Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Source of Data Collection
2.3. Information Processing
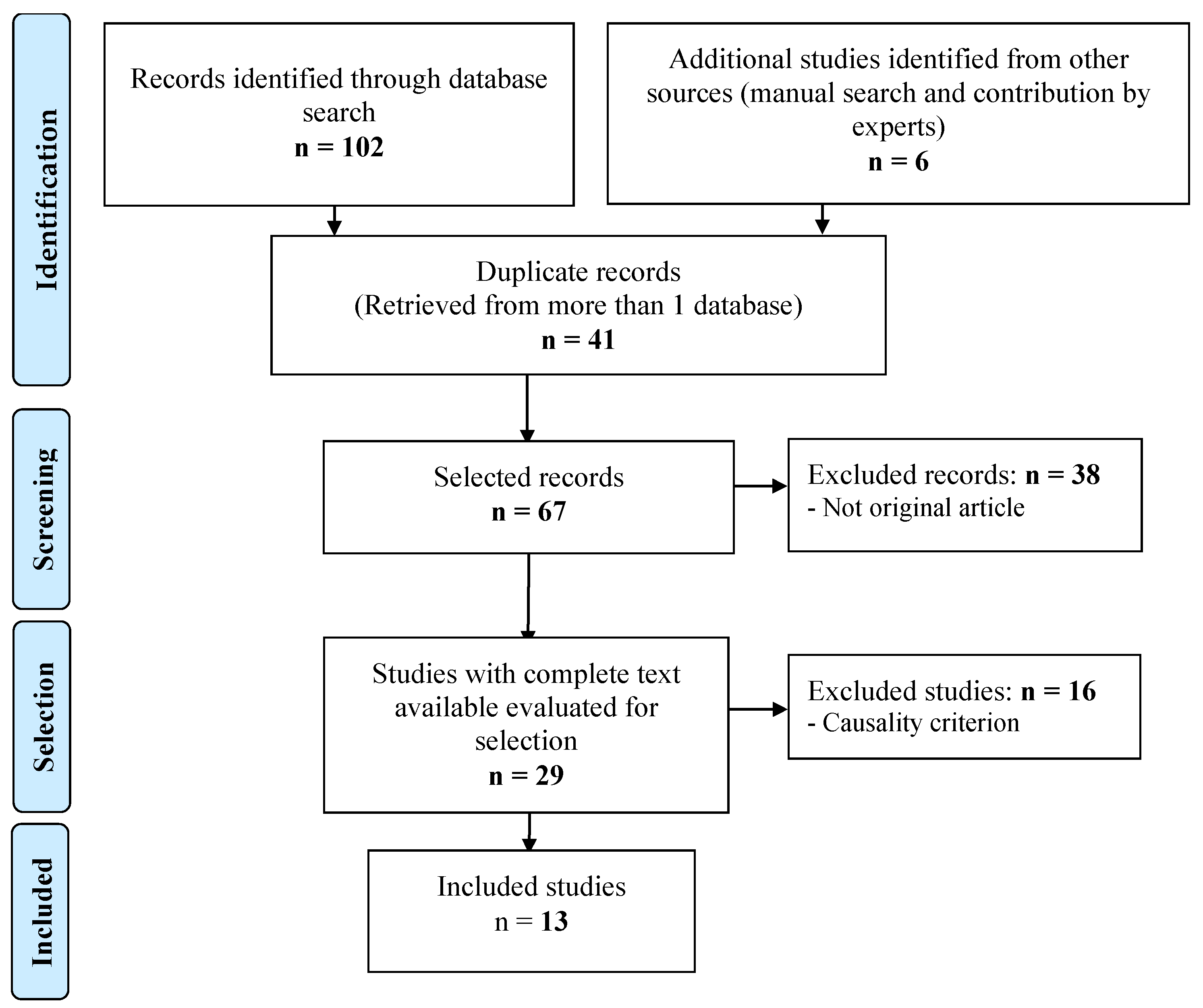
2.4. Final Selection of Articles
2.5. Quality Assessment, Level of Evidence and Grade of Recommendation
2.6. Data Extraction
3. Results
3.1. Study Design
3.2. Population
3.3. Interventions
3.4. TDM
3.5. Costs
3.6. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines inflammatory bowel disease. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef]
- Manninen, P.; Karvonen, A.L.; Huhtala, H.; Aitola, P.; Hyöty, M.; Nieminen, I.; Hemminki, H.; Collin, P. The risk of colorectal cancer in patients with inflammatory bowel diseases in Finland: A follow-up of 20 years. J. Crohn’s Colitis 2013, 7, e51–e57. [Google Scholar] [CrossRef]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of extra-intestinal cancer in inflammatory bowel disease: Meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef]
- Duricova, D.; Pedersen, N.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Overall and cause-specific mortality in Crohn’s disease: A metaanalysis of population-based studies. Inflamm. Bowel Dis. 2010, 16, 347–353. [Google Scholar] [CrossRef]
- Jussila, A.; Virta, L.J.; Pukkala, E.; Färkkilä, M.A. Mortality and causes of death in patients with inflammatory bowel disease: A nationwide register study in Finland. J. Crohn’s Colitis 2014, 8, 1088–1096. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). REMICADE: Summary of Product Characteristics. EMA. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf (accessed on 26 November 2021).
