Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Line
2.2. Preparation of Liposome-Encapsulated β-Sitosterol (LS)
2.3. Analysis of β-Sitosterol Concentration Using High-Performance Liquid Chromatography
2.4. Cell Viability Assay
2.5. Invasion Assay
2.6. Western Blotting
2.7. Establishment of Lung Metastatic Mouse Model
2.8. Assays of IL-12, IL-18, and IFN-γ in Small Intestines of Mice
2.9. Determination of T Cell Subsets by Flow Cytometry
2.10. In Vivo and Ex Vivo Bioluminescent Imaging (BLI)
2.11. Histopathological Examination
2.12. Statistical Analysis
3. Results
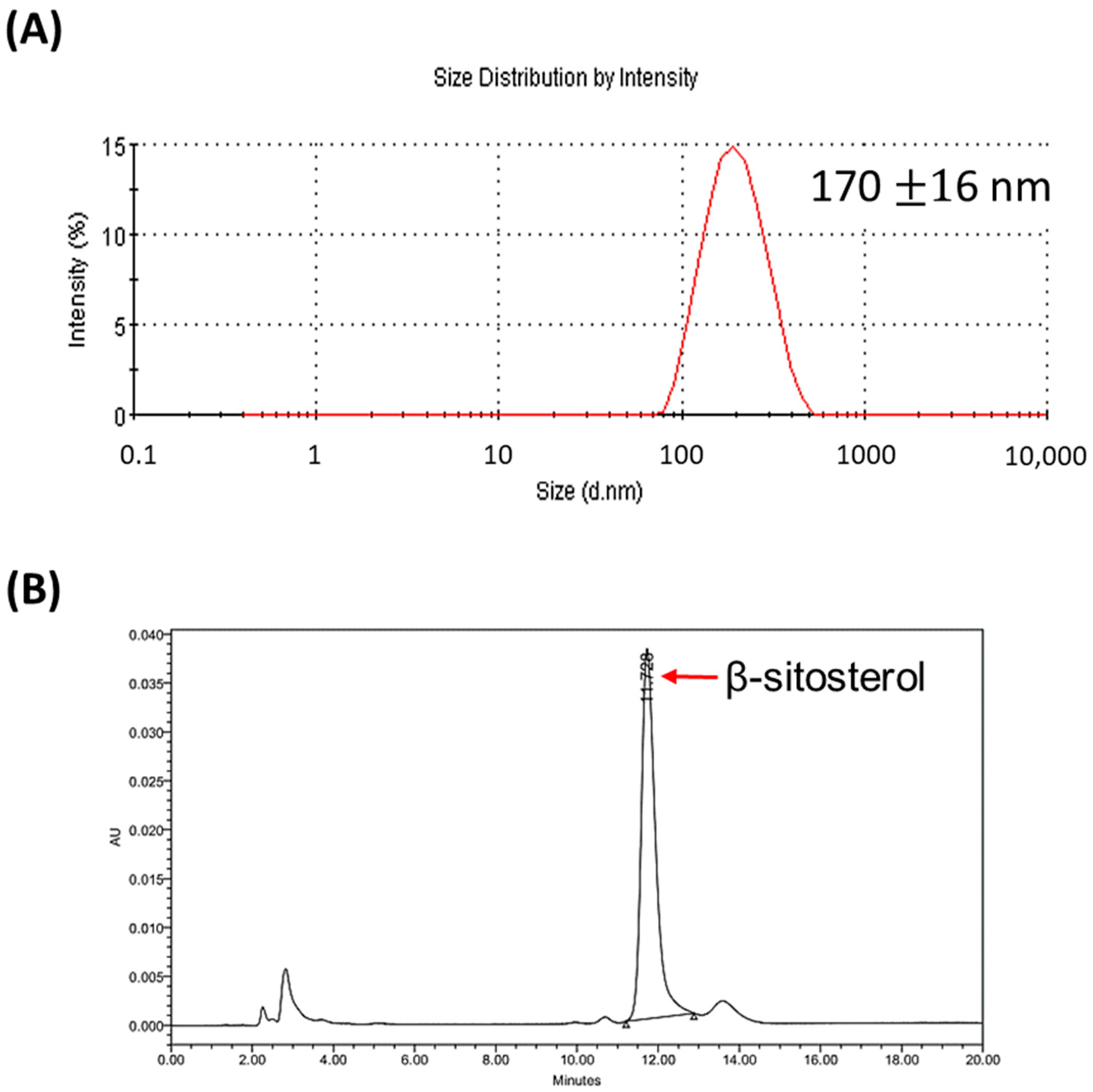
3.1. Characterization of Liposomal β-Sitosterol
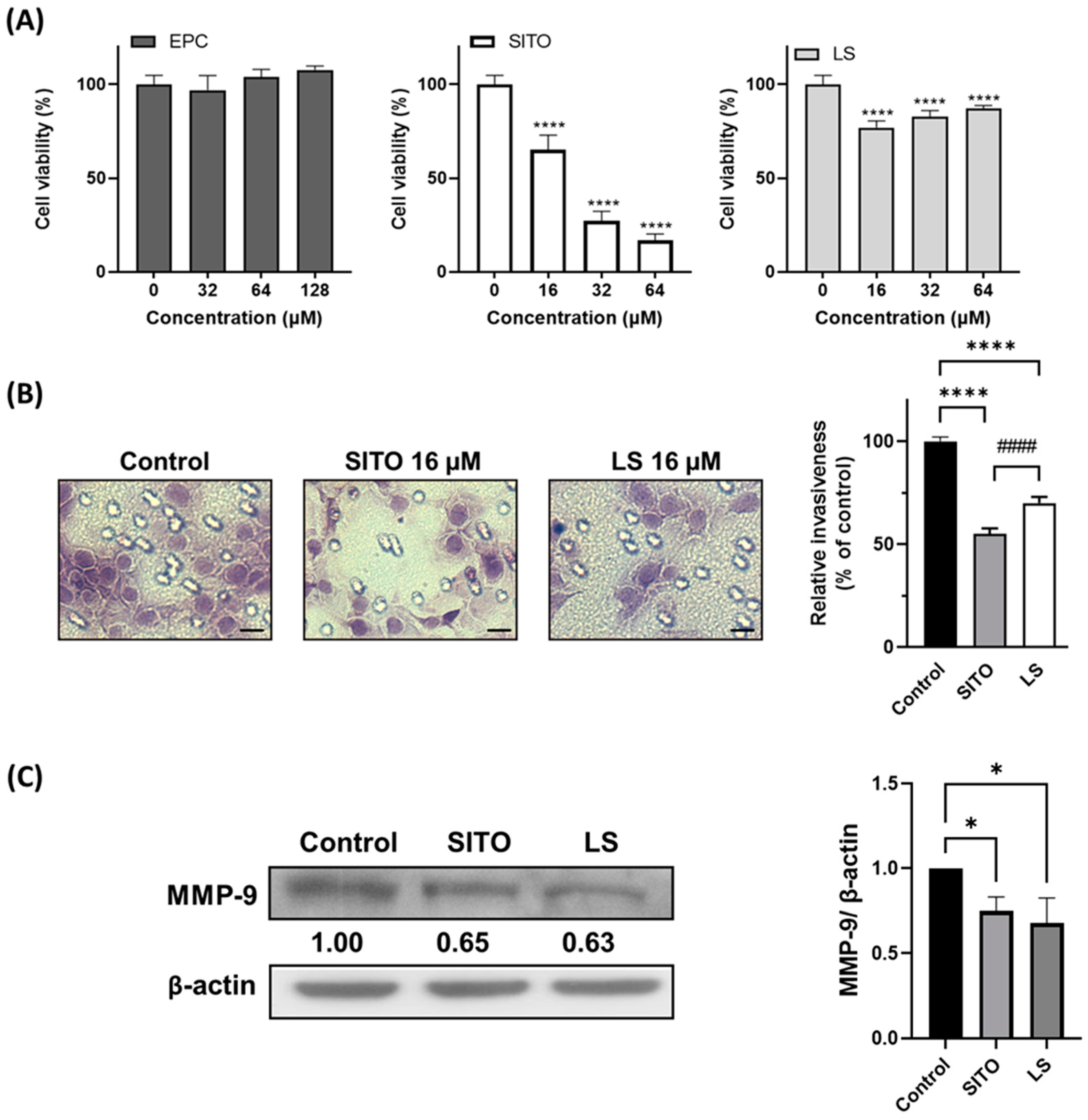
3.2. β-Sitosterol Suppresses Cell Growth and Invasion of CT26/luc Cells
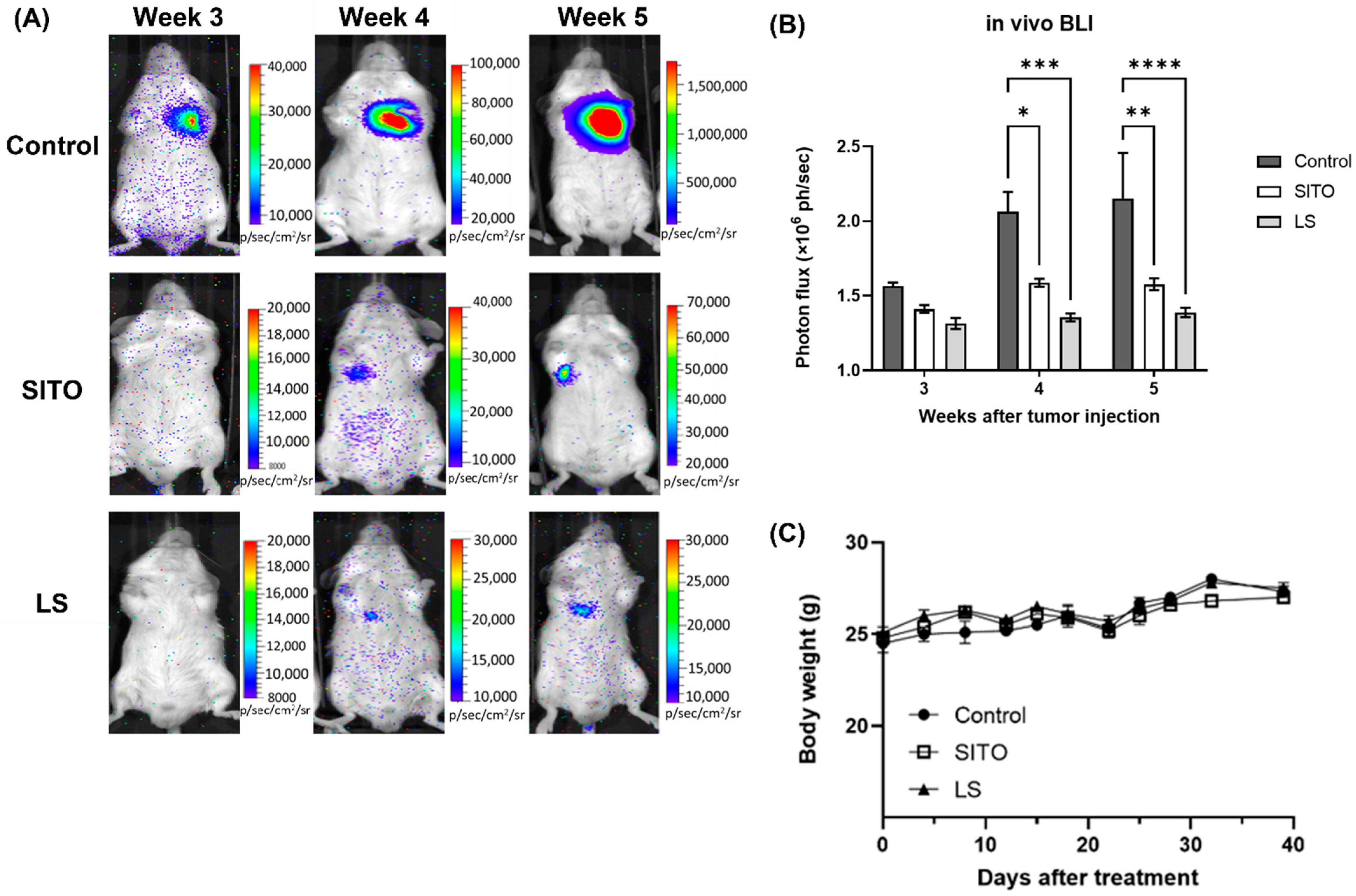
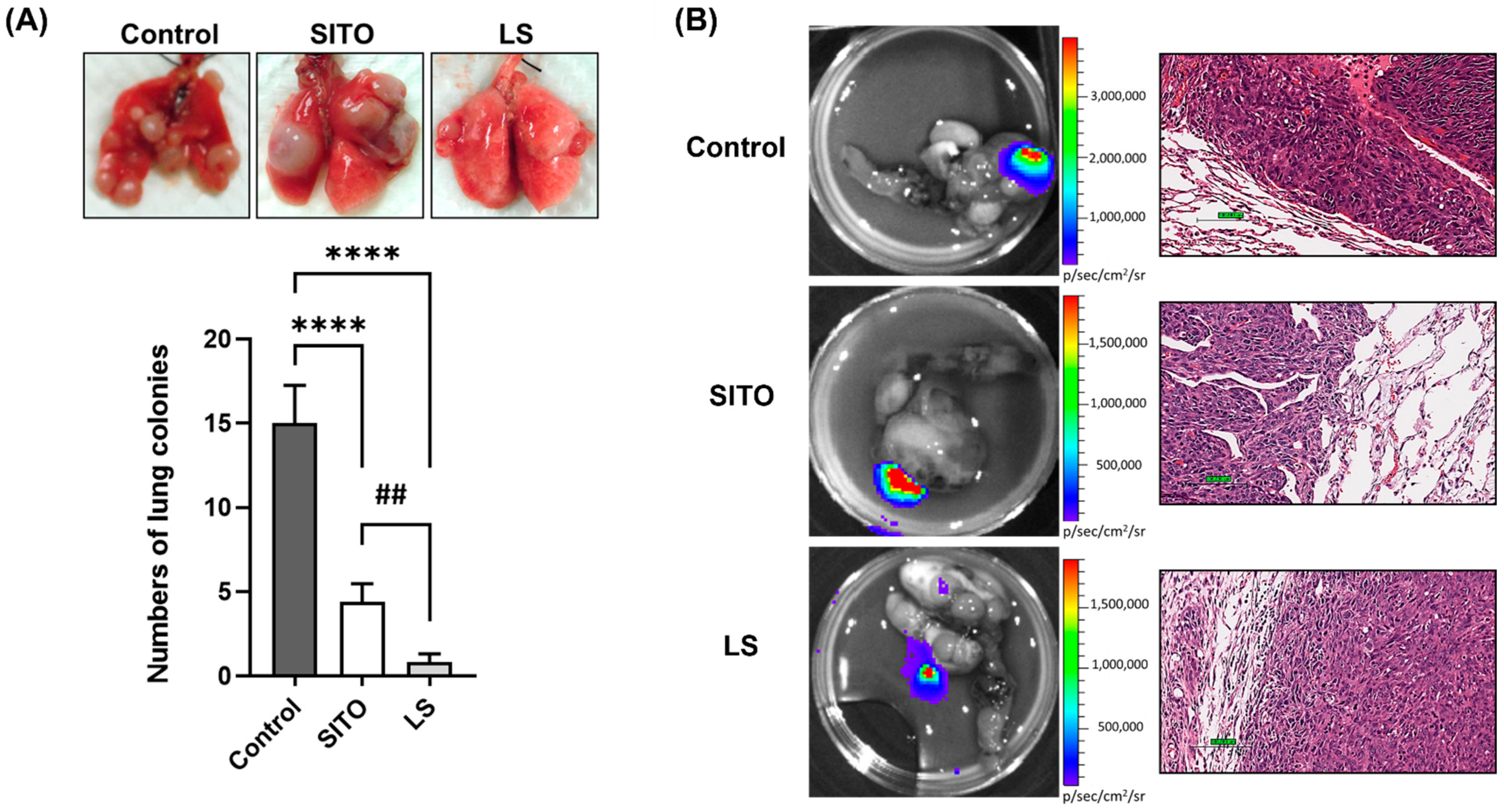
3.3. Liposomal β-Sitosterol Suppresses Tumor Progression In Vivo
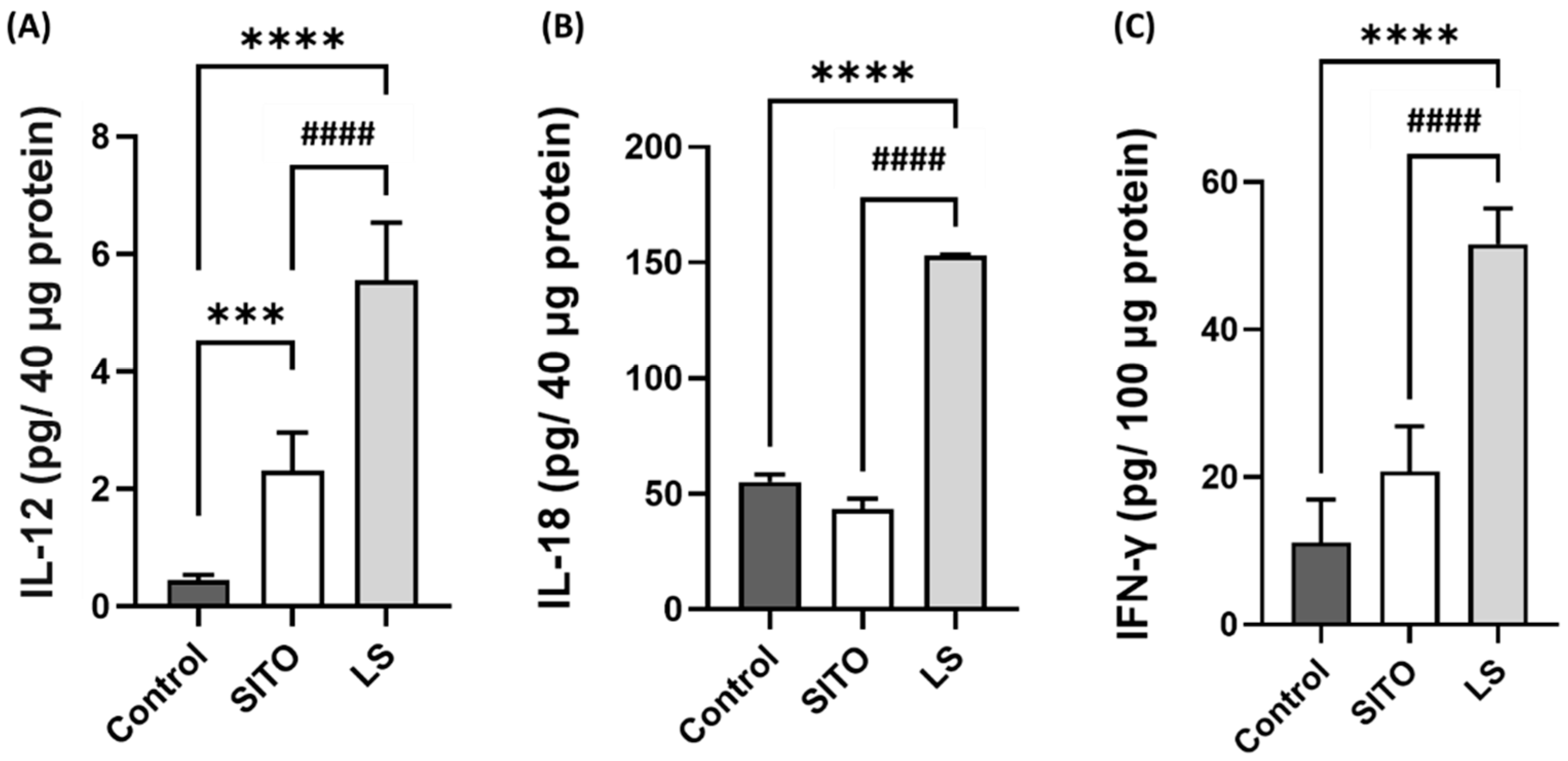
3.4. Liposomal β-Sitosterol Treatment Elevates the Expressions of IL-12, IL-18, and IFN-γ in the Small Intestine and Increases the CD4+/CD8+ T Cell Subsets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fakih, M.G. Metastatic Colorectal Cancer: Current State and Future Directions. J. Clin. Oncol. 2015, 33, 1809–1824. [Google Scholar] [CrossRef]
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Rao Pariti, R.K.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014, 35, 747–759. [Google Scholar] [CrossRef]
