Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Fabrication of PGA/SF and PGA/SF-DFO Nanofibrous Scaffolds
2.3. Physicochemical Characterization of PGA/SF and PGA/SF-DFO Nanofibrous Scaffolds
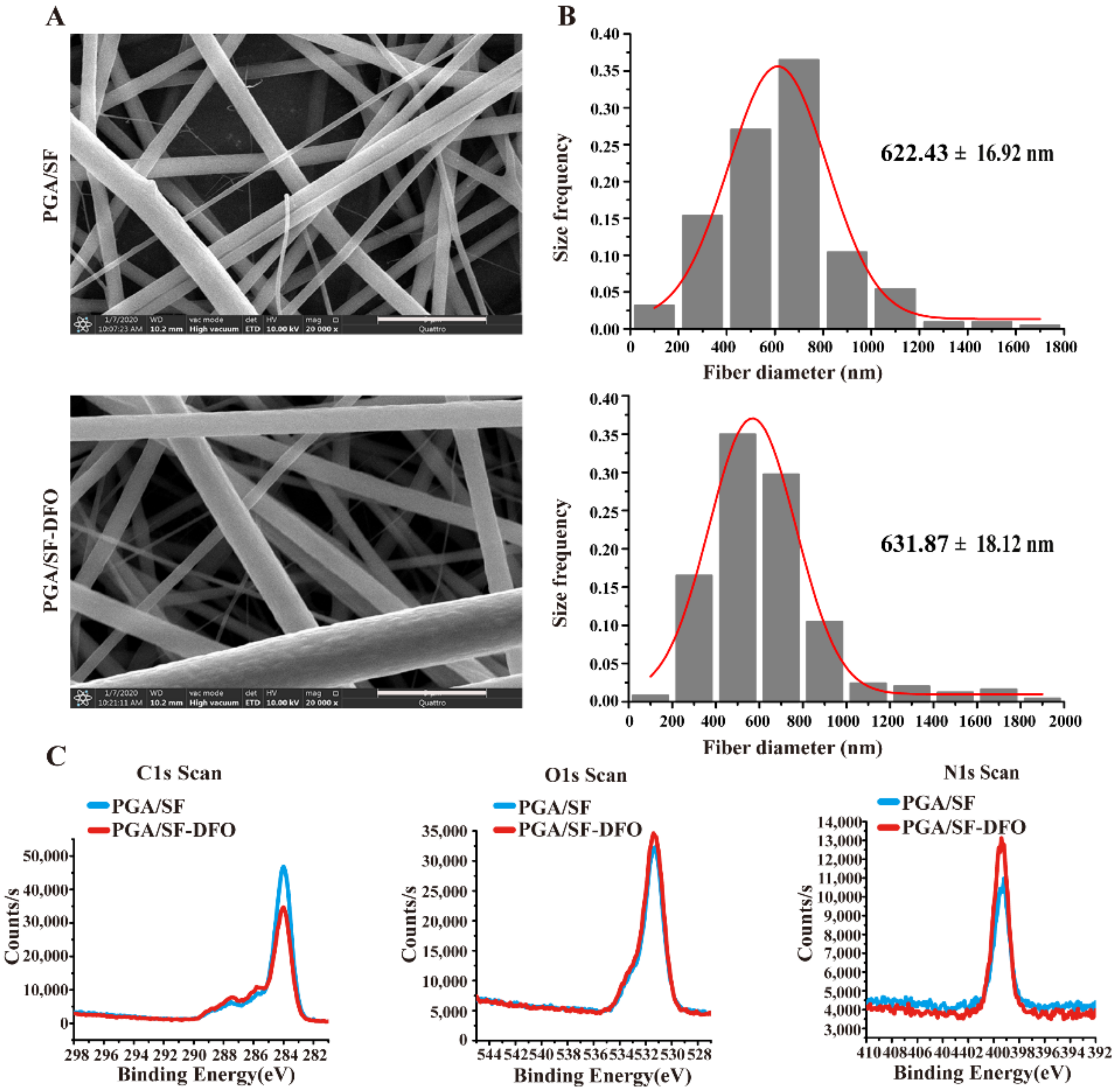
2.3.1. Surface Morphology Analysis
2.3.2. Hydrophilicity Analysis
2.3.3. Chemical Component Analysis
2.3.4. Mechanical Test In Vitro
2.3.5. Drug Release In Vitro
2.3.6. Scaffolds Degradation In Vitro
2.4. Effects of PGA/SF and PGA/SF-DFO Scaffolds on HUVECs In Vitro
2.4.1. HUVECs Adhesion In Vitro
2.4.2. Cell Viability and Proliferation
2.4.3. Migration Assay In Vitro
2.4.4. Tube Formation Assay In Vitro
2.5. Wound Healing Evaluation In Vivo
2.5.1. Induction of Diabetic Mice
2.5.2. Excisional Wound Model Preparation
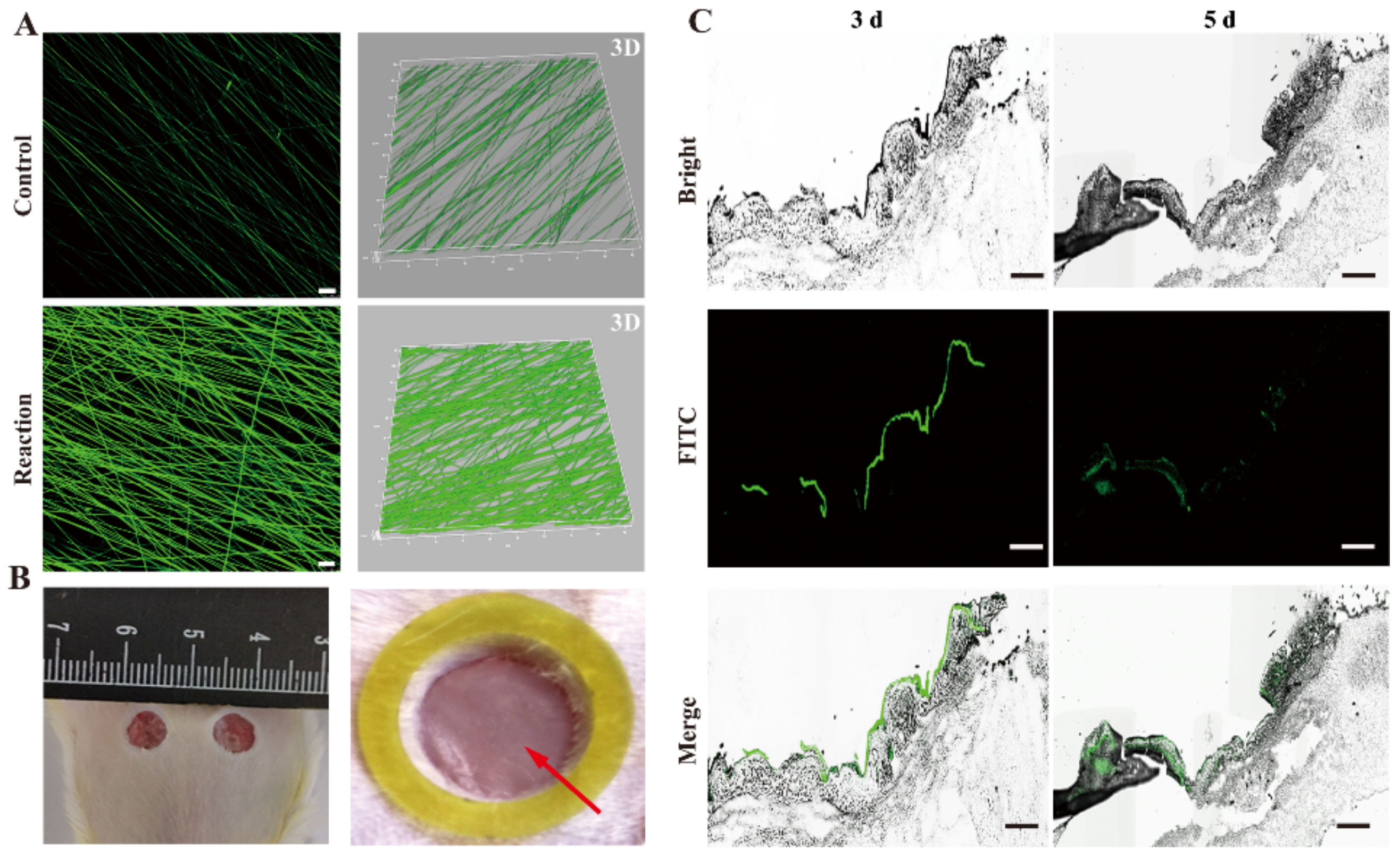
2.5.3. Scaffolds Degradation In Vivo
2.5.4. Wound Closure Measurement
2.5.5. Finite Element Modeling Analysis
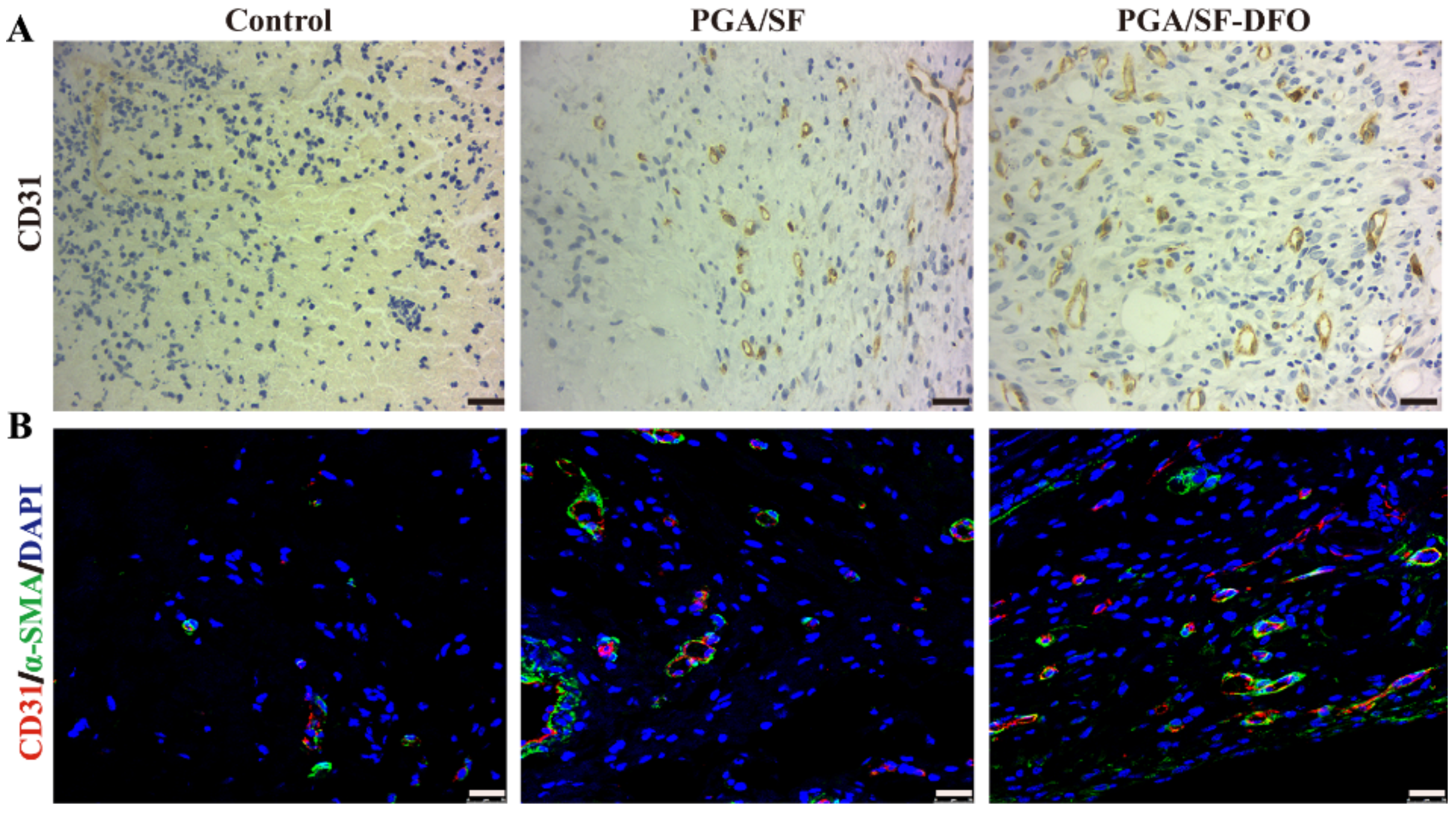
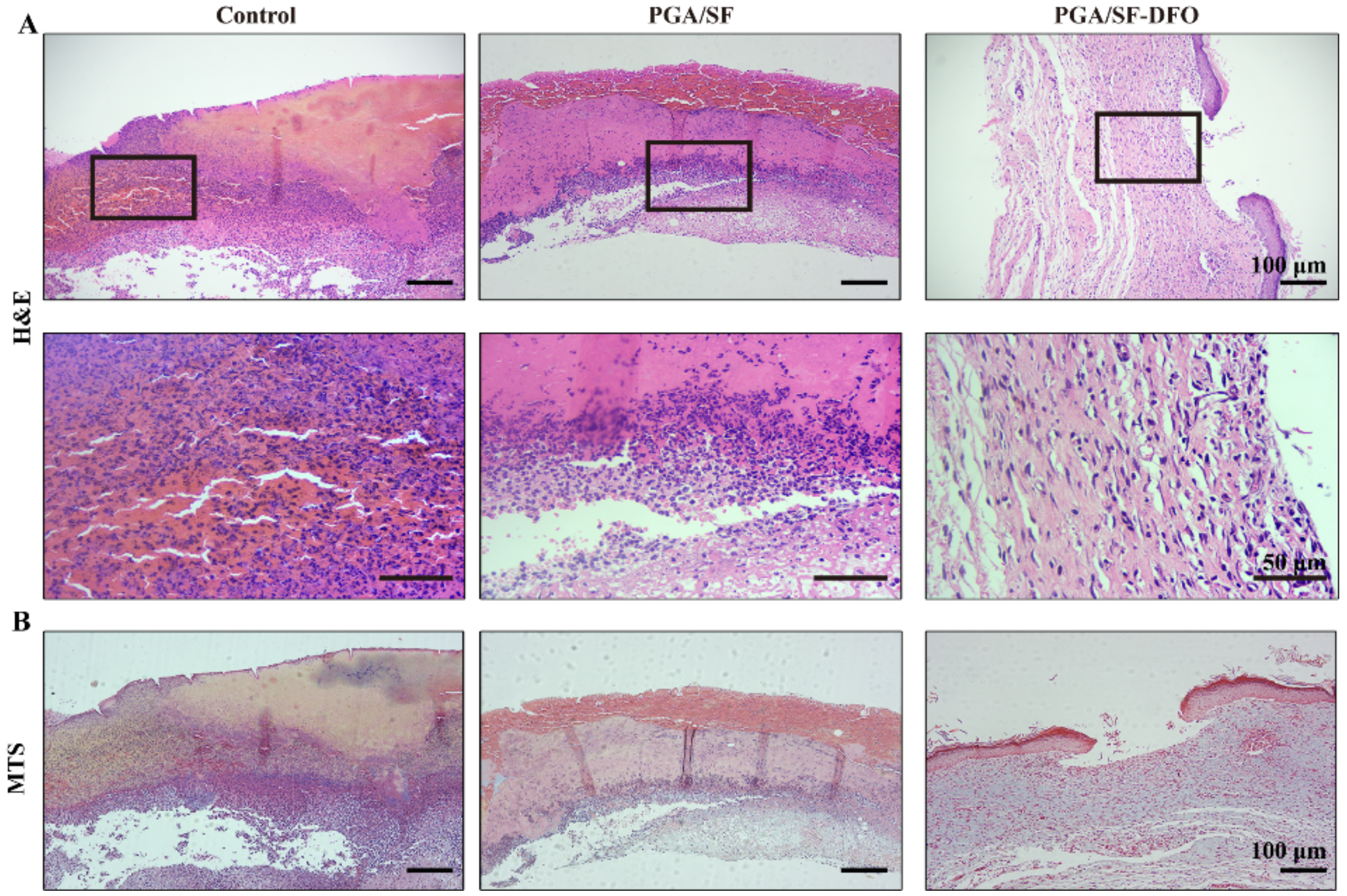
2.5.6. Histologic Analysis
2.5.7. Mechanical Property Evaluation In Vivo
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of PGA/SF-DFO Nanofibrous Scaffolds
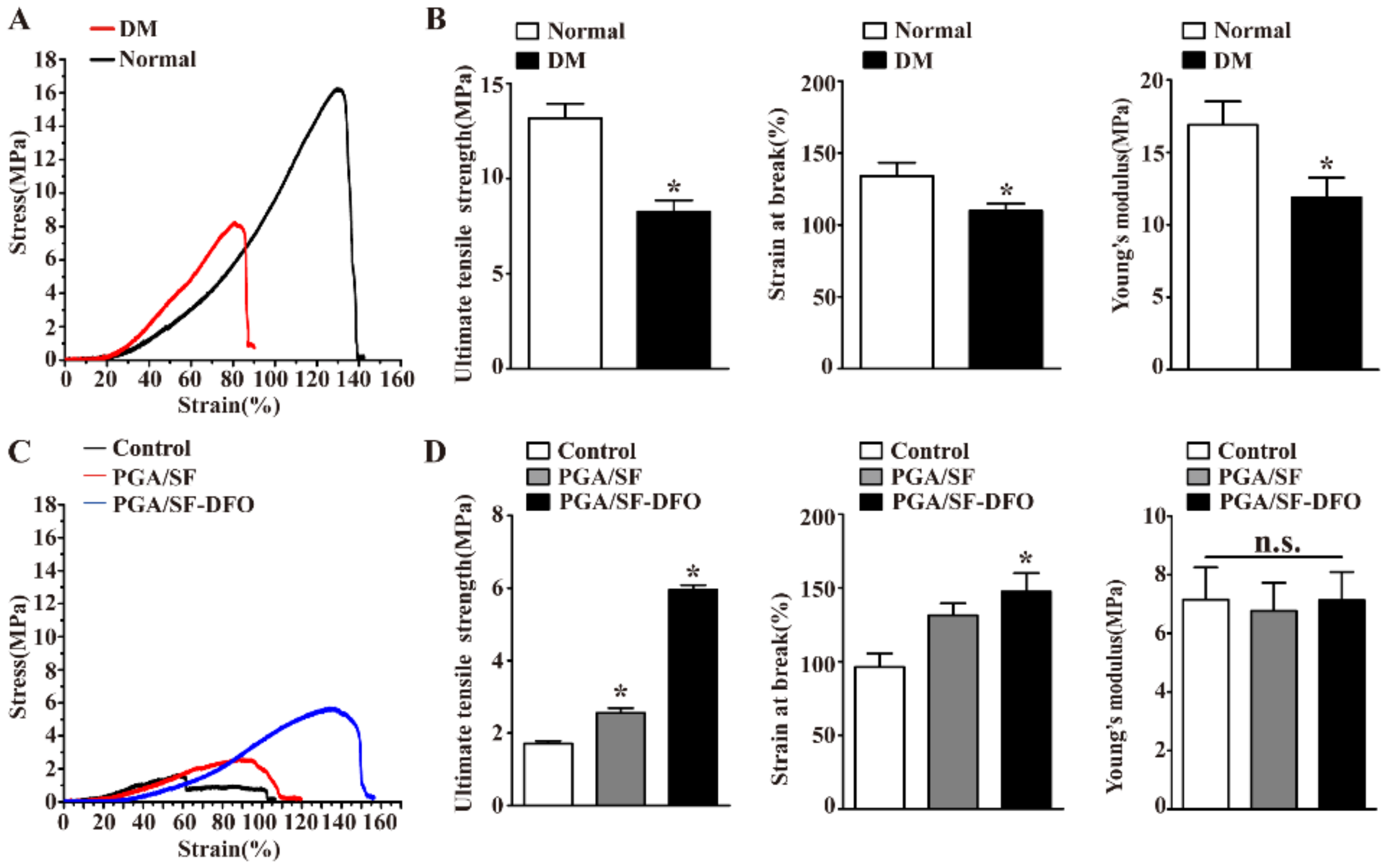
3.2. Mechanical Properties of Different Scaffolds
3.3. Drug Release Behavior and Biodegradable Profile of Different Scaffolds
3.4. Effects of PGA/SF and PGA/SF-DFO Scaffolds on Adhesion, Proliferation, Migration, and Tube Formation of HUVECs In Vitro
3.5. Scaffolds Degradation In Vivo
3.6. In Vivo Wound Healing
3.7. Silk Fibroin-Based Scaffolds Remodeled the Mechanical Properties of Wounds
3.8. Histopathological Analysis
3.9. Evaluation of Mechanical Properties of Impaired Wounds with Different Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Monteiro-Soares, M.; Russell, D.; Boyko, E.J.; Jeffcoate, W.; Mills, J.L.; Morbach, S.; Game, F. Guidelines on the classification of diabetic foot ulcers(IWGDF 2019). Diabetes. Metab. Res. Rev. 2020, 36, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Liu, D.; He, X.; Shen, Y.; Zhao, Y.; Hou, X.; Chen, B.; OuYang, W.; Dai, J.; Li, X. A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater Sci. 2020, 8, 6337–6349. [Google Scholar] [CrossRef]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K.; Gallagher, K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diab. Rep. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin Wound Healing Assisted by Angiogenic Targeted Tissue Engineering: A Comprehensive Review of Bioengineered Approaches. J. Biomed. Mater. Res. Part A 2020, 109, 453–478. [Google Scholar] [CrossRef]
- Caskey, R.C.; Zgheib, C.; Morris, M.; Allukian, M.; Dorsett-Martin, W.; Xu, J.; Wu, W.; Liechty, K.W. Dysregulation of collagen production in diabetes following recurrent skin injury: Contribution to the development of a chronic wound. Wound Repair Regen. 2014, 22, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, S.M.; Louiselle, A.E.; Hilton, S.A.; Dewberry, L.C.; Zhang, L.; Azeltine, M.; Xu, J.; Singh, S.; Sakthivel, T.S.; Seal, S.; et al. Nanosilk Increases the Strength of Diabetic Skin and Delivers CNP-miR146a to Improve Wound Healing. Front. Immunol. 2020, 11, 590285. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Choi, C.; Cheung, A.K.; Chun, M.; Ng, H.; Zheng, Y.; Jan, Y.; Lai, G.; Cheing, Y. Indentation Stiffness Measurement by an Optical Coherence Indentation System Can Reflect Type I Collagen Abundance and Organisation in Diabetic Wounds. Front Bioeng Biotechnol. 2021, 9, 648453. [Google Scholar] [CrossRef]
- Bayer, I.S. A review of sustained drug release studies from nanofiber hydrogels. Biomedicines 2021, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Han, Y.; Li, F.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2020, 17, 100247. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and Fabrication of Collagen-Coated Ostholamide Electrospun Nanofiber Scaffold for Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Bera, H.; Zhang, H.; Chen, Y.; Cun, D.; Foderà, V.; Yang, M. α-Lactalbumin Based Nanofiber Dressings Improve Burn Wound Healing and Reduce Scarring. ACS Appl. Mater. Interfaces 2020, 12, 45702–45713. [Google Scholar] [CrossRef]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G. Upregulating Hif-1α by Hydrogel Nanofibrous Scaffolds for Rapidly Recruiting Angiogenesis Relative Cells in Diabetic Wound. Adv Health Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef]
