Prevalence and Severity of Potential Drug–Drug Interactions in Patients with Multiple Sclerosis with and without Polypharmacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Gathered Data
2.3. Drug Characterization
2.4. Polypharmacy
2.5. Identification of Drug–Drug Interactions
2.6. Composite Rating of pDDI Severity Levels
2.7. Statistics
3. Results
3.1. Sociodemographic and Clinical Patient Profile
3.2. Polypharmacy
3.3. Comorbidities
3.4. Drug Profile
3.5. Drug–Drug Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Frahm, N.; Hecker, M.; Zettl, U.K. Polypharmacy among patients with multiple sclerosis: A qualitative systematic review. Expert Opin. Drug Saf. 2020, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.A. The epidemiology of polypharmacy. Clin. Med. 2016, 16, 465–469. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. Houndmills Basingstoke Engl. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primer 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Rommer, P.S.; Eichstädt, K.; Ellenberger, D.; Flachenecker, P.; Friede, T.; Haas, J.; Kleinschnitz, C.; Pöhlau, D.; Rienhoff, O.; Stahmann, A.; et al. Symptomatology and symptomatic treatment in multiple sclerosis: Results from a nationwide MS registry. Mult. Scler. Houndmills Basingstoke Engl. 2019, 25, 1641–1652. [Google Scholar] [CrossRef]
- Selmi, C.; Mix, E.; Zettl, U.K. A clear look at the neuroimmunology of multiple sclerosis and beyond. Autoimmun. Rev. 2012, 11, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, D.; Flachenecker, P.; Haas, J.; Hellwig, K.; Paul, F.; Stahmann, A.; Warnke, C.; Zettl, U.K.; Rommer, P.S.; Scientific Advisory Group by the German MS-Register of the German MS Society. Is benign MS really benign? What a meaningful classification beyond the EDSS must take into consideration. Mult. Scler. Relat. Disord. 2020, 46, 102485. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, D.; Flachenecker, P.; Fneish, F.; Frahm, N.; Hellwig, K.; Paul, F.; Stahmann, A.; Warnke, C.; Rommer, P.S.; Zettl, U.K. Aggressive multiple sclerosis: A matter of measurement and timing. Brain J. Neurol. 2020, 143, e97. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Rommer, P.S.; Zettl, U.K. Managing the side effects of multiple sclerosis therapy: Pharmacotherapy options for patients. Expert Opin. Pharmacother. 2018, 19, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Moiola, L.; Rommer, P.S.; Zettl, U.K. Prevention and management of adverse effects of disease modifying treatments in multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 286–294. [Google Scholar] [CrossRef]
- Rommer, P.; Zettl, U.K. Treatment options in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders. Curr. Pharm. Des. 2022, 28, 428–436. [Google Scholar] [CrossRef]
- Wiendl, H.; Gold, R.; Berger, T.; Derfuss, T.; Linker, R.; Mäurer, M.; Aktas, O.; Baum, K.; Berghoff, M.; Bittner, S.; et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): Position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther. Adv. Neurol. Disord. 2021, 14, 17562864211039648. [Google Scholar] [CrossRef]
- Hemmer, B. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis-Optica-Spektrum-Erkrankungen und MOG-IgG-assoziierten Erkrankungen; Leitlinien für Diagnostik und Therapie in der Neurologie; Deutsche Gesellschaft für Neurologie (Hrsg.): Berlin, Germany, 2021; Available online: www.dgn.org/leitlinien (accessed on 13 October 2021).
- Schiess, N.; Calabresi, P. Multiple Sclerosis. Semin. Neurol. 2016, 36, 350–356. [Google Scholar] [CrossRef]
- Frahm, N.; Hecker, M.; Zettl, U.K. Polypharmacy in Chronic Neurological Diseases: Multiple Sclerosis, Dementia and Parkinson’s Disease. Curr. Pharm. Des. 2021, 27, 4008–4016. [Google Scholar] [CrossRef]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef]
- Moura, C.S.; Acurcio, F.A.; Belo, N.O. Drug-drug interactions associated with length of stay and cost of hospitalization. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2009, 12, 266–272. [Google Scholar] [CrossRef]
- Rekić, D.; Reynolds, K.S.; Zhao, P.; Zhang, L.; Yoshida, K.; Sachar, M.; Piquette Miller, M.; Huang, S.-M.; Zineh, I. Clinical Drug-Drug Interaction Evaluations to Inform Drug Use and Enable Drug Access. J. Pharm. Sci. 2017, 106, 2214–2218. [Google Scholar] [CrossRef]
- Köhler, G.I.; Bode-Böger, S.M.; Busse, R.; Hoopmann, M.; Welte, T.; Böger, R.H. Drug-drug interactions in medical patients: Effects of in-hospital treatment and relation to multiple drug use. Int. J. Clin. Pharmacol. Ther. 2000, 38, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/visits associated with drug-drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Moss, B.P.; Rensel, M.R.; Hersh, C.M. Wellness and the Role of Comorbidities in Multiple Sclerosis. Neurother. J. Am. Soc. Exp. Neurother. 2017, 14, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Cohen, J.; Stuve, O.; Trojano, M.; Sørensen, P.S.; Reingold, S.; Cutter, G.; Reider, N. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Mult. Scler. Houndmills Basingstoke Engl. 2015, 21, 263–281. [Google Scholar] [CrossRef] [PubMed]
- mediQ-Startseite. Available online: https://www.mediq.ch/ (accessed on 13 October 2021).