- European Medicines Agency (EMA). HUMIRA: Summary of Product Characteristics. EMA. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (accessed on 26 November 2021).
- Qiu, Y.; Chen, B.L.; Mao, R.; Zhang, S.H.; He, Y.; Zeng, Z.R.; Ben-Horin, S.; Chen, M.H. Systematic review with meta-analysis:loss of response and requirement of anti-TNFa dose intensification in Crohn’s disease. J. Gastroenterol. 2017, 52, 535–554. [Google Scholar] [CrossRef]
- Adeboukun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014, 147, 1296–1307. [Google Scholar] [CrossRef]
- Cornillie, F.; Hanauer, S.B.; Diamond, R.H.; Wang, J.; Tang, K.L.; Xu, Z.; Rutgeerts, P.; Vermeire, S. Post-induction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: A retrospective analysis of the ACCENT I trial. Gut 2014, 63, 1721–1727. [Google Scholar] [CrossRef]
- van Hoeve, K.; Dreesen, E.; Hoffman, I.; Van Assche, G.; Ferrante, F.; Gils, A.; Vermeire, S. Higher Infliximab Trough Levels Are Associated with Better Outcome in Paediatric Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 1316–1325. [Google Scholar] [CrossRef]
- Juncadella, A.; Papamichael, K.; Vaughn, B.P.; Cheifetz, A.S. Maintenance Adalimumab Concentrations Are Associated with Biochemical, Endoscopic, and Histologic Remission in Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 3067–3073. [Google Scholar] [CrossRef]
- Aguas, M.; Bosó, V.; Navarro, B.; Marqués-Miñana, M.R.; Bastida, G.; Beltrán, B.; Iborra, M.; Sáez-González, E.; Monte-Boquet, E.; Poveda-Andrés, J.L.; et al. Serum Adalimumab Levels Predict Successful Remission and Safe Deintensification in Inflammatory Bowel Disease Patients in Clinical Practice. Inflamm. Bowel Dis. 2017, 23, 1454–1460. [Google Scholar] [CrossRef]
- Reinhold, I.; Blümel, S.; Schreiner, J.; Boyman, O.; Bögeholz, J.; Cheetham, M.; Rogler, G.; Biedermann, L.; Scharl, M. Clinical Relevance of Anti-TNF Antibody Trough Levels and Anti-Drug Antibodies in Treating Inflammatory Bowel Disease Patients. Inflamm. Intest. Dis. 2021, 6, 38–47. [Google Scholar] [CrossRef]
- Fine, S.; Papamichael, K.; Cheifetz, A.S. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2019, 15, 656–665. [Google Scholar]
- Papamichael, K.; Vajravelu, R.K.; Osterman, M.T.; Cheifetz, A.S. Long-Term Outcome of Infliximab Optimization for Overcoming Immunogenicity in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 761–767. [Google Scholar] [CrossRef]
- Yanai, H.; Lichtenstein, L.; Assa, A.; Mazor, Y.; Weiss, B.; Levine, A.; Ron, Y.; Kopylov, U.; Bujanover, Y.; Rosenbach, Y.; et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin. Gastroenterol. Hepatol. 2015, 13, 522–530. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Vande Casteele, N.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668. [Google Scholar] [CrossRef]
- Ungar, B.; Levy, I.; Yavne, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Loebstein, R.; Chowers, Y.; Eliakim, R.; Kopylov, U.; et al. Optimizing anti-TNF therapy: Serum levels of infliximab and adalimumab associate with mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 550–557. [Google Scholar] [CrossRef]
- Papamichael, K.; Juncadella, A.; Wong, D.; Rakowsky, S.; Sattler, L.A.; Campbell, J.P.; Vaughn, B.P.; Cheifetz, A.S. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared to standard of care in patients with inflammatory bowel disease. J. Crohn’s Colitis 2019, 13, 976–981. [Google Scholar] [CrossRef]
- Burisch, J.; Vardi, H.; Schwartz, D.; Friger, M.; Kiudelis, G.; Kupčinskas, J.; Fumery, M.; Gower-Rousseau, C.; Lakatos, L.; Lakatos, P.L.; et al. Epi-IBD group. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: A population-based study. Lancet Gastroenterol. Hepatol. 2020, 5, 454–464. [Google Scholar] [CrossRef]
- Martelli, L.; Olivera, P.; Roblin, X.; Attar, A.; Peyrin-Biroulet, L. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: A systematic review. J. Gastroenterol. 2017, 52, 19–25. [Google Scholar] [CrossRef]
- Zrubka, Z.; Gulácsi, L.; Brodszky, V.; Rencz, F.; Alten, R.; Szekanecz, Z.; Péntek, M. Long-term efficacy and cost-effectiveness of infliximab as first-line treatment in rheumatoid arthritis: Systematic review and meta-analysis. Expert Rev. Pharmacoecon. Outcomes Res. 2019, 19, 537–549. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, X.; You, J.H.S. A Systematic Review on Cost-effectiveness Analyses of Therapeutic Drug Monitoring for Patients with Inflammatory Bowel Disease: From Immunosuppressive to Anti-TNF Therapy. Inflamm. Bowel Dis. 2021, 27, 275–282. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic reviews in nutrition: Standardized methodology. Br. J. Nutr. 2012, 107, S3–S7. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 2013, 16, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ 2001, 323, 334–336. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, X.; You, J.S.H. Proactive therapeutic drug monitoring of adalimumab for pediatric Crohn’s disease patients: A cost-effectiveness analysis. J. Gastroenterol. Hepatol. 2021, 36, 2397–2407. [Google Scholar] [CrossRef]
