123I-BMIPP, a Radiopharmaceutical for Myocardial Fatty Acid Metabolism Scintigraphy, Could Be Utilized in Bacterial Infection Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Accumulation of Radiopharmaceuticals in E. coli EC-14
2.3. Accumulation of 123I-BMIPP in E. coli EC-14 under Low-Temperature Conditions
2.4. Accumulation of 123I-BMIPP in E. coli EC-14 in the Presence of a CD36 Inhibitor
2.5. Mouse Model of E. coli EC-14 Infection
2.6. Biological Distribution of 123I-BMIPP and 18F-FDG in E. coli EC-14 Infection Model Mice
2.7. Planar Imaging with 123I-BMIPP in E. coli EC-14 Infection Model Mice
2.8. Statistical Analysis
3. Results
3.1. Accumulation of Radiopharmaceuticals in E. coli EC-14
3.2. Accumulation of 123I-BMIPP in E. coli EC-14 under Low-Temperature Conditions
3.3. Accumulation of 123I-BMIPP in E. coli EC-14 in the Presence of a CD36 Inhibitor
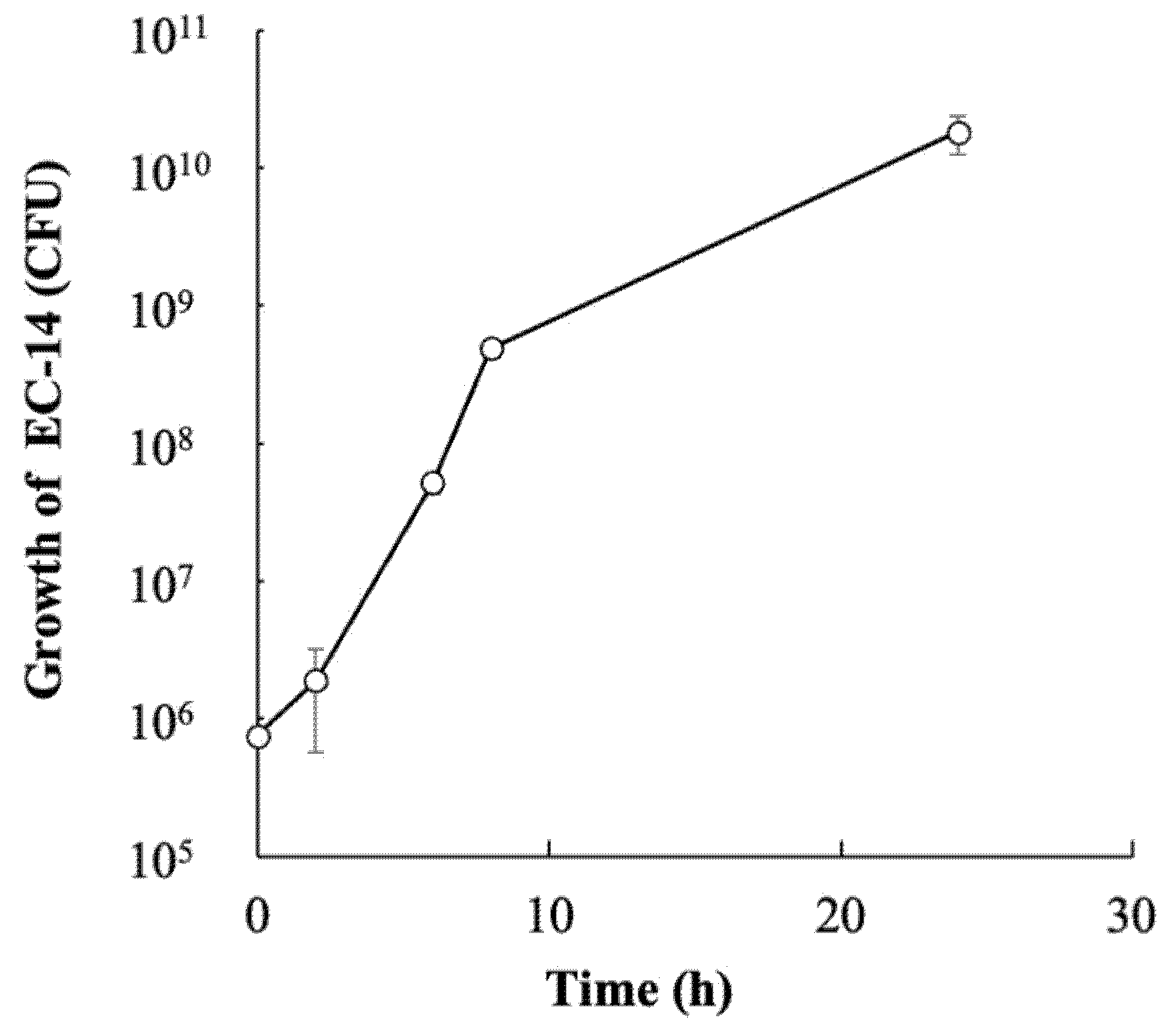
3.4. Growth of E. coli EC-14 in Infection Model Mice
3.5. Biological Distribution of 123I-BMIPP and 18F-FDG in EC-14 Infection Model Mice
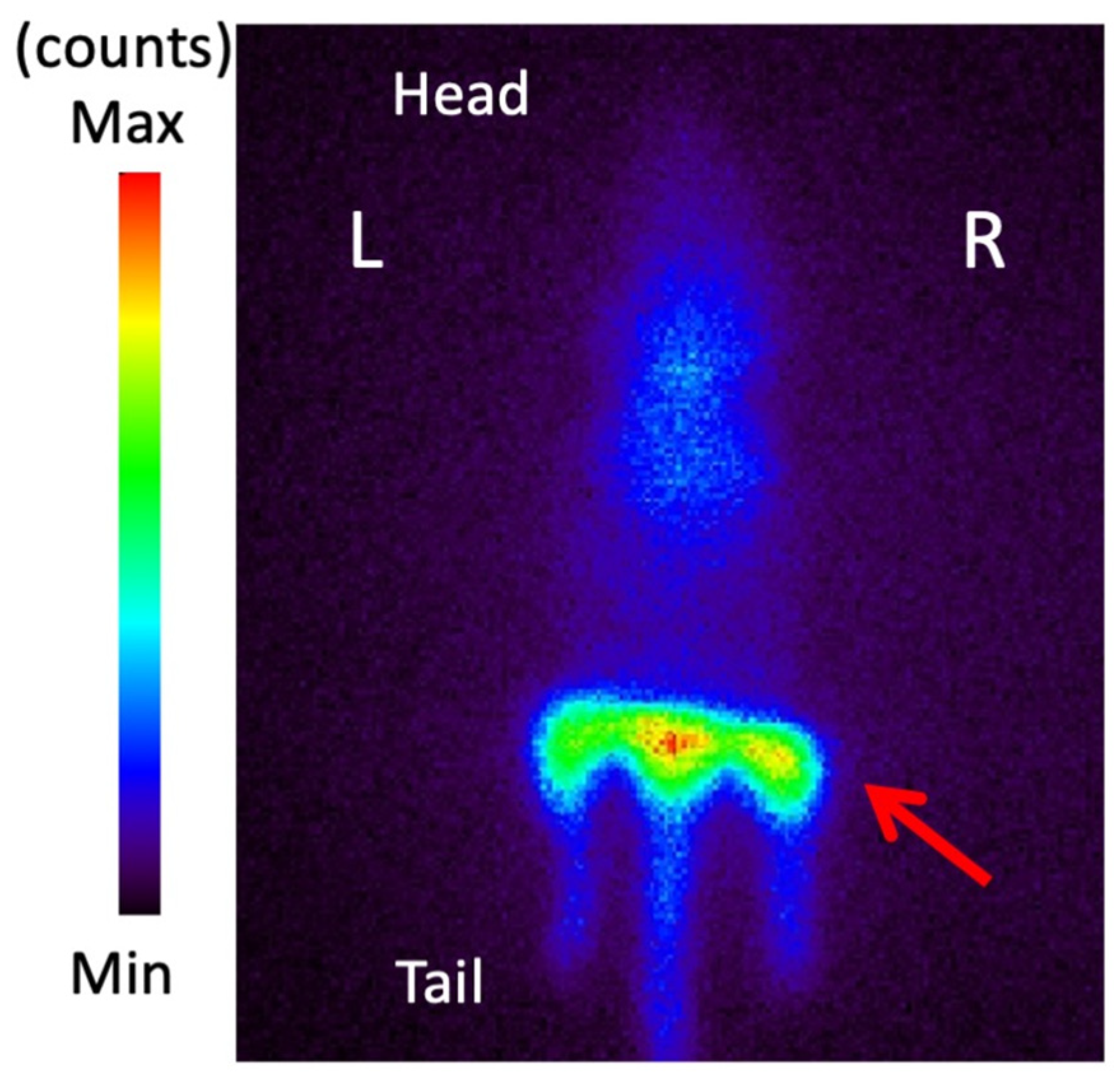
3.6. Planar Imaging with 123I-BMIPP in E. coli EC-14 Infection Model Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lastours, V.; Chau, F.; Roy, C.; Larroque, B.; Fantin, B. Emergence of quinolone resistance in the microbiota of hospitalized patients treated or not with a fluoroquinolone. J. Antimicrob. Chemother. 