- Alven, S.; Buyana, B.; Feketshane, Z.; Atim, A.B. Electrospun Nanofibers/Nanofibrous Scaffolds Loaded with Silver Nanoparticles as Effective Antibacterial Wound Dressing Materials. Pharmaceutics 2021, 13, 964. [Google Scholar] [CrossRef]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated Chitosan Rescues Dysfunctional Macrophages and Accelerates Wound Healing in Diabetic Mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, Z.; Wei, J.; Li, L.; Peng, H.; Yang, A.; Li, H.; Lv, G. Degradability and biocompatibility of bioglass/poly(amino acid) composites with different surface bioactivity as bone repair materials. J. Appl. Polym. Sci. 2021, 138, 49751. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, B.C.; Huang, W.; Kaltbeitzel, A.; Kizisavas, G.; Crespy, D.; Zhang, K.A.I.; Landfester, K. Visible light active nanofibrous membrane for antibacterial wound dressing. Nanoscale Horizons 2018, 3, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Jiang, L.; Xu, C.; Kai, D.; Fan, X.; You, M.; Hui, C.M.; Wu, C.; Wu, Y.L.; Li, Z. Engineered Janus amphipathic polymeric fiber films with unidirectional drainage and anti-adhesion abilities to accelerate wound healing. Chem. Eng. J. 2021, 421, 127725. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Qi, X.; Hang, C.; Sun, Y.; Peng, Z.; Wei, W.; Wang, Y. Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation. Acta Biomater. 2019, 91, 99–111. [Google Scholar] [CrossRef]
- Serban, M.A.; Panilaitis, B.; Kaplan, D.L. Silk fibroin and polyethylene glycol-based biocompatible tissue adhesives. J. Biomed. Mater. Res. A 2011, 98, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Tanaka, T.; Tanaka, R. Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk Vascular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 5561–5577. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Guo, P.; Duan, T.; Cheng, W.; Guo, Y.; Zheng, X.; Lu, G.; Lu, Q.; Kaplan, D.L. Injectable Desferrioxamine-Laden Silk Nanofiber Hydrogels for Accelerating Diabetic Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1147–1158. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Kim, B.N.; Ko, Y.G.; Yeo, T.; Kim, E.J.; Kwon, O.K.; Kwon, O.H. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber-Poly(glycolic acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, R.; Li, X.; Cao, Y.; Huang, K.; Ding, J.; Liu, M.; Feng, Z.; Yin, S.; Ma, J.; et al. CD271 promotes STZ-induced diabetic wound healing and regulates epidermal stem cell survival in the presence of the pTrkA receptor. Cell Tissue Res. 2020, 379, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chang, C.; Li, J.; Li, Y.; Niu, X.; Zhang, D.; Li, L.; Gao, J. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis. Drug Deliv. 2018, 25, 1779–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, P.; Nair, L.S. Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration. Tissue Eng. B Rev. 2019, 25, 461–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, Y.; Cornelia, R.; Swisher, S.; Kim, H. Regulation of VEGF expression by HIF-1α in the femoral head cartilage following ischemia osteonecrosis. Sci. Rep. 2012, 2, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Sun, T.; Zhong, R.; You, C.; Tian, M. PEGylation of Deferoxamine for Improving the Stability, Cytotoxicity, and Iron-Overload in an Experimental Stroke Model in Rats. Front. Bioeng. Biotechnol. 2020, 8, 592294. [Google Scholar] [CrossRef]
- Porter, J.B.; Faherty, A.; Stallibrass, L. A Trial to Investigate the Relationship between DFO Pharmacokinetics and Metabolism and DFO-Related Toxicity. Ann. N. Y. Acad. Sci. 1998, 850, 483–487. [Google Scholar] [CrossRef]
- Zafari, M.; Aghamohammady, A.; Mosavy, M. Renal function in thalassemia major patients who treated by Desferal. Bangladesh J. Med. Sci. 2018, 17, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, L.; Baquey, C.; Bareille, R.; Lefebvre, F.; Lauroua, C.; Guerin, V.; Rouais, F.; More, N.; Vergnes, C.; Anderson, J.M. Endothelial cell compatibility testing of three different Pellethanes. J. Biomed. Mater. Res. 1993, 27, 1367–1381. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Zhao, Y.; Ma, P.; Zha, S.; Chen, P.; Lu, H.; Jiang, X.; Wan, S.; Luo, J.; et al. Dual-Peptide-Functionalized Nanofibrous Scaffolds Recruit Host Endothelial Progenitor Cells for Vasculogenesis to Repair Calvarial Defects. ACS Appl. Mater. Interfaces 2020, 12, 3474–3493. [Google Scholar] [CrossRef]