- Negoescu, D.M.; Enns, E.A.; Swanhorst, B.; Baumgartner, B.; Campbell, J.P.; Osterman, M.T.; Papamichael, K.; Cheifetz, A.S.; Vaughn, B.P. Proactive vs. reactive therapeutic drug monitoring of infliximab in Crohn’s disease: A cost-effectiveness analysis in a simulated cohort. Inflamm. Bowel Dis. 2020, 26, 103–111. [Google Scholar] [CrossRef]
- Attar, A.; Duru, G.; Roblin, X.; Savarieau, B.; Brunel, P.; Lamure, M.; Peyrin-Biroulet, L. Cost savings using a test-based de-escalation strategy for patients with Crohn’s disease in remission on optimized infliximab: A discrete event model study. Dig. Liver Dis. 2019, 51, 112–119. [Google Scholar] [CrossRef]
- Freeman, K.; Connock, M.; Auguste, P.; Taylor-Phillips, S.; Mistry, H.; Shyangdan, D.; Arasaradnam, R.; Sutcliffe, P.; Clarke, A. Clinical effectiveness and cost-effectiveness of use of therapeutic monitoring of tumour necrosis factor alpha (TNF-α) inhibitors [LISA-TRACKER enzyme-linked immunosorbent assay (ELISA) kits, TNF-α-Blocker ELISA kits and Promonitor ELISA kits] versus standard care in patients with Crohn’s disease: Systematic reviews and economic modelling. Health Technol. Assess. 2016, 20, 1–288. [Google Scholar] [CrossRef]
- Roblin, X.; Attar, A.; Lamure, M.; Savarieau, B.; Brunel, P.; Duru, G.; Peyrin-Biroulet, L. Cost savings of anti-TNF therapy using a test-based strategy versus an empirical dose escalation in Crohn’s disease patients who lose response to infliximab. J. Mark. Access Health Policy 2015, 3, 29229. [Google Scholar] [CrossRef]
- Velayos, F.S.; Kahn, J.G.; Sandborn, W.J.; Feagan, B.G. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin. Gastroenterol. Hepatol. 2013, 11, 654–666. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, B.; Thilakanathan, C.; Lehmann, P.; Xuan, W.; Mohsen, W.; Toong, C.; Williams, A.J.; Ng, W.; Connor, S. Therapeutic drug monitoring in inflammatory bowel disease reduces unnecessary use of infliximab with substantial associated cost-savings. Intern. Med. J. 2021, 51, 739–745. [Google Scholar] [CrossRef]
- Ganesananthan, S.; Durai, D. Clinical value and cost saving of therapeutic drug monitoring of infliximab in adult patients with inflammatory bowel disease. Clin. Med. (Lond.) 2020, 20, s23–s24. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Pugliese, D.; Tonucci, T.P.; Berrino, A.; Tolusso, B.; Basile, M.; Cantoro, L.; Balestrieri, P.; Civitelli, F.; Bertani, L.; et al. Therapeutic Drug Monitoring is More Cost-Effective than a Clinically Based Approach in the Management of Loss of Response to Infliximab in Inflammatory Bowel Disease: An Observational Multicentre Study. J. Crohn’s Colitis. 2018, 12, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Taks, M.; Pijls, P.A.; Derijks, L.J.; Broeke, R.T.; Grouls, R.J.; Curvers, J.; Gilissen, L.P. The effect of implementation of a treatment algorithm for infliximab on remission rates and drug costs in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2017, 29, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015, 148, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Brynskov, J.; Thomsen, O.Ø.; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Pedersen, G.; Kjeldsen, J.; Jacobsen, B.A.; Oxholm, A.S.; et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn’s disease patients failing infliximab. Dig. Dis. Sci. 2015, 60, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Brynskov, J.; Thomsen, O.Ø.; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Pedersen, G.; Kjeldsen, J.; Jacobsen, B.A.; Oxholm, A.S.; et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut 2014, 63, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef]
- Dretzke, J.; Edlin, R.; Round, J.; Connock, M.; Hulme, C.; Czeczot, J.; Fry-Smith, C.; McCabe, C.; Meads, C. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol. Assess 2011, 15, 1–244. [Google Scholar] [CrossRef]
- Papamichael, K.; Van Stappen, T.; Casteele, N.V.; Gils, A.; Billiet, T.; Tops, S.; Claes, K.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 543–549. [Google Scholar] [CrossRef]
- Papamichael, K.; Chachu, K.A.; Vajravelu, R.K.; Vaughn, B.P.; Ni, J.; Osterman, M.T.; Cheifetz, A.S. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, 1580–1588. [Google Scholar] [CrossRef]