2014, 69, 3393–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliberti, S.; Cook, G.S.; Babu, B.L.; Reyes, L.F.; Rodriguez, A.H.; Sanz, F.; Soni, N.J.; Anzueto, A.; Faverio, P.; Sadud, R.F.; et al. International prevalence and risk factors evaluation for drug-resistant Streptococcus pneumoniae pneumonia. J. Infect. 2019, 79, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, Y.; Zhang, X. Estimating Factors Related to Fluoroquinolone Resistance Based on One Health Perspective: Static and Dynamic Panel Data Analyses from Europe. Front. Pharmacol. 2019, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 5 March 2022).
- O’Neill, J. The Review on Antimicrobial Resistance. Tackling Drug Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and the Department of Health of United Kingdom Government: London, UK, 2016.
- Signore, A.; Artiko, V.; Conserva, M.; Ferro-Flores, G.; Welling, M.; Jain, S.; Hess, S.; Sathekge, M. Imaging Bacteria with Radiolabelled Probes: Is It Feasible? J. Clin. Med. 2020, 9, 2372. [Google Scholar] [CrossRef] [PubMed]
- Bunschoten, A.; Welling, M.M.; Termaat, M.F.; Sathekge, M.; van Leeuwen, F.W.B. Development and Prospects of Dedicated Tracers for the Molecular Imaging of Bacterial Infections. Bioconjug. Chem. 2013, 24, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, Y.; Mizutani, A.; Kobayashi, M.; Nakamoto, K.; Matsue, M.; Nishi, K.; Yamazaki, K.; Nishii, R.; Shikano, N.; Okamoto, S.; et al. Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model. Int. J. Mol. Sci. 2022, 23, 2467. [Google Scholar] [CrossRef] [PubMed]
