Formation of Ciprofloxacin–Isonicotinic Acid Cocrystal Using Mechanochemical Synthesis Routes—An Investigation into Critical Process Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
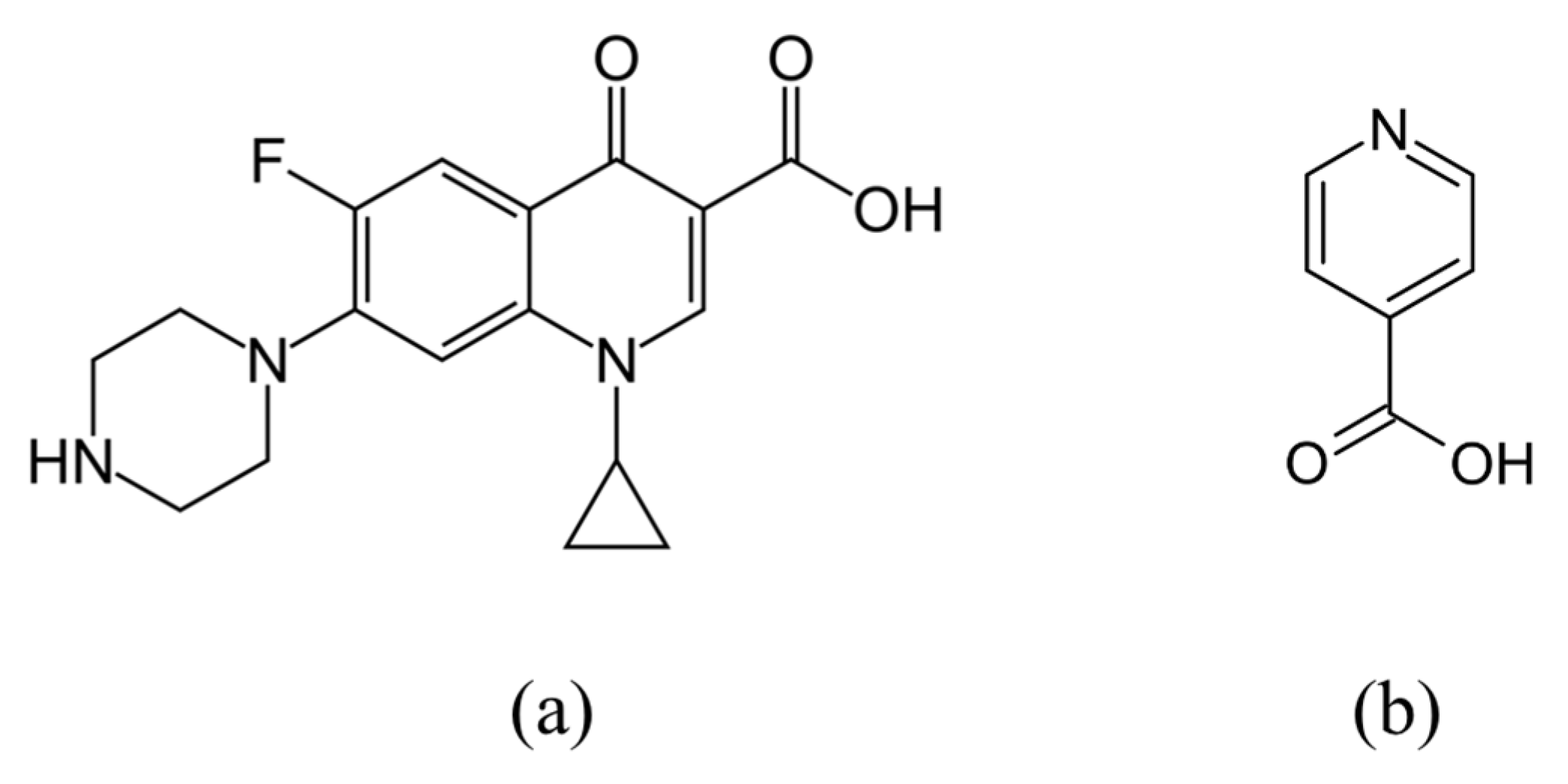
2.2. Materials Selection Rationale
2.3. Cocrystal Preparation Methods
2.3.1. Neat Grinding
2.3.2. Ball Milling
2.3.3. Hot-Melt Extrusion (HME)
2.4. Powder X-ray Diffraction (PXRD)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Scanning Electron Microscopy (SEM)
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Dissolution
3. Results and Discussion
3.1. Grinding
3.1.1. Powder X-ray Diffraction (PXRD)
3.1.2. Differential Scanning Calorimetry (DSC)
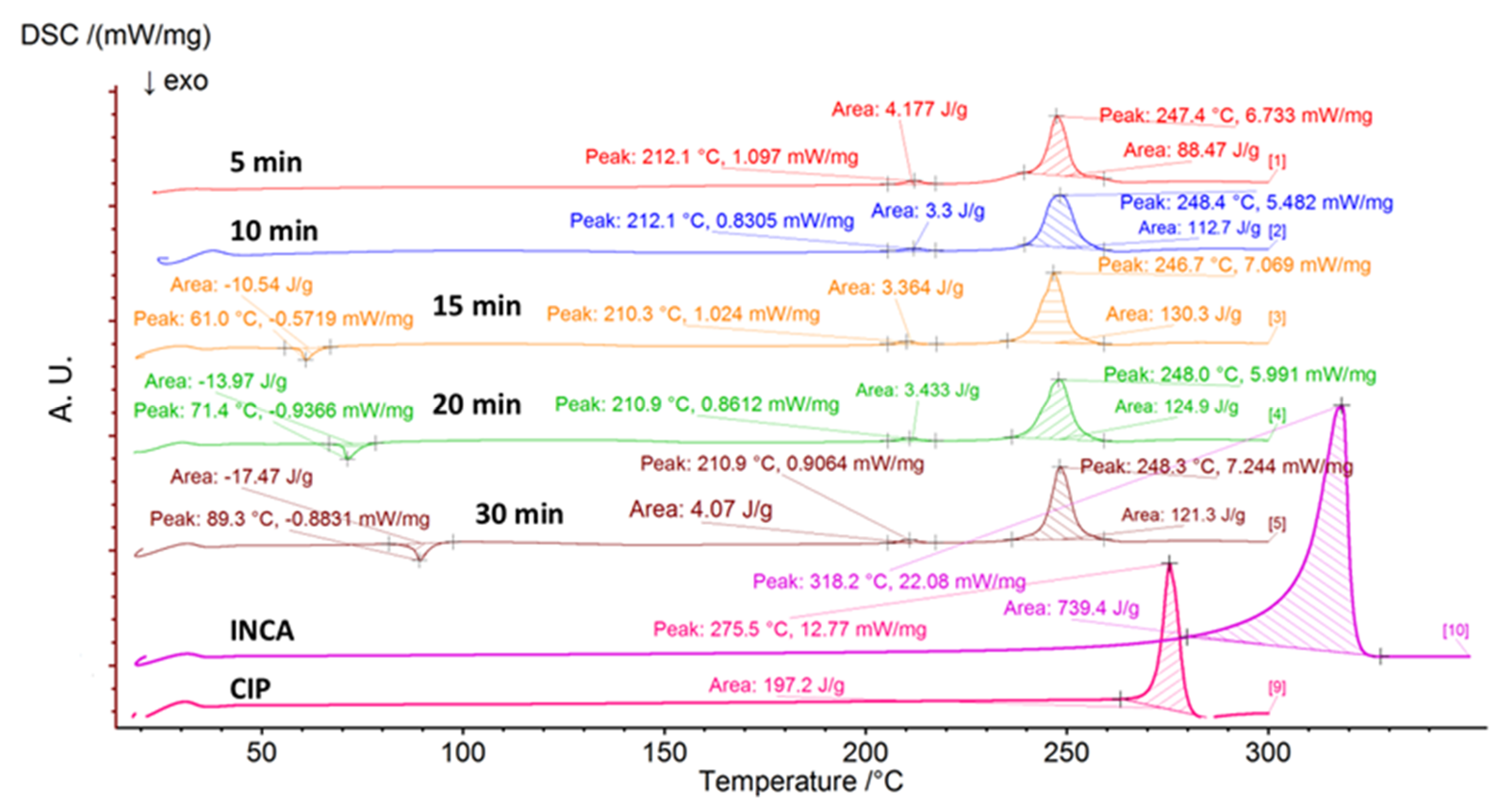
3.2. Ball Milling
3.2.1. Powder X-ray Diffraction (PXRD)
3.2.2. Differential Scanning Calorimetry (DSC)
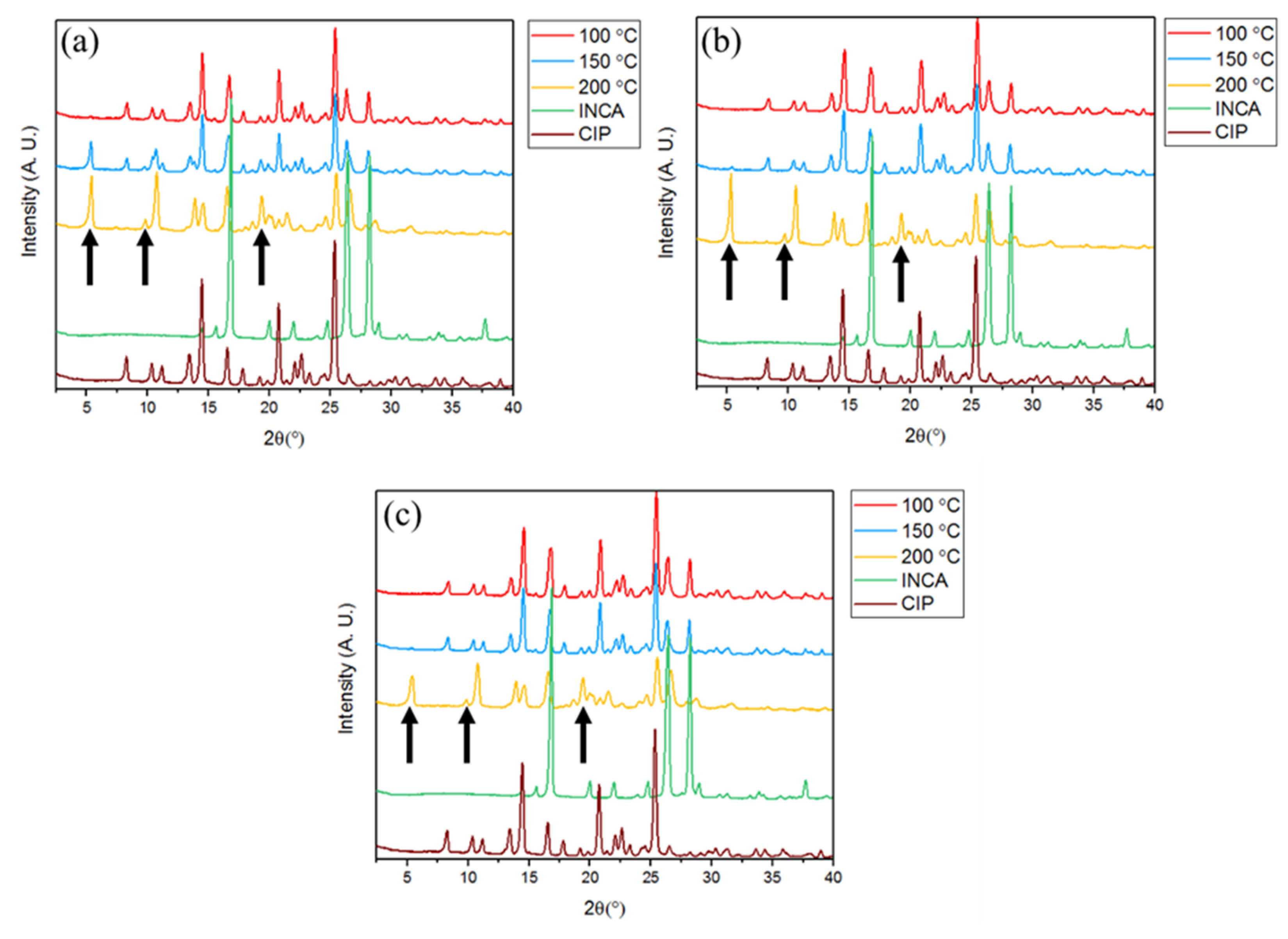
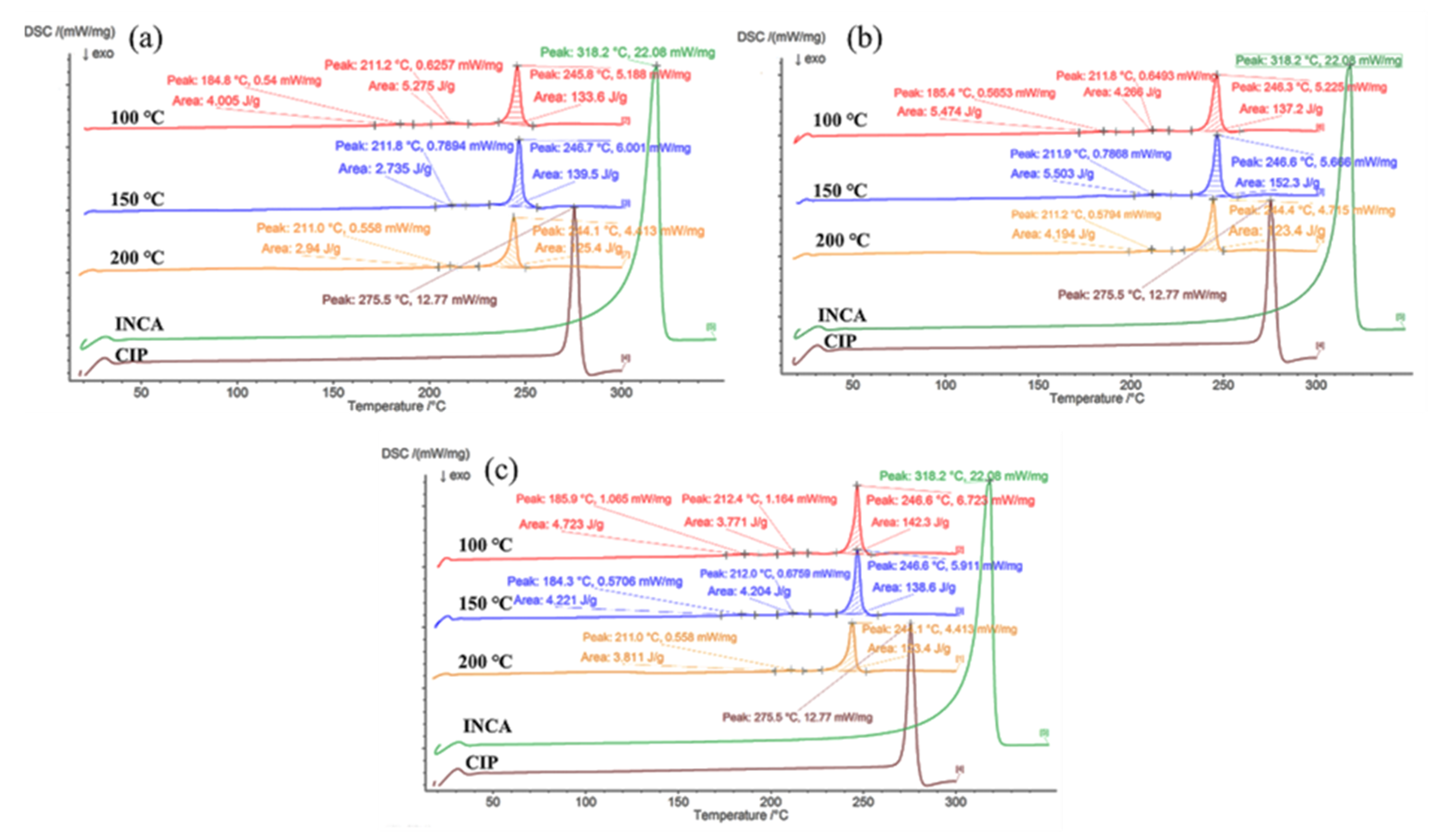
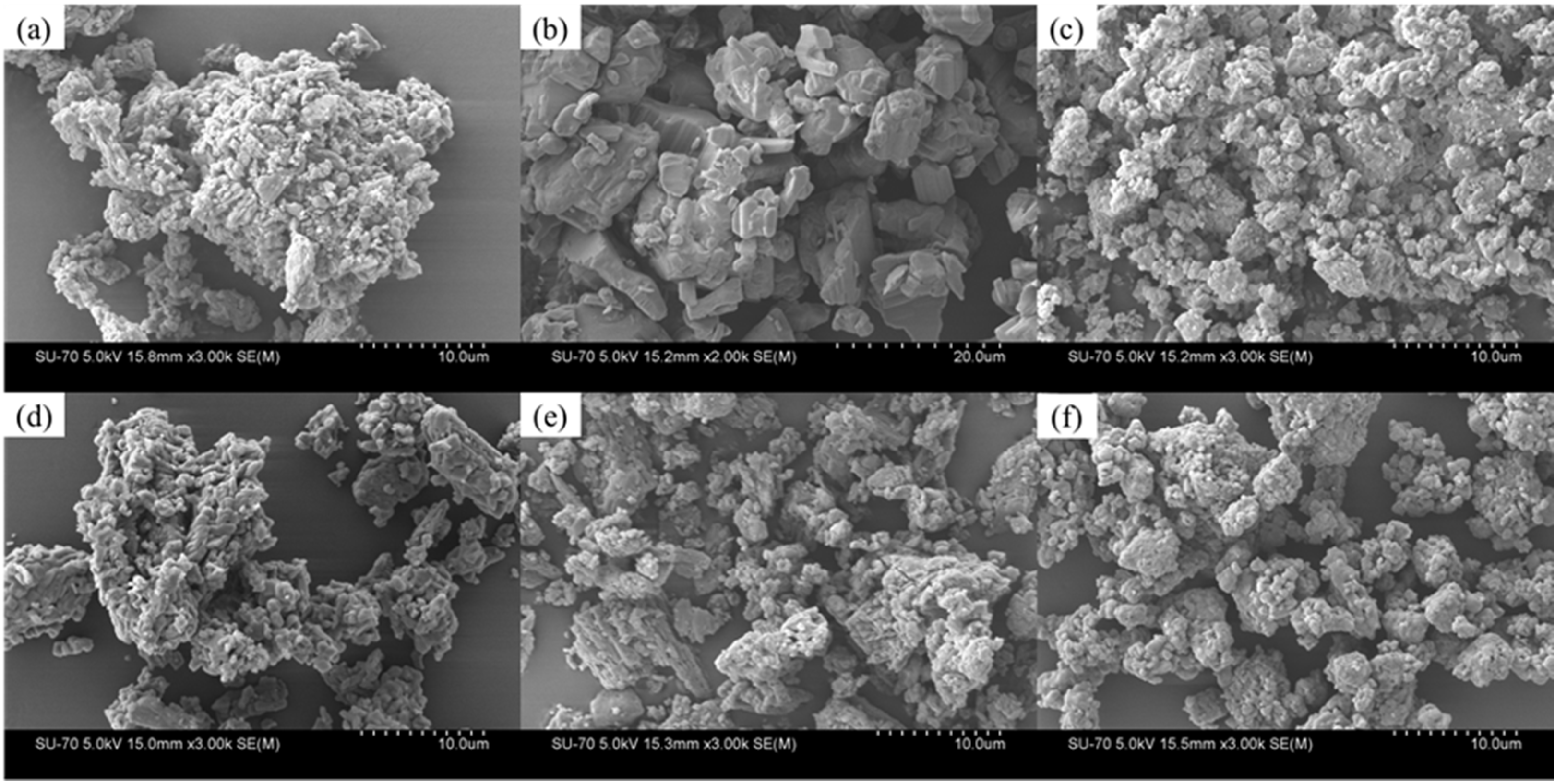
3.3. Hot-Melt Extrusion
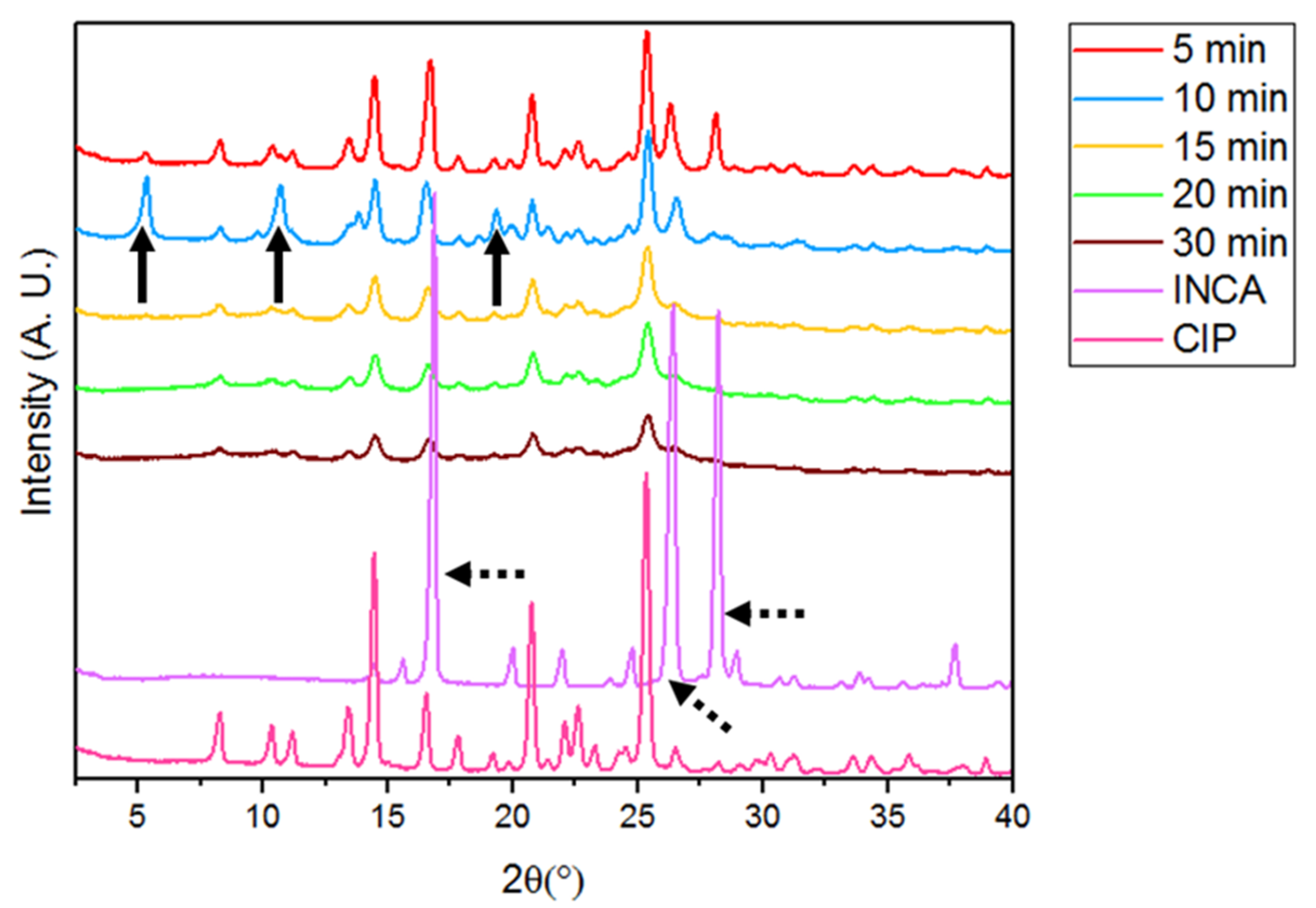
3.3.1. Powder X-ray Diffraction (PXRD)
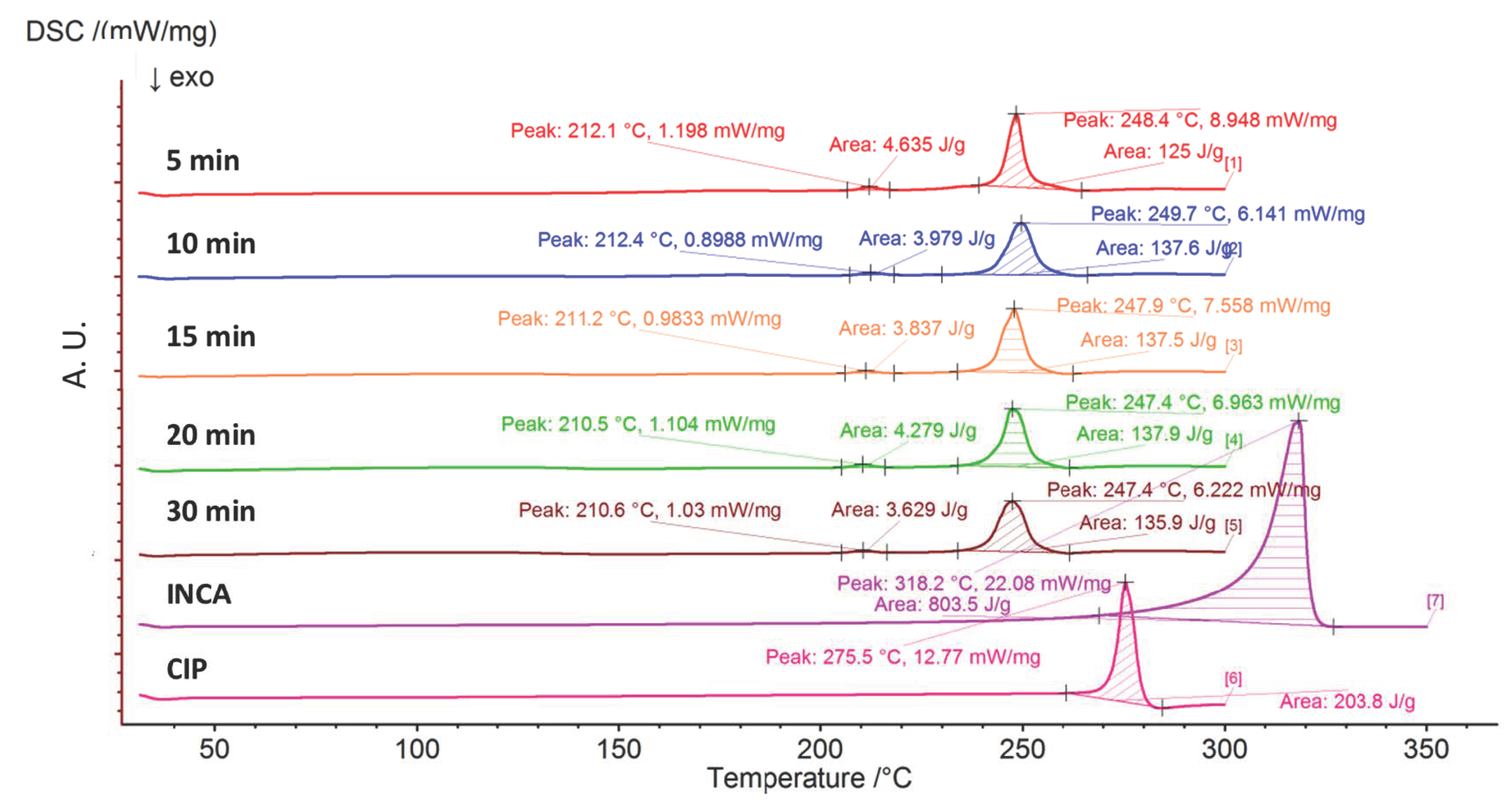
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. Scanning Electron Microscopy (SEM) and Morphological Analysis
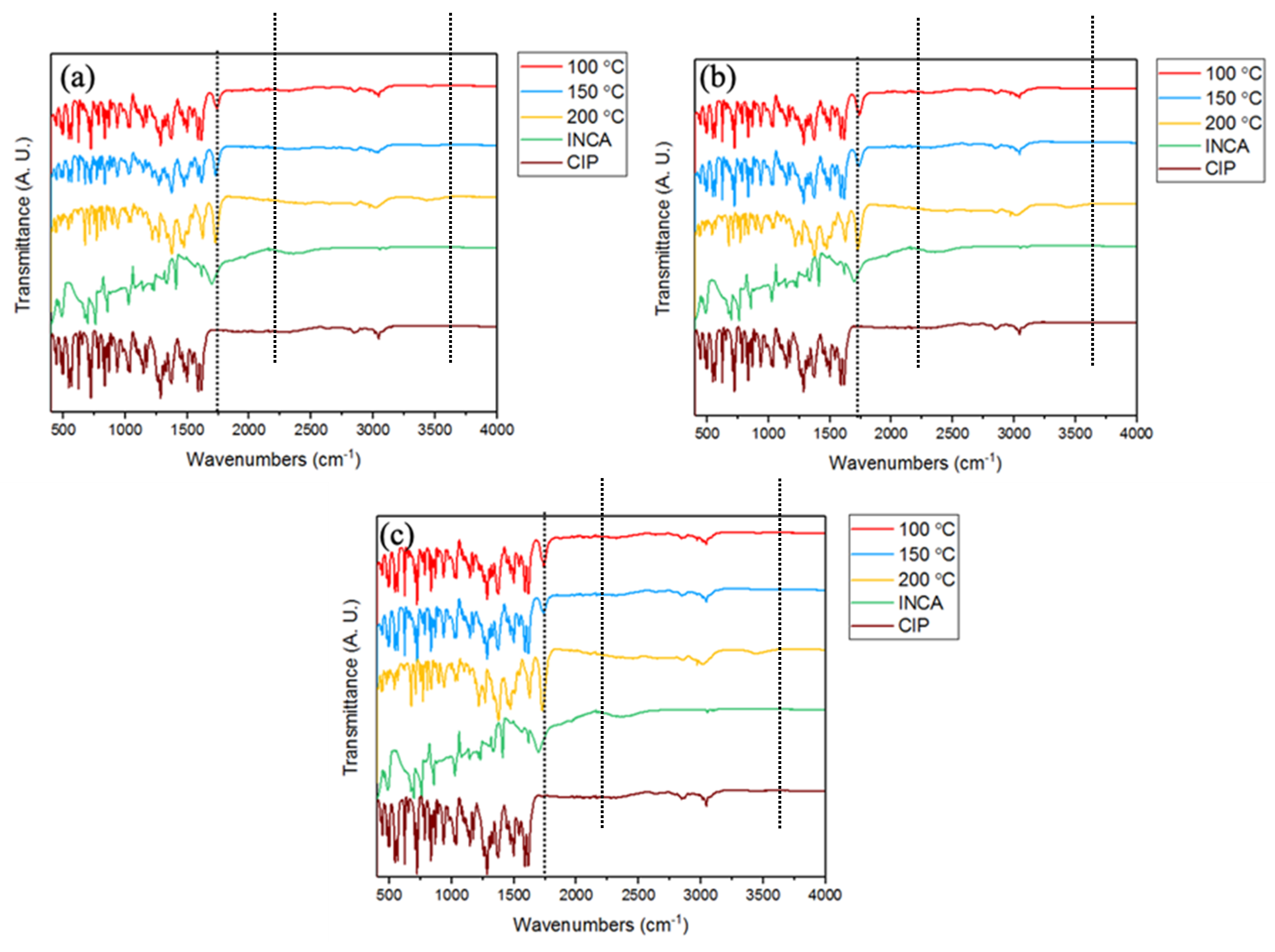
3.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.5. Statistical Analysis
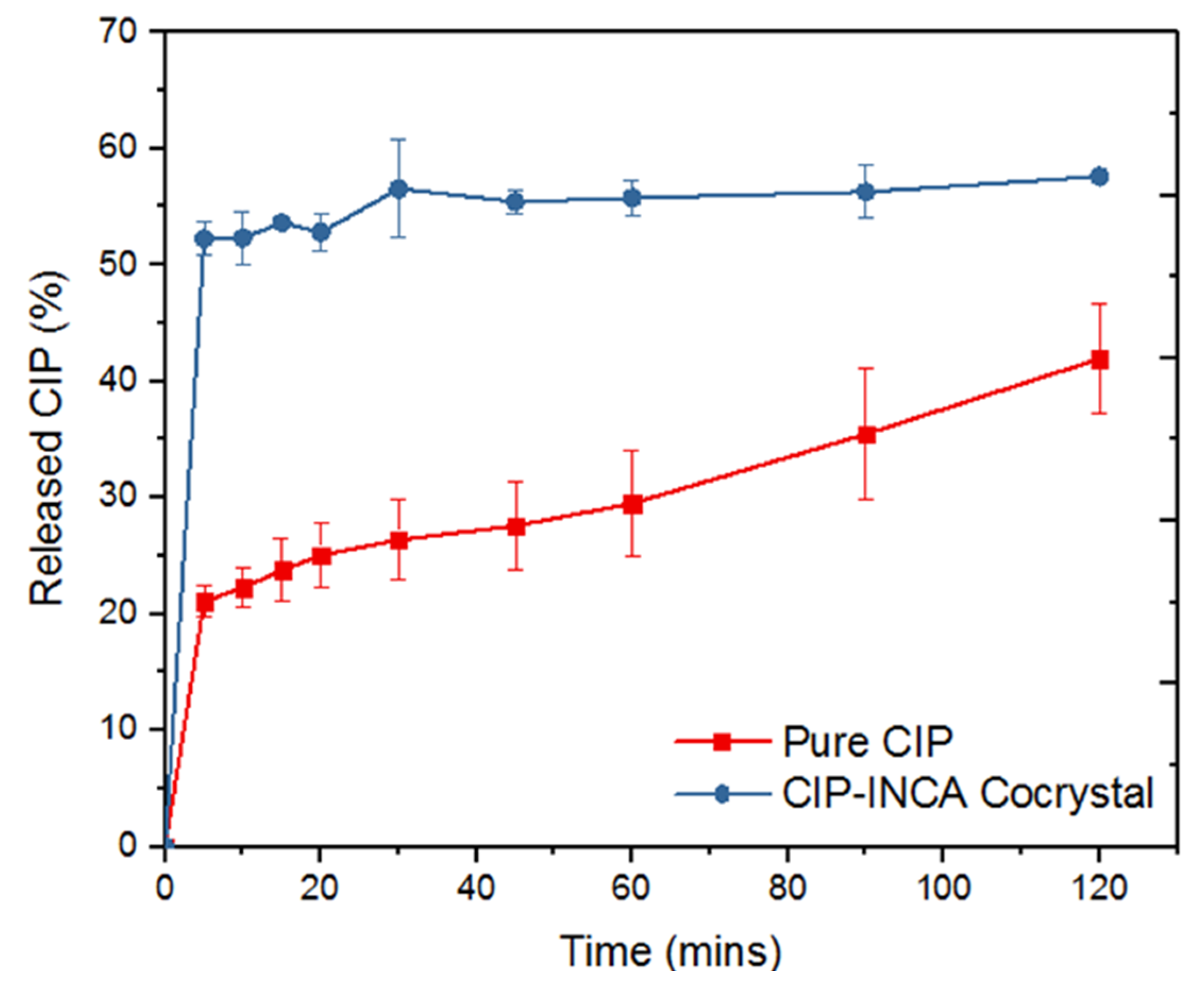
3.3.6. Dissolution Rate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Thayer, A.M. Finding Solutions. Chem. Eng. News Arch. 2010, 88, 13–18. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G.G.; David, J.R.; Myrdal, P.B. Effects of Polymorphism and Solid-State Solvation on Solubility and Dissolution Rate, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Khankari, R.K.; Grant, D.J. Pharmaceutical hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Braun, D.E.; Kahlenberg, V.; Gelbrich, T.; Ludescher, J.; Griesser, U.J. Solid state characterisation of four solvates of R-cinacalcet hydrochloride. CrystEngComm 2008, 10, 1617–1625. [Google Scholar] [CrossRef]
- Tong, H.H.Y.; Chow, A.S.; Chan, H.; Chow, A.H.; Wan, Y.K.; Williams, I.; Shek, F.L.; Chan, C.K. Process-induced phase transformation of berberine chloride hydrates. J. Pharm. Sci. 2010, 99, 1942–1954. [Google Scholar] [PubMed]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Ziaee, A.; Iqbal, J.; O’Reilly, E.; Croker, D.; Walker, G. Impact of polymeric excipient on cocrystal formation via hot-melt extrusion and subsequent downstream processing. Int. J. Pharm. 2019, 566, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ngilirabanga, J.B.; Samsodien, H. Pharmaceutical co-crystal: An alternative strategy for enhanced physicochemical properties and drug synergy. Nano Sel. 2021, 2, 512–526. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Remenar, J.F.; Morissette, S.L.; Peterson, M.L.; Moulton, B.; MacPhee, J.M.; Guzmán, A.R.; Almarsson, Ö. Crystal Engineering of Novel Cocrystals of a Triazole Drug with 1,4-Dicarboxylic Acids. J. Am. Chem. Soc. 2003, 125, 8456–8457. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Gao, Z.; Tian, N.; Li, Z.; Gong, J.; Wang, J.; Rohani, S. Review of melt crystallization in the pharmaceutical field, towards crystal engineering and continuous process development. Chem. Eng. Res. Des. 2021, 166, 268–280. [Google Scholar] [CrossRef]
