Silk Fibroin-Based Therapeutics for Impaired Wound Healing
Abstract
:1. Introduction
2. Physicochemical Properties of Silk Fibroin
3. SF Scaffolds
3.1. SF-Composite Scaffolds
3.2. Cellularized and Decellularized SF Scaffolds
4. SF Solution
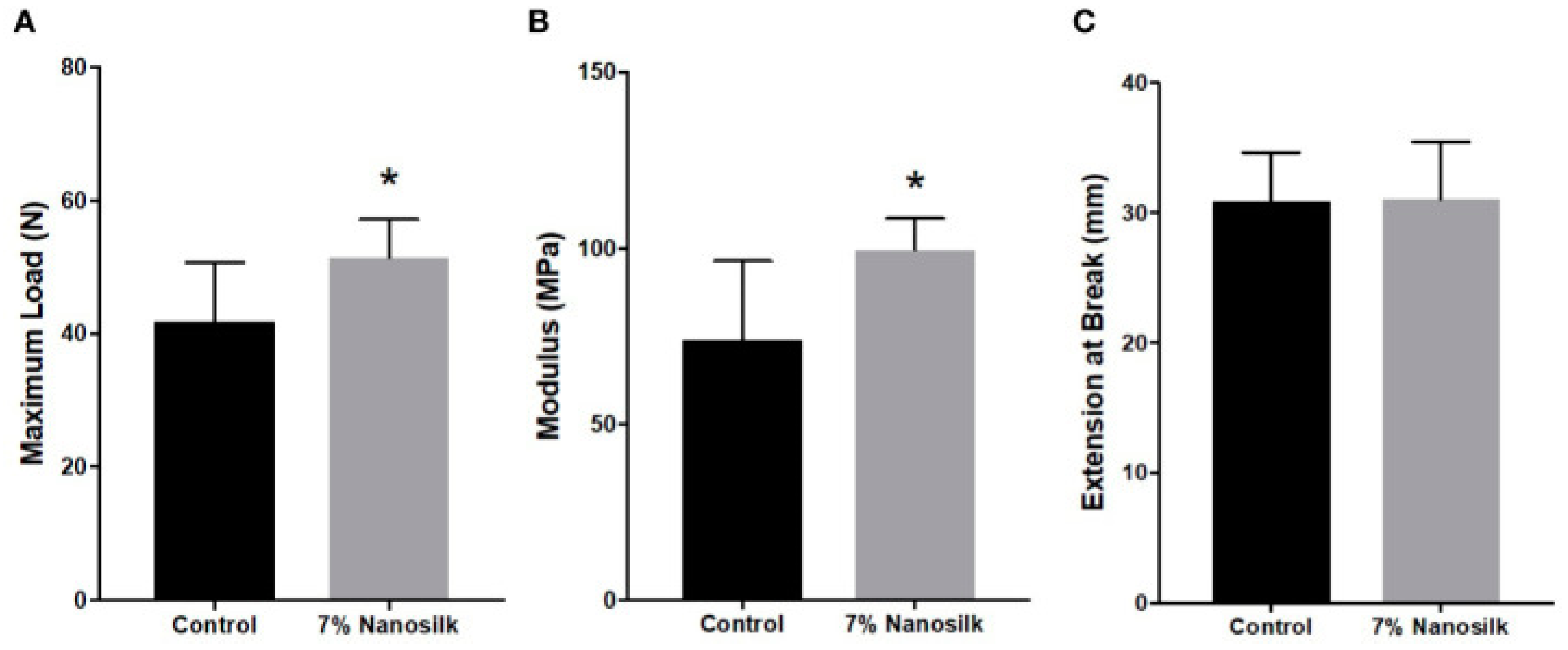
4.1. Nanosilk
4.2. SF Solution Delivery System
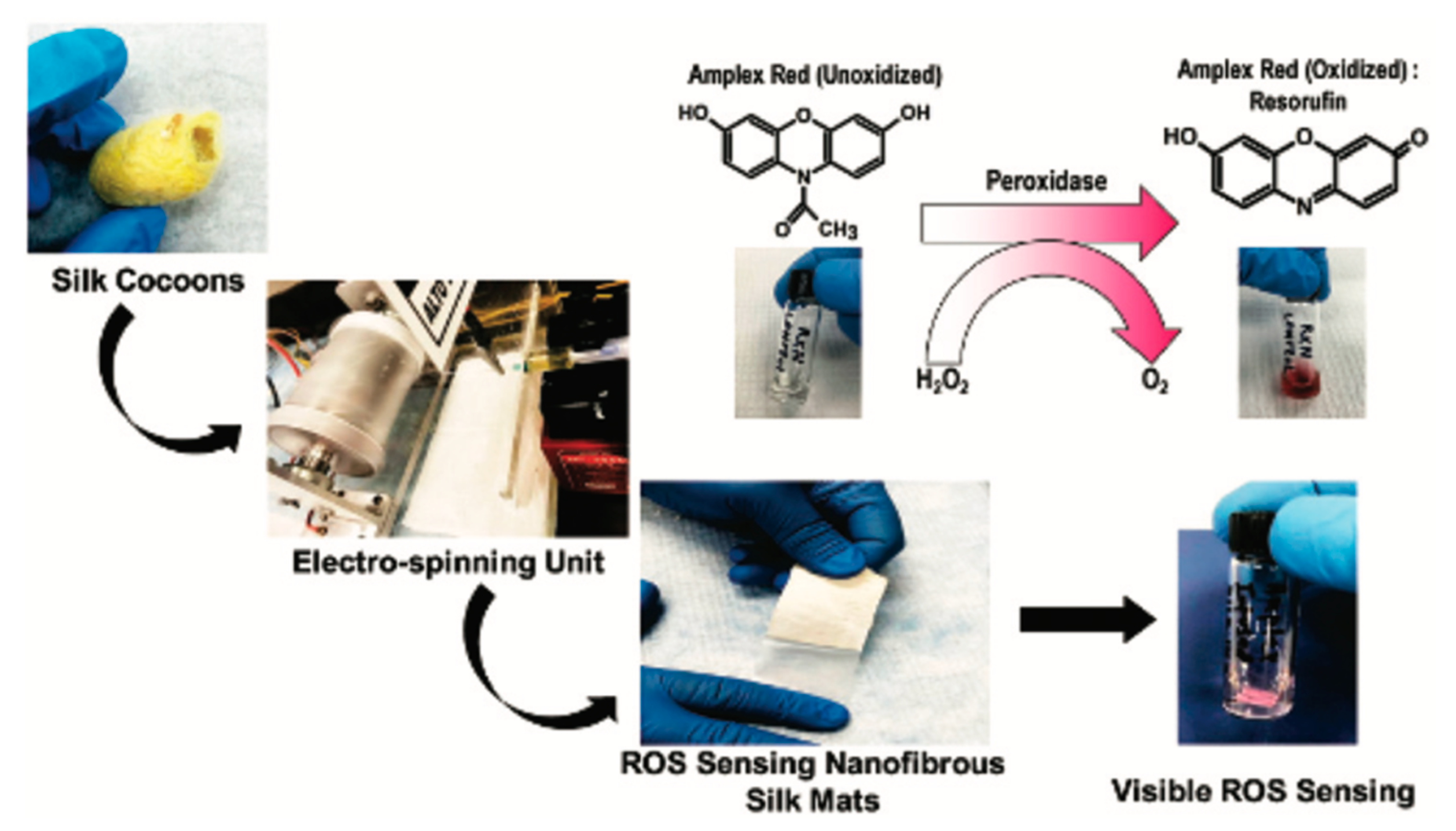
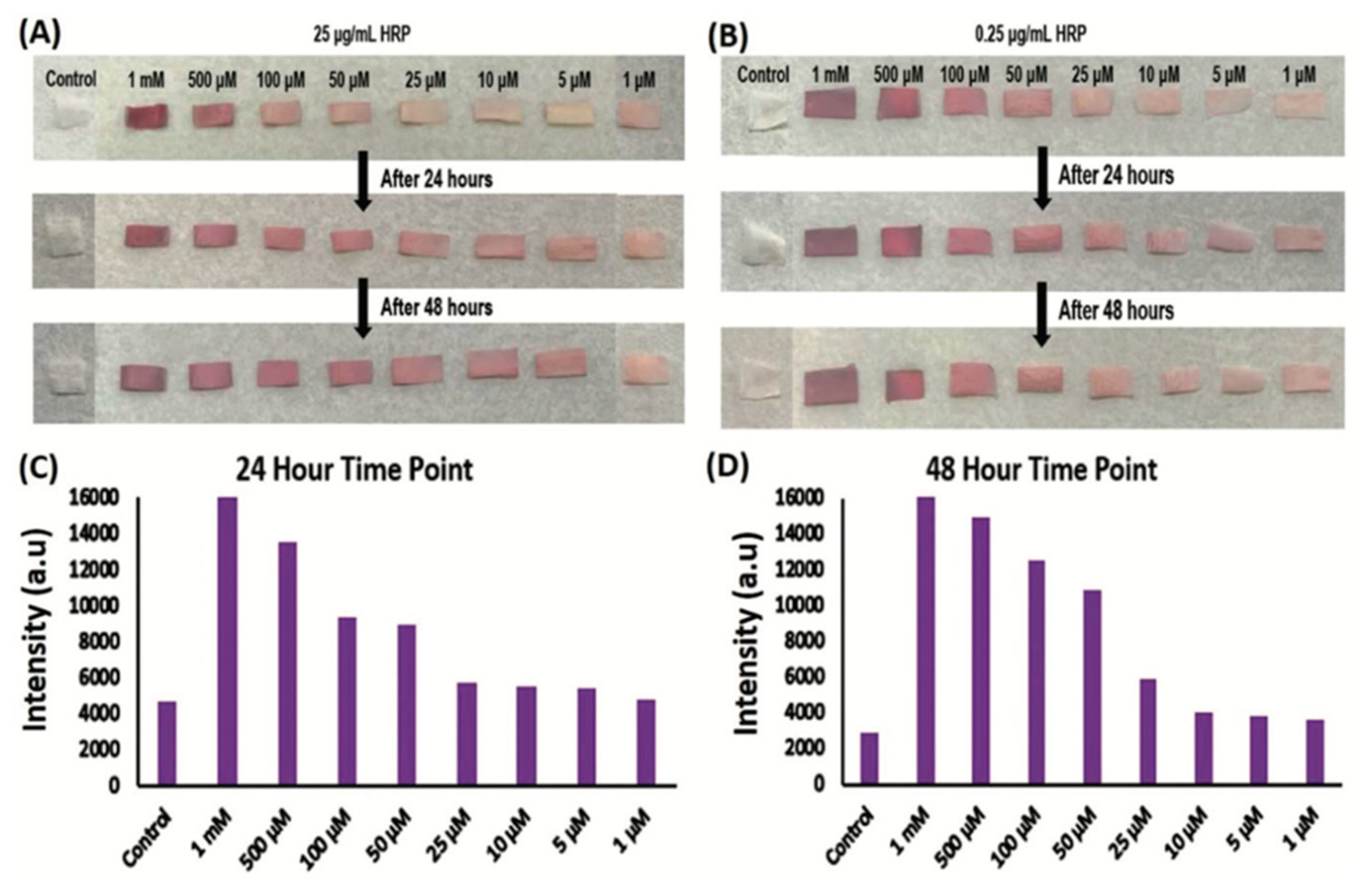
5. SF-Based Sensors
6. SF Nanoparticles
7. SF Hydrogels
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L. Type 2 diabetes mellitus and heart failure, a scientific statement from the American Heart Association and Heart Failure Society of America. J. Card. Fail. 2019, 25, 584–619. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.W.; LeRoith, D. The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Winkler, S.; Kaplan, D.L. Molecular biology of spider silk. Rev. Mol. Biotechnol. 2000, 74, 85–93. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Galateanu, B.; Hudita, A.; Zaharia, C.; Bunea, M.-C.; Vasile, E.; Buga, M.-R.; Costache, M. Polym. Polym. Compos., A Reference Series; Springer: Cham, Switzerland, 2019; pp. 1791–1817. [Google Scholar]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A. How to improve physico-chemical properties of silk fibroin materials for biomedical applications?—Blending and cross-linking of silk fibroin—A review. Materials 2021, 14, 1510. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, S.M.; Louiselle, A.E.; Hilton, S.A.; Dewberry, L.C.; Zhang, L.; Azeltine, M.; Xu, J.; Singh, S.; Sakthivel, T.S.; Seal, S.; et al. Nanosilk Increases the Strength of Diabetic Skin and Delivers CNP-miR146a to Improve Wound Healing. Front. Immunol. 2020, 11, 590285. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Lu, C.-L.; Coburn, J.; Kaplan, D.L. Impact of silk biomaterial structure on proteolysis. Acta Biomater. 2015, 11, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, S.M.; Percival, S.L. Proteases and delayed wound healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar] [CrossRef]
- Silva, S.S.; Santos, T.C.; Cerqueira, M.T.; Marques, A.P.; Reys, L.L.; Silva, T.H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. The use of ionic liquids in the processing of chitosan/silk hydrogels for biomedical applications. Green Chem. 2012, 14, 1463–1470. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Tadepalli, S.; Park, S.H.; Kazemi-Moridani, A.; Jiang, Q.; Singamaneni, S.; Lee, J.-H. Extreme mechanical behavior of nacre-mimetic graphene-oxide and silk nanocomposites. Nano Lett. 2018, 18, 987–993. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Martínez, C.M.; Romecín, P.A.; Aznar-Cervantes, S.D.; Carpes-Ruiz, M.; Cenis, J.L.; Moraleda, J.M.; Atucha, N.M.; García-Bernal, D. Silk fibroin scaffolds seeded with Wharton’s jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Res. Ther. 2019, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Q.; Shen, W.-D.; Gu, R.-A.; Zhu, J.; Xue, R.-Y. Amperometric biosensor for uric acid based on uricase-immobilized silk fibroin membrane. Anal. Chim. Acta 1998, 369, 123–128. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. Detection of hydrogen peroxide with Amplex Red: Interference by NADH and reduced glutathione auto-oxidation. Arch. Biochem. Biophys. 2004, 431, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Kiani, M.; Farokhi, M.; Kundu, S.C.; Reis, R.L.; Gholami, M.; Bardania, H.; Dinarvand, R.; Geramifar, P.; Beiki, D. Targeted delivery system based on gemcitabine-loaded silk fibroin nanoparticles for lung cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 31600–31611. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Gupta, V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 2010, 5, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Wang, Y.-J.; Wang, H.-Y.; Zhu, L.; Zhou, Z.-Z. Highly efficient processing of silk fibroin nanoparticle-l-asparaginase bioconjugates and their characterization as a drug delivery system. Soft Matter 2011, 7, 9728–9736. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Kaplan, D.L. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin. Drug Deliv. 2011, 8, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Mecham, R. The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatz, R.; von Jan, N.; Schildberg, F.-W. Mechanisms of action of collagenase in wound repair. In Wound Healing and Skin Physiology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 227–237. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Goh, J.C. Self-assembled silk fibroin particles: Tunable size and appearance. Powder Technol. 2012, 215, 85–90. [Google Scholar] [CrossRef]
- Cheng, Y.; Koh, L.-D.; Li, D.; Ji, B.; Han, M.-Y.; Zhang, Y.-W. On the strength of β-sheet crystallites of Bombyx mori silk fibroin. J. R. Soc. Interface 2014, 11, 20140305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.-J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horan, R.L.; Antle, K.; Collette, A.L.; Wang, Y.; Huang, J.; Moreau, J.E.; Volloch, V.; Kaplan, D.L.; Altman, G.H. In vitro degradation of silk fibroin. Biomaterials 2005, 26, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Han, H.; Huang, X.; Xu, W.; Kaplan, D.L.; Zhu, H.; Lu, Q. Silk scaffolds with tunable mechanical capability for cell differentiation. Acta Biomater. 