- Adams, F.G.; Trappetti, C.; Waters, J.K.; Zang, M.; Brazel, E.B.; Paton, J.C.; Snel, M.F.; Eijkelkamp, B.A. To Make or Take: Bacterial Lipid Homeostasis during infection. Mbio 2021, 12, e00928-21. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Rock, C.O. How Bacterial Pathogens Eat Host Lipids: Implications for the Development of Fatty Acid Synthesis Therapeutics. J. Biol. Chem. 2015, 290, 5940–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, O.; Kikuchi, T.; Scholte, A.; Mahdiui, M.; Nishii, R.; Zhang, M.R.; Suzuki, E.; Yoshinaga, K. Radiopharmaceutical tracers for cardiac imaging. J. Nucl. Cardiol. 2018, 25, 1204–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, K.; Tamaki, N. Current status of nuclear cardiology in Japan: Ongoing efforts to improve clinical standards and to establish evidence. J. Nucl. Cardiol. 2015, 22, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K. Normal values for nuclear cardiology: Japanese databases for myocardial perfusion, fatty acid and sympathetic imaging and left ventricular function. Ann. Nucl. Med. 2010, 24, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Nakajima, K.; Yamashina, S.; Sakata, K.; Momose, M.; Hashimoto, J.; Kumita, S.; Kawano, M.; Okuda, K. Characterization of Japanese standards for myocardial sympathetic and metabolic imaging in comparison with perfusion imaging. Ann. Nucl. Med. 2009, 23, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, M.; Morel, M.; Ferrand, N.; Ferrand, N.; Fellahi, S.; Bastard, J.P.; Lamaziere, A.; Larsen, A.K.; Bereziat, V.; Altan, M.; et