- Baumgartner, R.; Eitzlmayr, A.; Matsko, N.; Tetyczka, C.; Khinast, J.; Roblegg, E. Nano-extrusion: A promising tool for continuous manufacturing of solid nano-formulations. Int. J. Pharm. 2014, 477, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mortier, S.T.F.C.; Gernaey, K.V.; De Beer, T.; Nopens, I. Analysing drying unit performance in a continuous pharmaceutical manufacturing line by means of mass—Energy balances. Eur. J. Pharm. Biopharm. 2014, 86, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, A.; Li, X.; Evans, J.M.; Barton, P.I. Capacity planning for continuous pharmaceutical manufacturing facilities. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1135–1139. [Google Scholar]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. Int. J. Pharm. 2012, 438, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gernaey, K.V.; De Beer, T.; Nopens, I. Model-based characterisation of twin-screw granulation system for continuous solid dosage manufacturing. In Computer Aided Chemical Engineering; Gernaey, K.V., Huusom, J.K., Gani, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 2165–2170. [Google Scholar]
- Daurio, D.; Medina, C.; Saw, R.; Nagapudi, K.; Alvarez-Núñez, F. Application of Twin Screw Extrusion in the Manufacture of Cocrystals, Part I: Four Case Studies. Pharmaceutics 2011, 3, 582–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imre, S.; Dogaru, M.T.; Vari, C.-E.; Muntean, T.; Kelemen, L. Validation of an HPLC method for the determination of ciprofloxacin in human plasma. J. Pharm. Biomed. Anal. 2003, 33, 125–130. [Google Scholar] [CrossRef]
