Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data
2.3. Image Acquisition
2.4. Image Processing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Comparison of Injection Frequencies
3.2. Observed Adverse Events after FAc Implantation
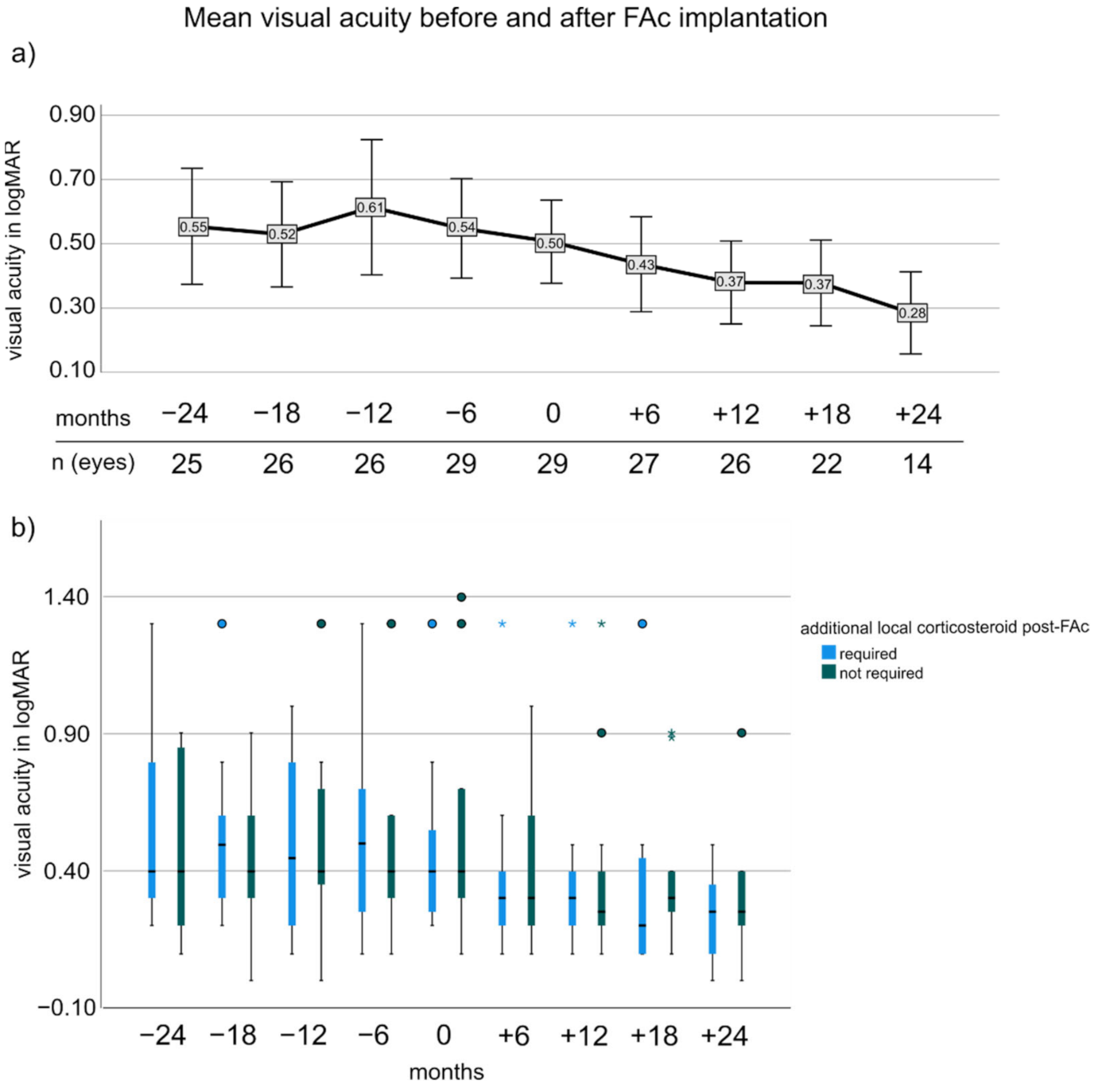
3.3. Visual Acuity before and after FAc Implantation
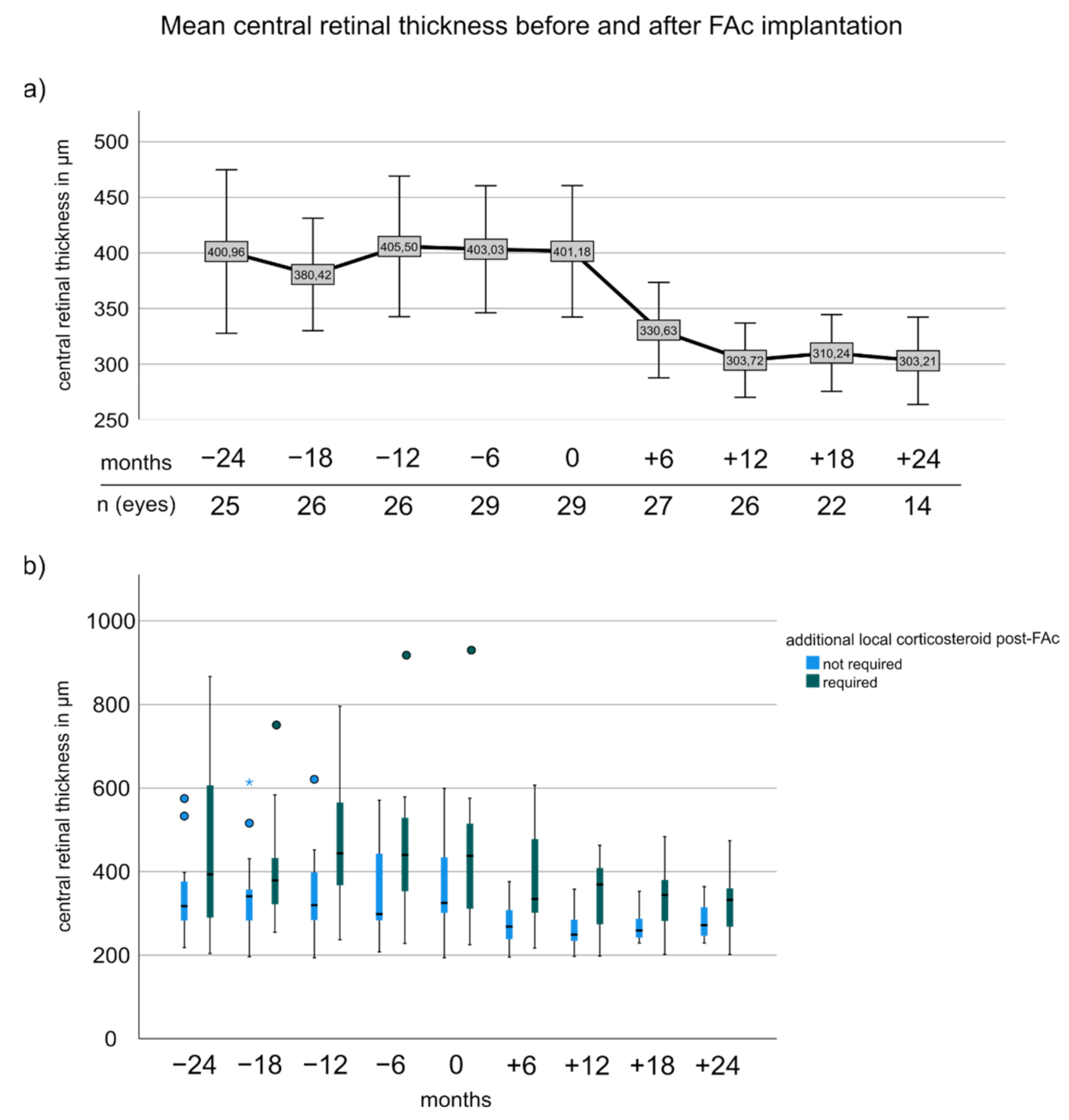
3.4. Central Retinal Thickness before and after FAc
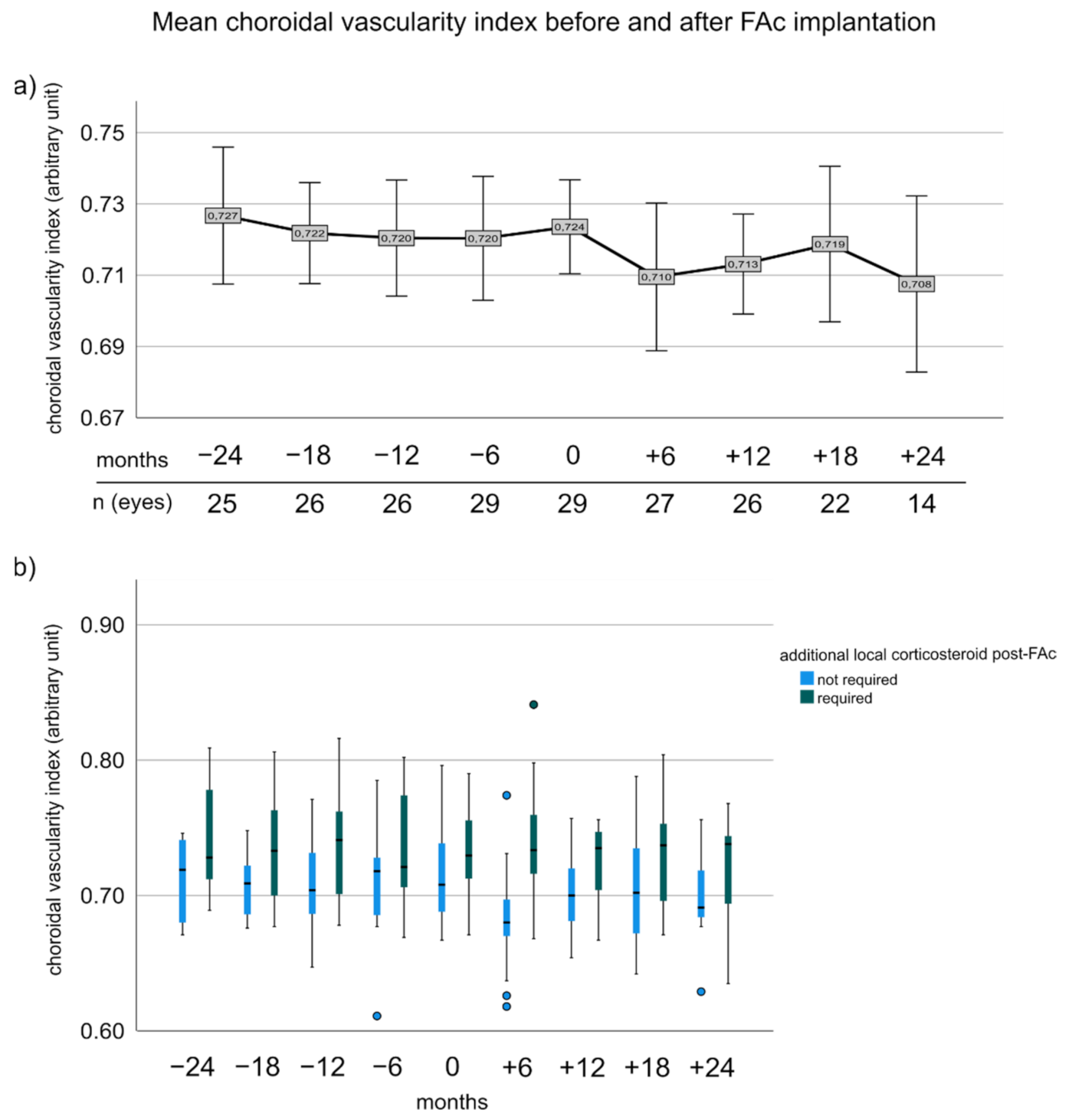
3.5. Choroidal Vascularity Index before and after FAc Implantation
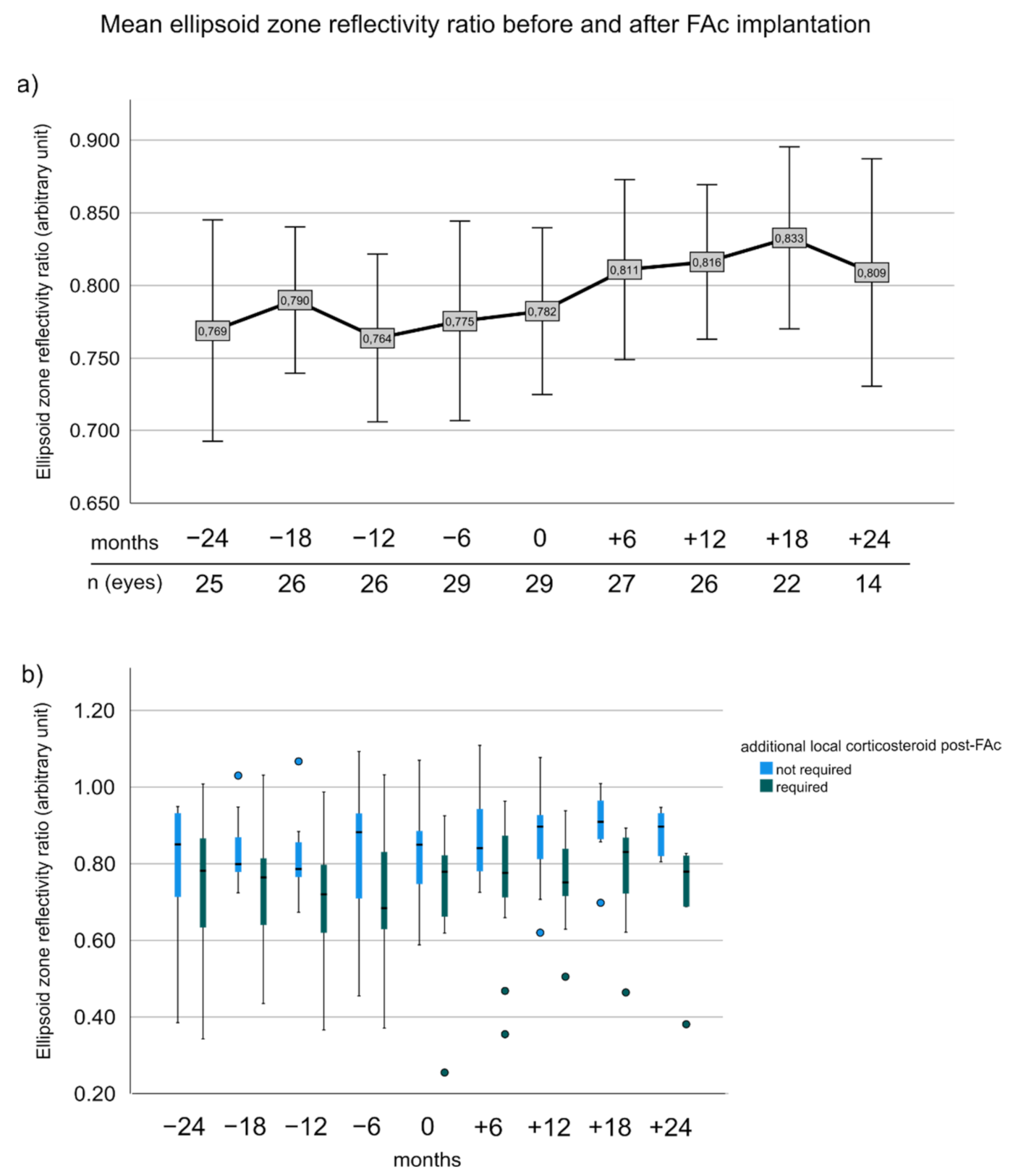
3.6. Ellipsoid Zone Reflectivity Ratio before and after FAc Implantation
3.7. Change of CVI and Number of CS Injections Prior to FAc Correlated to the Need of Additional CS after FAc
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsirouki, T.; Dastiridou, A.; Symeonidis, C.; Tounakaki, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A Focus on the Epidemiology of Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef]
- Yeung, I.Y.L.; Popp, N.A.; Chan, C.-C. The role of sex in uveitis and ocular inflammation. Int. Ophthalmol. Clin. 2015, 55, 111–131. [Google Scholar] [CrossRef]
- Acharya, N.R.; Tham, V.M.; Esterberg, E.; Borkar, D.S.; Parker, J.V.; Vinoya, A.C.; Uchida, A. Incidence and prevalence of uveitis: Results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013, 131, 1405–1412. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Bodaghi, B.; Couto, C.; Zierhut, M.; Acharya, N.; Pavesio, C.; Tay-Kearney, M.L.; Neri, P.; Douglas, K.; Pathai, S.; et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Semin. Arthritis Rheu. 2019, 49, 438–445. [Google Scholar] [CrossRef]