al. Breast-associated adipocytes secretome induce fatty acid uptake and invasiveness in breast cancer cells via CD36 independently of body mass index, menopausal status and mammary density. Cancers 2012, 11, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Vermot, T.S.; Kim, I.S.; Haldeman, P.; Mondal, C.; Gonzales, V.Y.; et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverstein, R.L.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemppainen, J.; Fujimoto, T.; Kalliokoski, K.; Viljanen, T.; Nuutila, P.; Knuuti, J. Myocardial and skeletal muscle glucose uptake during exercise in humans. J. Physiol. 2002, 542, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Fatty acids binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar] [CrossRef]
- Tsujimura, E.; Kusuoka, H.; Fukuchi, K. Changes in perfusion and fatty acid metabolism of rat heart with autoimmune myocarditis. Ann. Nucl. Med. 2000, 14, 361–367. [Google Scholar] [CrossRef] [PubMed]
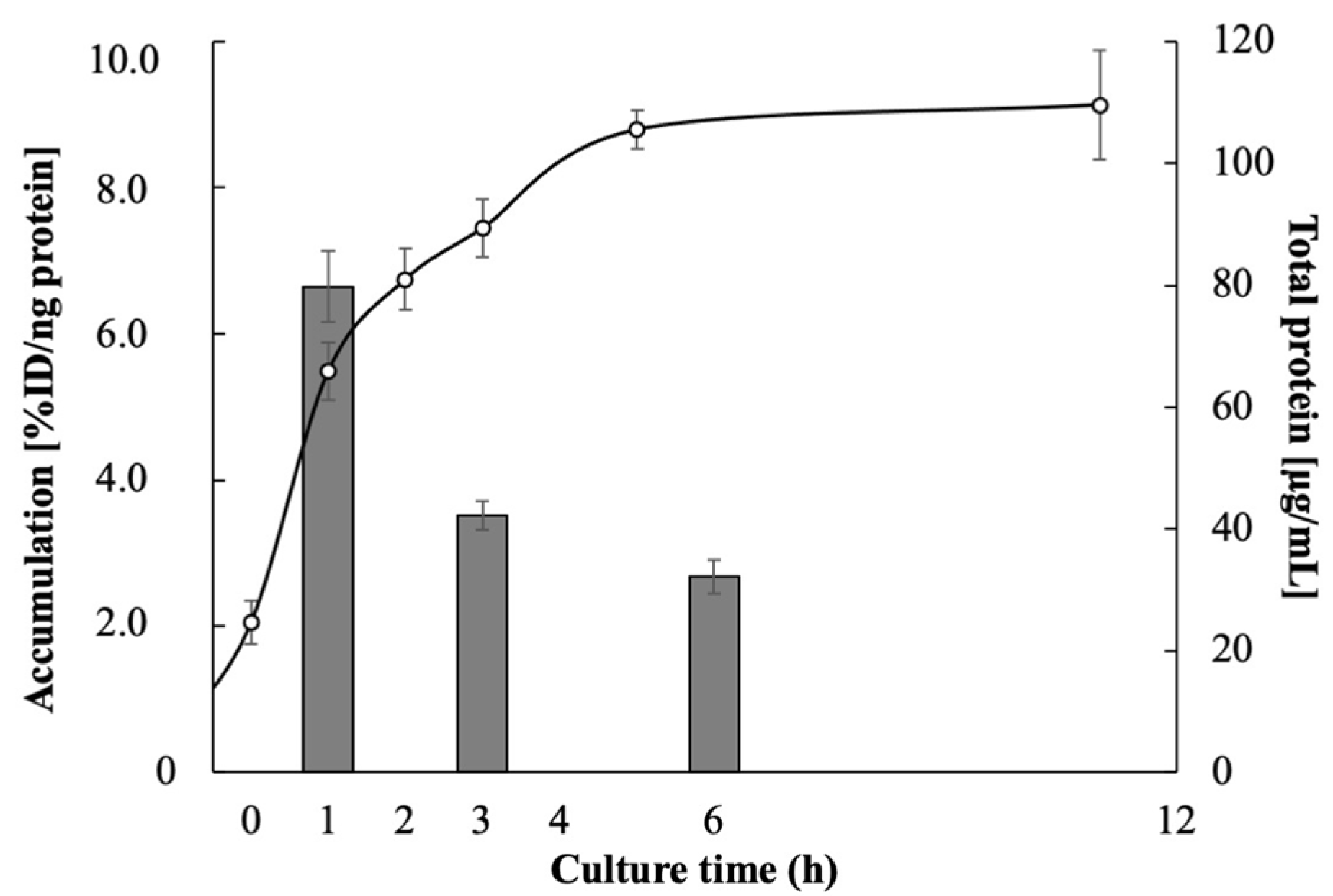


| Accumulation (%ID/ng Protein) | ||
|---|---|---|
| Culture Time (h) | 123I-BMIPP | 18F-FDG (0.1 mg/mL Glucose Concentration) |
| 1 | 6.65 ± 0.49 | 6.32 ± 2.31 |
| 3 | 3.52 ± 0.20 | 15.9 ± 1.37 |
| 6 | 2.68 ± 0.23 | 11.8 ± 0.49 |
| Accumulation of 123I-BMIPP (%ID/g) | ||||
|---|---|---|---|---|
| Time after Infection (h) | 2 | 8 | ||
| Time after 123I-BMIPP Injection (min) | 15 | 60 | 15 | 60 |