- Kaihara, T.; Kusaka, T.; Nishi, M.; Kawamata, H.; Imura, J.; Kitajima, K.; Itoh-Minami, R.; Aoyama, N.; Kasuga, M.; Oda, Y.; et al. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J. Exp. Clin. Cancer Res. 2003, 22, 117–123. [Google Scholar] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. Biomed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Cheng, M.; Wei, G.; Du, Y.; Fan, Y.; Li, J.; Li, H.; Deng, Z. Serum Cholesterol-Lowering Activity of β-Sitosterol Laurate Is Attributed to the Reduction of Both Cholesterol Absorption and Bile Acids Reabsorption in Hamsters. J. Agric. Food Chem. 2020, 68, 10003–10014. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, T.; Packiyaraj, P.; Suryanarayanan, V.; Singh, S.K.; Ruckmani, K.; Pandima Devi, K. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci. Rep. 2018, 8, 2071. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Attanzio, A.; Tesoriere, L.; Garcia-Llatas, G.; Barberá, R.; Cilla, A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. Int. J. Food Sci. Nutr. 2019, 70, 323–334. [Google Scholar] [CrossRef]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-Hepatocellular-Cancer Activity Exerted by β-Sitosterol and β-Sitosterol-Glucoside from Indigofera zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef]
- Lee, I.A.; Kim, E.J.; Kim, D.H. Inhibitory effect of β-sitosterol on TNBS-induced colitis in mice. Planta Med. 2012, 78, 896–898. [Google Scholar] [CrossRef]
- Kasirzadeh, S.; Ghahremani, M.H.; Setayesh, N.; Jeivad, F.; Shadboorestan, A.; Taheri, A.; Beh-Pajooh, A.; Azadkhah Shalmani, A.; Ebadollahi-Natanzi, A.; Khan, A.; et al. β-Sitosterol Alters the Inflammatory Response in CLP Rat Model of Sepsis by Modulation of NFκB Signaling. Biomed. Res. Int. 2021, 2021, 5535562. [Google Scholar] [CrossRef]