- Vaughn, B.P.; Martinez-Vazquez, M.; Patwardhan, V.R.; Moss, A.C.; Sandborn, W.J.; Adam, S.; Cheifetz, A.S. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: Results from a pilot observational study. Inflamm. Bowel Dis. 2014, 20, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Mitrev, N.; Vande Casteele, N.; Seow, C.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; et al. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 46, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Moors, G.; Bian, S.; Van Stappen, T.; Van Assche, G.; Vermeire, S.; Gils, A.; Ferrante, M. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: The usefulness of rapid testing. Aliment. Pharmacol. Ther. 2018, 48, 731–739. [Google Scholar] [CrossRef]
- Bots, S.J.; Parker, C.E.; Brandse, J.F.; Löwenberg, M.; Feagan, B.G.; Sandborn, W.J.; Jairath, V.; D’Haens, G.; Vande Casteele, N. Anti-Drug Antibody Formation Against Biologic Agents in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioDrugs 2021, 35, 715–733. [Google Scholar] [CrossRef]
- Jha, A.; Upton, A.; Dunlop, W.C.N.; Akehurst, R. The budget impact of biosimilar infliximab (Remsima(®)) for the treatment of autoimmune diseases in five European countries. Adv. Ther. 2015, 32, 742–756. [Google Scholar] [CrossRef]
- Rencz, F.; Gulácsi, L.; Péntek, M.; Gecse, K.B.; Dignass, A.; Halfvarson, J.; Gomollón, F.; Baji, P.; Peyrin-Biroulet, L.; Lakatos, P.L.; et al. Cost-utility of biological treatment sequences for luminal Crohn’s disease in Europe. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 597–606. [Google Scholar] [CrossRef]
- Papamichael, K.; Vajravelu, R.K.; Vaughn, B.P.; Osterman, M.T.; Cheifetz, A.S. Proactive Infliximab Monitoring Following Reactive Testing is Associated with Better Clinical Outcomes Than Reactive Testing Alone in Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2018, 12, 804–810. [Google Scholar] [CrossRef]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why has model-informed precision dosing not yet become common clinical reality? Lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef]
- Marquez-Megias, S.; Ramon-Lopez, A.; Más-Serrano, P.; Diaz-Gonzalez, M.; Candela-Boix, M.R.; Nalda-Molina, R. Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease. Pharmaceutics 2021, 13, 1244. [Google Scholar] [CrossRef]
- Konecki, C.; Feliu, C.; Cazaubon, Y.; Giusti, D.; Tonye-Libyh, M.; Brixi, H.; Cadiot, G.; Biron, A.; Djerada, Z. External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab. Pharmaceutics 2021, 13, 1191. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Tomislav, M. Biologic Therapy for Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 717–729. [Google Scholar] [CrossRef]
- Alsoud, D.; Vermeire, S.; Verstockt, B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: Hype or hope? Curr. Opin. Pharmacol. 2020, 55, 17–30. [Google Scholar] [CrossRef]
- Fobelo, M.J.; Serrano, R.; Sánchez, S. Therapeutic drug monitoring of infliximab in spondyloarthritis. A Review of the literature. Br. J. Clin. Pharmacol. 2019, 85, 2264–2279. [Google Scholar] [CrossRef]
- Felice, C.; Marzo, M.; Pugliese, D.; Papa, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Therapeutic drug monitoring of anti-TNF-α agents in inflammatory bowel diseases. Expert Opin. Biol. Ther. 2015, 15, 1107–1117. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Danese, S.; Cummings, F.; Atreya, R.; Greveson, K.; Pieper, B.; Kang, T. Anti-TNF biosimilars in Crohn’s Disease: A patient-centric interdisciplinary approach. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 731–738. [Google Scholar] [CrossRef]
- Mitrev, N.; Leong, R.W. Therapeutic drug monitoring of anti-tumour necrosis factor-alpha agents in inflammatory bowel disease. Expert Opin. Drug Saf. 2017, 16, 303–317. [Google Scholar] [CrossRef]
- Franco, D.L.; Click, B. Proactive Versus Reactive Therapeutic Drug Monitoring of Infliximab in Crohn’s Disease: Is the Juice Worth the Squeeze? Inflamm. Bowel Dis. 2020, 26, 112–113. [Google Scholar] [CrossRef]
- Hoseyni, H.; Xu, Y.; Zhou, H. Therapeutic Drug Monitoring of Biologics for Inflammatory Bowel Disease: An Answer to Optimized Treatment? J. Clin. Pharmacol. 2018, 58, 864–876. [Google Scholar] [CrossRef]
- Lega, S.; Bramuzzo, M.; Dubinsky, M.C. Therapeutic Drug Monitoring in Pediatric IBD: Current Application and Future Perspectives. Curr. Med. Chem. 2018, 25, 2840–2854. [Google Scholar] [CrossRef]
- Papamichael, K.; Van Stappen, T.; Jairath, V.; Gecse, K.; Khanna, R.; D’Haens, G.; Vermeire, S.; Gils, A.; Feagan, B.G.; Levesque, B.G.; et al. Review article: Pharmacological aspects of anti-TNF biosimilars in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 42, 1158–1169. [Google Scholar] [CrossRef]
