HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and HO-3867 Treatment
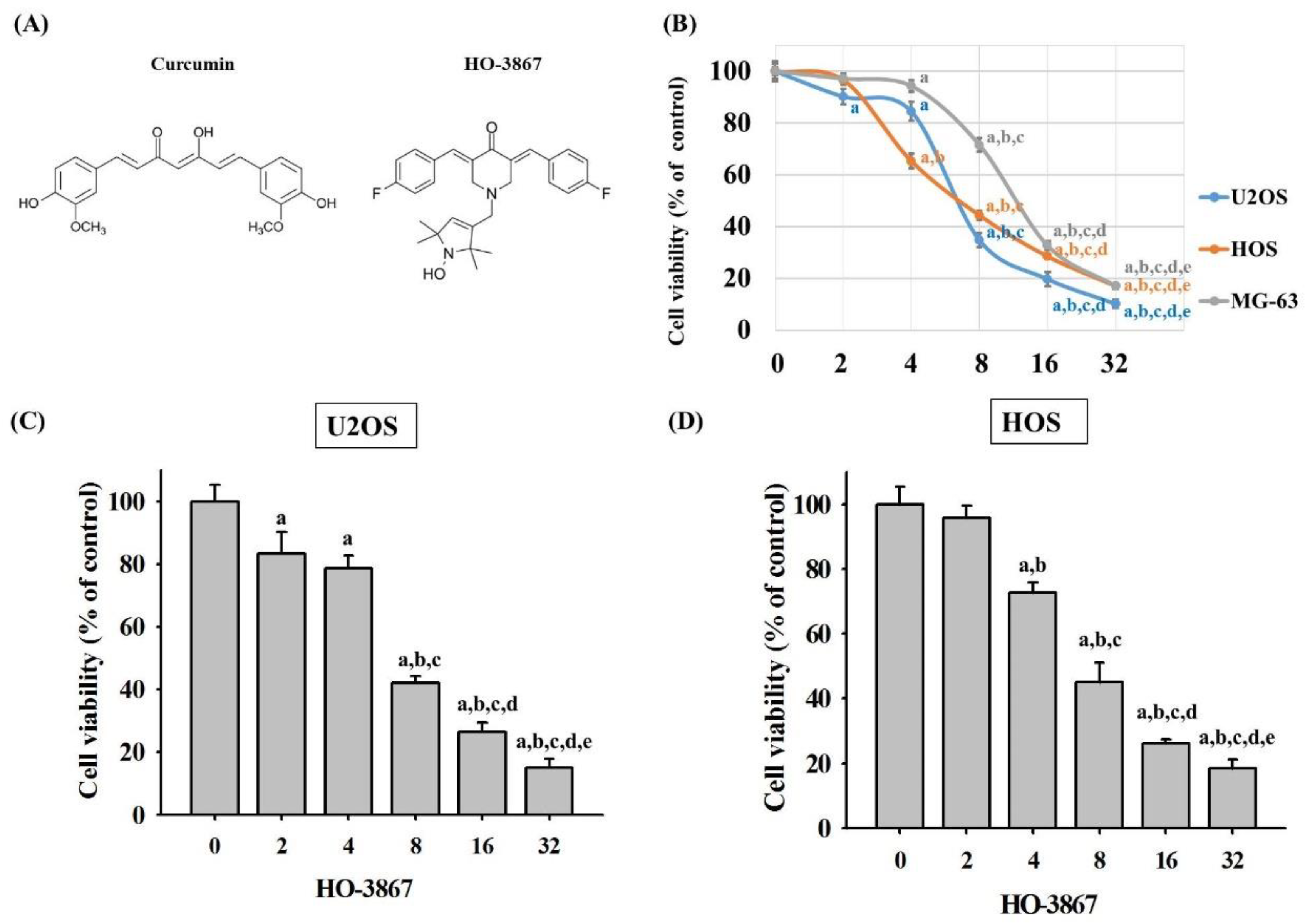
2.2. Microculture Tetrazolium Colorimetric (MTT) Assay
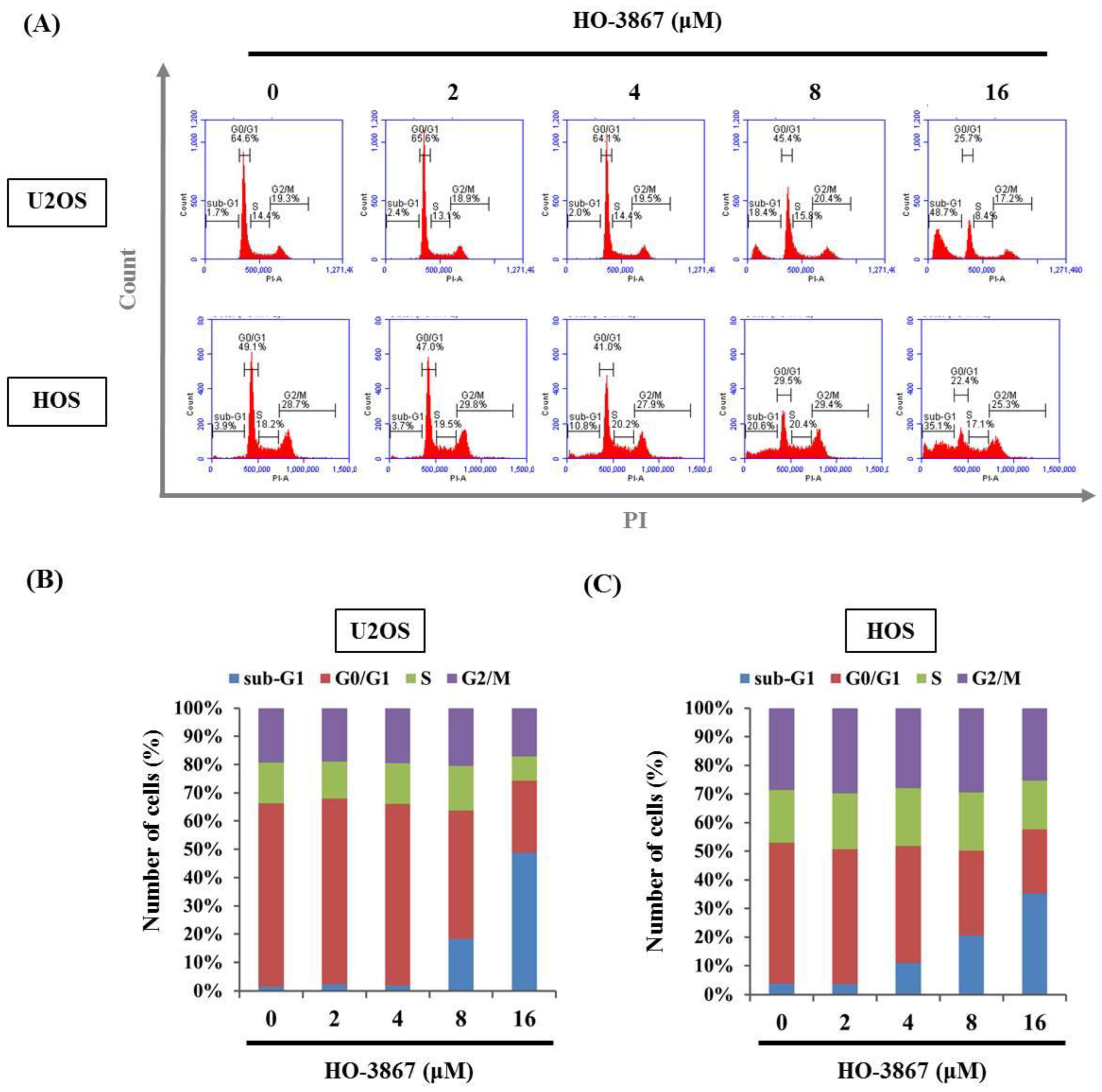
2.3. Flow Cytometric Analysis
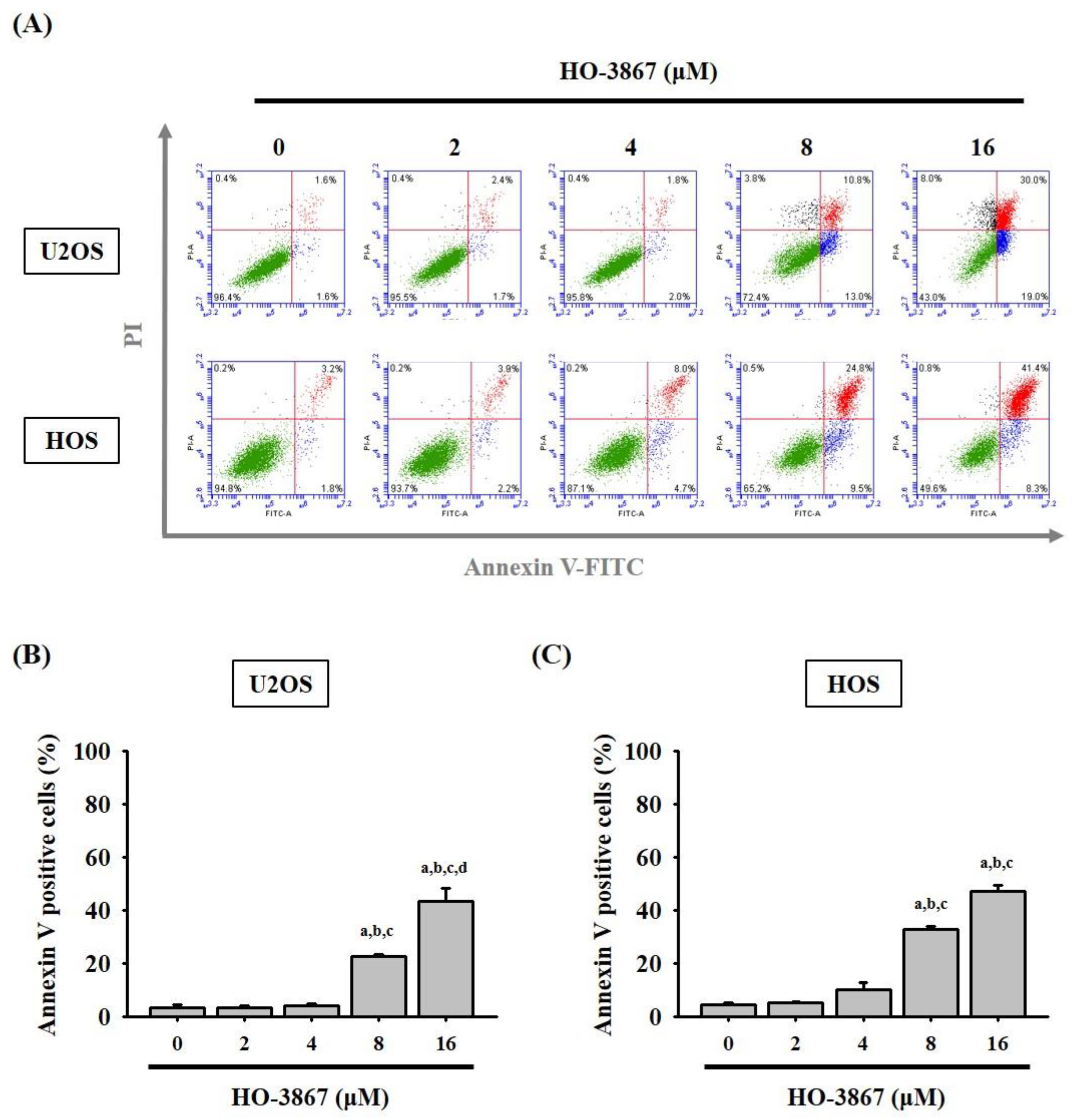
2.4. Annexin V-FITC Apoptosis Staining Assay
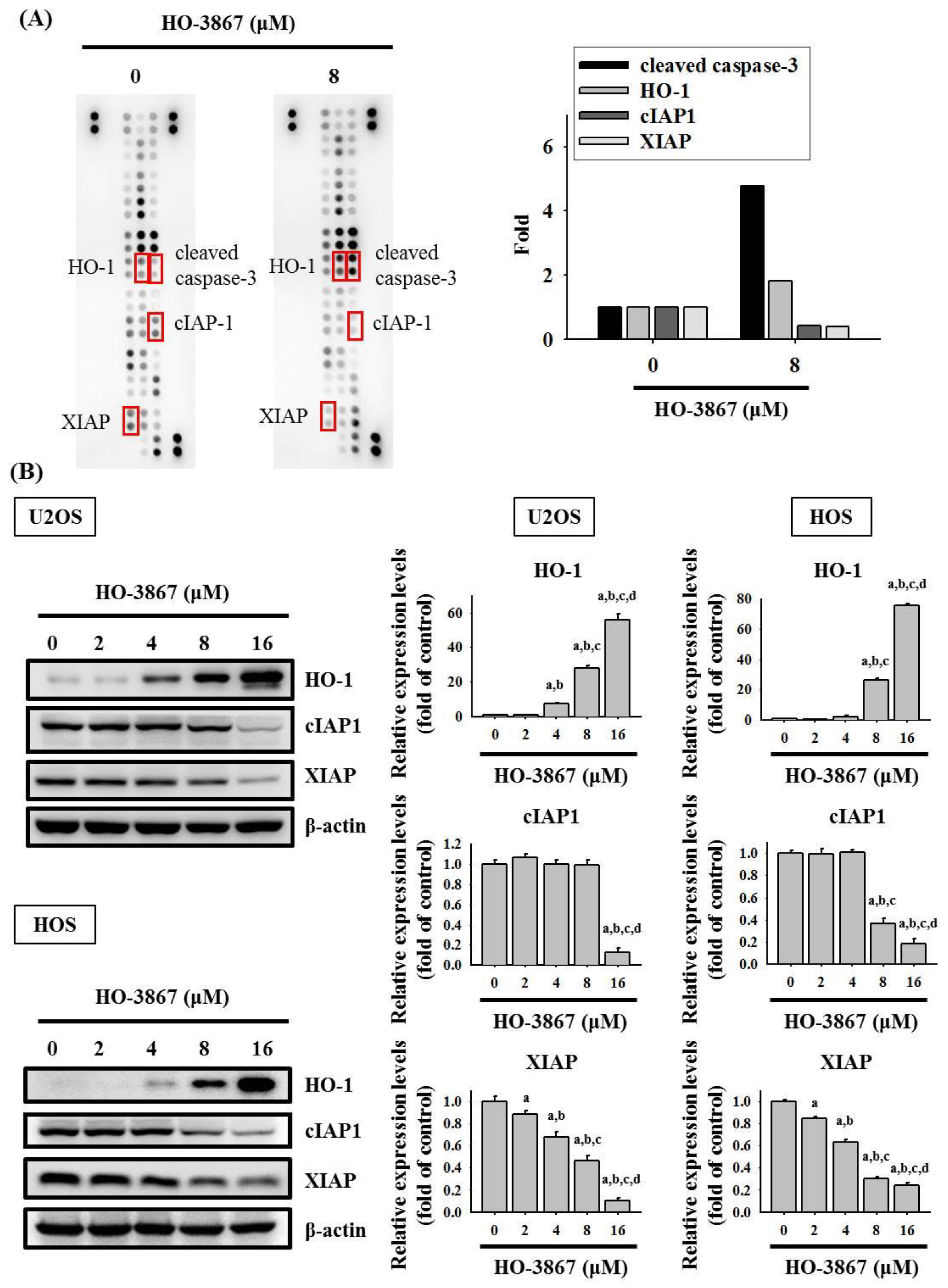
2.5. Human Apoptosis Array
2.6. Protein Extraction and Western Blot Analysis
2.7. Statistical Analyses
3. Results
3.1. HO-3867 Induces Cell Death in Human Osteosarcoma U2OS, HOS, and MG-63 Cells
3.2. HO-3867 Induces Cell Apoptosis and Arrest in the Sub-G1 Phase of U2OS and HOS Cells
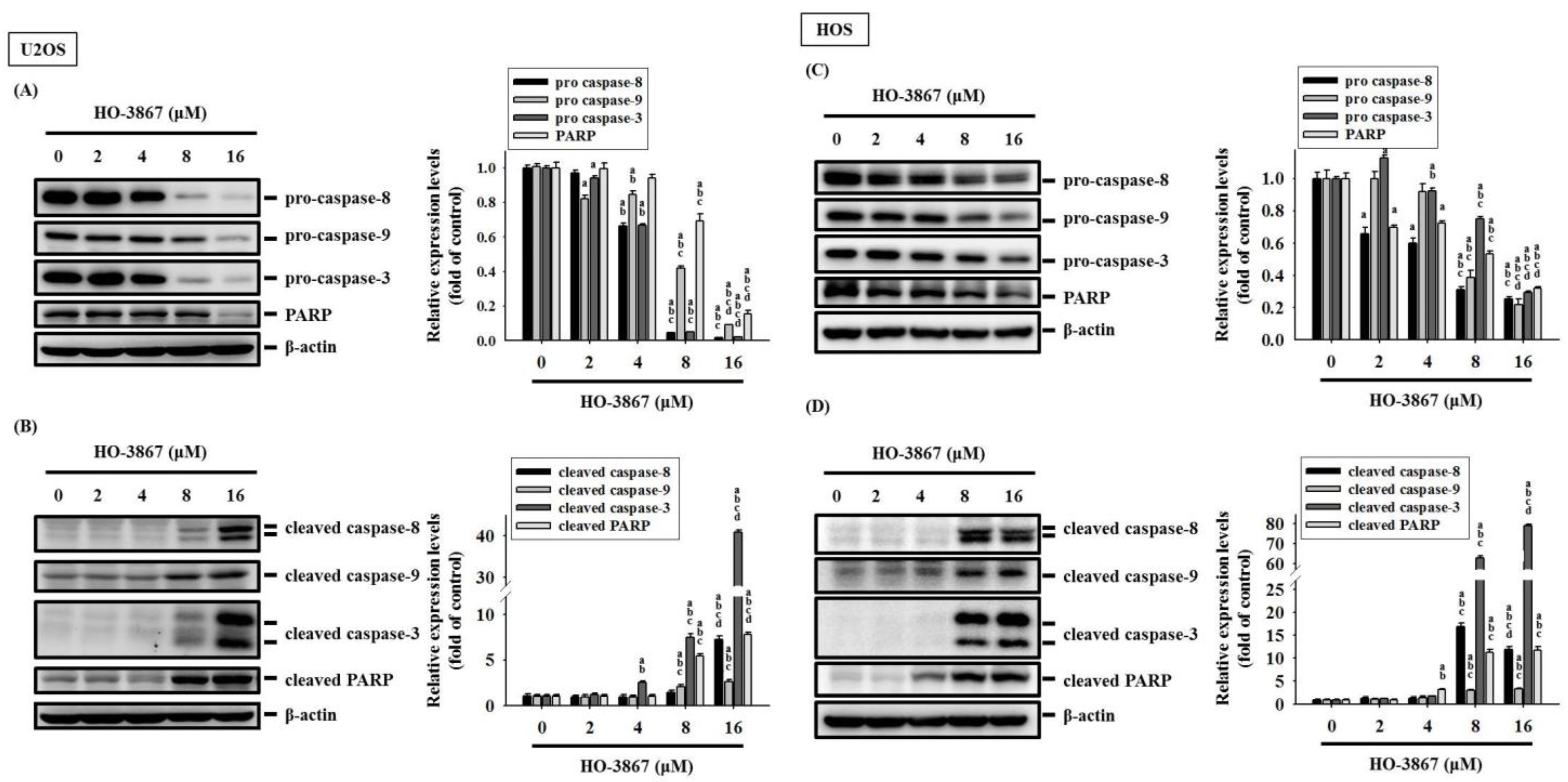
3.3. HO-3867 Increases the Cleaved Caspase 3 and Heme Oxygenase (HO)-1 Expression but Decreases XIAP and cIAP1 Expression in U2OS and HOS Cells
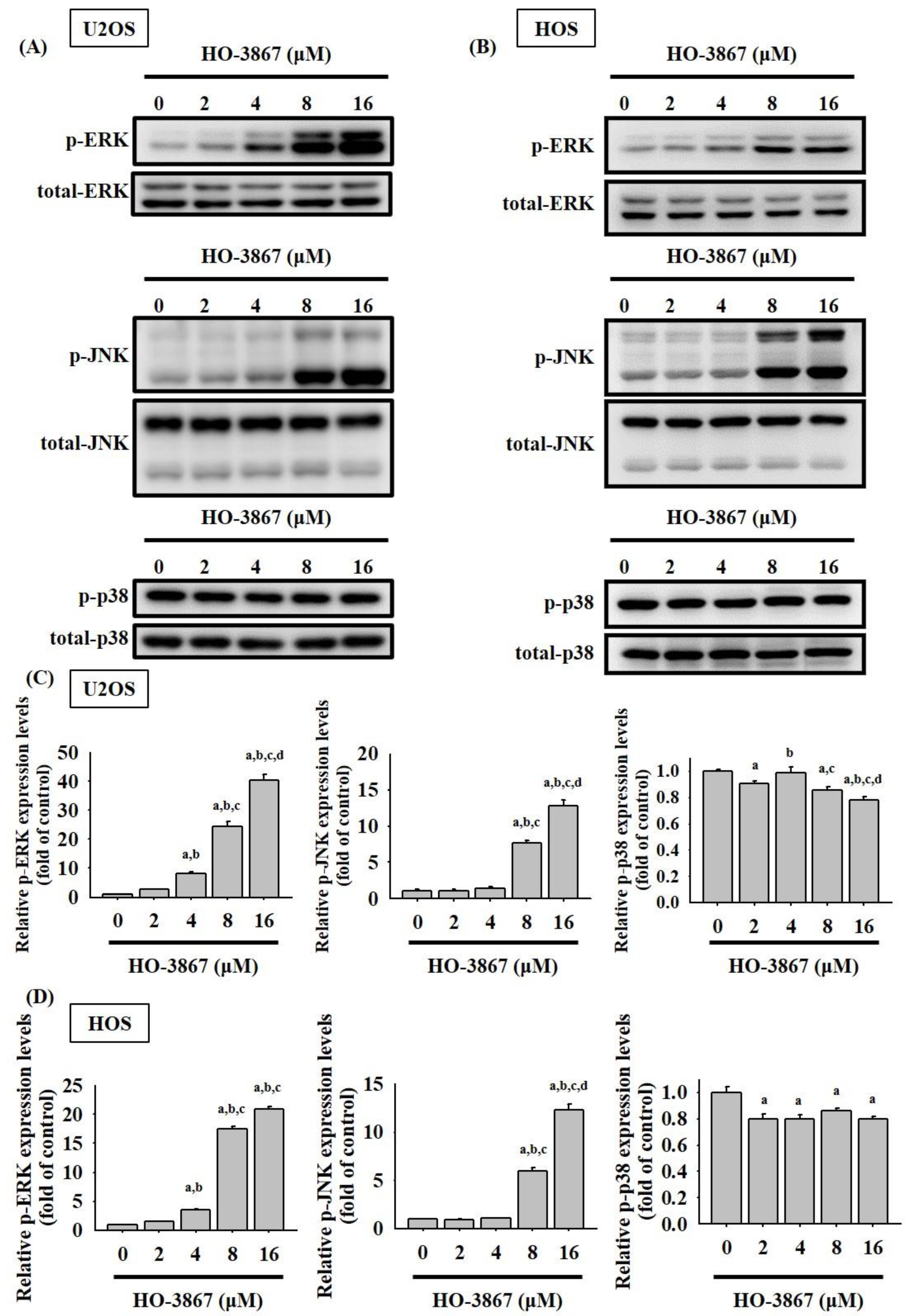
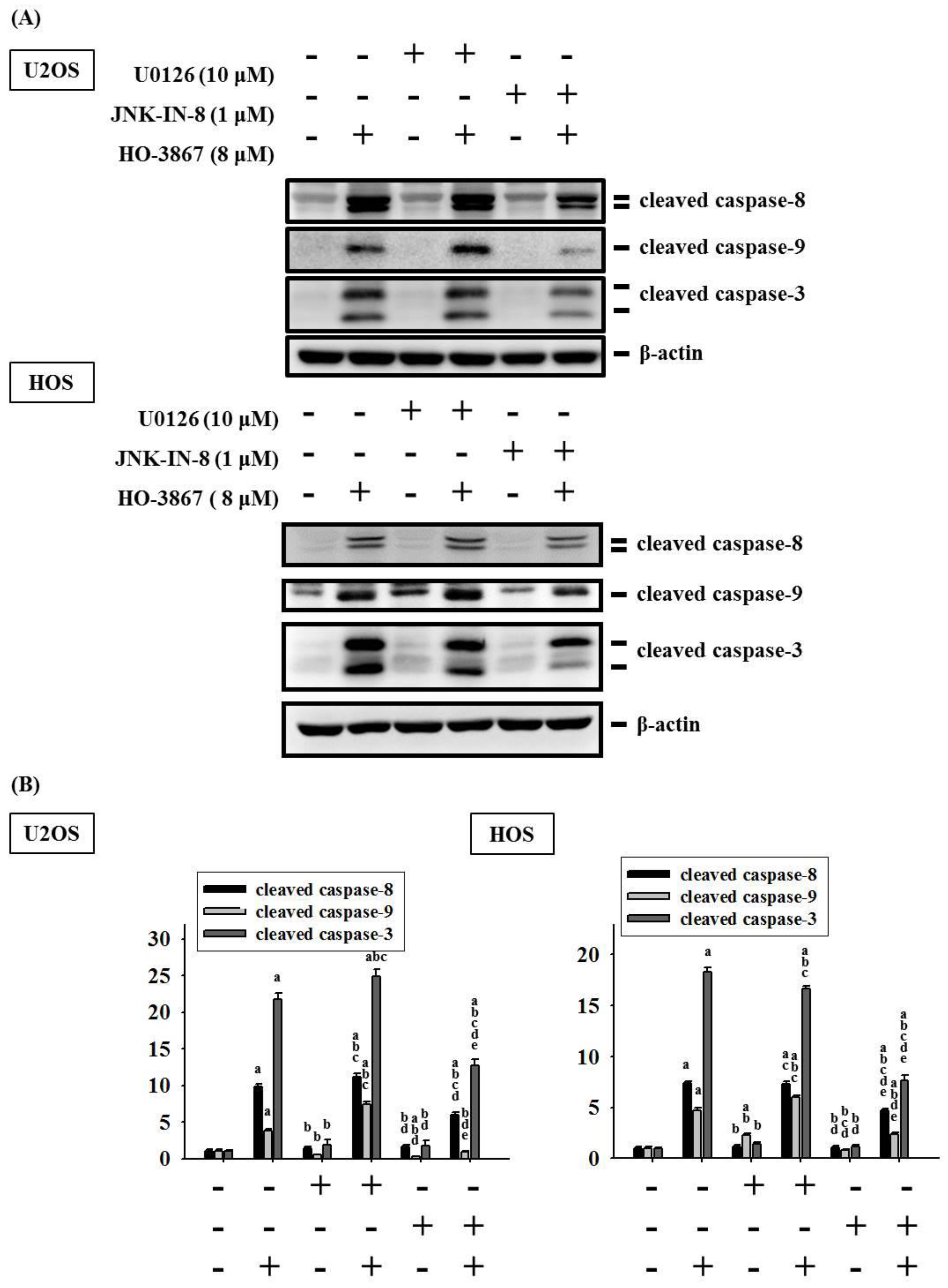
3.4. HO-3867 Activates Apoptotic Processes via the JNK-Signaling Pathway in U2OS and HOS Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Arndt, C.A.; Crist, W.M. Common musculoskeletal tumors of childhood and adolescence. N. Engl. J. Med. 1999, 341, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Lu, E.W.; Lin, C.W.; Yang, J.S.; Yang, S.F. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharmacol. Ther. 2020, 214, 107611. [Google Scholar] [CrossRef]