- Zhou, B.X.; Li, J.; Liang, X.L.; Pan, X.P.; Hao, Y.B.; Xie, P.F.; Jiang, H.M.; Yang, Z.F.; Zhong, N.S. β-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling. Acta Pharmacol. Sin. 2020, 41, 1178–1196. [Google Scholar] [CrossRef]
- Bouic, P.J.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; Van Jaarsveld, P.P. beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Alvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, J.; Hernández, N.; Flores-Mondragón, G.; Velasco, O. Cell protection induced by beta-sitosterol: Inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch. Toxicol. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Boubaker, J.; Ben Toumia, I.; Sassi, A.; Bzouich-Mokded, I.; Ghoul Mazgar, S.; Sioud, F.; Bedoui, A.; Safta Skhiri, S.; Ghedira, K.; Chekir-Ghedira, L. Antitumoral Potency by Immunomodulation of Chloroform Extract from Leaves of Nitraria retusa, Tunisian Medicinal Plant, via its Major Compounds β-sitosterol and Palmitic Acid in BALB/c Mice Bearing Induced Tumor. Nutr. Cancer 2018, 70, 650–662. [Google Scholar] [CrossRef]
- Ling, W.H.; Jones, P.J. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-S.; Tsai, Y.-L.; Hsu, K.-C. The feasibility of antihypertensive oligopeptides encapsulated in liposomes prepared with phytosterols-β-sitosterol or stigmasterol. Food Res. Int. 2010, 43, 133–139. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Lee, S.C. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.K.; Chen, H.W.; Chiang, I.T.; Chen, C.C.; Liao, J.F.; Su, T.P.; Tung, C.Y.; Uchitomi, Y.; Hwang, J.J. Mirtazapine inhibits tumor growth via immune response and serotonergic system. PLoS ONE 2012, 7, e38886. [Google Scholar] [CrossRef]