- Mesallati, H.; Mugheirbi, N.A.; Tajber, L. Two Faces of Ciprofloxacin: Investigation of Proton Transfer in Solid State Transformations. Cryst. Growth Des. 2016, 16, 6574–6585. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.; Wang, D.; Yang, Q.; Li, X.; Chen, H.; Sun, J.; An, H.; Wang, L.; Deng, Y.; Liu, J.; et al. Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci. Total Environ. 2017, 605–606, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Vasilev, N.A.; Churakov, A.V.; Stroh, J.; Emmerling, F.; Perlovich, G.L. Solid Forms of Ciprofloxacin Salicylate: Polymorphism, Formation Pathways, and Thermodynamic Stability. Cryst. Growth Des. 2019, 19, 2979–2990. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl. Clay Sci. 2015, 114, 69–76. [Google Scholar] [CrossRef]
- Kyriacos, S.B.; Boukarim, C.; Safi, W.; Mroueh, M.; Maroun, A.; El-Khoury, G.; Shehayeb, R. In vitro testing of ciprofloxacin formulations and preliminary study on BCS biowaiver. J. Food Drug Anal. 2009, 17, 78–84. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Roussel, P.; Perlovich, G.L. Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid. CrystEngComm 2018, 20, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Tehler, U.; Fagerberg, J.H.; Svensson, R.; Larhed, M.; Artursson, P.; Bergström, C.A.S. Optimizing Solubility and Permeability of a Biopharmaceutics Classification System (BCS) Class 4 Antibiotic Drug Using Lipophilic Fragments Disturbing the Crystal Lattice. J. Med. Chem. 2013, 56, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Ghosh, S.; Khan, H.; Devarapalli, R.; Reddy, C.M. Drug–drug salt forms of ciprofloxacin with diflunisal and indoprofen. CrystEngComm 2014, 16, 7393–7396. [Google Scholar] [CrossRef] [Green Version]
