Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosomes (Chitosan-Coated Anionic Liposomes) (CS-NPs)
2.2. Physicochemical Characterization of CS-NPs
2.2.1. Particle size, Zetapotential, and Polydispersity Index Measurement
2.2.2. Determination of Entrapment Efficiency (EE%)
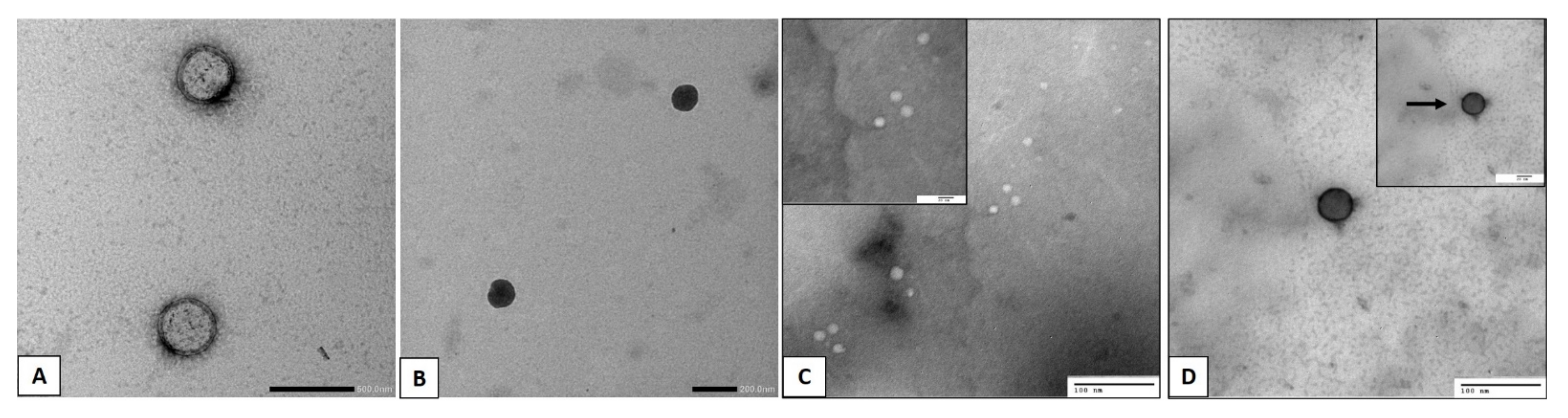
2.2.3. Transmission Electron Microscopy
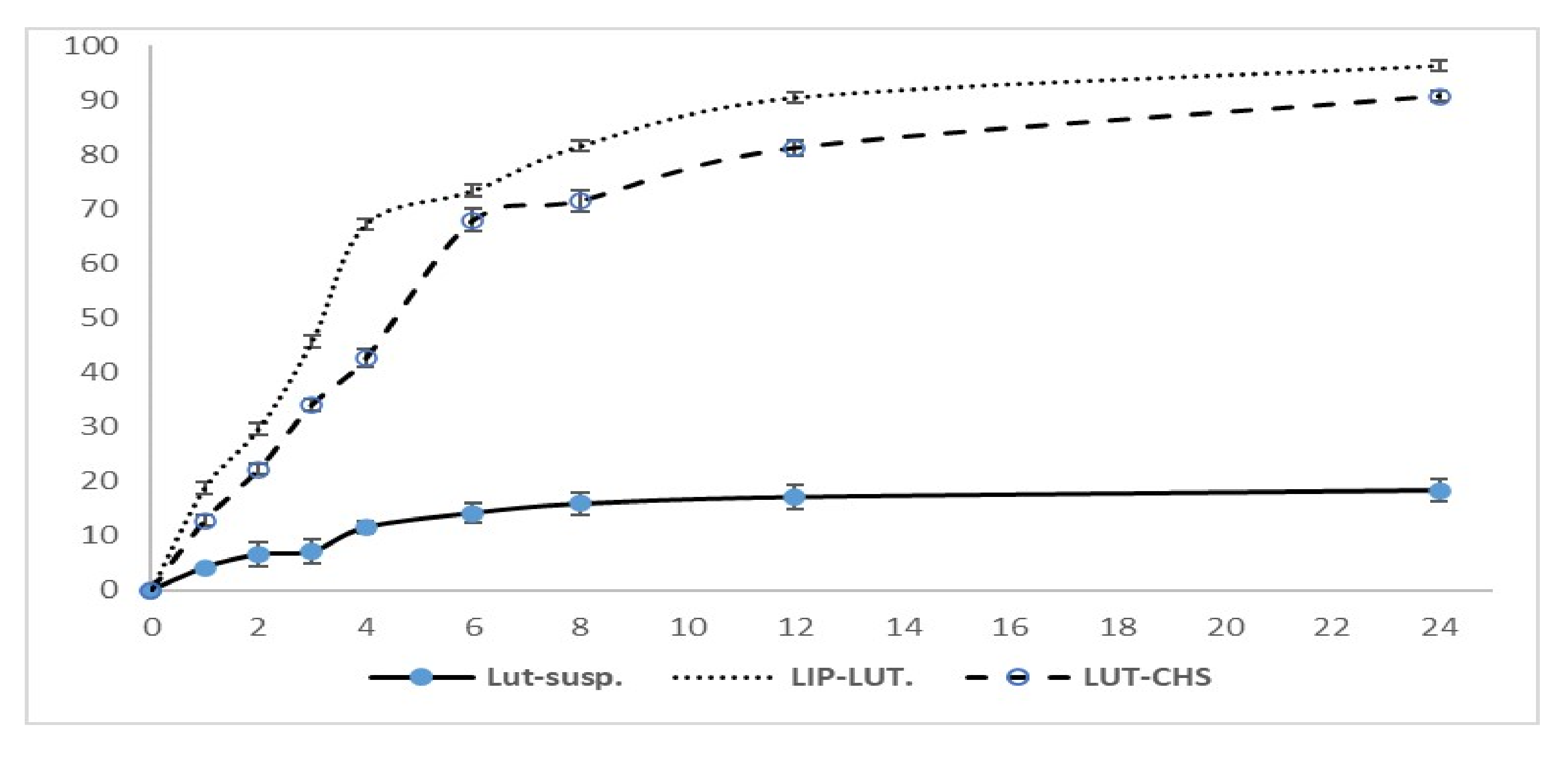
2.2.4. In Vitro Release Study
2.2.5. Release Kinetics of Chitosan Nanoparticles
2.2.6. Stability Study
2.2.7. In Vitro Mucoadhesion Test
2.3. In Vivo Studies
2.3.1. Animals
2.3.2. Induction of Sporadic Alzheimer’s Disease (SAD)
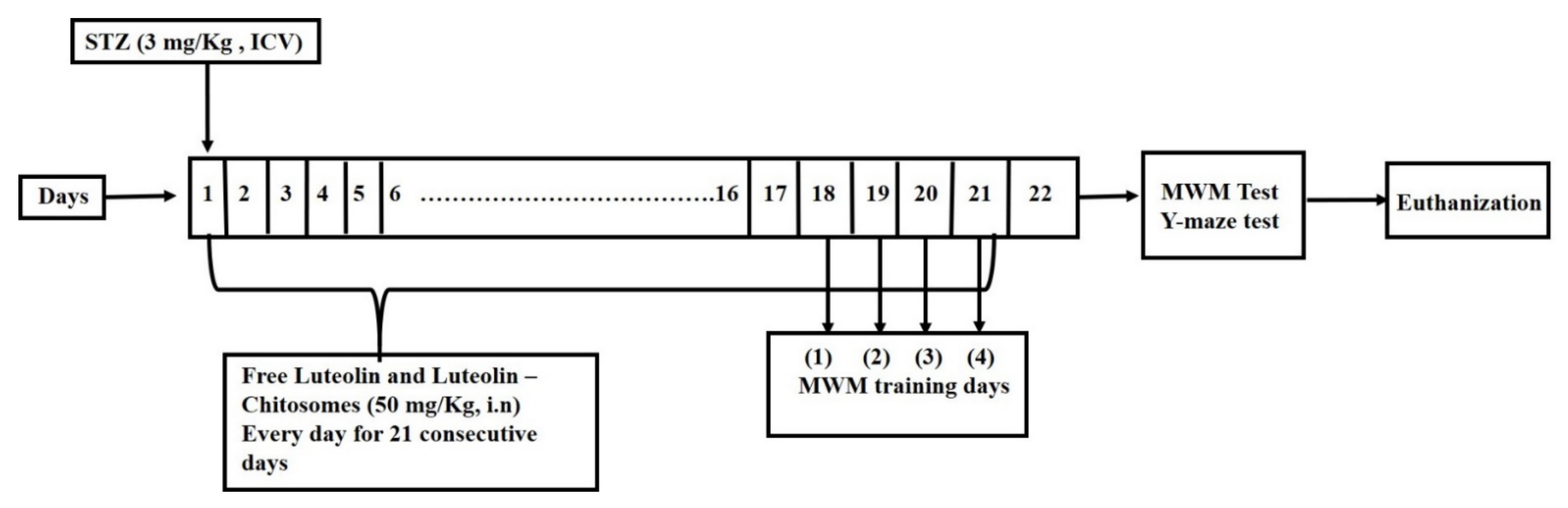
2.3.3. Experimental Design
2.3.4. Intranasal Administration
2.3.5. Behavioral Assessment
Y-Maze
Morris Water Maze (MWM)
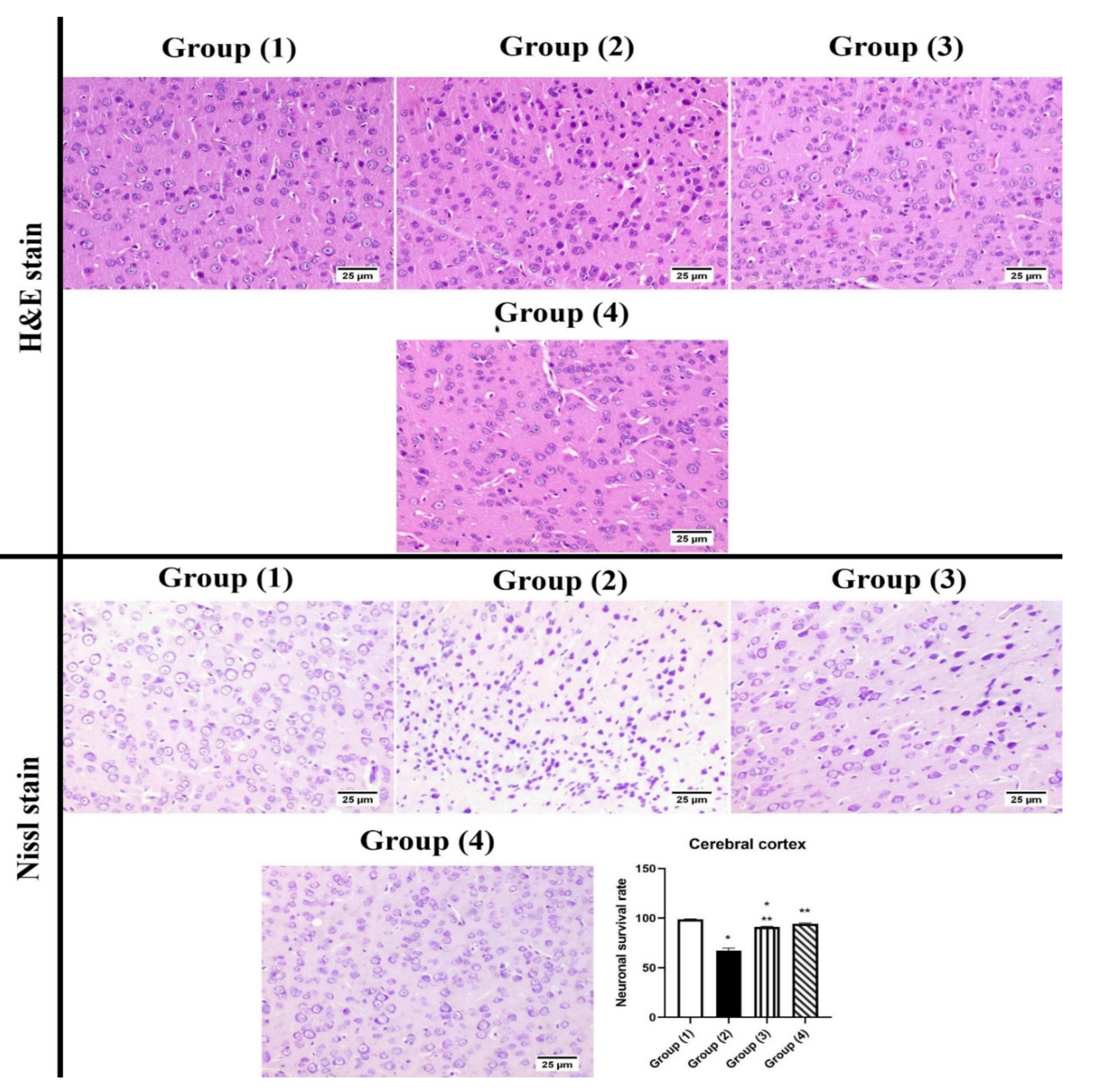
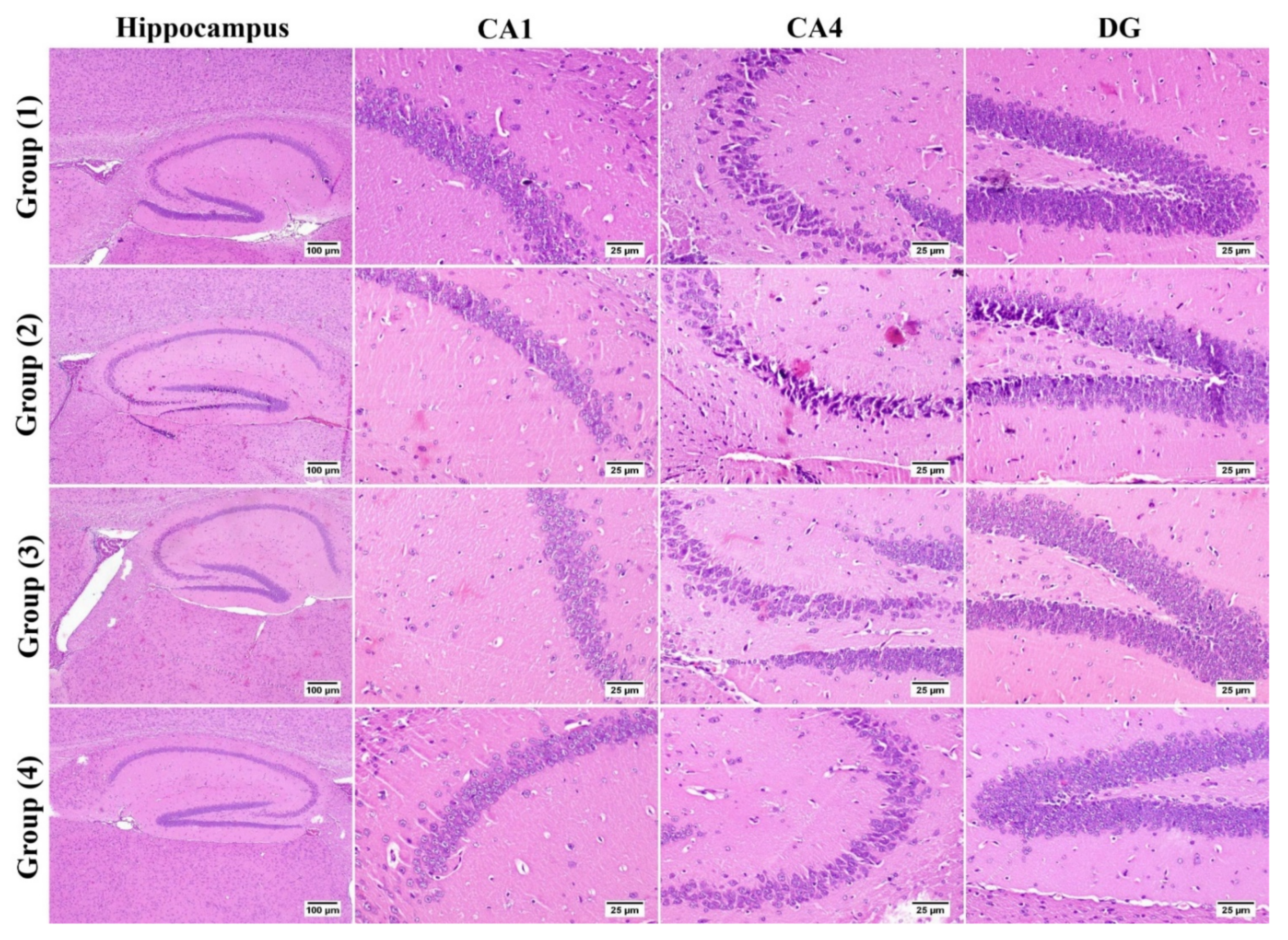
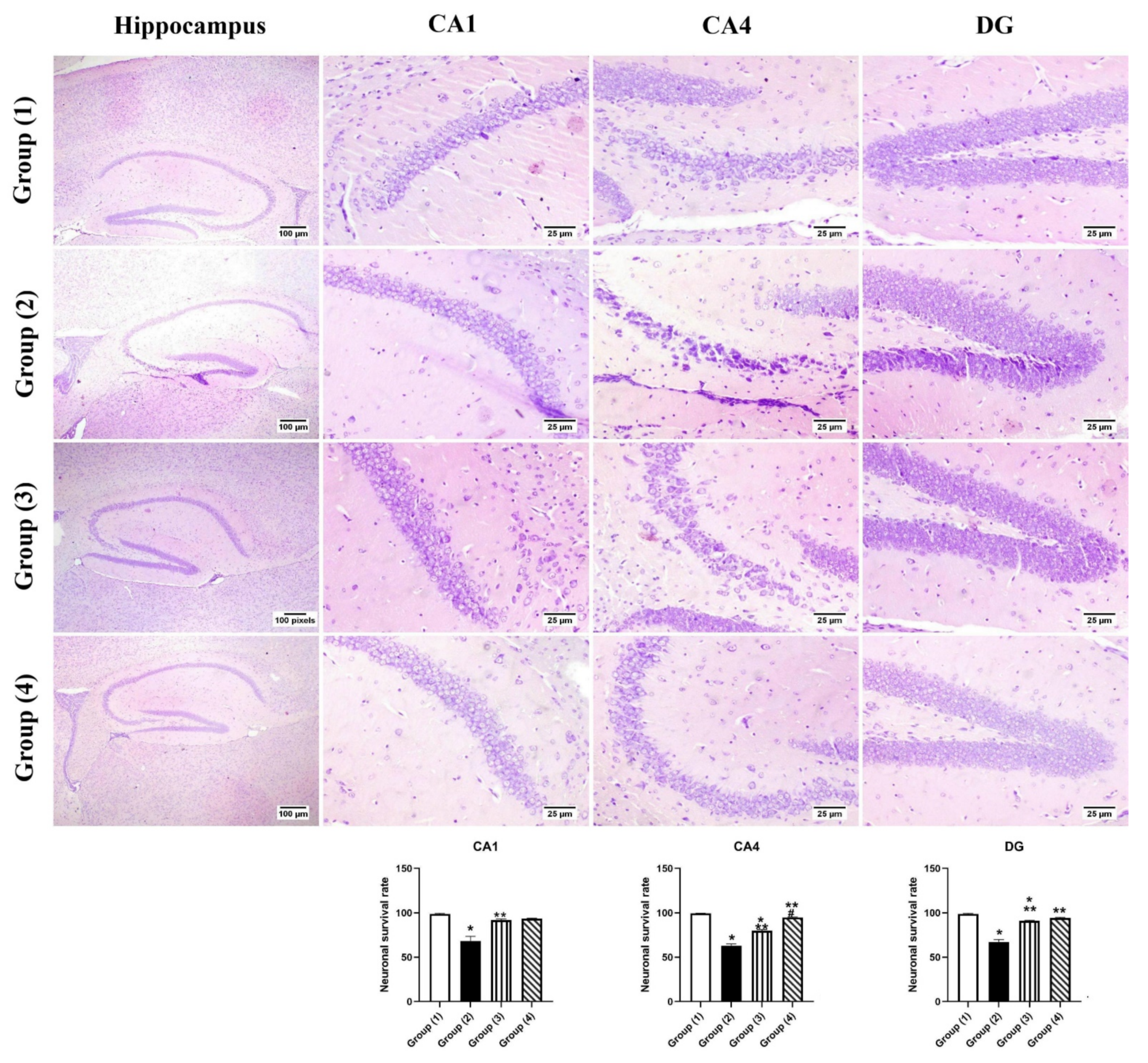
2.3.6. Histopathology
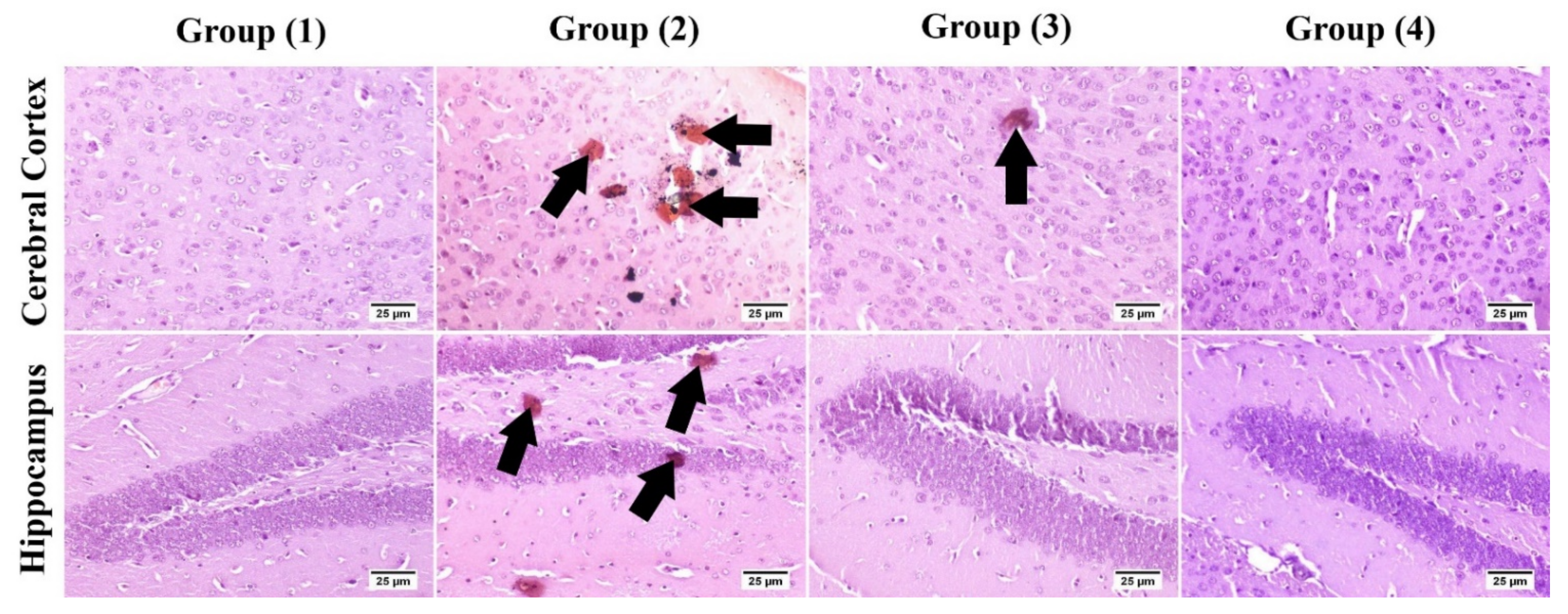
2.3.7. Immunohistochemistry
2.3.8. Measurement of Biochemical Parameters
Estimation of Oxidative Stress Markers (MDA, GSH, and NRF2)
Estimation of Pro-Inflammatory Mediators (NOS, COX-2, NF-Κβ, and TNF-α)
Determination of Mouse MMP-9(Matrix Metalloproteinase 9)
Estimation of Amyloidogenesis and Tauopathy (Aβ1-42 and Tau)
Estimation of The Mouse cAMP Response Element Binding Protein (CREB) Transcription Factors
2.3.9. Safety Studies
2.3.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Chitosomes (CHS)
3.2. Transmission Electron Microscopy (TEM)
3.3. In Vitro Release Study and Release Kinetics
3.4. In Vitro Mucoadhesion Test
3.5. In Vivo Study
3.5.1. Behavioral Test
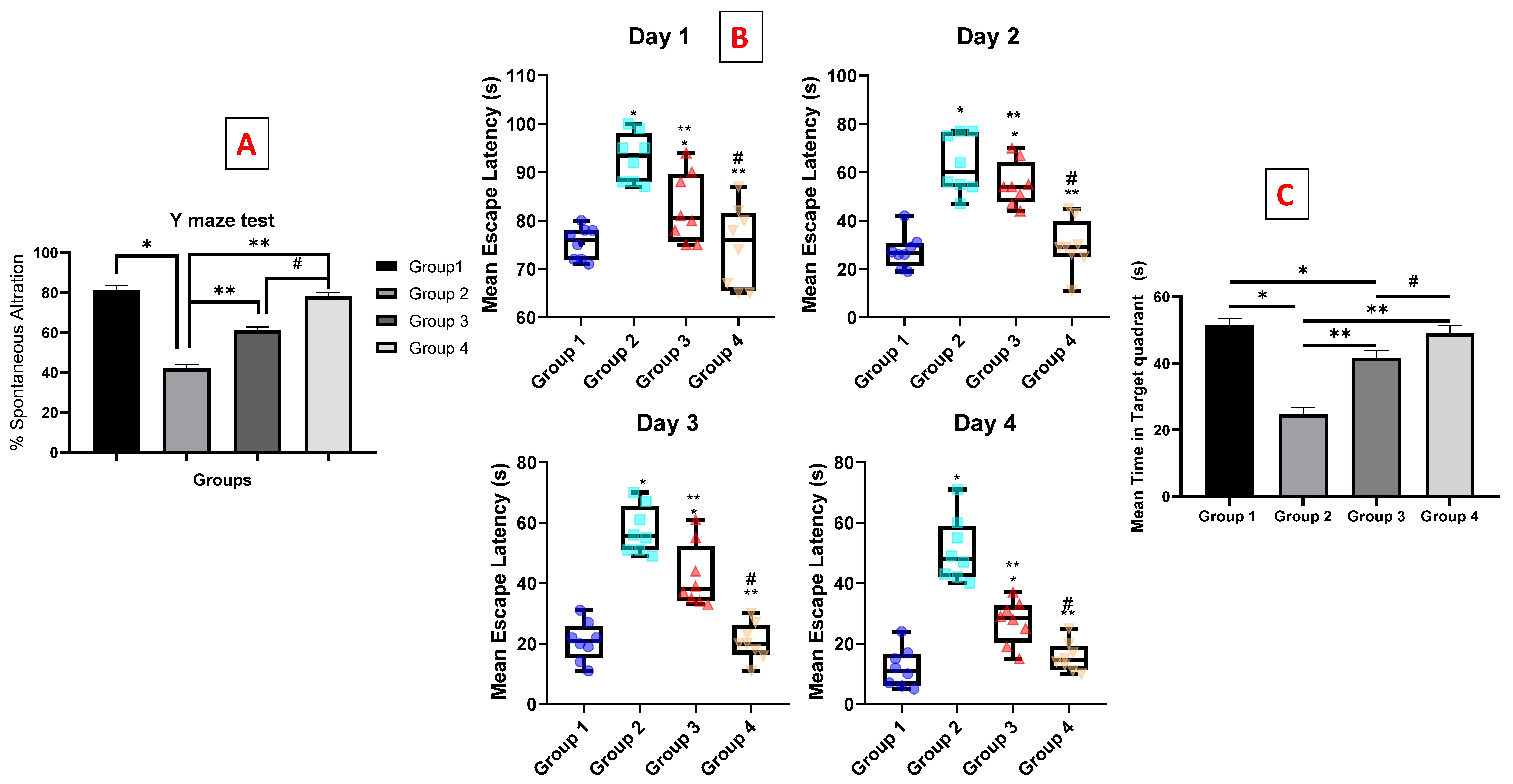
Y-Maze Test
Morris Water Maze (MWM) and Mean Escape Latency (MEL)
Morris Water Maze (MWM) and The Time Spent in The Target Quadrant
3.5.2. Histopathology
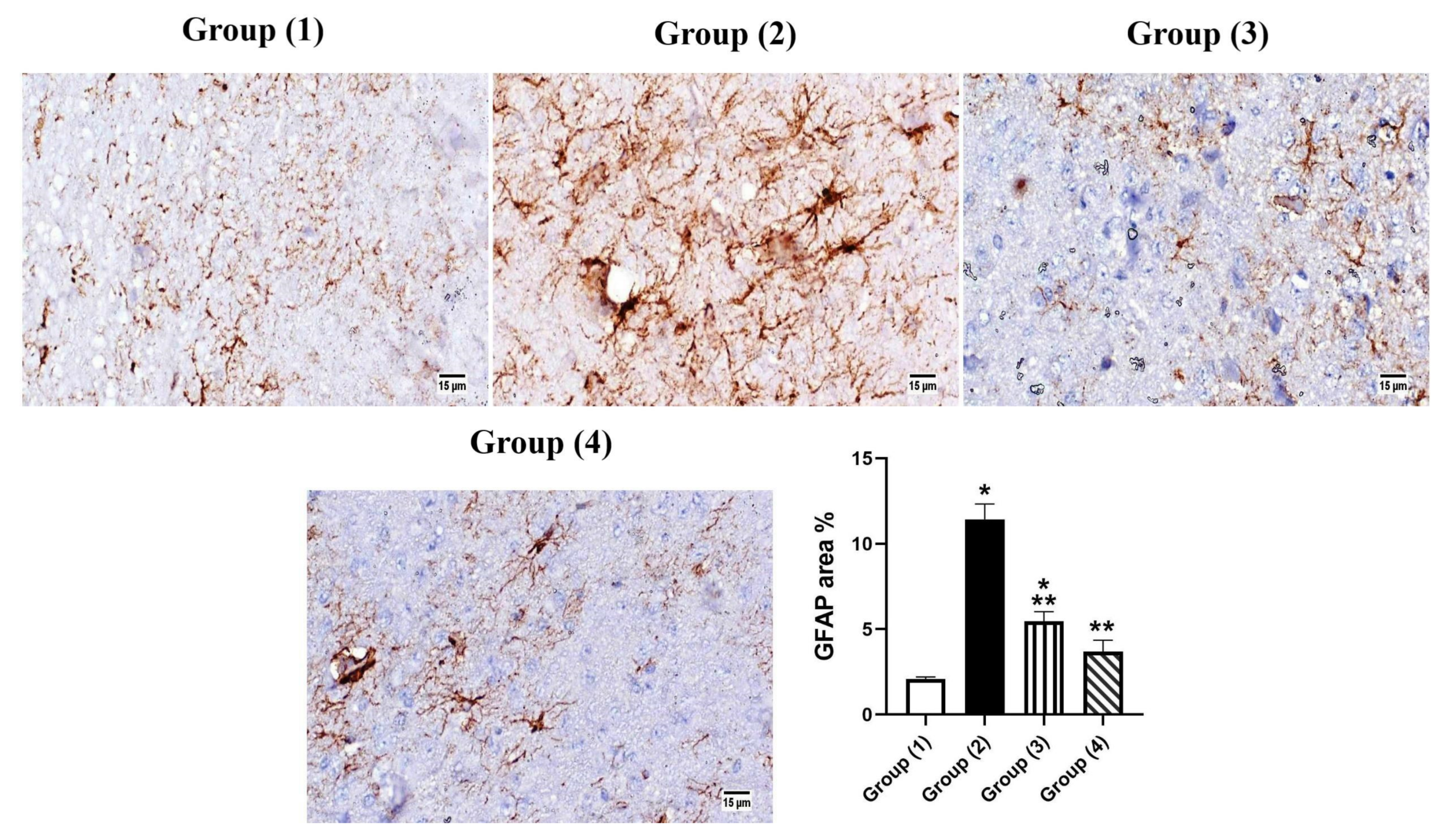
3.5.3. Immunohistochemistry
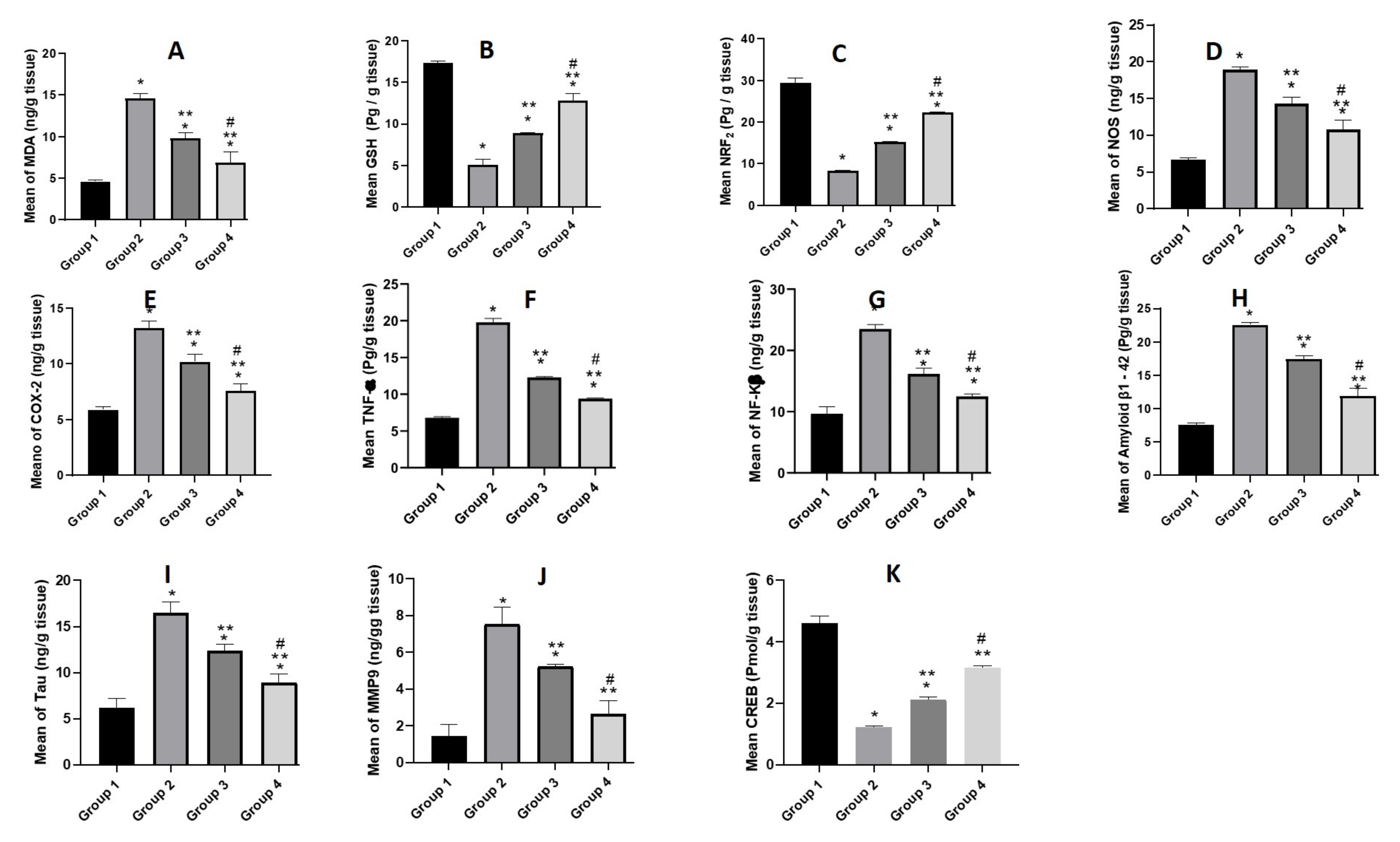
3.5.4. Biochemical Parameters
Estimation of Oxidative Stress Markers (MDA, GSH and NRF2)
Estimation of Pro-Inflammatory Mediators (NOS, COX-2, NF-κβ, and TNF-α)
Estimation of Aβ1-42 and Tau
Estimation of MMP9
Estimation of Transcription Factors (CREB)
3.5.5. Safety Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossini, P.M.; Di Iorio, R.; Vecchio, F.; Anfossi, M.; Babiloni, C.; Bozzali, M.; Bruni, A.C.; Cappa, S.F.; Escudero, J.; Fraga, F.J.; et al. Early diagnosis of Alzheimer’s disease: The role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin. Neurophysiol. 2020, 131, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Xing, J.G.; Wang, L.L.; Jiang, H.L.; Guo, S.L.; Liu, R. Luteolin Inhibits Fibrillary β-Amyloid(1-40)-Induced Inflammation in a Human Blood-Brain Barrier Model by Suppressing the p38 MAPK-Mediated NF-κB Signaling Pathways. Molecules 2017, 22, 334. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; A Elsheikh, M.; Abdallah, O.Y. Phytochylomicron as a dual nanocarrier for liver cancer targeting of luteolin: In vitro appraisal and pharmacodynamics. Nanomedicine 2018, 13, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Hatziagelaki, E. Brain “fog,” inflammation and obesity: Key aspects of neuropsychiatric disorders improved by luteolin. Front. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, F.; Xu, S.; Yun, K.; Wu, W.; Pan, W. Formulation and evaluation of luteolin supersaturatable self-nanoemulsifying drug delivery system (S-SNEDDS) for enhanced oral bioavailability. J. Drug Deliv. Sci. Technol. 2020, 58, 101783. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomed. 2019, 14, 7515–7531. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Xie, Y.; Chen, Y.; Liu, Y.; Yue, P.; Yang, M. Design and Evaluation of Novel Solid Self-Nanodispersion Delivery System for Andrographolide. AAPS PharmSciTech 2016, 18, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food Chem. 2013, 141, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-J.; Gao, X.; Qiu, J.-F.; Wang, B.-L.; Wei, X.-W.; Gou, M.-L.; Liu, X.; Guo, G.; Qian, Z.-Y.; Men, K. Preparation and characterization of monomethoxy poly(ethylene glycol)-poly(ε-caprolactone) micelles for the solubilization and in vivo delivery of luteolin. Int. J. Nanomed. 2013, 8, 3061–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmane, S.; Deshmane, S.; Shelke, S.; Biyani, K. Enhancement of solubility and bioavailability of ambrisentan by solid dispersion using Daucus carota as a drug carrier: Formulation, characterization, in vitro, and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1001–1011. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- El-Zaafarany, G.M.; Soliman, M.E.; Mansour, S.; Awad, G.A.S. Identifying lipidic emulsomes for improved oxcarbazepine brain targeting: In vitro and rat in vivo studies. Int. J. Pharm. 2016, 503, 127–140. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2015, 52, 774–784. [Google Scholar] [CrossRef]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.F.; Elnaggar, Y.S.; Abdallah, O.Y. Elaboration of polymersomes versus conventional liposomes for improving oral bioavailability of the anticancer flutamide. Nanomedicine 2018, 13, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Selvadoss, P.P.; Nellore, J.; Ravindrran, M.B.; Sekar, U.; Tippabathani, J. Enhancement of antimicrobial activity by liposomal oleic acid-loaded antibiotics for the treatment of multidrug-resistant Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 46, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Xu, K.; Xu, Z.; Rivas, M.D.L.; Wang, C.; Li, X.; Lu, J.; Zhou, Y.; Delso, I.; Merino, P.; et al. The small molecule luteolin inhibits N-acetyl-α-galactosaminyltransferases and reduces mucin-type O-glycosylation of amyloid precursor protein. J. Biol. Chem. 2017, 292, 21304–21319. [Google Scholar] [CrossRef] [Green Version]