- Hahn, M.; Roll, S.C. Validation of interaction databases in psychopharmacotherapy. Der Nervenarzt 2018, 89, 319–326. [Google Scholar] [CrossRef]
- Digital Medicines Information Suite. Available online: https://about.medicinescomplete.com/ (accessed on 13 October 2021).
- Frahm, N.; Hecker, M.; Langhorst, S.E.; Mashhadiakbar, P.; Haker, M.-C.; Zettl, U.K. The risk of polypharmacy, comorbidities and drug–drug interactions in women of childbearing age with multiple sclerosis. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420969501. [Google Scholar] [CrossRef]
- Rodrigues, M.C.S.; de Oliveira, C. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: An integrative review. Rev. Lat. Am. De Enferm. 2016, 24, e2800. [Google Scholar] [CrossRef]
- Astorp, J.; Gjela, M.; Jensen, P.; Bak, R.D.; Gazerani, P. Patterns and characteristics of polypharmacy among elderly residents in Danish nursing homes. Future Sci. OA 2020, 6, FSO590. [Google Scholar] [CrossRef]
- Merlo, J.; Liedholm, H.; Lindblad, U.; Björck-Linné, A.; Fält, J.; Lindberg, G.; Melander, A. Prescriptions with potential drug interactions dispensed at Swedish pharmacies in January 1999: Cross sectional study. BMJ 2001, 323, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Bogetti-Salazar, M.; González-González, C.; Juárez-Cedillo, T.; Sánchez-García, S.; Rosas-Carrasco, O. Severe potential drug-drug interactions in older adults with dementia and associated factors. Clinics 2016, 71, 17–21. [Google Scholar] [CrossRef]
- Dookeeram, D.; Bidaisee, S.; Paul, J.F.; Nunes, P.; Robertson, P.; Maharaj, V.R.; Sammy, I. Polypharmacy and potential drug-drug interactions in emergency department patients in the Caribbean. Int. J. Clin. Pharm. 2017, 39, 1119–1127. [Google Scholar] [CrossRef]
- Medication Safety in Polypharmacy: Technical Report. Available online: https://www.who.int/publications-detail-redirect/medication-safety-in-polypharmacy-technical-report (accessed on 17 October 2021).
- Soler, O.; Barreto, J.O.M. Community-Level Pharmaceutical Interventions to Reduce the Risks of Polypharmacy in the Elderly: Overview of Systematic Reviews and Economic Evaluations. Front. Pharmacol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Hanlon, J.T.; Pieper, C.F.; Hajjar, E.R.; Sloane, R.J.; Lindblad, C.I.; Ruby, C.M.; Schmader, K.E. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Maher, R.L.; Hanlon, J.; Hajjar, E.R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 2014, 13, 57–65. [Google Scholar] [CrossRef]
- Klauer, T.; Zettl, U.K. Compliance, adherence, and the treatment of multiple sclerosis. J. Neurol. 2008, 255 Suppl 6, 87–92. [Google Scholar] [CrossRef]
- Zettl, U.K.; Bauer-Steinhusen, U.; Glaser, T.; Hechenbichler, K.; Limmroth, V.; Study Group. Evaluation of an electronic diary for improvement of adherence to interferon beta-1b in patients with multiple sclerosis: Design and baseline results of an observational cohort study. BMC Neurol. 2013, 13, 117. [Google Scholar] [CrossRef]