- Massa, H.; Nagar, A.M.; Vergados, A.; Dadoukis, P.; Patra, S.; Panos, G.D. Intravitreal fluocinolone acetonide implant (ILUVIEN(R)) for diabetic macular oedema: A literature review. J. Int. Med. Res. 2019, 47, 31–43. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Ciulla, T.; Boyer, D.; Holz, F.G.; Tolentino, M.; Gupta, A.; Duarte, L.; Madreperla, S.; et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011, 118, 626–635.e622. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Lin, P.; Keenan, R.T.; Ashton, P.; Skalak, C.; Stinnett, S.S. Injectable Fluocinolone Acetonide Long-Acting Implant for Noninfectious Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Two-Year Results. Ophthalmology 2016, 123, 1940–1948. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Pavesio, C.E.; Study, I. Effect of a Fluocinolone Acetonide Insert on Recurrence Rates in Noninfectious Intermediate, Posterior, or Panuveitis: Three-Year Results. Ophthalmology 2020, 127, 1395–1404. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Cidlowski, J.A.; Csaky, K.G.; Ambati, J. Pharmacology of Corticosteroids for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1–12. [Google Scholar] [CrossRef]
- Augustin, A.J.; Bopp, S.; Fechner, M.; Holz, F.G.; Sandner, D.; Winkgen, A.M.; Khoramnia, R.; Neuhann, T.; Warscher, M.; Spitzer, M.S.; et al. The impact of vitrectomy on outcomes achieved with 0.19 mg fluocinolone acetonide implant in patients with diabetic macular edema. Eur. J. Ophthalmol. 2021, 32, 1101–1108. [Google Scholar] [CrossRef]
- Augustin, A.J.; Bopp, S.; Fechner, M.; Holz, F.; Sandner, D.; Winkgen, A.M.; Khoramnia, R.; Neuhann, T.; Warscher, M.; Spitzer, M.; et al. Three-year results from the Retro-IDEAL study: Real-world data from diabetic macular edema (DME) patients treated with ILUVIEN((R)) (0.19 mg fluocinolone acetonide implant). Eur. J. Ophthalmol. 2020, 30, 382–391. [Google Scholar] [CrossRef]
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. Iluvien: A new sustained delivery technology for posterior eye disease. Expert Opin. Drug Deliv. 2008, 5, 1039–1046. [Google Scholar] [CrossRef]