- Freag, M.S.; Elnaggar, Y.S.; A Abdelmonsif, D.; Abdallah, O.Y. Layer-by-layer-coated lyotropic liquid crystalline nanoparticles for active tumor targeting of rapamycin. Nanomedicine 2016, 11, 2975–2996. [Google Scholar] [CrossRef] [PubMed]
- Alomrani, A.; Badran, M.; Harisa, G.I.; Alshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.A.; Rizk, S.A.; Elnaggar, Y.S.R.; Abdallah, O.Y. Nanoemulsomes for Enhanced Oral Bioavailability of the Anticancer Phytochemical Andrographolide: Characterization and Pharmacokinetics. AAPS PharmSciTech 2021, 22, 246. [Google Scholar] [CrossRef]
- Elsheikh, M.; Elnaggar, Y.S.; Hamdy, D.A.; Abdallah, O.Y. Novel cremochylomicrons for improved oral bioavailability of the antineoplastic phytomedicine berberine chloride: Optimization and pharmacokinetics. Int. J. Pharm. 2018, 535, 316–324. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Elnaggar, Y.S.R.; Otify, D.Y.; Abdallah, O.Y. Bioactive-Chylomicrons for Oral Lymphatic Targeting of Berberine Chloride: Novel Flow-Blockage Assay in Tissue-Based and Caco-2 Cell Line Models. Pharm. Res. 2018, 35, 18. [Google Scholar] [CrossRef]
- Wilson, B.; Alobaid, B.N.M.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2020, 61, 102176. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic iron oxide loaded chitosan coated bilosomes for magnetic nose to brain targeting of resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, T.; Jain, A.; Kaur, H.; Katare, O.; Singh, B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids Surfaces B Biointerfaces 2021, 205, 111838. [Google Scholar] [CrossRef]
- Ztürk, A.A.; Kıyan, H.T. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef]
- Delan, W.K.; Zakaria, M.; Elsaadany, B.; ElMeshad, A.N.; Mamdouh, W.; Fares, A.R. Formulation of simvastatin chitosan nanoparticles for controlled delivery in bone regeneration: Optimization using Box-Behnken design, stability and in vivo study. Int. J. Pharm. 2020, 577, 119038. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Zayed, G.; Elshaier, Y.; Alsharif, F.M. Chitosan-Gelatin Hydrogel Crosslinked With Oxidized Sucrose for the Ocular Delivery of Timolol Maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- El-Sahar, A.E.; Shiha, N.A.; El Sayed, N.S.; Ahmed, L.A. Alogliptin Attenuates Lipopolysaccharide-Induced Neuroinflammation in Mice Through Modulation of TLR4/MYD88/NF-κB and miRNA-155/SOCS-1 Signaling Pathways. Int. J. Neuropsychopharmacol. 2021, 24, 158–169. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2013, 21, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Elkarray, S.M.; Farid, R.M.; Abd-Alhaseeb, M.M.; Omran, G.A.; Habib, D.A. Intranasal repaglinide-solid lipid nanoparticles integrated in situ gel outperform conventional oral route in hypoglycemic activity. J. Drug Deliv. Sci. Technol. 2022, 68, 103086. [Google Scholar] [CrossRef]