- Rouser, G.; Fkeischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Ma, L.; Ostlund, R.E., Jr. Natural Dietary Phytosterols. J. AOAC Int. 2015, 98, 679–684. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Brennan, P.; Boffetta, P.; Ronco, A.L.; Mendilaharsu, M.; Deneo-Pellegrini, H. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: A case-control study in Uruguay. Nutr. Cancer 2000, 38, 23–29. [Google Scholar] [CrossRef]
- McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Graham, S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J. Nutr. 2003, 133, 1937–1942. [Google Scholar] [CrossRef]
- Huang, J.; Xu, M.; Fang, Y.J.; Lu, M.S.; Pan, Z.Z.; Huang, W.Q.; Chen, Y.M.; Zhang, C.X. Association between phytosterol intake and colorectal cancer risk: A case-control study. Br. J. Nutr. 2017, 117, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ovesná, Z.; Vachálková, A.; Horváthová, K. Taraxasterol and beta-sitosterol: New naturally compounds with chemoprotective/chemopreventive effects. Neoplasma 2004, 51, 407–414. [Google Scholar]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The Story of Beta-sitosterol-A Review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of phytochemicals by liposome cargos: Recent progress, challenges and opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Stuart, D.D.; Allen, T.M. A new liposomal formulation for antisense oligodeoxynucleotides with small size, high incorporation efficiency and good stability. Biochim. Biophys. Acta 2000, 1463, 219–229. [Google Scholar] [CrossRef][Green Version]