- Schechet, S.A.; Adams, O.E.; Eichenbaum, D.A.; Hariprasad, S.M. Macular thickness amplitude changes when switching from discontinuous to continuous therapy for diabetic macular oedema. BMJ Open Ophthalmol. 2019, 4, e000271. [Google Scholar] [CrossRef]
- Riemann, C.D.; Eaton, A.M.; Cutino, A. Reduction in Retinal Thickness Fluctuations After Treatment With Fluocinolone Acetonide Implant for DME: A Post-Hoc Analysis of the USER Study. Ophthalmic. Surg. Lasers Imaging Retina 2020, 51, 298–306. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Foster, C.S.; Pavesio, C.E.; Paggiarino, D.A.; Riedel, G.E. Effect of an Injectable Fluocinolone Acetonide Insert on Recurrence Rates in Chronic Noninfectious Uveitis Affecting the Posterior Segment: Twelve-Month Results. Ophthalmology 2019, 126, 601–610. [Google Scholar] [CrossRef]
- Pockar, S.; Leal, I.; Chhabra, R.; Jones, N.P.; Steeples, L.R. Intravitreal Fluocinolone 0.19 mg Implant in the Management of Chronic Non-Infectious Uveitis: 12-Month Outcomes from a Single Tertiary Centre. Ocul. Immunol. Inflamm. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Arcinue, C.A.; Ceron, O.M.; Foster, C.S. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J. Ocul. Pharmacol. Ther. 2013, 29, 501–507. [Google Scholar] [CrossRef]
- Bodaghi, B.; Nguyen, Q.D.; Jaffe, G.; Khoramnia, R.; Pavesio, C. Preventing relapse in non-infectious uveitis affecting the posterior segment of the eye—Evaluating the 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN((R))). J. Ophthalmic. Inflamm. Infect. 2020, 10, 32. [Google Scholar] [CrossRef]
- Leinonen, S.; Immonen, I.; Kotaniemi, K. Fluocinolone acetonide intravitreal implant (Retisert((R))) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol. 2018, 96, 648–651. [Google Scholar] [CrossRef]
- Tao, L.W.; Wu, Z.C.; Guymer, R.H.; Luu, C.D. Ellipsoid zone on optical coherence tomography: A review. Clin. Exp. Ophthalmol. 2016, 44, 422–430. [Google Scholar] [CrossRef]