- Sorial, M.E.; Sayed, N.S.E.D.E. Protective effect of valproic acid in streptozotocin-induced sporadic Alzheimer’s disease mouse model: Possible involvement of the cholinergic system. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2017, 390, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.; Wojcik-Cwikla, J.; Andres-Mach, M.; Czuczwar, S.J. Pharmacological and Behavioral Characteristics of Interactions between Vigabatrin and Conventional Antiepileptic Drugs in Pentylenetetrazole-Induced Seizures in Mice: An Isobolographic Analysis. Neuropsychopharmacology 2004, 30, 958–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Hooge, R.; de Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, L.K. Improvement in long term and visuo-spatial memory following chronic pioglitazone in mouse model of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 102, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, B.; Jaggi, A.S.; Singh, N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: Possible involvement of PPAR-γ agonistic property. J. Renin-Angiotensin-Aldosterone Syst. 2012, 14, 124–136. [Google Scholar] [CrossRef]
- Blokland, A.; Geraerts, E.; Been, M. A detailed analysis of rats’ spatial memory in a probe trial of a Morris task. Behav. Brain Res. 2004, 154, 71–75. [Google Scholar] [CrossRef]
- Gamble, M. 9—The Hematoxylins and Eosin, in Theory and Practice of Histological Techniques, Sixth Edition; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone: Edinburgh, UK, 2008; pp. 121–134. [Google Scholar]
- A Pelleymounter, M.; Joppa, M.; Carmouche, M.; Cullen, M.J.; Brown, B.; Murphy, B.; E Grigoriadis, D.; Ling, N.; Foster, A.C. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J. Pharmacol. Exp. Ther. 2000, 293, 799–806. [Google Scholar]
- Pelleymounter, M.A.; Joppa, M.; Ling, N.; Foster, A.C. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J. Pharmacol. Exp. Ther. 2002, 302, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Warnock, G.I. Study of the Central Corticotrophin-Releasing Factor System Using the 2-Deoxyglucose Method for Measurement of Local Cerebral Glucose Utilisation; University of Bath: Bath, UK, 2007. [Google Scholar]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Hindam, M.O.; Sayed, R.; Skalicka-Woźniak, K.; Budzyńska, B.; ELSayed, N. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a streptozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytother Res. 2020, 34, 2351–2365. [Google Scholar] [CrossRef]