- Gaeta, T.J.; Fiorini, M.; Ender, K.; Bove, J.; Diaz, J. Potential drug-drug interactions in elderly patients presenting with syncope. J. Emerg. Med. 2002, 22, 159–162. [Google Scholar] [CrossRef]
- Rambhade, S.; Chakarborty, A.; Shrivastava, A.; Patil, U.K.; Rambhade, A. A survey on polypharmacy and use of inappropriate medications. Toxicol. Int. 2012, 19, 68–73. [Google Scholar] [CrossRef]
- Hovstadius, B.; Åstrand, B.; Persson, U.; Petersson, G. Acquisition cost of dispensed drugs in individuals with multiple medications--a register-based study in Sweden. Health Policy Amst. Neth. 2011, 101, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, L.; Cui, M.; Liu, X.; Gu, Z. Ticagrelor-induced life-threatening bleeding via the cyclosporine-mediated drug interaction: A case report. Medicine 2017, 96, e8065. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.I.; Rosencher, N.; Friedman, R.J.; Homering, M.; Dahl, O.E. Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb. Res. 2012, 130, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rochon, P.A.; Gurwitz, J.H. The prescribing cascade revisited. Lancet Lond. Engl. 2017, 389, 1778–1780. [Google Scholar] [CrossRef]
- Sarzynski, E.M.; Luz, C.C.; Rios-Bedoya, C.F.; Zhou, S. Considerations for using the “brown bag” strategy to reconcile medications during routine outpatient office visits. Qual. Prim. Care 2014, 22, 177–187. [Google Scholar]
- Nathan, A.; Goodyer, L.; Lovejoy, A.; Rashid, A. “Brown bag” medication reviews as a means of optimizing patients’ use of medication and of identifying potential clinical problems. Fam. Pract. 1999, 16, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Floor-Schreudering, A.; Heringa, M.; Buurma, H.; Bouvy, M.L.; De Smet, P.A.G.M. Missed drug therapy alerts as a consequence of incomplete electronic patient records in Dutch community pharmacies. Ann. Pharmacother. 2013, 47, 1272–1279. [Google Scholar] [CrossRef]
- Olesen, C.; Harbig, P.; Barat, I.; Damsgaard, E.M. Absence of “over-the-counter” medicinal products in on-line prescription records: A risk factor of overlooking interactions in the elderly. Pharmacoepidemiol. Drug Saf. 2013, 22, 145–150. [Google Scholar] [CrossRef]
- American Pharmacists Association. National Association of Chain Drug Stores Foundation Medication therapy management in pharmacy practice: Core elements of an MTM service model (version 2.0). J. Am. Pharm. Assoc. 2008, 48, 341–353. [Google Scholar] [CrossRef]
- Viswanathan, M.; Kahwati, L.C.; Golin, C.E.; Blalock, S.J.; Coker-Schwimmer, E.; Posey, R.; Lohr, K.N. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern. Med. 2015, 175, 76–87. [Google Scholar] [CrossRef]