2015, 20, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Z.-D.; Liu, W.-Q.; Feng, Q.-L. Preparation and cytocompatibility of silk fibroin/chitosan scaffolds. Front. Mater. Sci. China 2009, 3, 241–247. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Guang, S.; An, Y.; Ke, F.; Zhao, D.; Shen, Y.; Xu, H. Chitosan/silk fibroin composite scaffolds for wound dressing. J. Appl. Polym. Sci. 2015, 132, 42503–42510. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Yang, J.; Du, H.; Chai, N.; Sha, Z.; Geng, M.; Zhou, X.; He, C. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and characterization of electrospun silk fibroin/gelatin scaffolds crosslinked with glutaraldehyde vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- Ghalei, S.; Douglass, M.; Handa, H. Nitric Oxide-Releasing Gelatin Methacryloyl/Silk Fibroin Interpenetrating Polymer Network Hydrogels for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2021, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-based metal–organic framework as a controllable nitric oxide-releasing vehicle for enhanced diabetic wound healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef] [PubMed]
- Estes, L.M.; Singha, P.; Singh, S.; Sakthivel, T.S.; Garren, M.; Devine, R.; Brisbois, E.J.; Seal, S.; Handa, H. Characterization of a nitric oxide (NO) donor molecule and cerium oxide nanoparticle (CNP) interactions and their synergistic antimicrobial potential for biomedical applications. J. Colloid Interface Sci. 2021, 586, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Scott, P.G.; Tredget, E.E. Bone marrow-derived stem cells in wound healing: A review. Wound Repair Regen. 2007, 15, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, R.C.; Tredget, E.E. Concise review: Bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010, 28, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Ishikawa, H.; Kawai, K.; Tabata, Y.; Suzuki, S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 2013, 34, 9393–9400. [Google Scholar] [CrossRef] [Green Version]
- Ganier, C.; Gaucher, S. Emerging Technologies in Scar Management: The Role of Allogeneic Cells. In Textbook on Scar Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 451–455. [Google Scholar]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [Green Version]
- Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Hu, J.; Xu, J.; Liechty, K.W.; Zgheib, C. Mesenchymal stromal cells contract collagen more efficiently than dermal fibroblasts: Implications for cytotherapy. PLoS ONE 2019, 14, e0218536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombouts, W.; Ploemacher, R. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003, 17, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddappa, R.; Licht, R.; van Blitterswijk, C.; de Boer, J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Lei, X.; He, F.-L.; He, J.; Liu, Y.-L.; Ye, Y.-J.; Deng, X.; Duan, E.; Yin, D.-C. Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. Int. J. Biol. Macromol. 2017, 105, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.; Luo, C.; Zhang, C.; Li, Z.; Long, M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials 2014, 35, 3945–3955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Non-bioengineered silk fibroin protein 3D scaffolds for potential biotechnological and tissue engineering applications. Macromol. Biosci. 2008, 8, 807–818. [Google Scholar] [CrossRef]
- Altman, A.M.; Yan, Y.; Matthias, N.; Bai, X.; Rios, C.; Mathur, A.B.; Song, Y.H.; Alt, E.U. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 2009, 27, 250–258. [Google Scholar] [CrossRef]
- Navone, S.E.; Pascucci, L.; Dossena, M.; Ferri, A.; Invernici, G.; Acerbi, F.; Cristini, S.; Bedini, G.; Tosetti, V.; Ceserani, V. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshareddy, K.; Troyer, D.; Weiss, M.L. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord. Methods Cell Biol. 2008, 86, 101–119. [Google Scholar] [PubMed]
- Bermudez, D.M.; Herdrich, B.J.; Xu, J.; Lind, R.; Beason, D.P.; Mitchell, M.E.; Soslowsky, L.J.; Liechty, K.W. Impaired biomechanical properties of diabetic skin: Implications in pathogenesis of diabetic wound complications. Am. J. Pathol. 2011, 178, 2215–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, H.S.; Matsumoto, A.; Chin, I.J.; Jin, H.J.; Kaplan, D.L. Processing windows for forming silk fibroin biomaterials into a 3D porous matrix. Aust. J. Chem. 2005, 58, 716–720. [Google Scholar] [CrossRef]
- Hilton, S.A.; Zgheib, C.; Hodges, M.M.; Dewberry, L.C.; Seal, S.; Liechty, K.W. Nanosilk Improves the Biomechanical Properties of Human Diabetic Skin. J. Am. Coll. Surg. 2018, 227, S108. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.; Azeltine, M.; Mundra, L.; French, B.; Zgheib, C.; Liechty, K.W. Evaluation of skin care concerns and patient’s perception of the effect of NanoSilk Cream on facial skin. J. Cosmet. Dermatol. 2021, 21, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Perry, M.M.; Moschos, S.A.; Larner-Svensson, H.M.; Lindsay, M.A. Role of miRNA-146a in the regulation of the innate immune response and cancer. Biochem. Soc. Trans. 