- Oertel, S.; Blattmann, C.; Rieken, S.; Jensen, A.; Combs, S.E.; Huber, P.E.; Bischof, M.; Kulozik, A.; Debus, J.; Schulz-Ertner, D. Radiotherapy in the treatment of primary osteosarcoma—A single center experience. Tumori 2010, 96, 582–588. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Lu, K.H.; Lin, R.C.; Yang, J.S.; Yang, W.E.; Reiter, R.J.; Yang, S.F. Molecular and cellular mechanisms of melatonin in osteosarcoma. Cells 2019, 8, 1618. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Lu, K.H.; Chen, P.N.; Lue, K.H.; Lai, M.T.; Lin, M.S.; Hsieh, Y.S.; Chu, S.C. 2’-hydroxyflavanone induces apoptosis of human osteosarcoma 143 b cells by activating the extrinsic trail- and intrinsic mitochondria-mediated pathways. Nutr. Cancer 2014, 66, 625–635. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Targeting apoptosis pathways in cancer therapy. Curr. Cancer Drug Targets 2004, 4, 569–576. [Google Scholar] [CrossRef]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the jnk group of map kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Yue, J.; Lopez, J.M. Understanding mapk signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Bishr, M.K.; Almutairi, F.M.; Ali, A.G. Inhibitors of apoptosis: Clinical implications in cancer. Apoptosis 2017, 22, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Osaka, E.; Suzuki, T.; Osaka, S.; Yoshida, Y.; Sugita, H.; Asami, S.; Tabata, K.; Sugitani, M.; Nemoto, N.; Ryu, J. Survivin expression levels as independent predictors of survival for osteosarcoma patients. J. Orthop. Res. 2007, 25, 116–121. [Google Scholar] [CrossRef]