| Blood | 16.69 ± 0.93 | 16.10 ± 1.88 | 13.65 ± 3.57 | 19.43 ± 2.91 |
| Heart | 25.86 ± 6.46 | 29.11 ± 4.48 | 30.37 ± 3.90 | 30.66 ± 6.40 |
| Lung | 13.02 ± 2.00 | 11.84 ± 1.02 | 11.99 ± 1.10 | 12.01 ± 1.65 |
| Liver | 17.31 ± 3.86 | 11.04 ± 1.92 | 20.84 ± 3.32 | 12.02 ± 1.91 |
| Kidney | 14.01 ± 1.30 | 12.18 ± 1.27 | 12.00 ± 1.32 | 11.79 ± 1.88 |
| Accumulation of 18F-FDG (%ID/g) | ||||
|---|---|---|---|---|
| Time after Infection (h) | 2 | 8 | ||
| Time after 18F-FDG Injection (min) | 15 | 60 | 15 | 60 |
| Blood | 2.83 ± 0.90 | 0.69 ± 0.09 | 0.93 ± 0.11 | 0.18 ± 0.03 |
| Heart | 13.95 ± 5.47 | 15.05 ± 3.35 | 30.02 ± 7.33 | 28.57 ± 5.98 |
| Lung | 2.91 ± 0.76 | 2.96 ± 1.04 | 3.44 ± 0.39 | 4.04 ± 0.88 |
| Liver | 2.95 ± 1.07 | 1.10 ± 0.26 | 2.81 ± 0.49 | 2.10 ± 0.65 |
| Kidney | 5.43 ± 1.95 | 2.15 ± 0.52 | 5.77 ± 1.55 | 2.71 ± 0.67 |
| After Infection (h) | After Injection (min) | 123I-BMIPP | 18F-FDG | |||
|---|---|---|---|---|---|---|
| Accumulation (%ID/g) | Contrast | Accumulation (%ID/g) | Contrast | |||
| 2 | 15 | Infected | 6.09 ± 3.72 | 1.15 | 1.69 ± 0.50 | 1.05 |
| Control | 5.30 ± 0.72 | 1.61 ± 0.53 | ||||
| 60 | Infected | 6.13 ± 1.14 | 1.00 | 2.47 ± 0.69 | 0.97 | |
| Control | 6.10 ± 1.03 | 2.54 ± 0.81 | ||||
| 8 | 15 | Infected | 6.82 ± 2.02 * | 1.29 | 4.25 ± 0.92 | 0.93 |
| Control | 5.29 ± 0.63 | 4.58 ± 1.53 | ||||
| 60 | Infected | 8.64 ± 1.80 * | 1.31 | 3.14 ± 1.16 | 1.03 | |
