Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin
2.2.2. Physicochemical Characterization of Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin
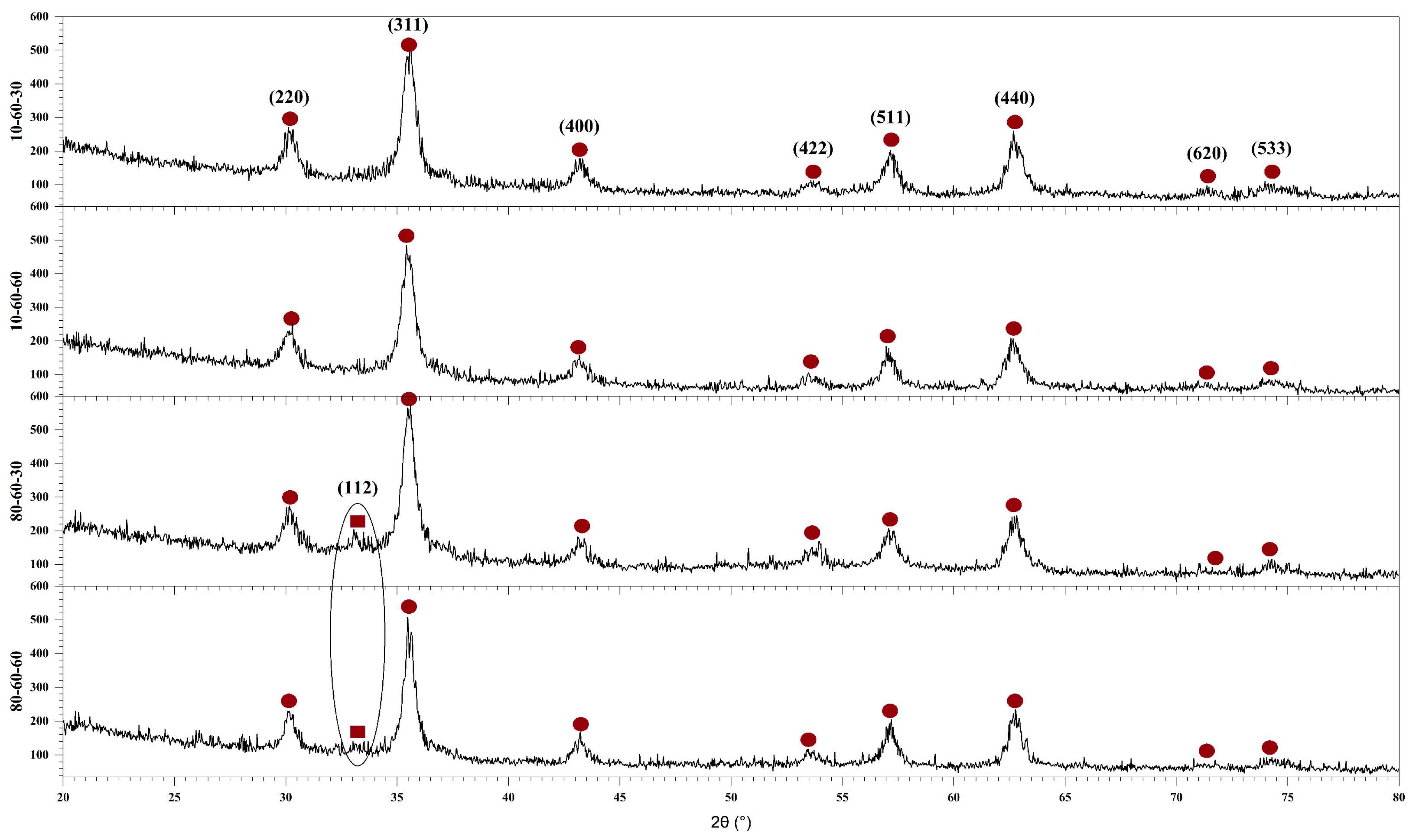
X-ray Diffraction (XRD) Coupled with Rietveld Refinement
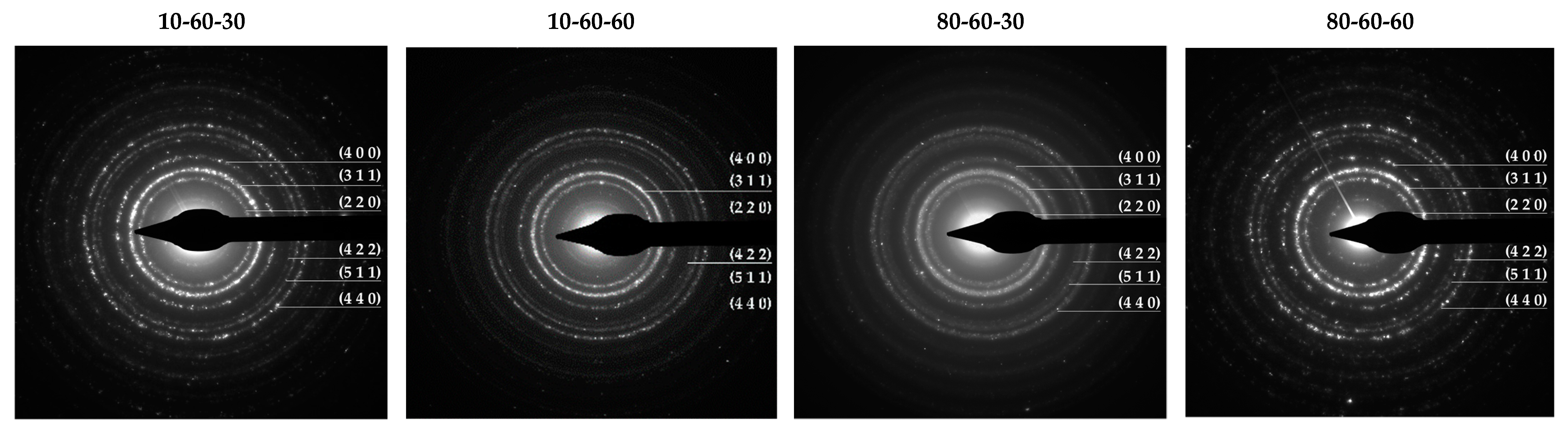
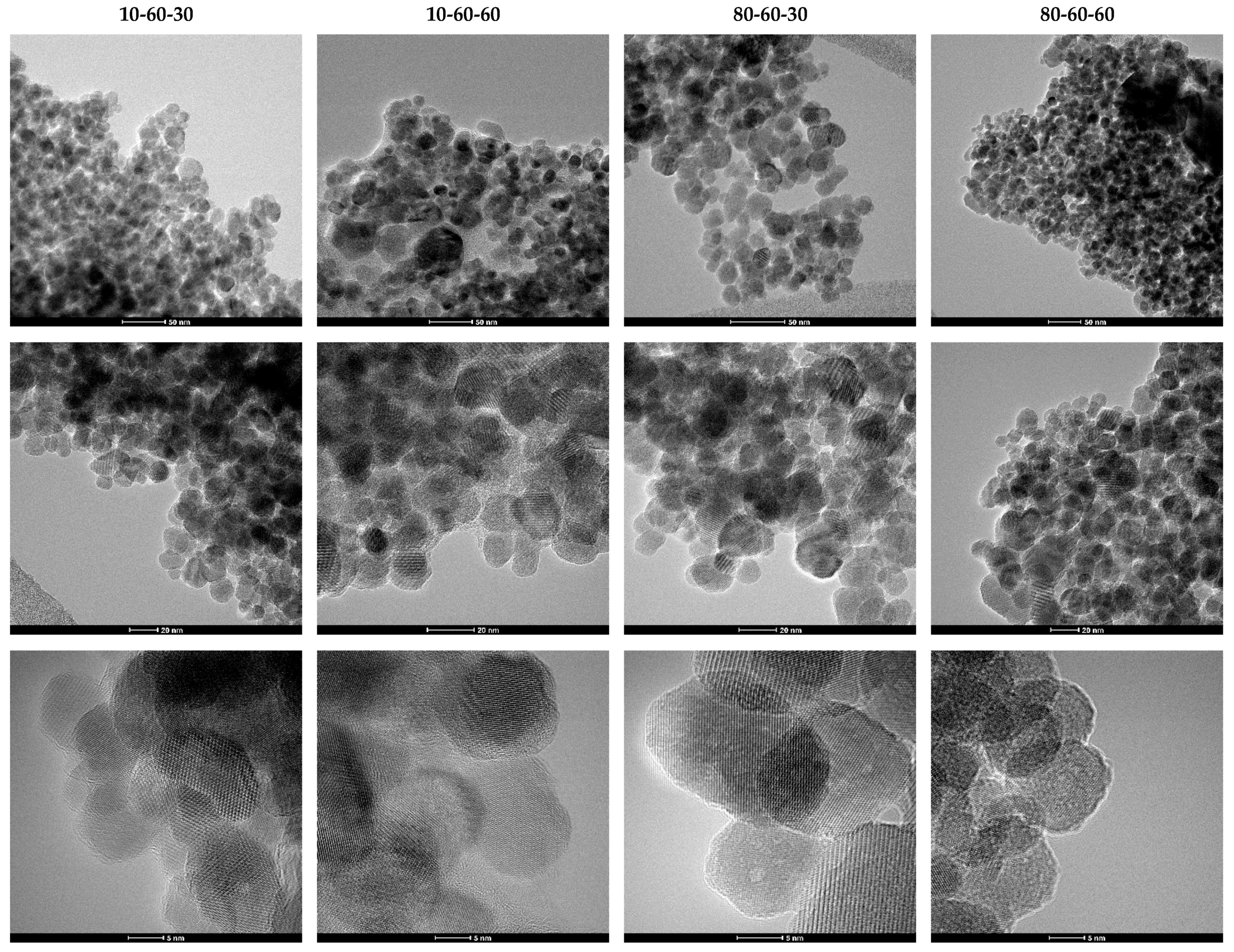
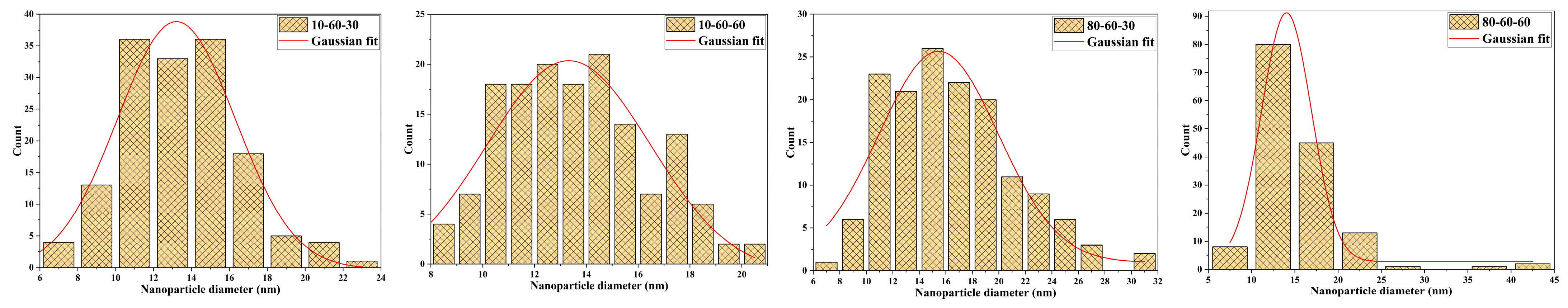
Transmission Electron Microscopy (TEM). High-Resolution TEM (HR-TEM). Selected Area Electron Diffraction (SAED)
Fourier Transform Infrared (FT-IR) Spectroscopy
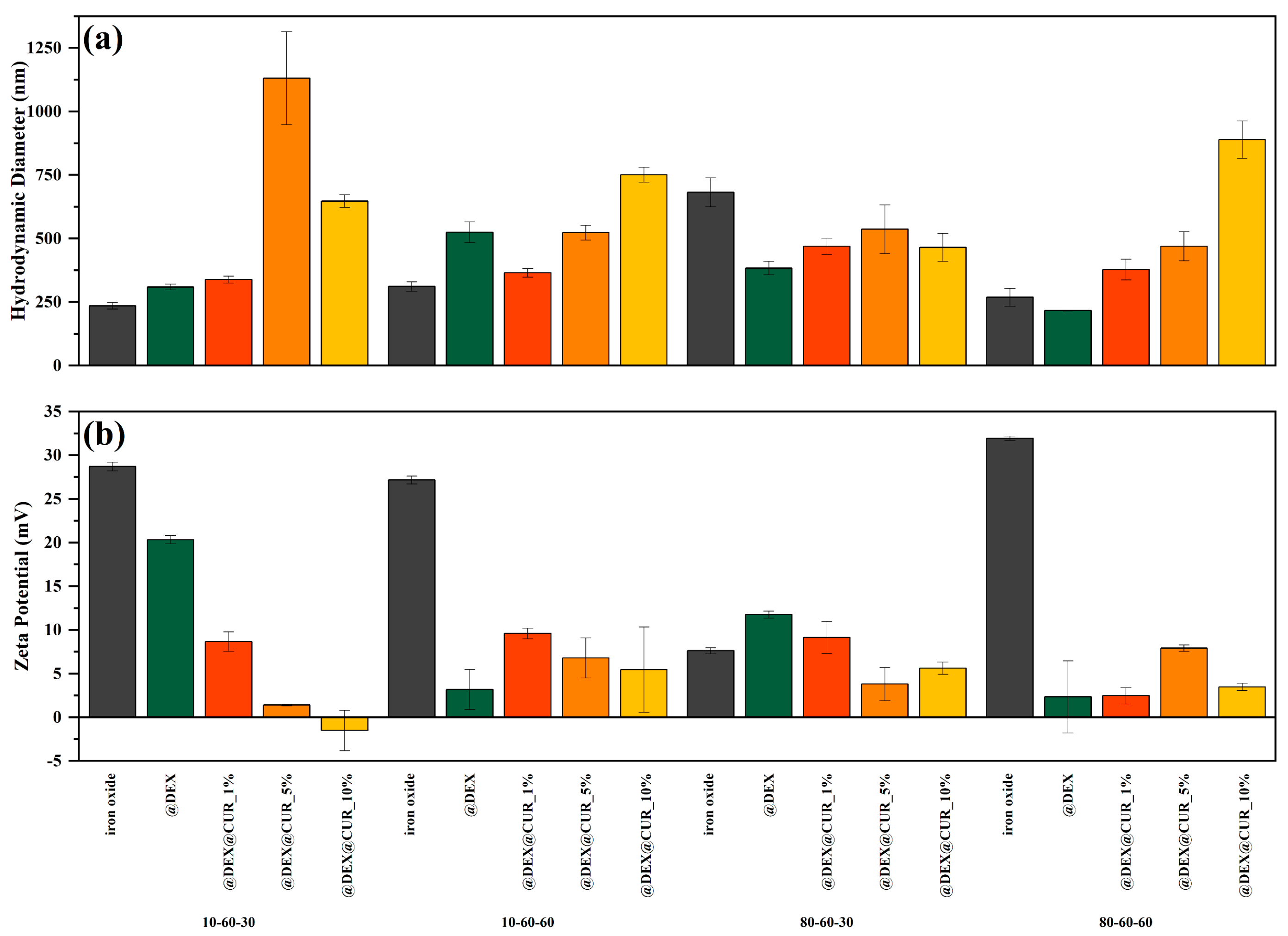
Dynamic Light Scattering (DLS) Zeta Potential
Thermogravimetry and Differential Scanning Calorimetry (TG-DSC)
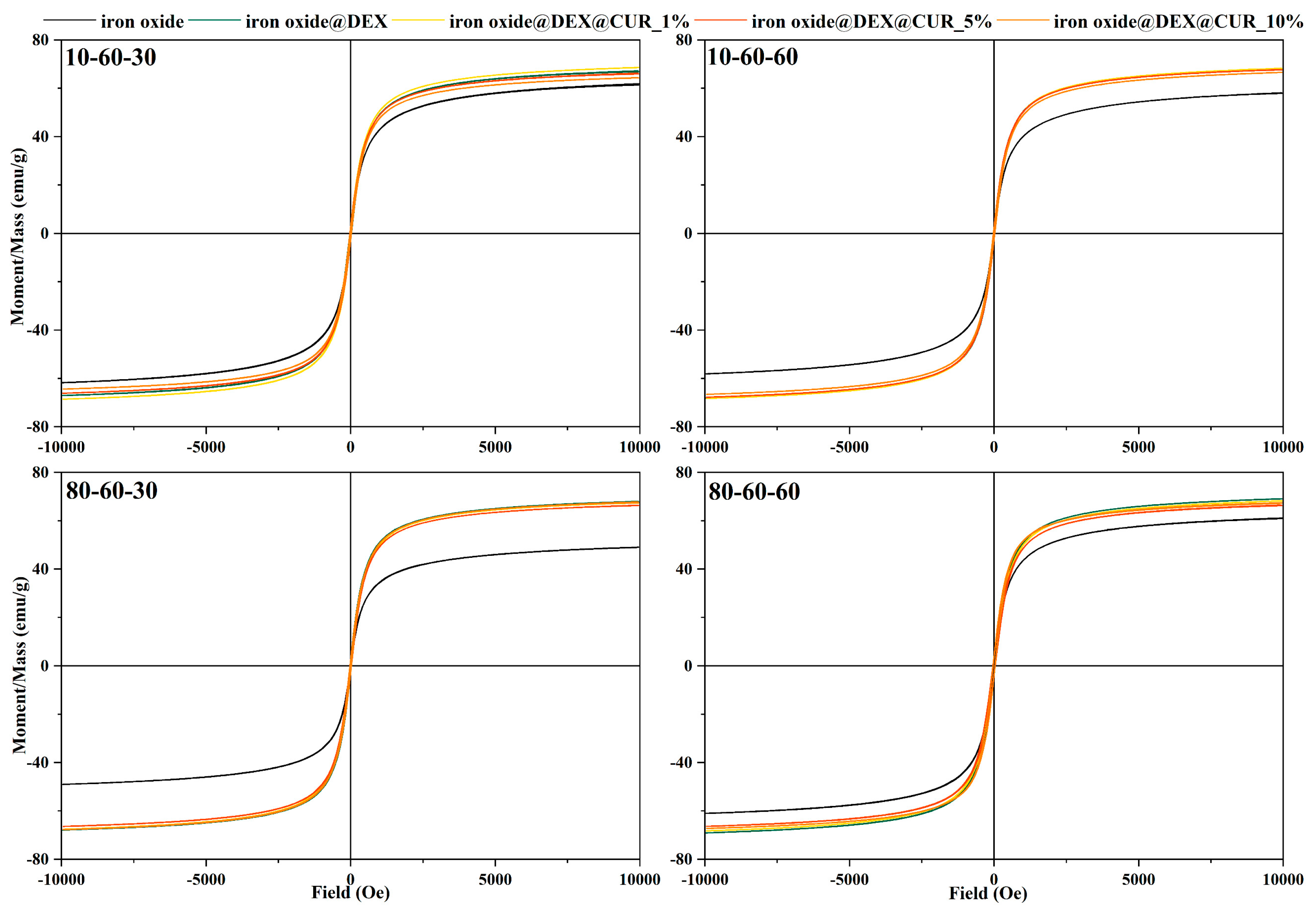
Vibrating Sample Magnetometry (VSM)
UV-Vis Spectrophotometry
Antimicrobial-Activity Assay
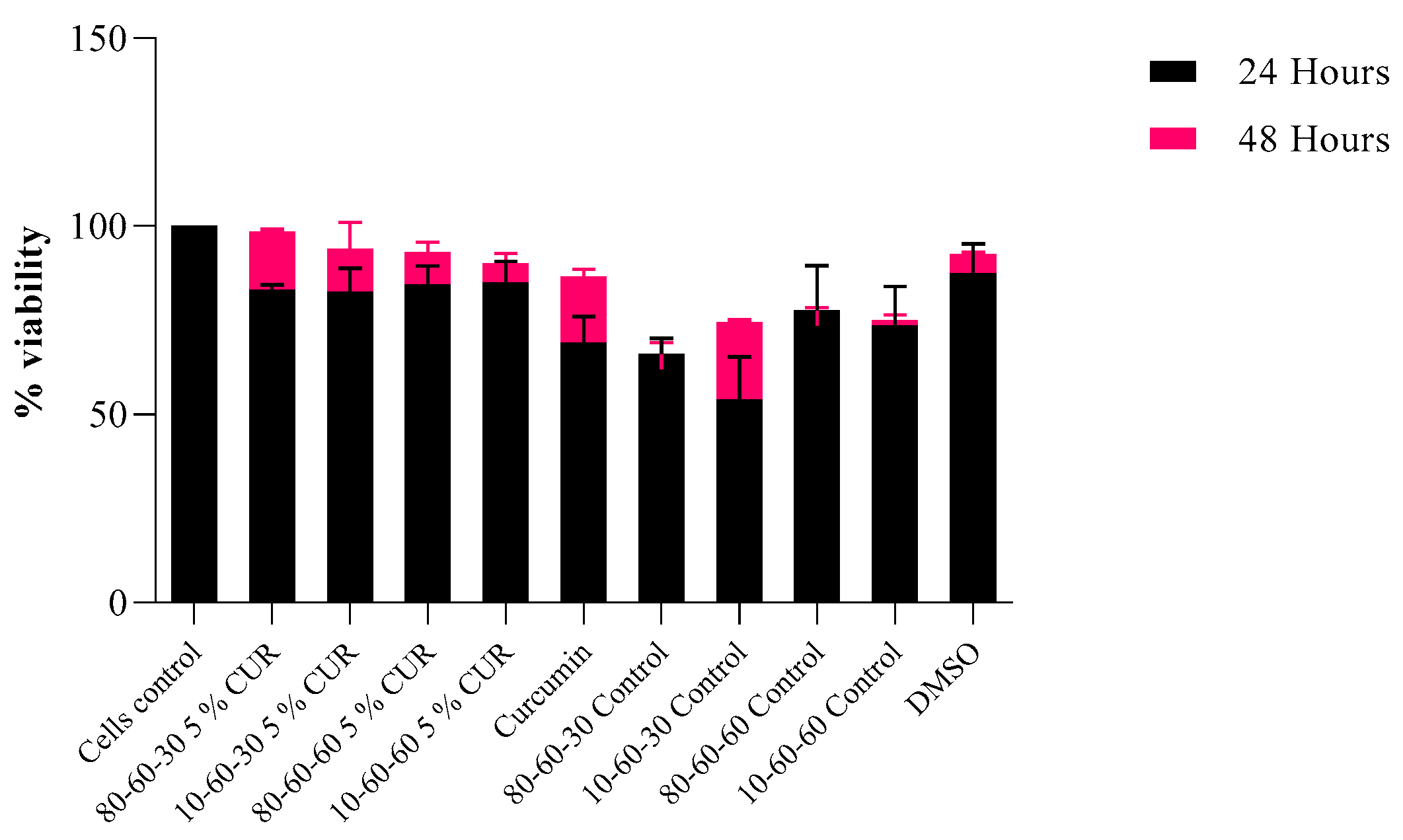
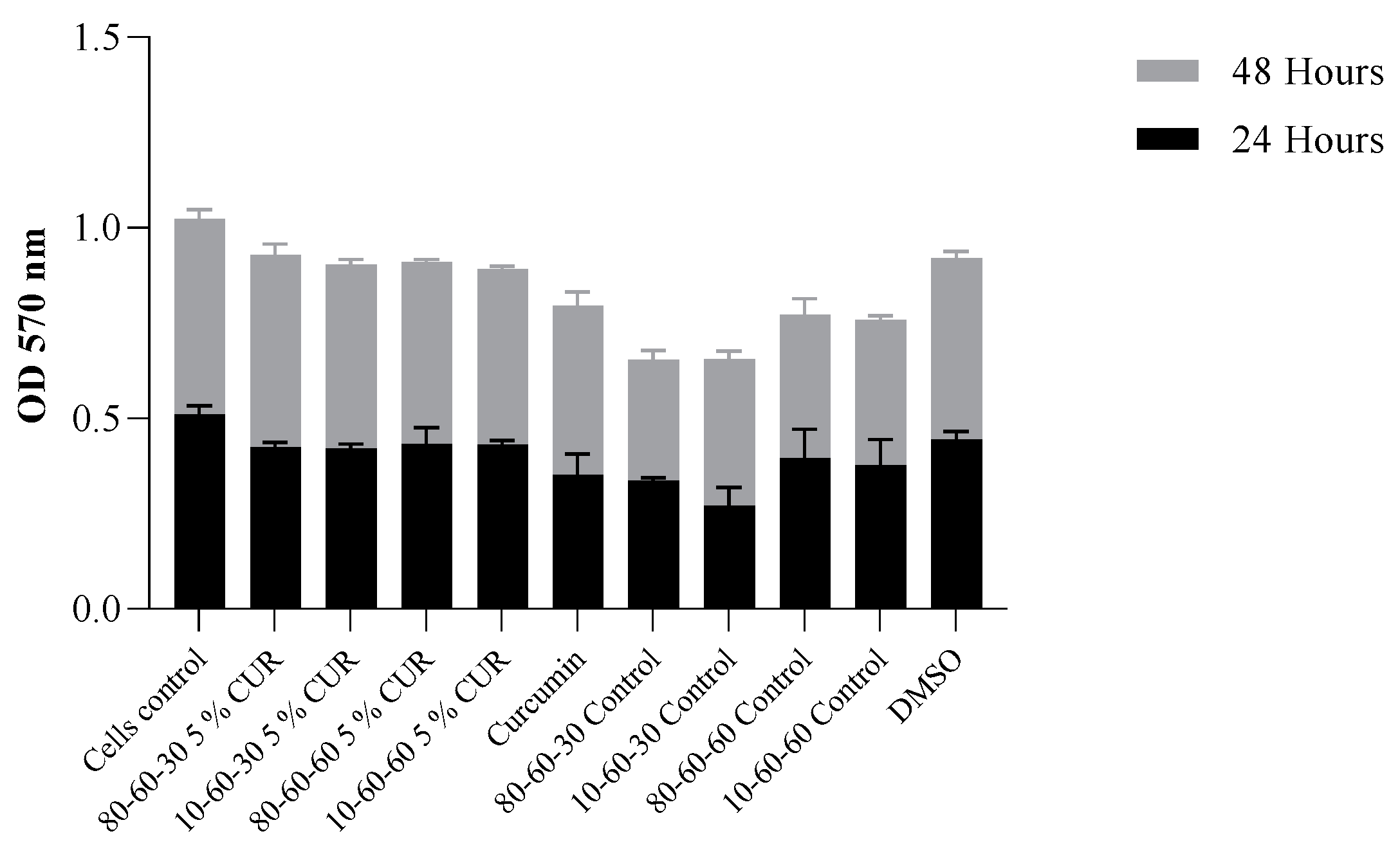
Biocompatibility Assay
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv. Healthc. Mater. 2018, 7, 1701392. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric nanoparticles for antimicrobial therapies: An up-to-date overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Devrim, B.; Bozkır, A. Nanocarriers and their potential application as antimicrobial drug delivery. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 169–202. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, G.; Li, W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem. Sci. 2019, 44, 961–972. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.-u.-R.; Quave, C.L. Antibacterial properties of medicinal plants from pakistan against multidrug-resistant eskape pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef] [Green Version]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Howes, M.-J.R. Chapter 28—Phytochemicals as anti-inflammatory nutraceuticals and phytopharmaceuticals. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 363–388. [Google Scholar] [CrossRef]
- Pastor-Villaescusa, B.; Sanchez Rodriguez, E.; Rangel-Huerta, O.D. Chapter 11—Polyphenols in obesity and metabolic syndrome. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 213–239. [Google Scholar] [CrossRef]