- Nedelcu, T.; Kubista, B.; Koller, A.; Sulzbacher, I.; Mosberger, I.; Arrich, F.; Trieb, K.; Kotz, R.; Toma, C.D. Livin and bcl-2 expression in high-grade osteosarcoma. J. Cancer Res. Clin. Oncol. 2008, 134, 237–244. [Google Scholar] [CrossRef]
- Qu, Y.; Xia, P.; Zhang, S.; Pan, S.; Zhao, J. Silencing xiap suppresses osteosarcoma cell growth, and enhances the sensitivity of osteosarcoma cells to doxorubicin and cisplatin. Oncol. Rep. 2015, 33, 1177–1184. [Google Scholar] [CrossRef][Green Version]
- Dayton, A.; Selvendiran, K.; Meduru, S.; Khan, M.; Kuppusamy, M.L.; Naidu, S.; Kalai, T.; Hideg, K.; Kuppusamy, P. Amelioration of doxorubicin-induced cardiotoxicity by an anticancer-antioxidant dual-function compound, ho-3867. J. Pharmacol. Exp. Ther. 2011, 339, 350–357. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Wang, Y.; He, A.; Hu, H.; Zhang, J.; Han, M.; Huang, Y. Curcumin inhibits the proliferation and invasion of mg-63 cells through inactivation of the p-jak2/p-stat3 pathway. Onco Targets Ther. 2019, 12, 2011–2021. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Zhu, Y.; Wang, D.; Shao, Y.; Zhang, J. Curcumin inhibits hypoxia-induced proliferation and invasion of mg-63 osteosarcoma cells via downregulating notch1. Mol. Med. Rep. 2017, 15, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Xing, J.; Yu, X. Curcumin induces osteosarcoma mg63 cells apoptosis via ros/cyto-c/caspase-3 pathway. Tumour Biol. 2014, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.K.; Muff, R.; Langsam, B.; Born, W.; Fuchs, B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Investig. New Drugs 2008, 26, 289–297. [Google Scholar] [CrossRef]
- Selvendiran, K.; Tong, L.; Bratasz, A.; Kuppusamy, M.L.; Ahmed, S.; Ravi, Y.; Trigg, N.J.; Rivera, B.K.; Kalai, T.; Hideg, K.; et al. Anticancer efficacy of a difluorodiarylidenyl piperidone (ho-3867) in human ovarian cancer cells and tumor xenografts. Mol. Cancer Ther. 2010, 9, 1169–1179. [Google Scholar] [CrossRef]
- Dayton, A.; Selvendiran, K.; Kuppusamy, M.L.; Rivera, B.K.; Meduru, S.; Kalai, T.; Hideg, K.; Kuppusamy, P. Cellular uptake, retention and bioabsorption of ho-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol. Ther. 2010, 10, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Yang, H.W.; Su, C.W.; Lue, K.H.; Yang, S.F.; Hsieh, Y.S. Phyllanthus urinaria suppresses human osteosarcoma cell invasion and migration by transcriptionally inhibiting u-pa via erk and akt signaling pathways. Food Chem. Toxicol. 2013, 52, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Chu, S.C.; Yang, S.F.; Chen, P.N.; Liu, Y.C.; Lu, K.H. Silibinin suppresses human osteosarcoma mg-63 cell invasion by inhibiting the erk-dependent c-jun/ap-1 induction of mmp-2. Carcinogenesis 2007, 28, 977–987. [Google Scholar] [CrossRef]