- Kessler, L.J.; Auffarth, G.U.; Bagautdinov, D.; Khoramnia, R. Ellipsoid Zone Integrity and Visual Acuity Changes during Diabetic Macular Edema Therapy: A Longitudinal Study. J. Diabetes Res. 2021, 2021, 8117650. [Google Scholar] [CrossRef]
- Etheridge, T.; Dobson, E.T.A.; Wiedenmann, M.; Oden, N.; VanVeldhuisen, P.; Scott, I.U.; Ip, M.S.; Eliceiri, K.W.; Blodi, B.A.; Domalpally, A. Ellipsoid Zone Defects in Retinal Vein Occlusion Correlates With Visual Acuity Prognosis: SCORE2 Report 14. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Zahid, R.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Meng, X.; Reese, J.; Le, T.K.; Lunasco, L.; Hu, M.; et al. Longitudinal Assessment of Ellipsoid Zone Integrity, Subretinal Hyperreflective Material, and Subretinal Pigment Epithelium Disease in Neovascular Age-Related Macular Degeneration. Ophthalmol. Retina 2021, 5, 1204–1213. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, A.G.; Lin, Y.H.; Huang, Y.M.; Lee, F.L.; Lee, S.M. Optical coherence tomography in resolution of photoreceptor damage in multiple evanescent white dot syndrome. J. Chin. Med. Assoc. 2012, 75, 663–666. [Google Scholar] [CrossRef][Green Version]
- Chai, Y.; Yamazaki, H.; Fujinami, K.; Tsunoda, K.; Yamamoto, S. Case of acute zonal occult outer retinopathy with abnormal pattern visual evoked potentials. Clin. Ophthalmol. 2011, 5, 1235–1241. [Google Scholar] [CrossRef][Green Version]
- Hua, R.; Liu, L.; Chen, L. Evaluation of the progression rate of atrophy lesions in punctate inner choroidopathy (PIC) based on autofluorescence analysis. Photodiagnosis Photodyn. Ther. 2014, 11, 565–569. [Google Scholar] [CrossRef]
- Agrawal, R.; Salman, M.; Tan, K.A.; Karampelas, M.; Sim, D.A.; Keane, P.A.; Pavesio, C. Choroidal Vascularity Index (CVI)—A Novel Optical Coherence Tomography Parameter for Monitoring Patients with Panuveitis? PLoS ONE 2016, 11, e0146344. [Google Scholar] [CrossRef]
- Hikal, M.; Celik, N.; Auffarth, G.U.; Kessler, L.J.; Mayer, C.S.; Khoramnia, R. Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Non-Infectious Uveitis. J. Clin. Med. 2021, 10, 3966. [Google Scholar] [CrossRef]