- Tsuyuki, R.T.; Johnson, J.A.; Teo, K.K.; Simpson, S.H.; Ackman, M.L.; Biggs, R.S.; Cave, A.; Chang, W.-C.; Dzavik, V.; Farris, K.B.; et al. A randomized trial of the effect of community pharmacist intervention on cholesterol risk management: The Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP). Arch. Intern. Med. 2002, 162, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Isetts, B.J.; Schondelmeyer, S.W.; Artz, M.B.; Lenarz, L.A.; Heaton, A.H.; Wadd, W.B.; Brown, L.M.; Cipolle, R.J. Clinical and economic outcomes of medication therapy management services: The Minnesota experience. J. Am. Pharm. Assoc. 2008, 48, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Aghili, M.; Kasturirangan, M.N. Management of Drug–Drug Interactions among Critically Ill Patients with Chronic Kidney Disease: Impact of Clinical Pharmacist’s Interventions. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2021, 25, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.B.; Petrakis, B.A.; Gillespie, C.; Solomon, J.L.; Park, A.M.; Ourth, H.; Morreale, A.; Rose, A.J. Knowing the Patient: A Qualitative Study on Care-Taking and the Clinical Pharmacist-Patient Relationship. Res. Soc. Adm. Pharm. 2016, 12, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Coe, A.; Kaylor-Hughes, C.; Fletcher, S.; Murray, E.; Gunn, J. Deprescribing intervention activities mapped to guiding principles for use in general practice: A scoping review. BMJ Open 2021, 11, e052547. [Google Scholar] [CrossRef]
- Carpenter, M.; Berry, H.; Pelletier, A.L. Clinically Relevant Drug-Drug Interactions in Primary Care. Am. Fam. Physician 2019, 99, 558–564. [Google Scholar]
- Weih, M.; Roßnagel, F.; Dikow, H.; Wehrle, K.; Braune, S.; Bergmann, A. Data on multiple sclerosis in Germany and their representation in the ambulatory registry NeuroTransData (NTD) network. Fortschr. Neurol. Psychiatr. 2020, 88, 379–385. [Google Scholar] [CrossRef]
- Flachenecker, P.; Eichstädt, K.; Berger, K.; Ellenberger, D.; Friede, T.; Haas, J.; Kleinschnitz, C.; Pöhlau, D.; Rienhoff, O.; Stahmann, A.; et al. Multiple sclerosis in Germany: Updated analysis of the German MS Registry 2014–2018. Fortschr. Neurol. Psychiatr. 2020, 88, 436–450. [Google Scholar] [CrossRef]
- Boström, I.; Stawiarz, L.; Landtblom, A.-M. Sex ratio of multiple sclerosis in the National Swedish MS Register (SMSreg). Mult. Scler. Houndmills Basingstoke Engl. 2013, 19, 46–52. [Google Scholar] [CrossRef]
- Ohle, L.-M.; Ellenberger, D.; Flachenecker, P.; Friede, T.; Haas, J.; Hellwig, K.; Parciak, T.; Warnke, C.; Paul, F.; Zettl, U.K.; et al. Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS registry. Sci. Rep. 2021, 11, 13340. [Google Scholar] [CrossRef]
- Tam, V.C.; Knowles, S.R.; Cornish, P.L.; Fine, N.; Marchesano, R.; Etchells, E.E. Frequency, type and clinical importance of medication history errors at admission to hospital: A systematic review. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 2005, 173, 510–515. [Google Scholar] [CrossRef]
