limitations of Current Antibiotic Therapies against Helicobacter pylori: Reviewing Amoxicillin Gastroretentive Formulations. Pharmaceutics 2022, 14, 1340. https://doi.org/10.3390/pharmaceutics14071340
Grosso R, de-Paz M-V. Scope and Limitations of Current Antibiotic Therapies against Helicobacter pylori: Reviewing Amoxicillin Gastroretentive Formulations. Pharmaceutics. 2022; 14(7):1340. https://doi.org/10.3390/pharmaceutics14071340
Chicago/Turabian StyleGrosso, Roberto, and M.-Violante de-Paz. 2022. "Scope and Limitations of Current Antibiotic Therapies against Helicobacter pylori: Reviewing Amoxicillin Gastroretentive Formulations" Pharmaceutics 14, no. 7: 1340. https://doi.org/10.3390/pharmaceutics14071340
APA StyleGrosso, R., & de-Paz, M.-V. (2022). Scope and Limitations of Current Antibiotic Therapies against Helicobacter pylori: Reviewing Amoxicillin Gastroretentive Formulations. Pharmaceutics, 14(7), 1340. https://doi.org/10.3390/pharmaceutics14071340