- Lavelle, E.C.; Sharif, S.; Thomas, N.W.; Holland, J.; Davis, S.S. The importance of gastrointestinal uptake of particles in the design of oral delivery systems. Adv. Drug Deliv. Rev. 1995, 18, 5–22. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Yang, T.; Cui, F.D.; Choi, M.K.; Cho, J.W.; Chung, S.J.; Shim, C.K.; Kim, D.D. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 338, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, J.; Tang, X.; Hu, Y. The Feasibility of Novel Liposome Consisted of Sphingomyelin and β-sitosterol for Gypenosides Delivery. Food Sci. Technol. Res. 2014, 20, 509–516. [Google Scholar] [CrossRef][Green Version]
- Tai, K.; Rappolt, M.; He, X.; Wei, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 2019, 293, 92–102. [Google Scholar] [CrossRef]
- Wang, Z.; Zhan, Y.; Xu, J.; Wang, Y.; Sun, M.; Chen, J.; Liang, T.; Wu, L.; Xu, K. β-Sitosterol Reverses Multidrug Resistance via BCRP Suppression by Inhibiting the p53-MDM2 Interaction in Colorectal Cancer. J. Agric. Food Chem. 2020, 68, 3850–3858. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Subash-Babu, P.; Nassrallah, A.; Alshatwi, A.A.; Alkaltham, M.S. Cyclotrisiloxan and β-Sitosterol rich Cassia alata (L.) flower inhibit HT-115 human colon cancer cell growth via mitochondrial dependent apoptotic stimulation. Saudi J. Biol. Sci. 2021, 28, 6009–6016. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, H.; Koide, H.; Shimizu, K.; Asai, T.; Kinouchi Shimizu, N.; Ishikado, A.; Makino, T.; Oku, N. Chemoprevention of tumor metastasis by liposomal beta-sitosterol intake. Biol. Pharm. Bull. 2008, 31, 400–404. [Google Scholar] [CrossRef]