- Basavoju, S.; Boström, D.; Velaga, S.P. Pharmaceutical Salts of Fluoroquinolone Antibacterial Drugs with Acesulfame Sweetener. Mol. Cryst. Liq. Cryst. 2012, 562, 254–264. [Google Scholar] [CrossRef]
- Reddy, J.S.; Ganesh, S.V.; Nagalapalli, R.; Dandela, R.; Solomon, K.A.; Kumar, K.A.; Goud, N.R.; Nangia, A. Fluoroquinolone salts with carboxylic acids. J. Pharm. Sci. 2011, 100, 3160–3176. [Google Scholar] [CrossRef]
- Paluch, K.J.; McCabe, T.; Müller-Bunz, H.; Corrigan, O.I.; Healy, A.M.; Tajber, L. Formation and Physicochemical Properties of Crystalline and Amorphous Salts with Different Stoichiometries Formed between Ciprofloxacin and Succinic Acid. Mol. Pharm. 2013, 10, 3640–3654. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Manin, A.N.; Voronin, A.P.; Drozd, K.V.; Simagina, A.A.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical salts of ciprofloxacin with dicarboxylic acids. Eur. J. Pharm. Sci. 2015, 77, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Golovnev, N.N.; Molokeev, M.S.; Lesnikov, M.K.; Atuchin, V.V. Two salts and the salt cocrystal of ciprofloxacin with thiobarbituric and barbituric acids: The structure and properties. J. Phys. Org. Chem. 2018, 31, e3773. [Google Scholar] [CrossRef] [Green Version]
- Pinto Vitorino, G.; Sperandeo, N.R.; Caira, M.R.; Mazzieri, M.R. A Supramolecular Assembly Formed by Heteroassociation of Ciprofloxacin and Norfloxacin in the Solid State: Co-Crystal Synthesis and Characterization. Cryst. Growth Des. 2013, 13, 1050–1058. [Google Scholar] [CrossRef]