- Baliga, M.S.; Rao, S.; Rao, P.; Pais, M.L.J.; Naik, T.S.; Adnan, M.; Palatty, P.L. Chapter 26—Hepatoprotective effects of curcumin in alcohol-induced hepatotoxicity: A memoir on the preclinical studies. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 313–317. [Google Scholar] [CrossRef]
- El-Monem, A.; Rahman, D.D.; Abdel, A.; Shereen HB, E. Nanocurcumin improves the therapeutic role of mesenchymal stem cells in liver fibrosis rats. Biointerface Res. Appl. Chem. 2021, 11, 14463–14479. [Google Scholar] [CrossRef]
- Larki, R.A.; Heiat, M.; Zayerzadeh, E. Therapeutic effects of curcumin on toxicity induced by permethrin in rat liver and sk-hep-1 cells. Biointerface Res. Appl. Chem. 2021, 11, 10885–10894. [Google Scholar] [CrossRef]
- Krisnamurti, G.C.; Bare, Y.; Amin, M.; Primiani, C.N. Combination of curcumin from curcuma longa and procyanidin from tamarindus indica in inhibiting cyclooxygenases for primary dysmenorrhea therapy: In silico study. Biointerface Res. Appl. Chem. 2021, 11, 7460–7467. [Google Scholar] [CrossRef]
- Afrida, I.R.; Fatchiyah, F.; Widodo, N.; Amin, M.; Djati, M.S. Shogaol, bisdemethoxycurcumin, and curcuminoid: Potential zingiber compounds against COVID-19. Biointerface Res. Appl. Chem. 2021, 11, 12869–12876. [Google Scholar] [CrossRef]
- Ronzio, R.A. 97—Naturally occurring antioxidants. In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: London, UK, 2020; pp. 731–751.e12. [Google Scholar] [CrossRef]
- Sekharan, T.R.; Chandira, R.M.; Rajesh, S.; Tamilvanan, S.; Vijayakumar, C.; Venkateswarlu, B. pH, viscosity of hydrophobic based natural deep eutectic solvents and the effect of curcumin solubility in it. Biointerface Res. Appl. Chem. 2021, 11, 14620–14633. [Google Scholar] [CrossRef]
- Ezealigo, U.S.; Ezealigo, B.N.; Aisida, S.O.; Ezema, F.I. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A comprehensive review of magnetic nanomaterials modern day theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Sadighian, S.; Sharifan, K.; Khanmohammadi, A.; Rohani, M.K. A facile synthesis of Fe3O4@ SiO2@ zno for curcumin delivery. Biointerface Res. Appl. Chem 2021, 12, 7994–8002. [Google Scholar] [CrossRef]