- El Sayed, N.S.; Ghoneum, M.H. Antia, a Natural Antioxidant Product, Attenuates Cognitive Dysfunction in Streptozotocin-Induced Mouse Model of Sporadic Alzheimer’s Disease by Targeting the Amyloidogenic, Inflammatory, Autophagy, and Oxidative Stress Pathways. Oxid. Med. Cell. Longev. 2020, 2020, 4386562. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Valero, J. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev. Neurosci. 2011, 22, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N. Superparamagnetic Iron Oxide–Loaded Lipid Nanocarriers Incorporated in Thermosensitive In Situ Gel for Magnetic Brain Targeting of Clonazepam. J. Pharm. Sci. 2018, 107, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.V.; Tschulik, K.; Batchelor-McAuley, C.; Jurkschat, K.; Compton, R.G. Reversible or Not? Distinguishing Agglomeration and Aggregation at the Nanoscale. Anal. Chem. 2015, 87, 10033–10039. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.; Baalousha, M.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K. Characterizing Manufactured Nanoparticles in the Environment: Multimethod Determination of Particle Sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Sharma, S.; Lohan, S.; Murthy, R.S.R. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev. Ind. Pharm. 2013, 40, 869–878. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Al-Sanea, M.M.; Hendawy, O.M.; Salama, A.; Ibrahim, M.F.; Ghoneim, M.M. Influence of Stabilizer on the Development of Luteolin Nanosuspension for Cutaneous Delivery: An In Vitro and In Vivo Evaluation. Pharmaceutics 2021, 13, 1812. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhao, Y.; He, M.; Zhang, X.; Niu, M.; Feng, N. Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int. J. Pharm. 2014, 476, 169–177. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Ha, J.H.; Park, S.N. Cytoprotective effects against UVA and physical properties of luteolin-loaded cationic solid lipid nanoparticle. J. Ind. Eng. Chem. 2016, 35, 54–62. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Chen, Y.; He, W.; Ni, L.; Xiong, P.; Wei, M. Vitamin E TPGS modified liposomes enhance cellular uptake and targeted delivery of luteolin: An in vivo/in vitro evaluation. Int. J. Pharm. 2016, 512, 262–272. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Turanlı, Y.; Tort, S.; Acartürk, F. Development and characterization of methylprednisolone loaded delayed release nanofibers. J. Drug Deliv. Sci. Technol. 2018, 49, 58–65. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2020, 167, 1176–1197. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Singla, A.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Zameer, S.; Ali, J.; Vohora, D.; Najmi, A.K.; Akhtar, M. Development, optimisation and evaluation of chitosan nanoparticles of alendronate against Alzheimer’s disease in intracerebroventricular streptozotocin model for brain delivery. J. Drug Target. 