- Abdolrasulnia, M.; Weichold, N.; Shewchuk, R.; Saag, K.; Cobaugh, D.J.; LaCivita, C.; Weissman, N.; Allison, J. Agreement between medical record documentation and patient-reported use of nonsteroidal antiinflammatory drugs. Am. J. Health Syst. Pharm. 2006, 63, 744–747. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Obreli Neto, P.R.; Nobili, A.; de Lyra, D.P.; Pilger, D.; Guidoni, C.M.; de Oliveira Baldoni, A.; Cruciol-Souza, J.M.; de Carvalho Freitas, A.L.; Tettamanti, M.; Gaeti, W.P.; et al. Incidence and predictors of adverse drug reactions caused by drug-drug interactions in elderly outpatients: A prospective cohort study. J. Pharm. Pharm. Sci. 2012, 15, 332–343. [Google Scholar] [CrossRef] [PubMed]
| Total Polypharmacy | Rx Polypharmacy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | PwP | Pw/oP | p | PwP | Pw/oP | p | ||||||
| N | 627 | 334 (53.3%) | 293 (46.7%) | 242 (38.6%) | 385 (61.4%) | |||||||
| Sociodemographic data | ||||||||||||
| Sex | 0.793 Fi | 0.720 Fi | ||||||||||
| Male | 186 (29.7%) | 101 (30.2%) | 85 (29.0%) | 74 (30.6%) | 112 (29.1%) | |||||||
| Female | 441 (70.3%) | 233 (69.8%) | 208 (71.0%) | 168 (69.4%) | 273 (70.9%) | |||||||
| Age (years) | 19–86 R | 48.6 (13.3) a | 20–86 R | 53.0 (12.7) a | 19–74 R | 43.6 (12.2) a | <0.001 t | 24–86 R | 54.8 (12.1) a | 19–75 R | 44.7 (12.5) a | <0.001 t |
| School years | 6–18 R | 10.5 (1.3) a | 6–14 R | 10.3 (1.2) a | 8–18 R | 10.7 (1.3) a | <0.001 t | 6–14 R | 10.2 (1.2) a | 8–18 R | 10.7 (1.3) a | <0.001 t |
| Educational level | 0.019 Chi | 0.002 Chi | ||||||||||
| No training | 19 (3.0%) | 8 (2.4%) | 11 (3.8%) | 7 (2.9%) | 12 (3.1%) | |||||||
| Skilled worker | 398 (63.5%) | 229 (68.6%) | 169 (57.7%) | 173 (71.5%) | 225 (58.4%) | |||||||
| Technical college | 89 (14.2%) | 46 (13.8%) | 43 (14.7%) | 33 (13.6%) | 56 (14.5%) | |||||||
| University | 121 (19.3%) | 51 (15.3%) | 70 (23.9%) | 29 (12.0%) | 92 (23.9%) | |||||||
| Employment status | <0.001 Chi | <0.001 Chi | ||||||||||
| In training | 7 (1.1%) | 1 (0.3%) | 6 (2.0%) | 0 (0.0%) | 7 (1.8%) | |||||||
| In studies | 6 (1.0%) | 0 (0.0%) | 6 (2.0%) | 0 (0.0%) | 6 (1.6%) | |||||||
| Employed | 269 (42.9%) | 92 (27.5%) | 177 (60.4%) | 53 (21.9%) | 216 (56.1%) | |||||||
| Unemployed | 25 (4.0%) | 10 (3.0%) | 15 (5.1%) | 7 (2.9%) | 18 (4.7%) | |||||||
| Disability-pensioned | 304 (48.5%) | 225 (67.4%) | 79 (27.0%) | 178 (73.6%) | 126 (32.7%) | |||||||
| Other | 16 (2.6%) | 6 (1.8%) | 10 (3.4%) | 4 (1.7%) | 12 (3.1%) | |||||||
| Partnership | 1.000 Fi | 0.305 Fi | ||||||||||
| No | 162 (25.8%) | 86 (25.7%) | 76 (25.9%) | 68 (28.1%) | 94 (24.4%) | |||||||
| Yes | 465 (74.2%) | 248 (74.3%) | 217 (74.1%) | 174 (71.9%) | 291 (75.6%) | |||||||
| Place of Residence | 0.288 Chi | 0.962 Chi | ||||||||||