- Awad, A.B.; Williams, H.; Fink, C.S. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr. Cancer 2001, 40, 157–164. [Google Scholar] [CrossRef]
- Zhong, W.; Myers, J.S.; Wang, F.; Wang, K.; Lucas, J.; Rosfjord, E.; Lucas, J.; Hooper, A.T.; Yang, S.; Lemon, L.A.; et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genom. 2020, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.-F.; Huang, J.; Shi, H.-S.; Chen, X.-c.; Wang, Y.-S. Chemo-Immunotherapy with Oxaliplatin and Interleukin-7 Inhibits Colon Cancer Metastasis in Mice. PLoS ONE 2014, 9, e85789. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Hainz, M.; Holzhausen, H.-J.; Arnold, D.; Katzer, M.; Schmidt, J.; Spielmann, R.P.; Behrmann, C. Skeletal muscle metastases: Primary tumours, prevalence, and radiological features. Eur. Radiol. 2010, 20, 649–658. [Google Scholar] [CrossRef]
- Jo, J.; Schiff, D. Chapter 26—Metastatic Disease and the Nervous System. In Aminoff’s Neurology and General Medicine, 6th ed.; Aminoff, M.J., Josephson, S.A., Eds.; Academic Press: Boston, MA, USA, 2021; pp. 475–498. [Google Scholar]
- Cao, Z.-Q.; Wang, X.-X.; Lu, L.; Xu, J.-W.; Li, X.-B.; Zhang, G.-R.; Ma, Z.-J.; Shi, A.-C.; Wang, Y.; Song, Y.-J. β-Sitosterol and Gemcitabine Exhibit Synergistic Anti-pancreatic Cancer Activity by Modulating Apoptosis and Inhibiting Epithelial-Mesenchymal Transition by Deactivating Akt/GSK-3β Signaling. Front. Pharmacol. 2019, 9, 1525. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef]
- Fraile, L.; Crisci, E.; Córdoba, L.; Navarro, M.A.; Osada, J.; Montoya, M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321. [Google Scholar] [CrossRef]