- Lu, K.H.; Lue, K.H.; Chou, M.C.; Chung, J.G. Paclitaxel induces apoptosis via caspase-3 activation in human osteogenic sarcoma cells (u-2 os). J. Orthop. Res. 2005, 23, 988–994. [Google Scholar] [CrossRef]
- Lu, P.W.; Lin, R.C.; Yang, J.S.; Lu, E.W.; Hsieh, Y.H.; Tsai, M.Y.; Lu, K.H.; Yang, S.F. Go-y078, a curcumin analog, induces both apoptotic pathways in human osteosarcoma cells via activation of jnk and p38 signaling. Pharmaceuticals 2021, 14, 497. [Google Scholar] [CrossRef]
- Yang, J.S.; Lin, R.C.; Hsieh, Y.H.; Wu, H.H.; Li, G.C.; Lin, Y.C.; Yang, S.F.; Lu, K.H. Clefma activates the extrinsic and intrinsic apoptotic processes through jnk1/2 and p38 pathways in human osteosarcoma cells. Molecules 2019, 24, 3280. [Google Scholar] [CrossRef]
- Lu, K.H.; Lin, C.W.; Hsieh, Y.H.; Su, S.C.; Reiter, R.J.; Yang, S.F. New insights into antimetastatic signaling pathways of melatonin in skeletomuscular sarcoma of childhood and adolescence. Cancer Metastasis Rev. 2020, 39, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Chen, C.W.; Hsieh, M.J.; Ju, P.C.; Hsieh, Y.H.; Su, C.W.; Chen, Y.L.; Yang, S.F.; Lin, C.W. Curcumin analog ho-3867 triggers apoptotic pathways through activating jnk1/2 signalling in human oral squamous cell carcinoma cells. J. Cell Mol. Med. 2022, 26, 2273–2284. [Google Scholar] [CrossRef]