- Teml, A.; Schaeffeler, E.; Herrlinger, K.R.; Klotz, U.; Schwab, M. Thiopurine treatment in inflammatory bowel disease: Clinical pharmacology and implication of pharmacogenetically guided dosing. Clin. Pharmacokinet. 2007, 46, 187–208. [Google Scholar] [CrossRef]
- Khanna, R.; Sattin, B.D.; Afif, W.; Benchimol, E.I.; Bernard, E.J.; Bitton, A.; Bressler, B.; Fedorak, R.N.; Ghosh, S.; Greenberg, G.R.; et al. Review article: A clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 447–459. [Google Scholar] [CrossRef]
- Ricciuto, A.; Dhaliwal, J.; Walters, T.D.; Griffiths, A.M.; Church, P.C. Clinical Outcomes with Therapeutic Drug Monitoring in Inflammatory Bowel Disease: A Systematic Review with Meta-Analysis. J. Crohn’s Colitis 2018, 12, 1302–1315. [Google Scholar] [CrossRef]
- Yanai, H.; Dotan, I. Therapeutic Drug Monitoring is More Cost-Effective than a Clinically Based Approach in the Management of Loss of Response to Infliximab in Inflammatory Bowel Disease: An Observational Multicentre Study. J. Crohn’s Colitis 2019, 13, 539–540. [Google Scholar] [CrossRef]
- McNeill, R.P.; Murray, L.; Barclay, M.L. Cost-effectiveness of therapeutic drug monitoring in inflammatory bowel disease. Curr. Opin. Pharmacol. 2020, 55, 41–46. [Google Scholar] [CrossRef]
- Papamichael, K.; Vermeire, S. Withdrawal of anti-tumour necrosis factor α therapy in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 4773–4778. [Google Scholar] [CrossRef]
- Andrews, J.M.; Travis, S.P.L.; Gibson, P.R.; Gasche, C. Systematic review: Does concurrent therapy with 5-ASA and immunomodulators in inflammatory bowel disease improve outcomes? Aliment. Pharmacol. Ther. 2009, 29, 459–469. [Google Scholar] [CrossRef]
- Travis, S. Advances in therapeutic approaches to ulcerative colitis and Crohn’s disease. Curr. Gastroenterol. Rep. 2005, 7, 475–484. [Google Scholar] [CrossRef]
- Rentsch, C.A.; Sparrow, M.; Ward, M.G.; Friedman, A.; Taylor, K.M.; Luber, R.P.; Su, H.Y.; Hopkins, R.; Headon, B.; Dooley, M.; et al. Pharmacist-led proactive therapeutic drug monitoring with infliximab (PROXIMO): Utility of and cost saving associated with the use of a rapid assay for assessing drug level. Gastroenterology 2018, 154 (Suppl. S1), S826. [Google Scholar] [CrossRef]
- Rentsch, C.; Sparrow, M.; Ward, M.; Friedman, A.; Taylor, K.; Luber, R.; Su, H.; Hopkins, R.; Headon, B.; Dooley, M.; et al. Pharmacist-led proactive therapeutic drug monitoring with infliximab (PROXIMO): Utility of and cost-saving associated with the use of a rapid assay for assessing drug level. J. Crohn’s Colitis 2018, 12 (Suppl. S1), S321–S322. [Google Scholar] [CrossRef][Green Version]
- Rentsch, C.; Sparrow, M.; Ward, M.; Friedman, A.; Taylor, K.; Luber, R.; Su, H.; Hopkins, R.; Headon, B.; Dooley, M.; et al. Pharmacist-led proactive therapeutic drug monitoring with infliximab (PROXIMO): Utility of and cost-saving associated with the use of a rapid assay for assessing drug level. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S2), 143–144. [Google Scholar] [CrossRef]
- Kozak, J.; El-Matary, W.; Huynh, H.Q.; El-Kalla, M. Therapeutic drug monitoring impacts decision-making for children with inflammatory bowel disease on infliximab. J. Pediatr. Gastroenterol. Nutr. 2016. [Google Scholar] [CrossRef]
- Janko, N.; Little, R.D.; Sparrow, M.P.; Gibson, P.R.; Ward, M.G. Uptake of drug monitoring of infliximab and adalimumab in inflammatory bowel disease in Australia. J. Gastroenterol. Hepatol. 2016, 31 (Suppl. S2), S136–S137. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; De Cruz, P.; Hamilton, A.L.; Ritchie, K.; Bell, S.J.; Brown, S.J.; Connell, W.R.; Desmond, P.V.; Liew, D. Structured post-operative treatment and monitoring to prevent Crohn’s disease recurrence is cost effective. Results from the POCER study. J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S3), S145. [Google Scholar] [CrossRef]
- Steenholdt, C.; Ainsworth, M.A. Letters: Authors’ response: Importance of defining loss of response before therapeutic drug monitoring. Gut 2015, 64, 1340–1341. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, J.J.; Altwerger, G.; Kohn, E.C. Proteomics and biomarkers in clinical trials for drug development. J. Proteomics 2011, 74, 2632–2641. [Google Scholar] [CrossRef]
- Doherty, J.; Varley, R.; Healy, M.; Dunne, C.; Mac Carthy, F.; Mc Kiernan, S.; Hartery, K.; Kevans, D. P449 Cost Effectiveness of a Proactive Therapeutic Drug Monitoring Strategy in Patients with Inflammatory Bowel Disease Receiving Infliximab. Poster presentations: Clinical: Therapy and Observation. 2021. Available online: https://www.ecco-ibd.eu/publications/congress-abstracts/item/p449-cost-effectiveness-of-a-proactive-therapeutic-drug-monitoring-strategy-in-patients-with-inflammatory-bowel-disease-receiving-infliximab.html (accessed on 29 March 2022).