- Ai, C.; Cai, J.; Zhu, J.; Zhou, J.; Jiang, J.; Chen, S. Effect of PET graft coated with silk fibroin: Via EDC/NHS crosslink on graft-bone healing in ACL reconstruction. RSC Adv. 2017, 7, 51303–51312. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Liu, Y.; Tao, J.; Baumgarten, K.M.; Sun, H. Hypoxia-Mimicking Nanofibrous Scaffolds Promote Endogenous Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 32450–32459. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Li, Q.; Wang, J.; Chen, P.; Jiang, S. Ellagic acid attenuates streptozocin induced diabetic nephropathy via the regulation of oxidative stress and inflammatory signaling. Food Chem. Toxicol. 2018, 123, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017, 8, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.; Zhao, L.; Zheng, Y. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 Diabetes mice induced by High-Fat Diet combining with Streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef]
- Shan, Q.; Zheng, G.; Zhu, A.; Cao, L.; Lu, J.; Wu, D.; Zhang, Z.F.; Fan, S.; Sun, C.; Hu, B.; et al. Epigenetic modification of miR-10a regulates renal damage by targeting CREB1 in type 2 diabetes mellitus. Toxicol. Appl. Pharmacol. 2016, 306, 134–143. [Google Scholar] [CrossRef]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green Tea Derivative Driven Smart Hydrogels with Desired Functions for Chronic Diabetic Wound Treatment. Adv. Funct. Mater. 2021, 3, 2009442. [Google Scholar] [CrossRef]
- Rebalka, I.A.; Cao, A.W.; Raleigh, M.J.; Henriksbo, B.D.; Coleman, S.K.; Schertzer, J.D.; Hawke, T.J. Statin therapy negatively impacts skeletal muscle regeneration and cutaneous wound repair in type 1 diabetic mice. Front. Physiol. 2017, 8, 1088. [Google Scholar] [CrossRef]
- Eshel, H.; Lanir, Y. Effects of strain level and proteoglycan depletion on preconditioning and viscoelastic responses of rat dorsal skin. Ann. Biomed. Eng. 2001, 29, 164–172. [Google Scholar] [CrossRef]
- Özyazgan, I.; Liman, N.; Dursun, N.; Güneş, I. The effects of ovariectomy on the mechanical properties of skin in rats. Maturitas 2002, 43, 65–74. [Google Scholar] [CrossRef]