| Control | 6.59 ± 0.80 | 3.05 ± 1.93 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muranaka, Y.; Mizutani, A.; Kobayashi, M.; Nakamoto, K.; Matsue, M.; Takagi, F.; Okazaki, K.; Nishi, K.; Yamazaki, K.; Nishii, R.; et al. 123I-BMIPP, a Radiopharmaceutical for Myocardial Fatty Acid Metabolism Scintigraphy, Could Be Utilized in Bacterial Infection Imaging. Pharmaceutics 2022, 14, 1008. https://doi.org/10.3390/pharmaceutics14051008
Muranaka Y, Mizutani A, Kobayashi M, Nakamoto K, Matsue M, Takagi F, Okazaki K, Nishi K, Yamazaki K, Nishii R, et al. 123I-BMIPP, a Radiopharmaceutical for Myocardial Fatty Acid Metabolism Scintigraphy, Could Be Utilized in Bacterial Infection Imaging. Pharmaceutics. 2022; 14(5):1008. https://doi.org/10.3390/pharmaceutics14051008
Chicago/Turabian StyleMuranaka, Yuka, Asuka Mizutani, Masato Kobayashi, Koya Nakamoto, Miki Matsue, Fumika Takagi, Kenichi Okazaki, Kodai Nishi, Kana Yamazaki, Ryuichi Nishii, and et al. 2022. "123I-BMIPP, a Radiopharmaceutical for Myocardial Fatty Acid Metabolism Scintigraphy, Could Be Utilized in Bacterial Infection Imaging" Pharmaceutics 14, no. 5: 1008. https://doi.org/10.3390/pharmaceutics14051008
APA StyleMuranaka, Y., Mizutani, A., Kobayashi, M., Nakamoto, K., Matsue, M., Takagi, F., Okazaki, K., Nishi, K., Yamazaki, K., Nishii, R., Shikano, N., Okamoto, S., Maki, H., & Kawai, K. (2022). 123I-BMIPP, a Radiopharmaceutical for Myocardial Fatty Acid Metabolism Scintigraphy, Could Be Utilized in Bacterial Infection Imaging. Pharmaceutics, 14(5), 1008. https://doi.org/10.3390/pharmaceutics14051008