2020, 29, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Pahuja, M.; Gupta, Y.K. Streptozotocin-Induced Sporadic Alzheimer’s Disease: Selection of Appropriate Dose. J. Alzheimer’s Dis. 2013, 33, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Lim, H.-S.; Kim, Y.J.; Kim, B.-Y.; Jeong, S.-J. Annona atemoya Leaf Extract Improves Scopolamine-Induced Memory Impairment by Preventing Hippocampal Cholinergic Dysfunction and Neuronal Cell Death. Int. J. Mol. Sci. 2019, 20, 3538. [Google Scholar] [CrossRef] [Green Version]
- Fronza, M.G.; Baldinotti, R.; Martins, M.C.; Goldani, B.; Dalberto, B.T.; Kremer, F.S.; Begnini, K.; Pinto, L.D.S.; Lenardão, E.J.; Seixas, F.K.; et al. Rational design, cognition and neuropathology evaluation of QTC-4-MeOBnE in a streptozotocin-induced mouse model of sporadic Alzheimer’s disease. Sci. Rep. 2019, 9, 7276. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Choi, J.H.; Kim, W.; Nam, S.M.; Jung, H.Y.; Kim, J.H.; Won, M.-H.; Yoon, Y.S.; Hwang, I.K. Effects of luteolin on spatial memory, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia model. Neurol. Res. 2013, 35, 813–820. [Google Scholar] [CrossRef]
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules 2020, 25, 4866. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris Water Maze Test for Learning and Memory Deficits in Alzheimer’s Disease Model Mice. J. Vis. Exp. 2011, e2920. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Dilger, R.N.; Johnson, R.W. Luteolin Inhibits Microglia and Alters Hippocampal-Dependent Spatial Working Memory in Aged Mice. J. Nutr. 2010, 140, 1892–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, T.V.; Luksys, G.; Sandi, C.; Vasilaki, E. Detailed classification of swimming paths in the Morris Water Maze: Multiple strategies within one trial. Sci. Rep. 2015, 5, 14562. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.K.; Brayer, S.W.; Hurwitz, M.; Niyonkuru, C.; Zou, H.; Failla, M.; Arenth, P.; Manole, M.D.; Skidmore, E.; Thiels, E. Non-spatial pre-training in the water maze as a clinically relevant model for evaluating learning and memory in experimental TBI. Neurobiol. Learn. Mem. 2013, 106, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Gupta, Y. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002, 71, 2489–2498. [Google Scholar] [CrossRef]
- Khalil, H.M.; Salama, H.H.; Al-Mokaddem, A.K.; Aljuaydi, S.H.; Edris, A.E. Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer’s disease in rats. J. Funct. Foods 2020, 75, 104296. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Bona, N.P.; Soares, M.S.P.; Teixeira, F.C.; Rahmeier, F.L.; Carvalho, F.B.; da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales , R.A.; et al. Tannic Acid Ameliorates STZ-Induced Alzheimer’s Disease-Like Impairment of Memory, Neuroinflammation, Neuronal Death and Modulates Akt Expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef]