- Mohamed, G.; Hassan, N.; Shahat, A.; El-Didamony, A.; Ashraf, A. Synthesis and characterization of porous magnetite nanosphere iron oxide as a novel adsorbent of anionic dyes removal from aqueous solution. Biointerface Res. Appl. Chem. 2021, 11, 13377–13401. [Google Scholar] [CrossRef]
- Chircov, C.; Vasile, B.S. New approaches in synthesis and characterization methods of iron oxide nanoparticles. In Iron Oxide Nanoparticles [Working Title]; Huang, X.-L., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Uthaman, S.; Muthiah, M.; Park, I.K.; Cho, C.S. 9—Fabrication and development of magnetic particles for gene therapy. In Polymers and Nanomaterials for Gene Therapy; Narain, R., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 215–230. [Google Scholar] [CrossRef]
- BeMiller, J.N. Dextran. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1772–1773. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Moreira, A.F.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Sousa, F.; Pichon, C.; Correia, I.J. Chapter 6—Multifunctional nanocarriers for codelivery of nucleic acids and chemotherapeutics to cancer cells. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 163–207. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron oxide–silica core–shell nanoparticles functionalized with essential oils for antimicrobial therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Leau, S.A.; Marin, S.; Holban, A.M.; Vasile, B.S.; Nicoara, A.I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-carboxymethylcellulose biocomposite wound-dressings with antimicrobial activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Caciandone, M.; Niculescu, A.-G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.Ș.; Bîrcă, A.C.; Grumezescu, A.M.; et al. Peg-functionalized magnetite nanoparticles for modulation of microbial biofilms on voice prosthesis. Antibiotics 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Villegas, V.A.R.; Ramírez, J.I.D.L.; Guevara, E.H.; Sicairos, S.P.; Ayala, L.A.H.; Sanchez, B.L. Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. J. Saudi Chem. Soc. 2020, 24, 223–235. [Google Scholar] [CrossRef]