2008, 36, 1211–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wu, W.; Zhang, L.; Dorset-Martin, W.; Morris, M.W.; Mitchell, M.E.; Liechty, K.W. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes 2012, 61, 2906–2912. [Google Scholar] [CrossRef] [Green Version]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S. Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct the diabetic wound healing impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, L.C.; Niemiec, S.M.; Hilton, S.A.; Louiselle, A.E.; Singh, S.; Sakthivel, T.S.; Hu, J.; Seal, S.; Liechty, K.W.; Zgheib, C. Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102483. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jönsson, U. Real-time biospecific interaction analysis. Biosensors 2016, 92, 260–267. [Google Scholar]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Duggan, C.A. Implanted electroenzymatic glucose sensors. Diabetes Care 1982, 5, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q. Natural silk fibroin as a support for enzyme immobilization. Biotechnol. Adv. 1998, 16, 961–971. [Google Scholar] [CrossRef]
- Delezuk, J.A.; Pavinatto, A.; Moraes, M.L.; Shimizu, F.M.; Rodrigues, V.C.; Campana-Filho, S.P.; Ribeiro, S.J.; Oliveira, O.N., Jr. Silk fibroin organization induced by chitosan in layer-by-layer films: Application as a matrix in a biosensor. Carbohydr. Polym. 2017, 155, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. The Lesch-Nyhan syndrome. Annu. Rev. Med. 1973, 24, 41–60. [Google Scholar] [CrossRef]
- Terkeltaub, R.A. Gout. N. Engl. J. Med. 2003, 349, 1647–1655. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Yu, H.-Y.; Abdalkarim, S.Y.H.; Ouyang, Z.; Zhu, J.; Yao, J. Multifunctional Biosensors Made with Self-Healable Silk Fibroin Imitating Skin. ACS Appl. Mater. Interfaces 2021, 13, 33371–33382. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Cortes, G.; Kumar, U.; Sakthivel, T.S.; Niemiec, S.M.; Louiselle, A.E.; Azeltine-Bannerman, M.; Zgheib, C.; Liechty, K.W.; Seal, S. Silk fibroin nanofibrous mats for visible sensing of oxidative stress in cutaneous wounds. Biomater. Sci. 2020, 8, 5900–5910. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Bai, D.; Abraham, A.N.; Jadhav, A.; Linklater, D.; Matusica, A.; Nguyen, D.; Murdoch, B.J.; Zakhartchouk, N.; Dekiwadia, C. Electrospun Nanodiamond–Silk Fibroin Membranes: A Multifunctional Platform for Biosensing and Wound-Healing Applications. ACS Appl. Mater. Interfaces 2020, 12, 48408–48419. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Karponis, D.; Mosahebi, A.; Seifalian, A.M. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front. Biosci. 2018, 23, 1038–1059. [Google Scholar]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef]