| Rural community | 224 (35.7%) | 119 (35.6%) | 105 (35.8%) | 89 (36.8%) | 135 (35.1%) | |||||||
| Provincial town | 108 (17.2%) | 63 (18.9%) | 45 (15.4%) | 42 (17.4%) | 66 (17.1%) | |||||||
| Medium-sized town | 112 (17.9%) | 64 (19.2%) | 48 (16.4%) | 43 (17.8%) | 69 (17.9%) | |||||||
| City | 183 (29.2%) | 88 (26.3%) | 95 (32.4%) | 68 (28.1%) | 115 (29.9%) | |||||||
| Number of children | 0–4 R | 1 b | 0–4 R | 1 b | 0–4 R | 1 b | 0.089 U | 0–4 R | 1 b | 0–4 R | 1 b | 0.056 U |
| 0 | 169 (27.0%) | 77 (23.1%) | 92 (31.4%) | 54 (22.3%) | 115 (29.9%) | |||||||
| 1 | 170 (27.1%) | 98 (29.3%) | 72 (24.6%) | 68 (28.1%) | 102 (26.5%) | |||||||
| ≥2 | 288 (45.9%) | 159 (47.6%) | 129 (44.0%) | 120 (49.6%) | 168 (43.6%) | |||||||
| Number of siblings | 0–13 R | 1 b | 0–13 R | 1 b | 0–11 R | 1 b | 0.081 U | 0–13 R | 1 b | 0–11 R | 1 b | 0.018 U |
| 0 | 71 (11.3%) | 33 (9.9%) | 38 (13.0%) | 26 (10.7%) | 45 (11.7%) | |||||||
| 1 | 305 (48.6%) | 160 (47.9%) | 145 (49.5%) | 103 (42.6%) | 202 (52.5%) | |||||||
| ≥2 | 251 (40.0%) | 141 (42.2%) | 110 (37.5%) | 113 (46.7%) | 138 (35.8%) | |||||||
| Clinical data | ||||||||||||
| EDSS | 0–9 R | 3.5 b | 0–9 R | 4.5 b | 0–7.5 R | 2.0 b | <0.001 U | 0–9 R | 5.0 b | 0–7.5 R | 2.5 b | <0.001 U |
| Disease duration (years) | 0–52 R | 10 b | 0–50 R | 12.5 b | 0–52 R | 9 b | <0.001 U | 0–50 R | 14 b | 0–52 R | 9 b | <0.001 U |
| Age at MS onset | 9–75 R | 35 b | 9–75 R | 38 b | 12–62 R | 32 b | <0.001 U | 9–75 R | 39 b | 12–69 R | 33 b | <0.001 U |
| Disease course | <0.001 Chi | <0.001 Chi | ||||||||||
| CIS/RRMS | 415 (66.2%) | 158 (47.3%) | 257 (87.7%) | 91 (37.6%) | 324 (84.2%) | |||||||
| SPMS | 154 (24.6%) | 125 (37.4%) | 29 (9.9%) | 109 (45.0%) | 45 (11.7%) | |||||||
| PPMS | 58 (9.3%) | 51 (15.3%) | 7 (2.4%) | 42 (17.4%) | 16 (4.2%) | |||||||
| Comorbidities | 0–9 R | 1 b | 0–9 R | 2 b | 0–5 R | 1 b | <0.001 U | 0–9 R | 3 b | 0–7 R | 1 b | <0.001 U |
| 0 | 184 (29.3%) | 46 (13.8%) | 138 (47.1%) | 24 (9.9%) | 160 (41.6%) | |||||||
| 1 | 150 (23.9%) | 60 (18.0%) | 90 (30.7%) | 39 (16.1%) | 111 (28.8%) | |||||||
| 2 | 122 (19.5%) | 76 (22.8%) | 46 (15.7%) | 50 (20.7%) | 72 (18.7%) | |||||||
| 3 | 82 (13.1%) | 71 (21.3%) | 11 (3.8%) | 58 (24.0%) | 24 (6.2%) | |||||||
| 4 | 50 (8.0%) | 44 (13.2%) | 6 (2.0%) | 35 (14.5%) | 15 (3.9%) | |||||||
| ≥5 | 39 (6.2%) | 37 (11.1%) | 2 (0.7%) | 36 (14.9%) | 3 (0.8%) | |||||||
| Pharmaceutical data | ||||||||||||
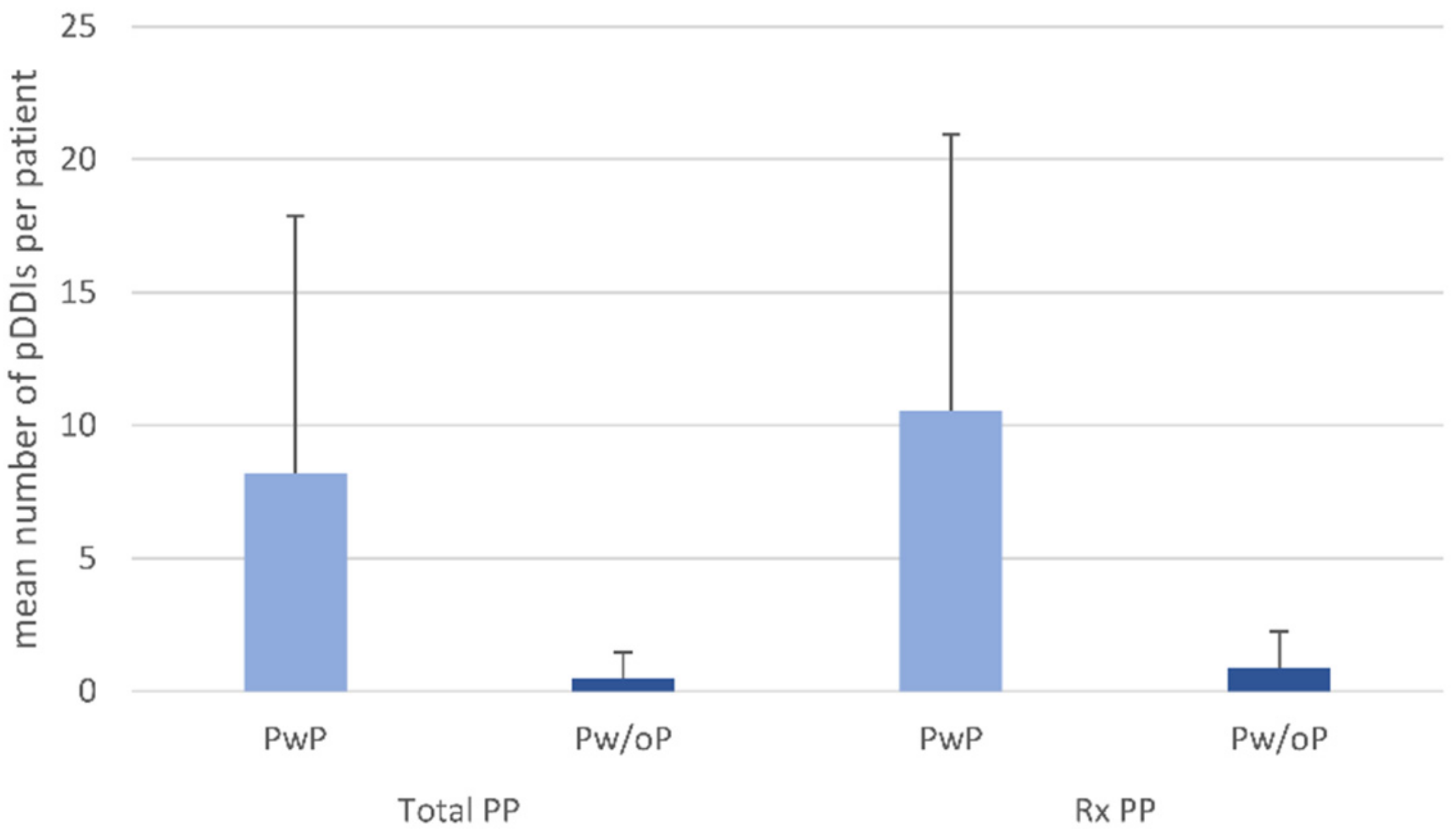
| Number of total drugs taken | 0–19 R | 5.3 (3.3) c | 5–19 R | 7.8 (2.7) c | 0–4 R | 2.6 (1.1) c | <0.001 t | 5–19 R | 8.5 (2.7) c | 0–9 R | 3.3 (1.7) c | <0.001 t |
| 0–4 | 293 (46.7%) | 0 (0.0%) | 293 (100.0%) | 0 (0.0%) | 293 (76.1%) | |||||||
| 5–9 | 261 (41.6%) | 261 (78.1%) | 0 (0.0%) | 169 (69.8%) | 92 (23.9%) | |||||||
| ≥10 | 73 (11.6%) | 73 (21.9%) | 0 (0.0%) | 73 (30.2%) | 0 (0.0%) | |||||||
| Duration of use | ||||||||||||