- Gunawan, P.; Mei, L.; Teo, J.; Ma, J.; Highfield, J.; Li, Q.; Zhong, Z. Ultrahigh sensitivity of au/1d α-Fe2O3 to acetone and the sensing mechanism. Langmuir 2012, 28, 14090–14099. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. Maple coatings embedded with essential oil-conjugated magnetite for anti-biofilm applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Niculescu, A.-G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Anti-biofilm coatings based on chitosan and lysozyme functionalized magnetite nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-functionalized magnetite nanoparticles modulate virulence and persistence in Pseudomonas aeruginosa clinical strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- Castro, M.A.M.; Portela, T.O.; Correa, G.S.; Oliveira, M.M.; Rangel, J.H.G.; Rodrigues, S.F.; Mercury, J.M.R. Synthesis of hydroxyapatite by hydrothermal and microwave irradiation methods from biogenic calcium source varying ph and synthesis time. Boletín de la Sociedad Española de Cerámica y Vidrio 2022, 61, 35–41. [Google Scholar] [CrossRef]
- Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Ueda, T.; Zhu, K.; Moreno-Perez, B. Preparation of silicon hydroxyapatite nanopowders under microwave-assisted hydrothermal method. Nanomaterials 2021, 11, 1548. [Google Scholar] [CrossRef]
- Fu, L.-H.; Liu, Y.-J.; Ma, M.-G.; Zhang, X.-M.; Xue, Z.-M.; Zhu, J.-F. Microwave-assisted hydrothermal synthesis of cellulose/hydroxyapatite nanocomposites. Polymers 2016, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-C.; Chou, Y.-H.; Liu, C.-M.; Liu, Y.-M.; Ger, M.-D.; Shu, Y.-Y. Microwave-assisted hydrothermal synthesis of zinc oxide particles starting from chloride precursor. Mater. Res. Bull. 2012, 47, 96–100. [Google Scholar] [CrossRef]
- Rana, A.u.H.S.; Kang, M.; Kim, H.-S. Microwave-assisted facile and ultrafast growth of zno nanostructures and proposition of alternative microwave-assisted methods to address growth stoppage. Sci. Rep. 2016, 6, 24870. [Google Scholar] [CrossRef] [PubMed]