- Redhead, H.; Davis, S.; Illum, L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: In vitro characterisation and in vivo evaluation. J. Control. Release 2001, 70, 353–363. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Roser, M.; Fischer, D.; Kissel, T. Surface-modified biodegradable albumin nano-and microspheres. II: Effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur. J. Pharm. Biopharm. 1998, 46, 255–263. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Tomkins, M.; Fajardo, R.; Meinel, L.; Snyder, B.; Wade, K.; Chen, J.; Vunjak-Novakovic, G.; Kaplan, D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. Part A 2006, 78, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Anumolu, R.; Gustafson, J.A.; Magda, J.J.; Cappello, J.; Ghandehari, H.; Pease, L.F., III. Fabrication of highly uniform nanoparticles from recombinant silk-elastin-like protein polymers for therapeutic agent delivery. Acs Nano 2011, 5, 5374–5382. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanoparticle Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Karaly, A.H.; Sarhan, W.A.; El-Sherbiny, I.M. Development of a silk fibroin-based multitask aerosolized nanopowder formula for efficient wound healing. Int. J. Biol. Macromol. 2021, 182, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Amirkaveei, S.; Behbahani, B.A. Antimicrobial effect of mangrove extract on Escherichia coli and Penicillium digitatum. In Proceedings of the International Conference on Food Engineering and Biotechnology, Bangkok, Thailand, 7–9 May 2011; pp. 185–188. [Google Scholar]
- Blanchard, C.; Brooks, L.; Beckley, A.; Colquhoun, J.; Dewhurst, S.; Dunman, P.M. Neomycin sulfate improves the antimicrobial activity of mupirocin-based antibacterial ointments. Antimicrob. Agents Chemother. 2016, 60, 862–872. [Google Scholar] [CrossRef] [Green Version]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short Iii, G.F.; Staunton, J.E.; Jin, X. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Meng, Y.; Chen, L.; Zhang, Q. Development of a dual drug-loaded hydrogel delivery system for enhanced cancer therapy: In situ formation, degradation and synergistic antitumor efficiency. J. Mater. Chem. B 2017, 5, 8487–8497. [Google Scholar] [CrossRef]
- Numata, K. Silk hydrogels for tissue engineering and dual-drug delivery. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 503–518. [Google Scholar]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Focus: Infectious diseases: Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269. [Google Scholar]