- Alappat, L.; Valerio, M.; Awad, A.B. Effect of vitamin D and β-sitosterol on immune function of macrophages. Int. Immunopharmacol. 2010, 10, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front. Immunol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Li, Q.; Carr, A.L.; Donald, E.J.; Skitzki, J.J.; Okuyama, R.; Stoolman, L.M.; Chang, A.E. Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern. Cancer Res. 2005, 65, 1063–1070. [Google Scholar]
- Meng, S.; Li, L.; Zhou, M.; Jiang, W.; Niu, H.; Yang, K. Distribution and prognostic value of tumor-infiltrating T cells in breast cancer. Mol. Med. Rep. 2018, 18, 4247–4258. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, B.; Yuan, P.; Zhou, J.; Qin, P.; Han, L.; Chen, G.; Wang, Z.; Run, Z.; Zhao, P.; et al. Tumor-infiltrating CD4+ T cells in patients with gastric cancer. Cancer Cell Int. 2017, 17, 114. [Google Scholar] [CrossRef]
- Kee, J.Y.; Han, Y.H.; Park, J.; Kim, D.S.; Mun, J.G.; Ahn, K.S.; Kim, H.J.; Um, J.Y.; Hong, S.H. β-Lapachone Inhibits Lung Metastasis of Colorectal Cancer by Inducing Apoptosis of CT26 Cells. Integr. Cancer Ther. 2017, 16, 585–596. [Google Scholar] [CrossRef]
- Kaemmer, C.A.; Umesalma, S.; Maharjan, C.K.; Moose, D.L.; Narla, G.; Mott, S.L.; Zamba, G.K.D.; Breheny, P.; Darbro, B.W.; Bellizzi, A.M.; et al. Development and comparison of novel bioluminescent mouse models of pancreatic neuroendocrine neoplasm metastasis. Sci. Rep. 2021, 11, 10252. [Google Scholar] [CrossRef]
- Ma, H.; Yu, Y.; Wang, M.; Li, Z.; Xu, H.; Tian, C.; Zhang, J.; Ye, X.; Li, X. Correlation between microbes and colorectal cancer: Tumor apoptosis is induced by sitosterols through promoting gut microbiota to produce short-chain fatty acids. Apoptosis 2019, 24, 168–183. [Google Scholar] [CrossRef]