- Steen, J.; McCormack, M.; McShane, C.; Healy, M.; Crowley, V.; Kennedy, U.; Hayes, O.; Dunne, C.; Hartery, K.; McKiernan, M.; et al. Cost-effectiveness of utilising proactive Infliximab therapeutic drug monitoring for inflammatory bowel disease in routine clinical practice. J. Crohn’s Colitis 2019, 13 (Suppl. S1), S307–S308. [Google Scholar] [CrossRef]
- Yang, S.K. Personalizing IBD Therapy: The Asian Perspective. Dig. Dis. 2016, 34, 165–174. [Google Scholar] [CrossRef]
- Mehta, P.; Manson, J.J. What Is the Clinical Relevance of TNF Inhibitor Immunogenicity in the Management of Patients with Rheumatoid Arthritis? Front. Immunol. 2020, 11, 589. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.G.; Rebollo, N.; Munoz, F.; Martin-Suarez, A.; Calvo, M.V. Therapeutic drug monitoring of tumour necrosis factor inhibitors in the management of chronic inflammatory diseases. Ann. Clin. Biochem. 2019, 56, 28–41. [Google Scholar] [CrossRef]
- Zandvliet, M.L.; van Bezooijen, J.S.; Bos, M.A.; Prens, E.P.; van Doorn, M.; Bijen, I.; Schreurs, M.W.J.; van der Velden, V.H.J.; Koch, B.C.P.; van Gelder, T. Monitoring antigen-specific biologics: Current knowledge and future prospects. Ther. Drug Monit. 2013, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N.; Nigam, G.B.; Jatale, R.G.; Desai, D.; Makharia, G.; Ahuja, V.; Limdi, J.K. An Indian national survey of therapeutic drug monitoring with anti-tumor necrosis (TNF) medications in inflammatory bowel disease. Indian J. Gastroenterol. 2020, 39, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Syversen, S.W.; Goll, G.L.; Jørgensen, K.K.; Olsen, I.C.; Sandanger, Ø.; Gehin, J.E.; Warren, D.J.; Sexton, J.; Mørk, C.; Jahnsen, J.; et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: Study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials 2020, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Drobne, D.; Kurent, T.; Golob, S.; Svegl, P.; Rajar, P.; Terzic, S.; Kozelj, M.; Novak, G.; Smrekar, N.; Plut, S.; et al. Success and safety of high infliximab trough levels in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Selinger, C.P.; Lenti, M.V.; Clark, T.; Rafferty, H.; Gracie, D.; Ford, A.C.; O’Connor, A.; Ahmad, T.; Hamlin, P.J. Infliximab Therapeutic Drug Monitoring Changes Clinical Decisions in a Virtual Biologics Clinic for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2083–2088. [Google Scholar] [CrossRef]
- Thomas, P.W.A.; Chin, P.K.L.; Barclay, M.L. A nationwide survey on therapeutic drug monitoring of anti-tumour necrosis factor agents for inflammatory bowel disease. Intern. Med. J. 2021, 51, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, L.B.; Papamichael, K.; Feuerstein, J.D.; Siegel, C.A.; Ullman, T.A.; Cheifetz, A.S. A Survey Study of Gastroenterologists’ Attitudes and Barriers Toward Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 24, 191–197. [Google Scholar] [CrossRef]
- Nigam, G.B.; Nayeemuddin, S.; Kontopantelis, E.; Hayee, B.; Limdi, J.K. UK National Survey of Gastroenterologists’ attitudes and barriers toward therapeutic drug monitoring of anti-TNF therapy in inflammatory bowel disease. Frontline Gastroenterol. 2020, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Langford, T.; Arkir, Z.; Chalkidou, A.; Goddard, K.; Kaftantzi, L.; Samaan, M.; Irving, P. The Clinical and Cost-Effectiveness of 4 Enzyme-Linked Immunosorbent Assay Kits for Monitoring Infliximab in Crohn Disease Patients: Protocol for a Validation Study. JMIR Res. Protoc. 2018, 7, e11218. [Google Scholar] [CrossRef] [PubMed]
- Crane, H.; Wu, N.; Chan, P.; Nguyen, P.; Williams, A.J.; Ng, W.; Connor, S.J. Safety, satisfaction, and cost savings of accelerated infusions of standard and intensified-dose infliximab for inflammatory bowel disease. Intern. Med. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.N.; Pellish, R.S.; Thompson, K.D.; Baptista, V.; Siegel, C.A. Using Therapeutic Drug Monitoring to Identify Variable Infliximab Metabolism in an Individual Patient with Ulcerative Colitis. J. Clin. Gastroenterol. 2016, 50, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, M. What are the treatment targets in inflammatory bowel disease (IBD) in 2020?: Session one summary. J. Gastroenterol. Hepatol. 2021, 36 (Suppl. S1), 4–5. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Crandall, W.V.; Fridge, J.; Leibowitz, I.H.; Tsou, M.; Dykes, D.M.H.; Hoffenberg, E.J.; Kappelman, M.D.; Colletti, R.B. Implementable strategies and exploratory considerations to reduce costs associated with anti-TNF therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 946–951. [Google Scholar] [CrossRef]