| Long-term drugs | 0–16 R | 4.6 (3.1) c | 1–16 R | 6.7 (2.7) c | 0–4 R | 2.2 (1.1) c | <0.001 t | 1–16 R | 7.4 (2.7) c | 0–9 R | 2.8 (1.7) c | <0.001 t |
| PRN drugs | 0–7 R | 0.8 (1.2) c | 0–7 R | 1.1 (1.4) c | 0–4 R | 0.4 (0.7) c | <0.001 t | 0–7 R | 1.2 (1.4) c | 0–6 R | 0.6 (0.9) c | <0.001 t |
| Rx vs. OTC | ||||||||||||
| Rx drugs | 0–18 R | 4.2 (3.0) c | 1–18 R | 6.2 (2.8) c | 0–4 R | 1.9 (1.0) c | <0.001 t | 5–18 R | 7.3 (2.4) c | 0–4 R | 2.2 (1.2) c | <0.001 t |
| OTC drugs | 0–8 R | 1.1 (1.3) c | 0–8 R | 1.6 (1.4) c | 0–3 R | 0.6 (0.8) c | <0.001 t | 0–6 R | 1.2 (1.3) c | 0–8 R | 1.1 (1.3) c | 0.206 t |
| Drug purpose | ||||||||||||
| DMD | 0–2 R | 0.9 (0.4) c | 0–2 R | 0.9 (0.4) c | 0–2 R | 0.8 (0.4) c | 0.004 t | 0–2 R | 0.9 (0.4) c | 0–2 R | 0.8 (0.4) c | <0.001 t |
| Symptomatic drugs | 0–9 R | 2.0 (2.0) c | 0–9 R | 3.1 (2.0) c | 0–3 R | 0.7 (0.9) c | <0.001 t | 0–9 R | 3.3 (2.0) c | 0–9 R | 1.2 (1.4) c | <0.001 t |
| Comorbidity drugs | 0–14 R | 2.5 (2.4) c | 0–14 R | 3.8 (2.6) c | 0–4 R | 1.0 (0.9) c | <0.001 t | 0–14 R | 4.3 (2.7) c | 0–7 R | 1.3 (1.3) c | <0.001 t |
| Total Polypharmacy | Rx Polypharmacy | ||||||
|---|---|---|---|---|---|---|---|
| Drug Category | Total Number of Drugs | PwP | Pw/oP | p | PwP | Pw/oP | p |
| All | 3341 (100%) | 2591 (77.6%) | 750 (22.4%) | 2060 (61.7%) | 1281 (38.3%) | ||
| Duration of use | 0.176 Fi | 0.013 Fi | |||||
| Long-term drugs | 2855 (85.5%) | 2226 (85.9%) | 629 (83.9%) | 1785 (86.7%) | 1070 (83.5%) | ||
| PRN drugs | 486 (14.5%) | 365 (14.1%) | 121 (16.1%) | 275 (13.3%) | 211 (16.5%) | ||
| Rx vs. OTC | 0.011 Fi | <0.001 Fi | |||||
| Rx drugs | 2630 (78.7%) | 2065 (79.7%) | 565 (75.3%) | 1766 (85.7%) | 864 (67.4%) | ||
| OTC drugs | 711 (21.3%) | 526 (20.3%) | 185 (24.7%) | 294 (14.3%) | 417 (32.6%) | ||
| Drug purpose | <0.001 Chi | <0.001 Chi | |||||
| DMD | 530 (15.9%) | 297 (11.5%) | 233 (31.1%) | 223 (10.8%) | 307 (24.0%) | ||
| Symptomatic drugs | 1253 (37.5%) | 1035 (39.9%) | 218 (29.0%) | 796 (38.6%) | 457 (35.7%) | ||
| Comorbidity drugs | 1558 (46.6%) | 1259 (48.6%) | 299 (39.9%) | 1041 (50.6%) | 517 (40.3%) | ||
| Total Polypharmacy | Rx Polypharmacy | ||||||
|---|---|---|---|---|---|---|---|
| All Patients | PwP | Pw/oP | pFi | PwP | Pw/oP | pFi | |
| N | 627 | 334 (53.3%) | 293 (46.7%) | 242 (38.6%) | 385 (61.4%) | ||
| Most used non-DMDs | |||||||
| Cholecalciferol | 261 (41.6%) | 178 (53.3%) | 83 (28.3%) | <0.001 | 125 (51.7%) | 136 (35.3%) | <0.001 |
| Pantoprazole | 178 (28.4%) | 155 (46.4%) | 23 (7.8%) | <0.001 | 144 (59.5%) | 34 (8.8%) | <0.001 |
| Enoxaparin | 127 (20.3%) | 114 (34.1%) | 13 (4.4%) | <0.001 | 105 (43.3%) | 22 (5.7%) | <0.001 |
| Ibuprofen | 105 (16.7%) | 61 (18.3%) | 44 (15.0%) | 0.286 | 41 (16.9%) | 64 (16.6%) | 0.913 |
| Baclofen | 78 (12.4%) | 72 (21.6%) | 6 (2.0%) | <0.001 | 68 (28.1%) | 10 (2.6%) | <0.001 |