- Rath, K.S.; Naidu, S.K.; Lata, P.; Bid, H.K.; Rivera, B.K.; McCann, G.A.; Tierney, B.J.; Elnaggar, A.C.; Bravo, V.; Leone, G.; et al. Ho-3867, a safe stat3 inhibitor, is selectively cytotoxic to ovarian cancer. Cancer Res. 2014, 74, 2316–2327. [Google Scholar] [CrossRef]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Kuppusamy, M.L.; Tazi, M.; Bratasz, A.; Tong, L.; Rivera, B.K.; Kalai, T.; Hideg, K.; et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: Differential cytotoxicity in healthy and cancer cells. Free Radic. Biol. Med. 2010, 48, 1228–1235. [Google Scholar] [CrossRef]
- Tierney, B.J.; McCann, G.A.; Naidu, S.; Rath, K.S.; Saini, U.; Wanner, R.; Kuppusamy, P.; Suarez, A.; Goodfellow, P.J.; Cohn, D.E.; et al. Aberrantly activated pstat3-ser727 in human endometrial cancer is suppressed by ho-3867, a novel stat3 inhibitor. Gynecol. Oncol. 2014, 135, 133–141. [Google Scholar] [CrossRef][Green Version]
- Saini, U.; Naidu, S.; ElNaggar, A.C.; Bid, H.K.; Wallbillich, J.J.; Bixel, K.; Bolyard, C.; Suarez, A.A.; Kaur, B.; Kuppusamy, P.; et al. Elevated stat3 expression in ovarian cancer ascites promotes invasion and metastasis: A potential therapeutic target. Oncogene 2017, 36, 168–181. [Google Scholar] [CrossRef]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Ravi, Y.; Kuppusamy, M.L.; Bratasz, A.; Rivera, B.K.; Kalai, T.; Hideg, K.; Kuppusamy, P. Ho-3867, a