- Vogel, H.G. Antagonistic effect of aminoacetonitrile and prednisolone on mechanical properties of rat skin. Biochim. Biophys. Acta 1971, 252, 580–585. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Solouk, A.; Mirzadeh, H. Improvement of the Electrospinnability of Silk Fibroin Solution by Atmospheric Pressure Plasma Treatment. Fibers Polym. 2019, 20, 1594–1600. [Google Scholar] [CrossRef]
- Lee, H.; Jang, C.H.; Kim, G.H. A polycaprolactone/silk-fibroin nanofibrous composite combined with human umbilical cord serum for subacute tympanic membrane perforation; An in vitro and in vivo study. J. Mater. Chem. B 2014, 2, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, L.; Dong, L.; Wang, S.; Huang, Z.; Qian, Y.; Wang, C.; Liu, W.; Yang, L. Gradient Biomineralized Silk Fibroin Nanofibrous Scaffold with Osteochondral Inductivity for Integration of Tendon to Bone. ACS Biomater. Sci. Eng. 2021, 7, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Kumar, A.; Poddar, S.; Kumar, S. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Boehm, R.D.; Daniels, J.; Stafslien, S.; Nasir, A.; Lefebvre, J.; Narayan, R.J. Polyglycolic acid microneedles modified with inkjet-deposited antifungal coatings. Biointerphases 2015, 10, 011004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignesh, S.; Sivashanmugam, A.; Annapoorna, M.; Janarthanan, R.; Subramania, I.; Shantikumar V., N.; Jayakumar, R. Injectable deferoxamine nanoparticles loaded chitosan-hyaluronic acid coacervate hydrogel for therapeutic angiogenesis. Colloids Surfaces B Biointerfaces 2018, 161, 129–138. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Bhutto, M.A.; Zhu, T.; Yu, K.; Bao, J.; Morsi, Y.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Fabrication and characterization of Antheraea pernyi silk fibroin-blended P(LLA-CL) nanofibrous scaffolds for peripheral nerve tissue engineering. Front. Mater. Sci. 2017, 11, 22–32. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X.; Xu, Y.; Liu, J.; Lv, X. Fabrication of hydrophilic and underwater superoleophobic SiO2/silk fibroin coated mesh for oil/water separation. J. Environ. Chem. Eng. 2021, 9, 105085. [Google Scholar] [CrossRef]