- Hardwicke, J.; Schmaljohann, D.; Boyce, D.; Thomas, D. Epidermal growth factor therapy and wound healing—Past, present and future perspectives. Surgeon 2008, 6, 172–177. [Google Scholar] [CrossRef]
- Rezaei, F.; Damoogh, S.; Reis, R.L.; Kundu, S.C.; Mottaghitalab, F.; Farokhi, M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 2020, 13, 015005. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52. [Google Scholar]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Numata, K.; Yamazaki, S.; Naga, N. Biocompatible and biodegradable dual-drug release system based on silk hydrogel containing silk nanoparticles. Biomacromolecules 2012, 13, 1383–1389. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from ultrasonication-induced silk fibroin−hyaluronic acid hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.u.; Samudrala, P.K.; Mandal, B.B. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv. Healthc. Mater. 2018, 7, 1801092. [Google Scholar] [CrossRef]
| SF Biomaterial | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|
| tissue scaffolds | ECM mimic dynamic properties biomechanical strength | invasive treatment | tissue repair strengthens skin | [7,19,20] |
| solutions | topical application biomechanical strength | decreased solubility | drug delivery strengthens skin wound repair | [9,14] |
| biosensors | biomarker detection | minimal therapeutic value | wound monitoring | [21,22] |
| nanoparticles | customizable size short-term drug release | degrades over time unsuitable for long-term release | drug delivery | [23,24,25] |
| hydrogels | efficient drug delivery | swelling decreased mechanical strength | wound healing drug delivery | [11,26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, T.; Vaughn, A.E.; Seal, S.; Liechty, K.W.; Zgheib, C. Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics 2022, 14, 651. https://doi.org/10.3390/pharmaceutics14030651
Lehmann T, Vaughn AE, Seal S, Liechty KW, Zgheib C. Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics. 2022; 14(3):651. https://doi.org/10.3390/pharmaceutics14030651
Chicago/Turabian StyleLehmann, Tanner, Alyssa E. Vaughn, Sudipta Seal, Kenneth W. Liechty, and Carlos Zgheib. 2022. "Silk Fibroin-Based Therapeutics for Impaired Wound Healing" Pharmaceutics 14, no. 3: 651. https://doi.org/10.3390/pharmaceutics14030651
APA StyleLehmann, T., Vaughn, A. E., Seal, S., Liechty, K. W., & Zgheib, C. (2022). Silk Fibroin-Based Therapeutics for Impaired Wound Healing. Pharmaceutics, 14(3), 651. https://doi.org/10.3390/pharmaceutics14030651