- Martínez-Alejo, J.M.; Domínguez-Chávez, J.G.; Rivera-Islas, J.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H.; Senosiain, J.P. A Twist in Cocrystals of Salts: Changes in Packing and Chloride Coordination Lead to Opposite Trends in the Biopharmaceutical Performance of Fluoroquinolone Hydrochloride Cocrystals. Cryst. Growth Des. 2014, 14, 3078–3095. [Google Scholar] [CrossRef]
- Ràfols, C.; Fael, H.; Fuguet, E.; Outhwaite, B.; Lee, S.; Ruiz, R. Dissolution rates of ciprofloxacin and its cocrystal with resorcinol. ADMET DMPK 2018, 6, 61–70. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, A.C.; Torquetti, C.; Ferreira, P.O.; Fernandes, R.P.; dos Santos, E.C.; Kogawa, A.C.; Caires, F.J. Cocrystals of ciprofloxacin with nicotinic and isonicotinic acids: Mechanochemical synthesis, characterization, thermal and solubility study. Thermochim. Acta 2020, 685, 178346. [Google Scholar] [CrossRef]
- Torquetti, C.; Ferreira, P.O.; de Almeida, A.C.; Fernandes, R.P.; Caires, F.J. Thermal study and characterization of new cocrystals of ciprofloxacin with picolinic acid. J. Therm. Anal. Calorim. 2021, 147, 1299–1306. [Google Scholar] [CrossRef]
- de Almeida, A.C.; Ferreira, P.O.; Torquetti, C.; Ekawa, B.; Carvalho, A.C.S.; dos Santos, E.C.; Caires, F.J. Mechanochemical synthesis, characterization and thermal study of new cocrystals of ciprofloxacin with pyrazinoic acid and p-aminobenzoic acid. J. Therm. Anal. Calorim. 2020, 140, 2293–2303. [Google Scholar] [CrossRef]