- Ridha, N.J.; Umar, A.A.; Alosfur, F.; Jumali, M.H.; Salleh, M.M. Microwave assisted hydrothermal method for porous zinc oxide nanostructured-films. J. Nanosci. Nanotechnol. 2013, 13, 2667–2674. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.-L.; Wu, K.-D.; Zhang, D.-Z.; Debliquy, M.; Zhang, C. Microwave-assisted hydrothermal synthesis of copper oxide-based gas-sensitive nanostructures. Rare Met. 2021, 40, 1477–1493. [Google Scholar] [CrossRef]
- Quirino, M.R.; Lucena, G.L.; Medeiros, J.A.; Santos, I.M.G.d.; Oliveira, M.J.C.d. Cuo rapid synthesis with different morphologies by the microwave hydrothermal method. Mater. Res. 2018, 21, 6. [Google Scholar] [CrossRef]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2018, 8, 048201–048214. [Google Scholar] [CrossRef] [Green Version]
- Kostyukhin, E.M.; Kustov, L.M. Microwave-assisted synthesis of magnetite nanoparticles possessing superior magnetic properties. Mendeleev Commun. 2018, 28, 559–561. [Google Scholar] [CrossRef]
- Brollo, M.E.F.; Veintemillas-Verdaguer, S.; Salván, C.M.; Morales, M.d.P. Key parameters on the microwave assisted synthesis of magnetic nanoparticles for mri contrast agents. Contrast Media Mol. Imaging 2017, 2017, 8902424. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Narayanaswamy, V.; Sambasivam, S.; Saj, A.; Alaabed, S.; Issa, B.; Al-Omari, I.A.; Obaidat, I.M. Role of magnetite nanoparticles size and concentration on hyperthermia under various field frequencies and strengths. Molecules 2021, 26, 796. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Chapter 18—Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; Ajitha, R.A., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalkhali, M.; Sadighian, S.; Rostamizadeh, K.; Khoeini, F.; Naghibi, M.; Bayat, N.; Habibizadeh, M.; Hamidi, M. Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. Bioimpacts 2015, 5, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899. [Google Scholar] [CrossRef] [Green Version]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and mri application of dextran-coated fe3o4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as mri contrast agents: A comparative study. Daru 2015, 23, 45. [Google Scholar] [CrossRef] [Green Version]