- Kessler, L.J.; Bagautdinov, D.; Łabuz, G.; Auffarth, G.U.; Khoramnia, R. Semi-Automated Quantification of Retinal and Choroidal Biomarkers in Retinal Vascular Diseases: Agreement of Spectral-Domain Optical Coherence Tomography with and without Enhanced Depth Imaging Mode. Diagnostics 2022, 12, 333. [Google Scholar]
- Gin, T.J.; Wu, Z.; Chew, S.K.; Guymer, R.H.; Luu, C.D. Quantitative Analysis of the Ellipsoid Zone Intensity in Phenotypic Variations of Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2079–2086. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, L.J.; Pang, C.P.; Chen, H. Ellipsoid zone optical intensity reduction as an early biomarker for retinitis pigmentosa. Acta Ophthalmol. 2021, 99, e215–e221. [Google Scholar] [CrossRef]
- Midena, E.; Torresin, T.; Velotta, E.; Pilotto, E.; Parrozzani, R.; Frizziero, L. OCT Hyperreflective Retinal Foci in Diabetic Retinopathy: A Semi-Automatic Detection Comparative Study. Front. Immunol. 2021, 12, 613051. [Google Scholar] [CrossRef]
- Jacob, C. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1965. [Google Scholar]
- Battista, M.; Starace, V.; Cicinelli, M.V.; Capone, L.; Marchese, A.; Modorati, G.; Bandello, F.; Miserocchi, E. Efficacy of 0.19 mg Fluocinolone Acetonide Implant in Non-infectious Posterior Uveitis Evaluated as Area Under the Curve. Ophthalmol. Ther. 2021, 11, 215–224. [Google Scholar] [CrossRef]
- Weber, L.F.; Marx, S.; Auffarth, G.U.; Scheuerle, A.F.; Tandogan, T.; Mayer, C.; Khoramnia, R. Injectable 0.19-mg fluocinolone acetonide intravitreal implant for the treatment of non-infectious uveitic macular edema. J. Ophthalmic. Inflamm. Infect 2019, 9, 3. [Google Scholar] [CrossRef]