- Roy, P.; Ghosh, A. Progress on cocrystallization of poorly soluble NME’s in the last decade. CrystEngComm 2020, 22, 6958–6974. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Kan, S.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In Vitro Drug Release, Permeability, and Structural Test of Ciprofloxacin-Loaded Nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Jensen, K.T.; Löbmann, K.; Rades, T.; Grohganz, H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino Acid, proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Grohganz, H.; Rades, T.; Löbmann, K. Development of a screening method for co-amorphous formulations of drugs and amino acids. Eur. J. Pharm. Sci. 2016, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C.; Nanda, U.N.; Naik, S. FTIR and XRD investigations of some fluoroquinolones. Int. J. Pharm. Pharm. Sci. 2011, 3, 165–170. [Google Scholar]
- Polishchuk, A.V.; Karaseva, E.T.; Emelina, T.; Cramariuc, O.; Karasev, V.E. Polymorphism and Intramolecular Proton Transfer in Fluoroquinolone Compounds. J. Fluoresc. 2011, 21, 2117. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Dobrowolski, J.; Lewandowski, W.; Mazurek, A. Experimental and theoretical IR and Raman spectra of picolinic, nicotinic and isonicotinic acids. J. Mol. Struct. 2003, 655, 89–95. [Google Scholar] [CrossRef]
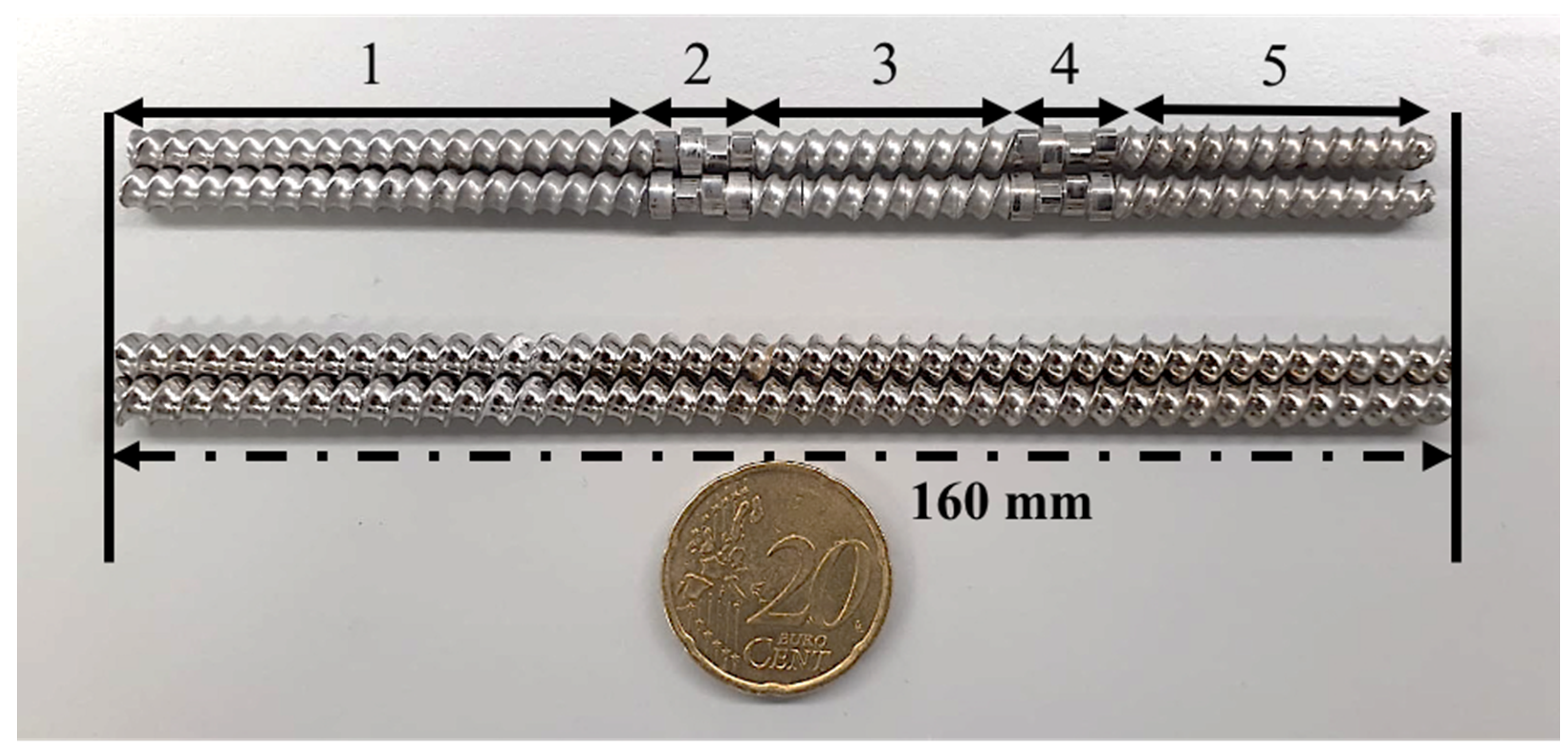



| Number of Experiments | Temperature (°C) | Screw Speed (rpm) | Screw Configuration | Relative CC Yield (%) |
|---|---|---|---|---|
| 1 | 100 | 55 | conveying | 41.3 |
| 2 | 100 | 10 | conveying | 43.5 |
| 3 | 150 | 10 | conveying | 61.9 |
| 4 | 200 | 55 | kneading | _ |
| 5 | 200 | 10 | kneading | _ |
| 6 | 200 | 100 | conveying | 81.2 |
| 7 | 100 | 55 | kneading | 51.4 |
| 8 | 100 | 100 | conveying | 42.3 |