- Abdolrahimi, M.; Vasilakaki, M.; Slimani, S.; Ntallis, N.; Varvaro, G.; Laureti, S.; Meneghini, C.; Trohidou, K.N.; Fiorani, D.; Peddis, D. Magnetism of nanoparticles: Effect of the organic coating. Nanomaterials 2021, 11, 1787. [Google Scholar] [CrossRef]
- Karaagac, O.; Köçkar, H. Improvement of the saturation magnetization of peg coated superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2022, 551, 169140. [Google Scholar] [CrossRef]
- Shehzad, A.; Shahzad, R.; Lee, Y.S. Chapter eight—Curcumin: A potent modulator of multiple enzymes in multiple cancers. In The Enzymes; Bathaie, S.Z., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 149–174. [Google Scholar] [CrossRef]
- Obulesu, M. Chapter 5—Curcumin: A promising therapeutic in parkinson’s disease treatment. In Parkinson’s Disease Therapeutics; Obulesu, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–63. [Google Scholar] [CrossRef]
- Răducanu, A.E.; Tihăuan, B.-M.; Marinaș, I.C.; Ciupercă, O.T.; Țebrencu, C.E.; Ionescu, E.; Onisei, T. The biological effects of novel nutraceuticals with curcuminoids and other plant-derived immunomodulators and pre-probiotics. Pharmaceutics 2021, 13, 666. [Google Scholar] [CrossRef]
- Baldi, A.; De Luca, A.; Maiorano, P.; D’Angelo, C.; Giordano, A. Curcumin as an anticancer agent in malignant mesothelioma: A review. Int. J. Mol. Sci. 2020, 21, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi-Ghadi, Z.; Behjou, N.; Ebrahimnejad, P.; Mahkam, M.; Goli, H.R.; Lam, M.; Nokhodchi, A. Improving antibacterial efficiency of curcumin in magnetic polymeric nanocomposites. J. Pharm. Innov. 2022. [Google Scholar] [CrossRef]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R. Selective antimicrobial effects of curcumin@halloysite nanoformulation: A caenorhabditis elegans study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, D.E.; Rozik, N.N.; Soliman, A.M.; Helaly, F. Study the release kinetics of curcumin released from pva/curcumin composites and its evaluation towards hepatocarcinoma. J. Appl. Pharm. Sci. 2016, 6, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]