- Wang, L.; Peng, F.; Peng, C.; Du, J.-R. Gut Microbiota in Tumor Microenvironment: A Critical Regulator in Cancer Initiation and Development as Potential Targets for Chinese Medicine. Am. J. Chin. Med. 2021, 49, 609–626. [Google Scholar] [CrossRef]

| Metastasis Site | Control | SITO | LS |
|---|---|---|---|
| Lungs | 9/10 | 5/10 | 3/10 |
| Muscle | 4/10 | 2/10 | 0/10 |
| Bone | 1/10 | 1/10 | 0/10 |
| Liver | 1/10 | 0/10 | 0/10 |
| Total metastatic lesions | 15 | 8 | 3 |
| Group | CD4+ T Cells (%) | CD8+ T Cells (%) |
|---|---|---|
| Control | 29.0 ± 0.8% | 45.8 ± 1.8% |
| SITO | 31.7 ± 0.4% * | 50.5 ± 0.8% * |
| LS | 34.7 ± 0.6% **,# | 53.7 ± 0.7% **,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-Y.; Lee, C.-F.; Chou, W.-T.; Hwang, J.-J.; Tyan, Y.-S.; Chuang, H.-Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics 2022, 14, 1214. https://doi.org/10.3390/pharmaceutics14061214
Shen C-Y, Lee C-F, Chou W-T, Hwang J-J, Tyan Y-S, Chuang H-Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics. 2022; 14(6):1214. https://doi.org/10.3390/pharmaceutics14061214
Chicago/Turabian StyleShen, Chao-Yu, Chia-Fen Lee, Wei-Taur Chou, Jeng-Jong Hwang, Yeu-Sheng Tyan, and Hui-Yen Chuang. 2022. "Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System" Pharmaceutics 14, no. 6: 1214. https://doi.org/10.3390/pharmaceutics14061214
APA StyleShen, C.-Y., Lee, C.-F., Chou, W.-T., Hwang, J.-J., Tyan, Y.-S., & Chuang, H.-Y. (2022). Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Evoke of Immune System. Pharmaceutics, 14(6), 1214. https://doi.org/10.3390/pharmaceutics14061214