- Kelly, O.B.; O’Donnell, S.; Stempak, J.M.; Steinhart, A.H.; Silverberg, M.S. Therapeutic Drug Monitoring to Guide Infliximab Dose Adjustment is Associated with Better Endoscopic Outcomes than Clinical Decision Making Alone in Active Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1202–1209. [Google Scholar] [CrossRef]
- Scharnhorst, V.; Schmitz, E.M.H.; van de Kerkhof, D.; Derijks, L.J.J.; Broeren, M.A.C. A value proposition for trough level-based anti-TNFα drug dosing. Clin. Chim. Acta 2019, 489, 89–95. [Google Scholar] [CrossRef]
- Jourdil, J.F.; Némoz, B.; Gautier-Veyret, E.; Romero, C.; Stanke-Labesque, F. Simultaneous Quantification of Adalimumab and Infliximab in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Ther. Drug Monit. 2018, 40, 417–424. [Google Scholar] [CrossRef]
| Study | Authors, Year | Country | Study Design | Drug Studied | Study Population (n, m/f, Age) | Study Duration | Measure of Outcomes | Intervention | CHEERS (n, %) |
|---|---|---|---|---|---|---|---|---|---|
| ST1 | Yao et al., 2021 [36] | US | Modeling approach | Adalimumab | 20,000 simulated CD pediatric biologic-naïve patients | 3 years and 4 weeks | Costs Cost savings QALY ICER | Proactive TDM (n = 10,000) vs. Reactive TDM (n = 10,000) | 21 (87.5%) Excellent |
| ST2 | Negoescu et al., 2020 [37] | US | Modeling approach | Infliximab | 100,000 CD simulated patients | 5 years | Costs Cost savings QALY ICER | Proactive TDM (n = NA) vs. Reactive TDM (n = NA) vs. Empirical strategy (n = NA) | 19 (79.2%) Very good |
| ST3 | Attar et al., 2019 [38] | France | Modeling approach | Infliximab | 40,000 CD simulated adult patients | 2 years | Costs Cost savings | Proactive TDM (n = 20,000) vs. Empirical strategy (n = 20,000) | 7 (29.2%) Insufficient |
| ST4 | Freeman et al., 2016 [39] | UK | Modeling approach | Infliximab | Simulations of CD patients in maintenance treatment of infliximab | 10 years | Costs Cost savings QALY ICER | Proactive TDM (n = NA) vs. Empirical strategy (n = NA) | 23 (95.8%) Excellent |
| ST5 | Roblin et al., 2015 [40] | France | Modeling approach | Infliximab | 10,000 Simulations of CD patients with LOR to infliximab | 1, 3 and 5 years | Cost savings | Reactive TDM (n = NA) vs. Empirical strategy (n = NA) | 10 (41.7%) Insufficient |
| ST6 | Velayos et al., 2013 [41] | US | Modeling approach | Infliximab | 10,000 simulations of CD patients with LOR to infliximab | 1 year | Costs Cost savings QALY ICER | Reactive TDM (n = NA) vs. Empirical strategy (n = NA) | 17 (70.8%) Very good |
| ST7 | Wu et al., 2021 [42] | Australia | Prospective observational study | Infliximab | 428 IBD patients (322/296, 36 ± 18.7 yo) | 56 weeks | Cost savings | Proactive TDM (n = 181) vs. Reactive TDM (n = 247) | 12 (50.0%) Insufficient |
| ST8 | Ganesnanthan et al., 2020 [43] | UK | Retrospective observational study | Infliximab | 85 IBD patients (54/31, 39.13 ± 14.25 yo) | NA | Cost savings | Proactive TDM (n = NA) vs. Reactive TDM (n = NA) vs. Proactive TDM post-induction (n = NA) | 7 (29.2%) Insufficient |
| ST9 | Guidi et al., 2018 [44] | Italy | Prospective observational study | Infliximab | 148 IBD patients in treatment for at least 4 months with LOR to infliximab (75/73, 40.8 (37.05–42.5) yo) | 12 weeks | Costs Cost savings | Reactive TDM (n = 96) vs. Empirical strategy (n = 52) | 17 (70.8%) Very good |
| ST10 | Taks et al., 2017 [45] | The Netherlands | Non-randomized clinical trial | Infliximab | 33 IBD adult patients (20/13, 43 (32–59) yo) | 1 year | Cost savings | Proactive TDM (n = 28) vs. Reactive TDM (n = 33) | 4 (16.7%) Insufficient |