| Sample Code | Pressure [bar] | Temperature [°C] | Treatment Time [min] | Stirring [%] |
|---|---|---|---|---|
| 10-60-30 | 10 | 60 | 30 | 10 |
| 10-60-60 | 10 | 60 | 60 | |
| 80-60-30 | 80 | 60 | 30 | |
| 80-60-60 | 80 | 60 | 60 |
| Sample | Crystalline Phase Proportions [%] | Average Crystallite Size ± SD [nm] | Crystallinity [%] |
|---|---|---|---|
| 10-60-30 | magnetite–100 | 9.42 ± 0.22 | 20.56 |
| 10-60-60 | magnetite–100 | 9.04 ± 0.23 | 18.55 |
| 80-60-30 | magnetite–66.60 goethite–33.40 | 9.04 ± 0.68 2.09 ± 0.19 | 27.99 |
| 80-60-60 | magnetite–94.10 goethite–5.90 | 11.16 ± 0.42 415.93 ± 1115.23 | 18.23 |
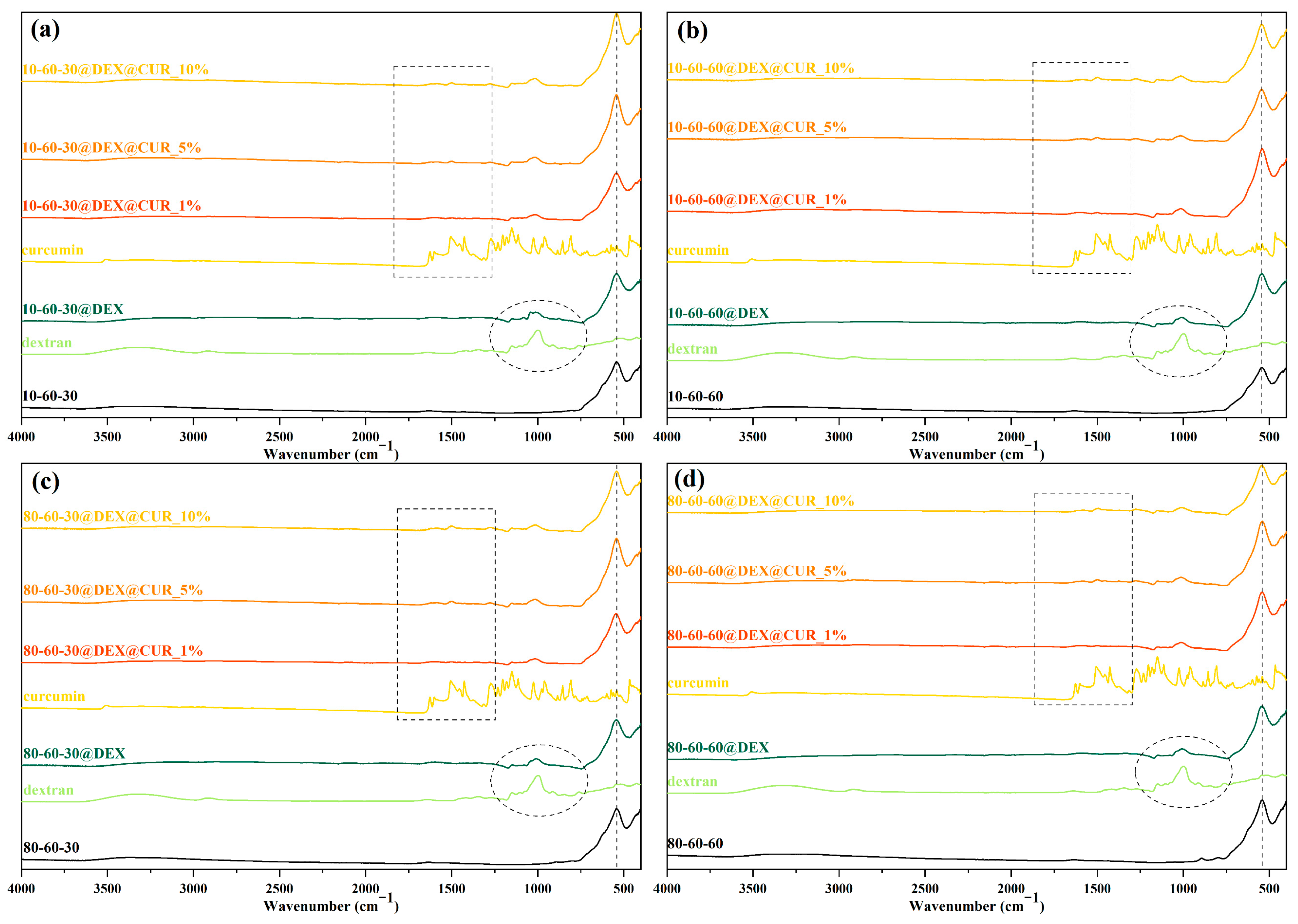
| Type of Bond | Wavenumber (cm−1) |
|---|---|
| Fe-O | 541 |
| C-O stretching (primary alcohol) | 1078, 1146 |
| C-O stretching (alkyl aryl ether) | 1276 |
| C=C stretching (alkene) | 1583 |
| O-H (phenol) | 1427 |
| C-H bending | 1495 |
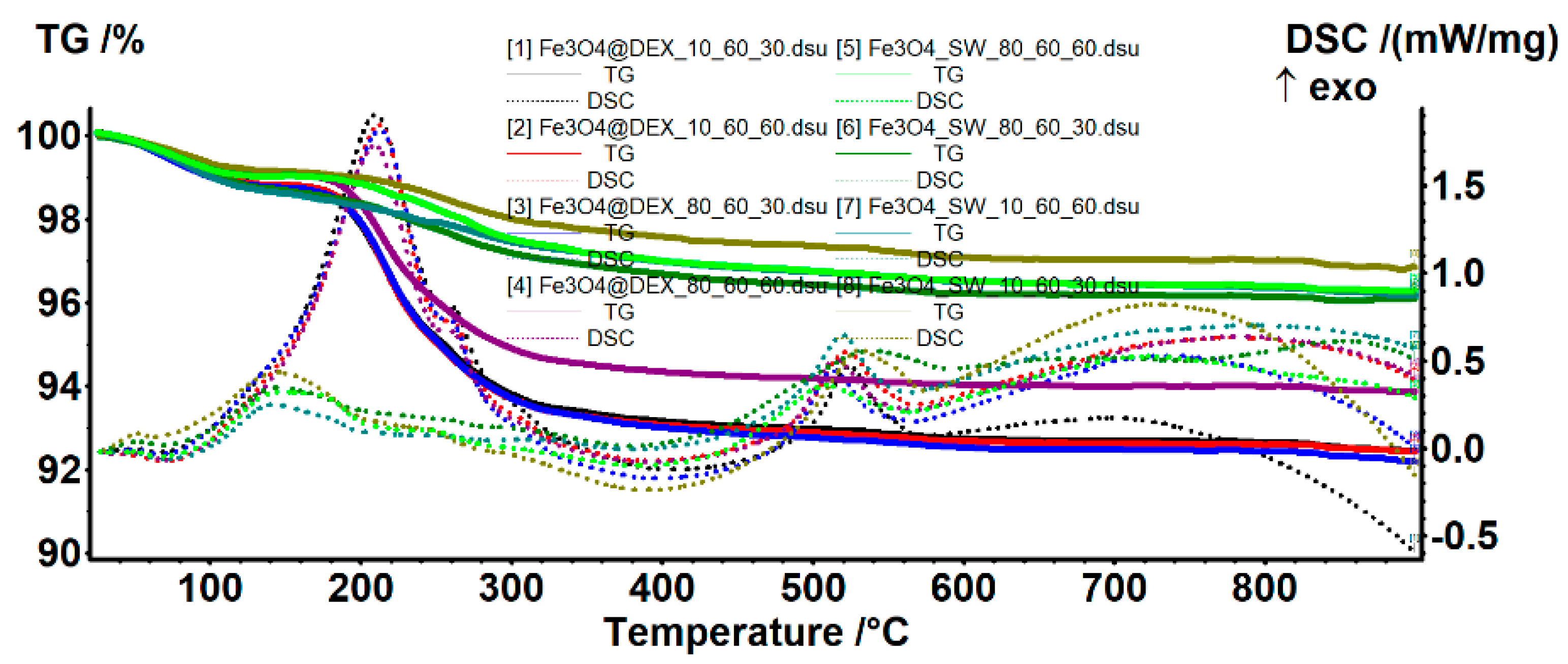
| Sample | Mass Loss (%) | Dextran-Loaded (%) | Thermal Effects (°C) | ||||
|---|---|---|---|---|---|---|---|
| 25–135 °C | 135–400 °C | Residual | Endothermic | Exothermic—Maghemite | Exothermic—Hematite | ||
| 10_60_30 | 0.85 | 1.58 | 96.84 | - | 66.5 | 143.6 | 532.4 |
| 10_60_30@DEX | 1.20 | 5.66 | 92.46 | 4.52 | 67.2 | 209.9 | 524.5 |
| 10_60_60 | 1.34 | 1.70 | 96.16 | - | 71.4 | 142.8 | 520.1 |
| 10_60_60@DEX | 1.19 | 5.77 | 92.41 | 3.90 | 70.3 | 212.2 | 521.8 |
| 80_60_30 | 1.31 | 2.06 | 96.10 | - | 70.2 | 146.0 | 538.7 |
| 80_60_30@DEX | 1.22 | 5.78 | 92.17 | 4.09 | 68.1 | 213.1 | 512.8 |
| 80_60_60 | 1.01 | 2.03 | 96.28 | - | 69.9 | 147.9 | 508.9 |
| 80_60_60@DEX | 0.99 | 4.70 | 93.87 | 2.50 | 70.1 | 209.4 | 515.0 |
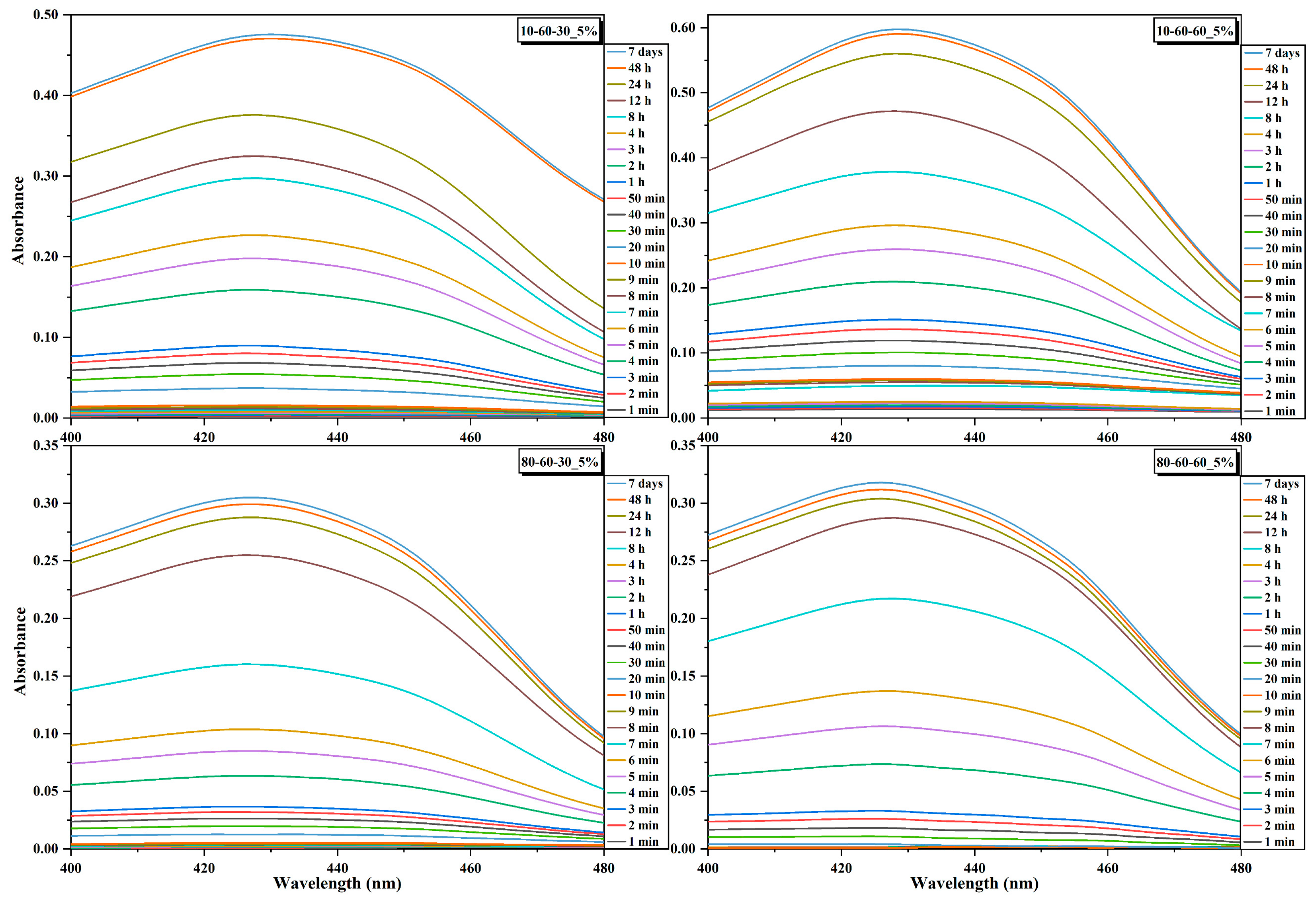
| Sample | Amount of Loaded Curcumin (mg) | Loading Efficiency (%) | Curcumin Released after 7 Days (%) | |
|---|---|---|---|---|
| 10-60-30 | 1% | 5.5 | 55 | - |
| 5% | 12.5 | 25 | 27.02 | |
| 10% | 60.0 | 60 | - | |
| 10-60-60 | 1% | 4.0 | 40 | - |