- Kriegel, M.; Heiligenhaus, A.; Heinz, C. Comparing the Efficacy of Intravitreal Dexamethasone and Time-displaced Fluocinolone Acetonide on Central Retinal Thickness in Patients with Uveitis. Ocul. Immunol. Inflamm. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Kapik, B.; Barakat, M.R.; Khurana, R.N.; Nguyen, Q.D.; Grewal, D.S.; Albini, T.; Cunningham, E.T., Jr.; Goldstein, D.A. OCT Anatomic and Temporal Biomarkers in Uveitic Macular Edema. Am. J. Ophthalmol. 2021, 237, 310–324. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Zollet, P.; Capone, L.L.; Lattanzio, R.; Bandello, F. Persistent or Recurrent Diabetic Macular Edema After Fluocinolone Acetonide 0.19 mg Implant: Risk Factors and Management. Am. J. Ophthalmol. 2020, 215, 14–24. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rosenblatt, A.; Grosso, D.; Zollet, P.; Capone, L.; Rabiolo, A.; Lattanzio, R.; Loewenstein, A.; Bandello, F.; International Retina, C. The outcome of fluocinolone acetonide intravitreal implant is predicted by the response to dexamethasone implant in diabetic macular oedema. Eye 2021, 35, 3232–3242. [Google Scholar] [CrossRef]
- Kongwattananon, W.; Kumar, A.; Oyeniran, E.; Sen, H.N.; Kodati, S. Changes in Choroidal Vascularity Index (CVI) in Intermediate Uveitis. Transl. Vis. Sci. Technol. 2021, 10, 33. [Google Scholar] [CrossRef]
- Kim, M.; Kim, R.Y.; Park, Y.H. Choroidal Vascularity Index and Choroidal Thickness in Human Leukocyte Antigen-B27-Associated Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 1280–1287. [Google Scholar] [CrossRef]
- Arrigo, A.; Capone, L.; Lattanzio, R.; Aragona, E.; Zollet, P.; Bandello, F. Optical Coherence Tomography Biomarkers of Inflammation in Diabetic Macular Edema Treated by Fluocinolone Acetonide Intravitreal Drug-Delivery System Implant. Ophthalmol. Ther. 2020, 9, 971–980. [Google Scholar] [CrossRef]
- Figueiredo, N.; Sarraf, D.; Gunnemann, F.; Sadda, S.R.; Bansal, A.; Berger, A.R.; Wong, D.T.; Kohly, R.P.; Kertes, P.J.; Hillier, R.J.; et al. Longitudinal Assessment of Ellipsoid Zone Recovery Using En Face Optical Coherence Tomography After Retinal Detachment Repair. Am. J. Ophthalmol. 2021, 236, 212–220. [Google Scholar] [CrossRef]