- Cevik, B.; Solmaz, V.; Yigitturk, G.; Cavusoğlu, T.; Peker, G.; Erbas, O. Neuroprotective effects of erythropoietin on Alzheimer’s dementia model in rats. Adv. Clin. Exp. Med. 2017, 26, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Fomeshi, M.K.; Azizi, M.G.; Esmaeili, M.R.; Gol, M.; Kazemi, S.; Ashrafpour, M.; Moghadamnia, A.A.; Hosseinzadeh, S. Piperine restores streptozotocin-induced cognitive impairments: Insights into oxidative balance in cerebrospinal fluid and hippocampus. Behav. Brain Res. 2017, 337, 131–138. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Kenawy, S.; Hegazy, R.; Hassan, A.; El-Shenawy, S.; Gomaa, N.; Zaki, H.; Attia, A. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: Protective role of metformin and saxagliptin. PLoS ONE 2017, 12, e0183565. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Lin, S.; Wu XI, N.; Esterman, M.; Koger, D.; Hanson, J.; Paul, S.M. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-S.; Yang, W.-N.; Jin, H.; Ma, K.-G.; Feng, G.-F. Puerarin attenuates learning and memory impairments and inhibits oxidative stress in STZ-induced SAD mice. NeuroToxicology 2015, 51, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Moosavi, F.; Hosseini, R.; Saso, L. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liang, Z.; Blanchard, J.; Dai, C.L.; Sun, S.; Lee, M.H.; Grundke-Iqbal, I.; Iqbal, K.; Liu, F.; Gong, C.X. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: Similarities to and differences from the transgenic model (3xTg-AD mouse). Mol. Neurobiol. 2013, 47, 711–725. [Google Scholar] [CrossRef]
- Theoharides, T.; Asadi, S.; Panagiotidou, S. A Case Series of a Luteolin Formulation (Neuroprotek®) in Children with Autism Spectrum Disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Manczak, M.; Yin, X.; Wang, R.; Reddy, P.H. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2018, 27, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2015, 53, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Adeli, S.; Zahmatkesh, M.; Dezfouli, M.A. Simvastatin Attenuates Hippocampal MMP-9 Expression in the Streptozotocin-Induced Cognitive Impairment. Iran. Biomed. J. 2019, 23, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Siddique, Y.H. Bioavailability and Pharmaco-therapeutic Potential of Luteolin in Overcoming Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, L.; Yang, C.; Li, Z.; Rong, S. Procyanidins and Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.-L.; Yu, S.-J.; Huang, W.-C.; Yen, H.-R. Luteolin Attenuates Allergic Nasal Inflammation via Inhibition of Interleukin-4 in an Allergic Rhinitis Mouse Model and Peripheral Blood From Human Subjects With Allergic Rhinitis. Front. Pharmacol. 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, B.; Davis, T. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%w/v) | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Phosphatidyl choline | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Cholesterol | 25 | 25 | 25 | 25 | 25 | 25 |