| 5% | 11.5 | 23 | 36.77 | |
| 10% | 30.0 | 30 | - | |
| 80-60-30 | 1% | 6.0 | 60 | - |
| 5% | 7.5 | 15 | 30.08 | |
| 10% | 15.0 | 15 | - | |
| 80-60-60 | 1% | 3.5 | 35 | - |
| 5% | 8.0 | 16 | 29.24 | |
| 10% | 15.0 | 15 | - | |
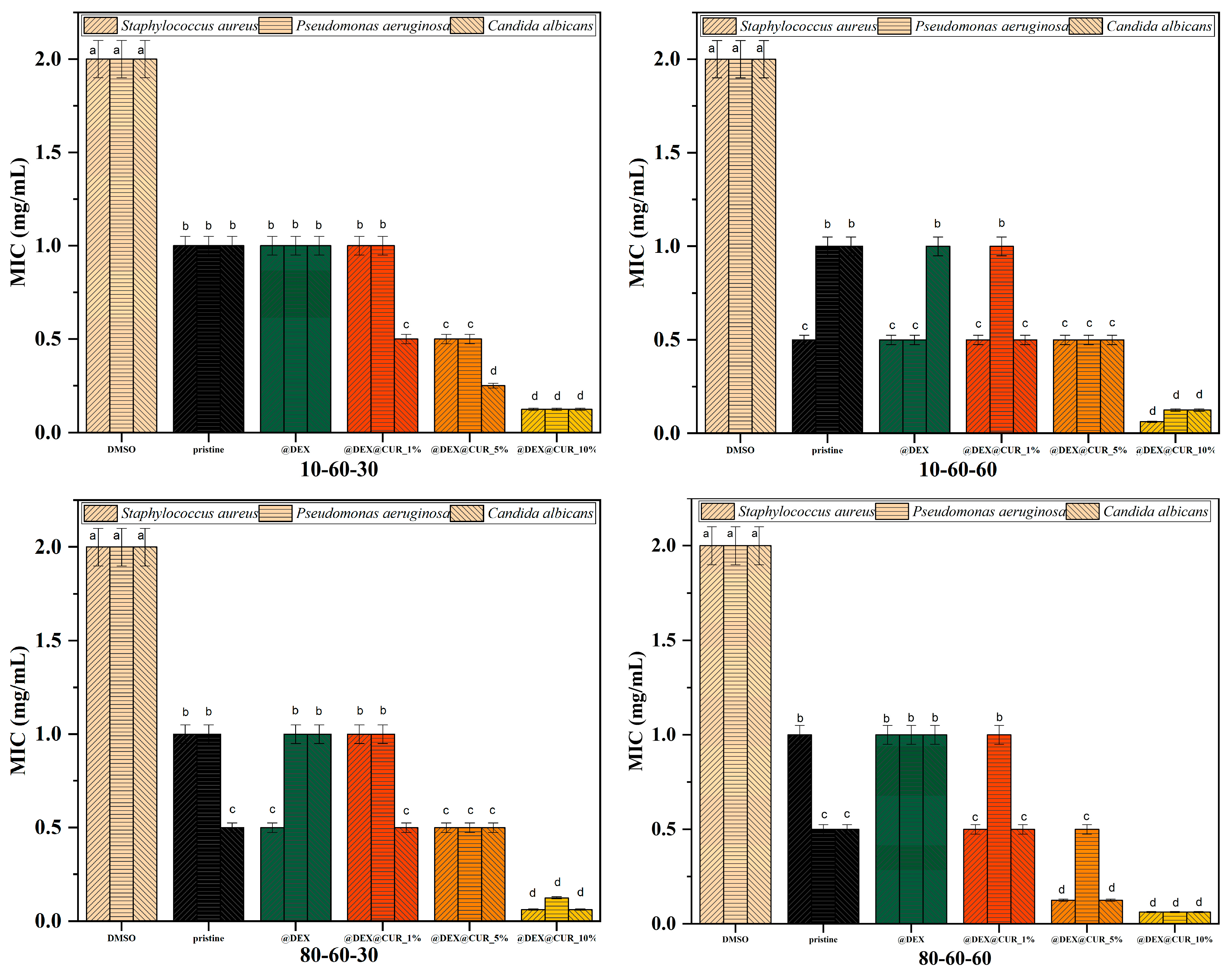
| Sample | Inhibition Zone (mm) | ||
|---|---|---|---|
| Staphylococcus aureus | Pseudomonas aeruginosa | Candida albicans | |
| DMSO | 1 | 1 | 1 |
| 10-60-30 | 6 | 5 | 6 |
| 10-60-30@DEX | 3 | 5 | 5 |
| 10-60-30@DEX@CUR_1% | 6 | 7 | 6 |
| 10-60-30@DEX@CUR_5% | 7 | 7 | 7 |
| 10-60-30@DEX@CUR_10% | 7 | 7 | 7 |
| 10-60-60 | 6 | 5 | 6 |
| 10-60-60@DEX | 6 | 6 | 6 |
| 10-60-60@DEX@CUR_1% | 5 | 5 | 7 |
| 10-60-60@DEX@CUR_5% | 6 | 6 | 5 |
| 10-60-60@DEX@CUR_10% | 7 | 7 | 8 |
| 80-60-30 | 6 | 6 | 6 |
| 80-60-30@DEX | 6 | 5 | 6 |
| 80-60-30@DEX@CUR_1% | 5 | 7 | 6 |
| 80-60-30@DEX@CUR_5% | 6 | 6 | 7 |
| 80-60-30@DEX@CUR_10% | 8 | 7 | 8 |
| 80-60-60 | 7 | 5 | 6 |
| 80-60-60@DEX | 6 | 6 | 6 |
| 80-60-60@DEX@CUR_1% | 8 | 7 | 7 |
| 80-60-60@DEX@CUR_5% | 7 | 6 | 6 |
| 80-60-60@DEX@CUR_10% | 8 | 7 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Ștefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacșu, I.A.; Tihăuan, B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057. https://doi.org/10.3390/pharmaceutics14051057
Chircov C, Ștefan R-E, Dolete G, Andrei A, Holban AM, Oprea O-C, Vasile BS, Neacșu IA, Tihăuan B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics. 2022; 14(5):1057. https://doi.org/10.3390/pharmaceutics14051057
Chicago/Turabian StyleChircov, Cristina, Raluca-Elena Ștefan, Georgiana Dolete, Adriana Andrei, Alina Maria Holban, Ovidiu-Cristian Oprea, Bogdan Stefan Vasile, Ionela Andreea Neacșu, and Bianca Tihăuan. 2022. "Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies" Pharmaceutics 14, no. 5: 1057. https://doi.org/10.3390/pharmaceutics14051057
APA StyleChircov, C., Ștefan, R.-E., Dolete, G., Andrei, A., Holban, A. M., Oprea, O.-C., Vasile, B. S., Neacșu, I. A., & Tihăuan, B. (2022). Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics, 14(5), 1057. https://doi.org/10.3390/pharmaceutics14051057