- Özcan, B.; Sezgintürk, M.K. A novel and disposable GP- based impedimetric biosensor using electropolymerization process with PGA for highly sensitive determination of leptin: Early diagnosis of childhood obesity. Talanta 2021, 225, 121985. [Google Scholar] [CrossRef]
- Onnizzo, B.R.K.C.; Hatt, P.A.R.B.; Iechty, K.E.W.L.; Oslowsky, L.O.J.S. Diabetes Alters Mechanical Properties and Collagen Fiber Re-Alignment in Multiple Mouse Tendons. Ann. Biomed. Eng. 2014, 42, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Guo, W.; Wu, P.; Yang, W.; Hu, S.; Xia, Y.; Feng, P. A graphene oxide-Ag co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018, 347, 322–333. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2012, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 5, 518–524. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- An, Y.; Liu, W.J.; Xue, P.; Ma, Y.; Zhang, L.Q.; Zhu, B.; Qi, M.; Li, L.Y.; Zhang, Y.J.; Wang, Q.T.; et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion article. Cell Death Dis. 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three Dimensional Printing Bilayer Membrane Scaffold Promotes Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yin, X.; Ma, N.; Lin, F.; Kong, X.; Chi, J.; Feng, Z. Desferrioxamine regulates HIF-1 alpha expression in neonatal rat brain after hypoxia-ischemia. Am. J. Transl. Res. 2014, 6, 377–383. [Google Scholar] [PubMed]
- Xie, T.; Ding, J.; Han, X.; Jia, H.; Yang, Y.; Liang, S.; Wang, W.; Liu, W.; Wang, W. Wound dressing change facilitated by spraying zinc ions. Mater. Horizons 2020, 7, 605–614. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Villa-Camacho, J.C.; Ghobril, C.; Perez-Viloriab, M.; Tevisa, K.M.; Blessing, W.A.; Nazarian, A.; Rodriguez, E.K.; Grinstaff, M.W. On-demand dissolution of a dendritic hydrogel-based dressing for second-degree burn wounds via thiol-thioester exchange reaction. Angew. Chem. Int. Ed. Engl. 2017, 55, 9984–9987. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yuan, L.; Yu, X.; Wu, C.; He, D.; Deng, J. Recent advances of on-demand dissolution of hydrogel dressings. Burn. Trauma 2018, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Cankova, Z.; Iwanaszko, M.; Lichtor, S.; Mrksich, M. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, 6816–6821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Song, Q.; Xu, J.; Serpe, M.J.; Zhang, X. Supramolecular Hydrogels Fabricated from Supramonomers: A Novel Wound Dressing Material. ACS Appl. Mater. Interfaces 2017, 9, 11368–11372. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.S.; Kano, G.E.; de Almeida Paulo, L.; Lopes, P.S.; de Moraes, M.A. Silk fibroin/chitosan/alginate multilayer membranes as a system for controlled drug release in wound healing. Int. J. Biol. Macromol. 2020, 152, 803–811. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019, 52, 272–281. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek incorporated silk fibroin nanofibers-A potential antioxidant scaffold for enhanced wound healing. ACS Appl. Mater. Interfaces. 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Qian, Y.; Huang, Y.; Ding, F.; Qi, X.; Shen, J. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 2021, 6, 2647–2657. [Google Scholar] [CrossRef]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, D.M.; Herdrich, B.J.; Xu, J.; Lind, R.; Beason, D.P.; Mitchell, M.E.; Soslowsky, L.J.; Liechty, K.W. Impaired Biomechanical Properties of Diabetic Skin Implications in Pathogenesis of Diabetic Wound. Am. J. Pathol. 2011, 178, 2215–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, S.; Utomo, Y.K.S.; Yang, L.; Liang, G.; Liu, W. Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing. Pharmaceutics 2022, 14, 601. https://doi.org/10.3390/pharmaceutics14030601
Zha S, Utomo YKS, Yang L, Liang G, Liu W. Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing. Pharmaceutics. 2022; 14(3):601. https://doi.org/10.3390/pharmaceutics14030601
Chicago/Turabian StyleZha, Shenfang, Yohanes Kristo Sugiarto Utomo, Li Yang, Guizhao Liang, and Wanqian Liu. 2022. "Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing" Pharmaceutics 14, no. 3: 601. https://doi.org/10.3390/pharmaceutics14030601
APA StyleZha, S., Utomo, Y. K. S., Yang, L., Liang, G., & Liu, W. (2022). Mechanic-Driven Biodegradable Polyglycolic Acid/Silk Fibroin Nanofibrous Scaffolds Containing Deferoxamine Accelerate Diabetic Wound Healing. Pharmaceutics, 14(3), 601. https://doi.org/10.3390/pharmaceutics14030601