synthetic compound, inhibits the migration and invasion of ovarian carcinoma cells through downregulation of fatty acid synthase and focal adhesion kinase. Mol. Cancer Res. 2010, 8, 1188–1197. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, S.; Bennett, S.; Tang, H.; Song, D.; Wood, D.; Zhan, X.; Xu, J. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021, 54, e12974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hui, C.; Xie, Y. Natural STAT3 inhibitors: A mini perspective. Bioorg. Chem. 2021, 115, 105169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, M.; Xu, Z.; Wang, H.; Wang, H.; Yu, X.; Li, Z.; Teng, L. HJC0152, a novel STAT3 inhibitor with promising anti-tumor effect in gastric cancer. Cancer Manag. Res. 2018, 10, 6857–6867. [Google Scholar] [CrossRef] [PubMed]
- Madan, E.; Parker, T.M.; Bauer, M.R.; Dhiman, A.; Pelham, C.J.; Nagane, M.; Kuppusmy, M.L.; Holmes, M.; Holmes, T.R.; Shaik, K.; et al. The curcumin analog HO-3867 selectively kills cancer cells by converting mutant p53 protein to transcriptionally active wildtype p53. J. Biol. Chem. 2018, 293, 4262–4276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, L.; Chen, W.; Xu, Y.; Wu, M.; Todorova, D.; Tang, Q.; Feng, B.; Jiang, L.; He, J.; et al. Wild-Type p53 Promotes Cancer Metabolic Switch. by Inducing PUMA-Dependent Suppression of Oxidative Phosphorylation. Cancer Cell 2019, 35, 191–203.e8. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Sasaki, Y.; Negishi, H.; Idogawa, M.; Toyota, M.; Yamashita, T.; Wada, T.; Nagoya, S.; Kawaguchi, S.; Yamashita, T.; et al. Antitumor effect of adenovirus-mediated p53 family gene transfer on osteosarcoma cell lines. Cancer Biol. Ther. 2007, 6, 1058–1066. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.W.-A.; Chou, C.-H.; Yang, J.-S.; Hsieh, Y.-H.; Tsai, M.-Y.; Lu, K.-H.; Yang, S.-F. HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells. Pharmaceutics 2022, 14, 1257. https://doi.org/10.3390/pharmaceutics14061257
Lu PW-A, Chou C-H, Yang J-S, Hsieh Y-H, Tsai M-Y, Lu K-H, Yang S-F. HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells. Pharmaceutics. 2022; 14(6):1257. https://doi.org/10.3390/pharmaceutics14061257
Chicago/Turabian StyleLu, Peace Wun-Ang, Chia-Hsuan Chou, Jia-Sin Yang, Yi-Hsien Hsieh, Meng-Ying Tsai, Ko-Hsiu Lu, and Shun-Fa Yang. 2022. "HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells" Pharmaceutics 14, no. 6: 1257. https://doi.org/10.3390/pharmaceutics14061257
APA StyleLu, P. W.-A., Chou, C.-H., Yang, J.-S., Hsieh, Y.-H., Tsai, M.-Y., Lu, K.-H., & Yang, S.-F. (2022). HO-3867 Induces Apoptosis via the JNK Signaling Pathway in Human Osteosarcoma Cells. Pharmaceutics, 14(6), 1257. https://doi.org/10.3390/pharmaceutics14061257