- Zhou, M.; Gu, R.P.; Sun, Z.; Jiang, C.H.; Chang, Q.; Xu, G.Z. Differences in photoreceptor recovery among patients and between different parts of the posterior pole in Vogt-Koyanagi-Harada disease. Eye 2018, 32, 572–578. [Google Scholar] [CrossRef]
- Starr, M.R.; Salabati, M.; Mahmoudzadeh, R.; Patel, L.G.; Ammar, M.J.; Hsu, J.; Garg, S.; Ho, A.C.; Kuriyan, A.E. Fluctuations in Central Subfield Thickness Associated With Worse Visual Outcomes in Patients With Diabetic Macular Edema in Clinical Trial Setting. Am. J. Ophthalmol. 2021, 232, 90–97. [Google Scholar] [CrossRef]
- Thorne, J.E.; Sugar, E.A.; Holbrook, J.T.; Burke, A.E.; Altaweel, M.M.; Vitale, A.T.; Acharya, N.R.; Kempen, J.H.; Jabs, D.A.; Multicenter Uveitis Steroid Treatment Trial Research Group. Periocular Triamcinolone vs. Intravitreal Triamcinolone vs. Intravitreal Dexamethasone Implant for the Treatment of Uveitic Macular Edema: The PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) Trial. Ophthalmology 2019, 126, 283–295. [Google Scholar] [CrossRef]
| Demographic Characteristics | n (in %) or Mean (±Standard Deviation) |
|---|---|
| Numbers of patients (one eye per patient) | 29 |
| Right eye as study eye | 11 (38%) |
| Age at FAc implantation (in years) | 60.03 ± 16.01 |
| Mean observation time (in years) | 3.51 ± 0.51 |
| Male | 10 (34%) |
| Disease duration until FAc implantation (in years) | 8.55 ± 6.20 |
| Uveitis classification | Numbers of eyes (in %) |
| Uveitis anterior with ME | 2 (7%) |
| Uveitis intermedia with ME | 18 (62%) |
| Uveitis posterior with ME | 5 (17%) |
| Panuveitis with ME | 4 (14%) |
| Etiology of non-infectious uveitis | Numbers of eyes (in %) |
| Idiopathic | 18 (63%) |
| Sarcoidosis | 3 (11%) |
| Birdshot retinopathy | 2 (7%) |
| Ocular tuberculosis (non-infectious when treated) | 1 (3%) |
| Multifocale chorioretinitis and panuveitis | 1 (3%) |