| 9 | 150 | 100 | Conveying | 81.2 |
| 10 | 200 | 55 | conveying | 100 |
| 11 | 150 | 55 | conveying | 45.6 |
| 12 | 150 | 55 | conveying | 48.2 |
| 13 | 150 | 10 | kneading | 89.5 |
| 14 | 200 | 100 | kneading | _ |
| 15 | 100 | 10 | kneading | 39.5 |
| 16 | 100 | 100 | kneading | 56.7 |
| 17 | 150 | 100 | kneading | 98.6 |
| 18 | 150 | 55 | kneading | 91.7 |
| 19 | 200 | 10 | conveying | 93.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi-Jafari, M.; Ziaee, A.; O’Reilly, E.; Croker, D.; Walker, G. Formation of Ciprofloxacin–Isonicotinic Acid Cocrystal Using Mechanochemical Synthesis Routes—An Investigation into Critical Process Parameters. Pharmaceutics 2022, 14, 634. https://doi.org/10.3390/pharmaceutics14030634
Karimi-Jafari M, Ziaee A, O’Reilly E, Croker D, Walker G. Formation of Ciprofloxacin–Isonicotinic Acid Cocrystal Using Mechanochemical Synthesis Routes—An Investigation into Critical Process Parameters. Pharmaceutics. 2022; 14(3):634. https://doi.org/10.3390/pharmaceutics14030634
Chicago/Turabian StyleKarimi-Jafari, Maryam, Ahmad Ziaee, Emmet O’Reilly, Denise Croker, and Gavin Walker. 2022. "Formation of Ciprofloxacin–Isonicotinic Acid Cocrystal Using Mechanochemical Synthesis Routes—An Investigation into Critical Process Parameters" Pharmaceutics 14, no. 3: 634. https://doi.org/10.3390/pharmaceutics14030634
APA StyleKarimi-Jafari, M., Ziaee, A., O’Reilly, E., Croker, D., & Walker, G. (2022). Formation of Ciprofloxacin–Isonicotinic Acid Cocrystal Using Mechanochemical Synthesis Routes—An Investigation into Critical Process Parameters. Pharmaceutics, 14(3), 634. https://doi.org/10.3390/pharmaceutics14030634