| Phosphatidyl serine | 3 | 3 | 3 | 3 | 3 | 3 |
| Luteolin | ----- | 20 a | 40 b | 20 | 20 | 20 |
| Chitosan | ----- | ----- | ----- | 2 | 4 | 8 |
| Formulation Code | Particle Size (nm) ± SD | PDI± SD | Zeta Potential (mV) ± SD | Entrapment Efficiency% ± SD |
|---|---|---|---|---|
| F1 (Empty Lip.) | 184.6 ± 1.85 | 0.3014 ± 0.06 | −24.9 ± 4.21 | NA |
| F2 (Lip-LUT20 a) | 212.3 ± 2.18 | 0.398 ± 0.09 | −33.1 ± 2.11 | 80.6 ± 1.28 |
| F3 (Lip-LUT40 b) | 320.0 ± 4.23 | 0.407 ± 0.25 | −31.6 ± 1.48 | 77.9 ± 1.33 |
| F4 (LUT-CHS2% c) | 347.2 ± 1.84 | 0.419 ± 0.04 | 28 ± 2.94 | 79.2 ± 2.54 |
| F5 (LUT-CHS4% d) | 412.8 ± 3.28 | 0.378 ± 0.07 | 37.4 ± 2.13 | 86.6 ± 2.05 |
| F6 (LUT-CHS8% e) | 473.1 ± 2.14 | 0.274 ± 0.03 | 36.2 ± 3.04 | 81.5 ± 1.77 |
| Months | Size (nm) | PDI | Zeta (mv) | EE (%) |
|---|---|---|---|---|
| 0 | 412.8 ± 3.28 | 0.378 ± 0.07 | 37.4 ± 2.13 | 86.6 ± 2.05 |
| 3 | 426.1 ± 3.01 | 0.400 ± 0.11 | 35.8 ± 3.17 | 85.1 ± 3.01 |
| 6 | 435.3 ± 2.81 | 0.415 ± 0.56 | 34.3 ± 3.04 | 82.8 ± 1.15 |
| Model Name and Equation | Parameters | Values |
|---|---|---|
| Higuchi * F = kH √t | Rsqr_adj | 0.909 |
| MSE | 94.59 | |
| kH | 21.38 | |
| MSC | 1.86 | |
| Hixson-Crowell * | Rsqr_adj | 0.975 |
| MSE | 27.49 | |
| k1/3 | 0.043 | |
| MSC | 3.09 | |
| Korsmeyer-Peppas * | Rsqr_adj | 0.900 |
| MSE | 103.7 | |
| n | 0.462 | |
| kp | 23.43 | |
| MSC | 1.68 | |
| Weibull * ) | Rsqr_adj | 0.992 |
| MSE | 16.49 | |
| 0.900 | ||
| MSC | 3.478 |
| Number of Amyloid Plaques (High Microscopic Field) | |
|---|---|
| Group (1) | ــــــــــــــــــــــ |
| Group (2) | 6.2 ± 0.41 c |
| Group (3) | 2.6 ± 0.4 b |
| Group (4) | 1.4 ± 0.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, H.; Sayed, N.S.E.; Youssef, N.A.H.A.; M. E. Gaafar, P.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. https://doi.org/10.3390/pharmaceutics14051003
Abbas H, Sayed NSE, Youssef NAHA, M. E. Gaafar P, Mousa MR, Fayez AM, Elsheikh MA. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics. 2022; 14(5):1003. https://doi.org/10.3390/pharmaceutics14051003
Chicago/Turabian StyleAbbas, Haidy, Nesrine S El Sayed, Nancy Abdel Hamid Abou Youssef, Passent M. E. Gaafar, Mohamed R. Mousa, Ahmed M. Fayez, and Manal A Elsheikh. 2022. "Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways" Pharmaceutics 14, no. 5: 1003. https://doi.org/10.3390/pharmaceutics14051003
APA StyleAbbas, H., Sayed, N. S. E., Youssef, N. A. H. A., M. E. Gaafar, P., Mousa, M. R., Fayez, A. M., & Elsheikh, M. A. (2022). Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics, 14(5), 1003. https://doi.org/10.3390/pharmaceutics14051003