| Vogt-Koyanagi-Harada syndrome | 1 (3%) |
| Acute zonal outer occult retinopathy (AZOOR) | 1 (3%) |
| Multiple sclerosis | 2 (7%) |
| Lens and vitreous status, n (%) | Numbers of eyes (in %) |
| Pseudophakic | 26 (89%) |
| Vitrectomized eyes | 4 (14%) |
| Local treatments (dexamethasone implants or peribulbar corticosteroids) | Mean (±standard deviation)[range] |
| Total number of local corticosteroid injections within 2 years prior to FAc | 4.52 ± 1.90 [1; 9] |
| Total number of local corticosteroid injections in the 2 years after FAc | 1.45 ± 1.94 [0; 6] |
| Local corticosteroid injection per year prior to FAc | 2.40 ± 0.88 [1.0; 4.5] |
| Local corticosteroid injection per year after FAc | 0.94 ± 1.33 [0; 5] |
| Covariates | Coefficient | Standard Error | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| CS per year before FAc | 0.97 | 0.37 | 2.65 | 1.21; 5.78 | 0.017 * |
| EZR at baseline | −0.49 | 0.32 | 0.61 | 0.31; 1.21 | 0.148 |
| CRT at baseline | −0.40 | 0.29 | 0.67 | 0.36; 1.23 | 0.183 |
| CVI reduction from baseline to 6 months | −0.20 | 0.05 | 0.82 | 0.73; 0.93 | 0.004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, L.J.; Łabuz, G.; Auffarth, G.U.; Khoramnia, R. Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema. Pharmaceutics 2022, 14, 688. https://doi.org/10.3390/pharmaceutics14040688
Kessler LJ, Łabuz G, Auffarth GU, Khoramnia R. Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema. Pharmaceutics. 2022; 14(4):688. https://doi.org/10.3390/pharmaceutics14040688
Chicago/Turabian StyleKessler, Lucy Joanne, Grzegorz Łabuz, Gerd U. Auffarth, and Ramin Khoramnia. 2022. "Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema" Pharmaceutics 14, no. 4: 688. https://doi.org/10.3390/pharmaceutics14040688
APA StyleKessler, L. J., Łabuz, G., Auffarth, G. U., & Khoramnia, R. (2022). Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema. Pharmaceutics, 14(4), 688. https://doi.org/10.3390/pharmaceutics14040688