| ST11 | Vande Castelee et al., 2015 [46] | Belgium | Randomized controlled clinical trial | Infliximab | 251 IBD adult patients with a stable clinical response for at least 14 weeks (138/113, 41 (34.5–49.0) yo) | 2 years and 16 weeks | Costs Cost savings QALY ICER | Proactive TDM (n = 128) vs. Empirical strategy (123) | 18 (75.0%) Very good |
| ST12 | Steenholdt et al., 2015 [47] | Denmark | Randomized controlled clinical trial | Infliximab | 69 CD adult patients with LOR to infliximab (27/69, 37 (19–81) yo) | 20 and 52 weeks | Costs Cost savings | Reactive TDM (n = 33) vs. Empirical strategy (n = 36) | 17 (70.8%) Very good |
| ST13 | Steenholdt et al., 2014 [48] | 12 weeks | 17 (70.8%) Very good |
| Study | Authors, Year | Total Cost | Cost Savings | Average Cost Savings per Patient | QALY | ICER |
|---|---|---|---|---|---|---|
| ST1 | Yao et al., 2021 [36] | PA: USD 110,851.18 (EUR 94,223.50) RA: USD 111,508.01 (EUR 94,781.81) | PA: USD 656.83 (EUR 558.31) compared to RA | PA: EUR 0.06 compared to RA | PA: 0.81 RA: 0.74 | RA-PA: Dominated by PA |
| ST2 | Negoescu et al., 2020 [37] | PA: USD 16,585.42 (EUR 14,926.88) RA: USD 15,847.69 (EUR 14,262.92) ES: USD 15,853.68 (EUR 14,268.31) | RA: USD 737.73 (EUR 663.96) compared to PA and USD 5.99 (EUR 5.39) compared to ES | NA | PA: 0.74 RA: 0.73 ES: 0.73 | RA-PA: USD 146,509.12 (EUR 131,858.21) ES-RA: Dominated by RA |
| ST3 | Attar et al., 2019 [38] | PA: EUR 186,635,650 ES: EUR 201,879,000 | PA: EUR 15,243,350 compared to ES | PA: EUR 0.76 compared to ES | NA | NA |
| ST4 | Freeman et al., 2016 [39] | PA: GBP 13,980 (EUR 18,174) ES: GBP 15,050 (EUR 19,565) | PA: GBP 1070 (EUR 1391) compared to ES | NA | PA: 0.63 ES: 0.65 | ES-PA: GBP 43,727.01 (EUR 56,845.12) |
| ST5 | Roblin et al., 2015 [40] | NA | RA: EUR 26,260,058.60 compared to ES | NA | NA | NA |
| ST6 | Velayos et al., 2013 [41] | RA: USD 31,870 (EUR 23,902.5) ES: USD 37,266 (EUR 27,949.5) | RA: USD 5396 (EUR 4047) compared to ES | NA | RA: 0.80 ES: 0.80 | ES-RA: Dominated by RA |
| ST7 | Wu et al., 2021 [42] | NA | PA: AUD 304,916.95 (EUR 196,394.48) compared to RA | NA | NA | NA |
| ST8 | Ganesnanthan et al., 2020 [43] | NA | PA: GBP 56,865 (EUR 62,551) compared to ES PA post-induction: GBP 51,595 (EUR 56,754.50) compared to ES RA: GBP 27,081.85 (EUR 29,790.04) compared to ES | NA | NA | NA |
| ST9 | Guidi et al., 2018 [44] | RA: EUR 3,230,810.44 ES: EUR 3,788,285.67 | RA: EUR 557,475.23 compared to ES | RA: EUR 39,197.38 compared to ES | NA | NA |
| ST10 | Taks et al., 2017 [45] | NA | PA: EUR 47,026 compared to RA | NA | NA | NA |
| ST11 | Vande Castelee et al., 2015 [46] | PA: EUR 5,201,473 ES: EUR 5,276,773 | PA: EUR 75,300 compared to ES | PA: EUR 300 compared to ES | PA: 0.82 ES: 0.84 | ES-PA: EUR 3,901,554.40 |
| ST12 | Steenholdt et al., 2015 [47] | RA: USD 22,066 (EUR 17,652.80) ES: USD 29,072 (EUR 23,257.60) | RA: USD 7006 (EUR 5604.8) compared to ES | RA: EUR 111.11 compared to ES | NA | NA |
| ST13 | Steenholdt et al., 2014 [48] | RA: USD 26,164.67 (EUR 19,623.5) ES: USD 39,771.33 (EUR 29,828.5) | RA: USD 13,606.67 (EUR 10,205) compared to ES | RA: EUR 233.92 compared to ES | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez-Megias, S.; Nalda-Molina, R.; Sanz-Valero, J.; Más-Serrano, P.; Diaz-Gonzalez, M.; Candela-Boix, M.R.; Ramon-Lopez, A. Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics 2022, 14, 1009. https://doi.org/10.3390/pharmaceutics14051009
Marquez-Megias S, Nalda-Molina R, Sanz-Valero J, Más-Serrano P, Diaz-Gonzalez M, Candela-Boix MR, Ramon-Lopez A. Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics. 2022; 14(5):1009. https://doi.org/10.3390/pharmaceutics14051009
Chicago/Turabian StyleMarquez-Megias, Silvia, Ricardo Nalda-Molina, Javier Sanz-Valero, Patricio Más-Serrano, Marcos Diaz-Gonzalez, Maria Remedios Candela-Boix, and Amelia Ramon-Lopez. 2022. "Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review" Pharmaceutics 14, no. 5: 1009. https://doi.org/10.3390/pharmaceutics14051009
APA StyleMarquez-Megias, S., Nalda-Molina, R., Sanz-Valero, J., Más-Serrano, P., Diaz-Gonzalez, M., Candela-Boix, M. R., & Ramon-Lopez, A. (2022). Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics, 14(5), 1009. https://doi.org/10.3390/pharmaceutics14051009