| Levothyroxine | 75 (12.0%) | 51 (15.3%) | 24 (8.2%) | 0.007 | 41 (16.9%) | 34 (8.8%) | 0.003 |
| Cyanocobalamin | 66 (10.5%) | 46 (13.8%) | 20 (6.8%) | 0.006 | 27 (11.2%) | 39 (10.1%) | 0.690 |
| Zopiclone | 65 (10.4%) | 58 (17.4%) | 7 (2.4%) | <0.001 | 53 (21.9%) | 12 (3.1%) | <0.001 |
| Magnesium | 60 (9.6%) | 45 (13.5%) | 15 (5.1%) | <0.001 | 21 (8.7%) | 39 (10.1%) | 0.580 |
| Acetylsalicylic acid | 55 (8.8%) | 48 (14.4%) | 7 (2.4%) | <0.001 | 41 (16.9%) | 14 (3.6%) | <0.001 |
| DMDs (all, incl. methylprednisolone) | |||||||
| Methylprednisolone | 123 (19.6%) | 110 (32.9%) | 13 (4.4%) | <0.001 | 101 (41.7%) | 22 (5.7%) | <0.001 |
| Interferon beta-1a | 64 (10.2%) | 25 (7.5%) | 39 (13.3%) | 0.018 | 14 (5.8%) | 50 (13.0%) | 0.004 |
| Glatiramer acetate | 57 (9.1%) | 21 (6.3%) | 36 (12.3%) | 0.012 | 14 (5.8%) | 43 (11.2%) | 0.023 |
| Natalizumab | 47 (7.5%) | 18 (5.4%) | 29 (9.9%) | 0.034 | 9 (3.7%) | 38 (9.9%) | 0.005 |
| Fingolimod | 41 (6.5%) | 21 (6.3%) | 20 (6.8%) | 0.872 | 15 (6.2%) | 26 (6.8%) | 0.869 |
| Teriflunomide | 36 (5.7%) | 19 (5.7%) | 17 (5.8%) | 1.000 | 11 (4.5%) | 25 (6.5%) | 0.379 |
| Dimethyl fumarate | 32 (5.1%) | 10 (3.0%) | 22 (7.5%) | 0.011 | 8 (3.3%) | 24 (6.2%) | 0.135 |
| Mitoxantrone | 28 (4.5%) | 15 (4.5%) | 13 (4.4%) | 1.000 | 11 (4.5%) | 17 (4.4%) | 1.000 |
| Ocrelizumab | 27 (4.3%) | 25 (7.5%) | 2 (0.7%) | <0.001 | 19 (7.9%) | 8 (2.1%) | 0.001 |
| Interferon beta-1b | 23 (3.7%) | 9 (2.7%) | 14 (4.8%) | 0.203 | 7 (2.9%) | 16 (4.2%) | 0.515 |
| Alemtuzumab | 20 (3.4%) | 5 (1.5%) | 15 (5.1%) | 0.012 | 2 (0.8%) | 18 (4.7%) | 0.009 |
| Immunoglobulin G | 7 (1.1%) | 3 (0.3%) | 4 (1.4%) | 0.711 | 0 (0.0%) | 7 (1.8%) | 0.047 |
| Cladribine | 6 (1.0%) | 2 (0.6%) | 4 (1.4%) | 0.426 | 2 (0.8%) | 4 (1.0%) | 1.000 |
| Azathioprine | 4 (0.6%) | 2 (0.6%) | 2 (0.7%) | 1.000 | 1 (0.4%) | 3 (0.8%) | 1.000 |
| Rituximab | 2 (0.3%) | 1 (0.3%) | 1 (0.3%) | 1.000 | 0 (0.0%) | 2 (0.5%) | 0.525 |
| Total Polypharmacy | Rx Polypharmacy | ||||||
|---|---|---|---|---|---|---|---|
| All Patients | PwP | Pw/oP | pFi | PwP | Pw/oP | pFi | |
| N | 627 | 334 (53.3%) | 293 (46.7%) | 242 (38.6%) | 385 (61.4%) | ||
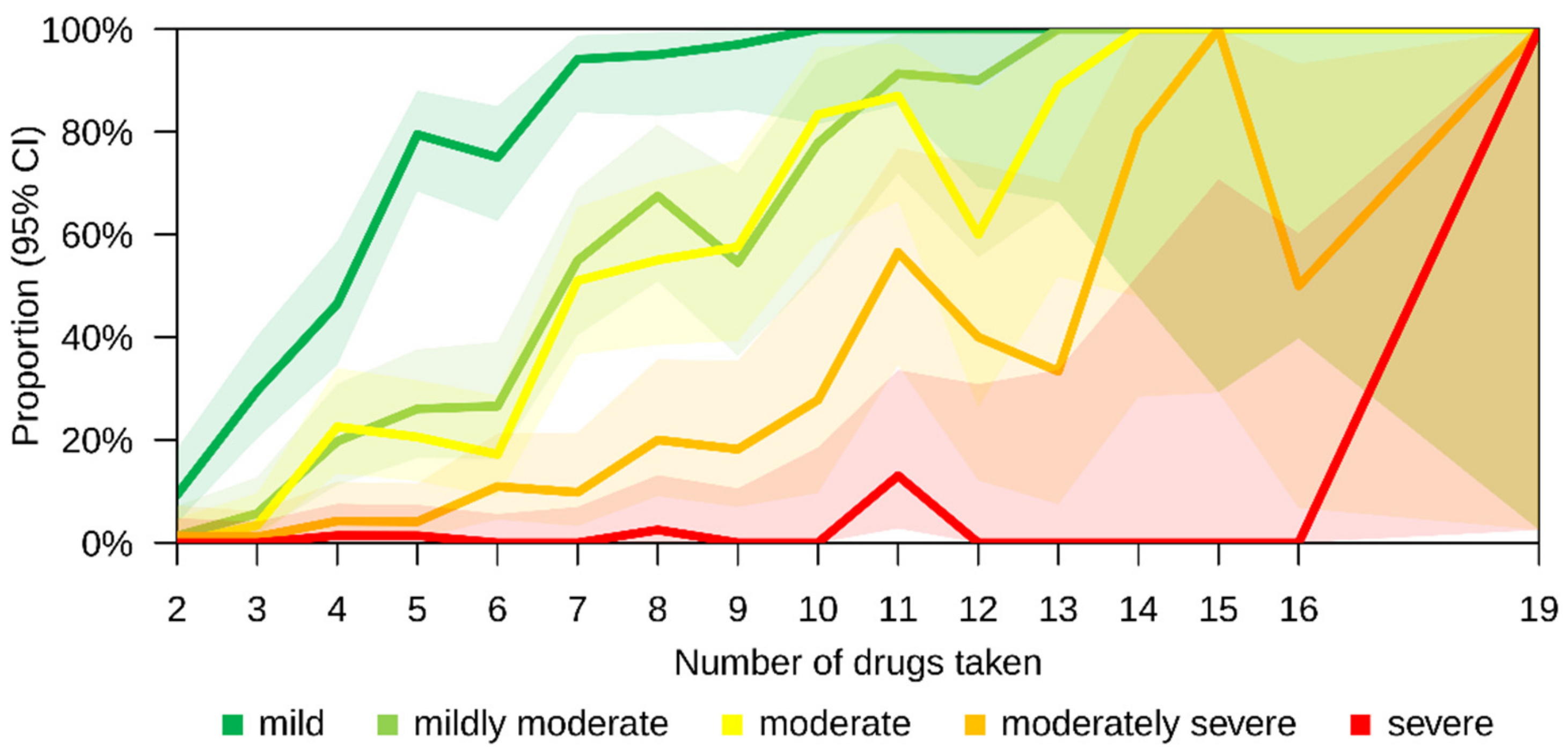
| Severity level | |||||||
| Mild | 363 (57.9%) | 297 (88.9%) | 66 (22.5%) | <0.001 | 225 (93.0%) | 138 (35.8%) | <0.001 |
| Mildly moderate | 195 (31.1%) | 175 (52.4%) | 20 (6.8%) | <0.001 | 157 (64.9%) | 38 (9.9%) | <0.001 |
| Moderate | 174 (27.8%) | 155 (46.4%) | 19 (6.5%) | <0.001 | 140 (57.9%) | 34 (8.8%) | <0.001 |
| Moderately severe | 69 (11.0%) | 64 (19.2%) | 5 (1.7%) | <0.001 | 61 (25.2%) | 8 (2.1%) | <0.001 |
| Severe | 7 (1.1%) | 6 (1.8%) | 1 (0.3%) | 0.129 | 5 (2.1%) | 2 (0.5%) | 0.114 |
| No pDDI at all | 227 (36.2%) | 22 (6.6%) | 205 (70.0%) | <0.001 | 7 (2.9%) | 220 (57.1%) | <0.001 |
| Total Number of pDDIs Recorded | Rx-Rx | Rx-OTC | OTC-OTC | pChi | |
|---|---|---|---|---|---|
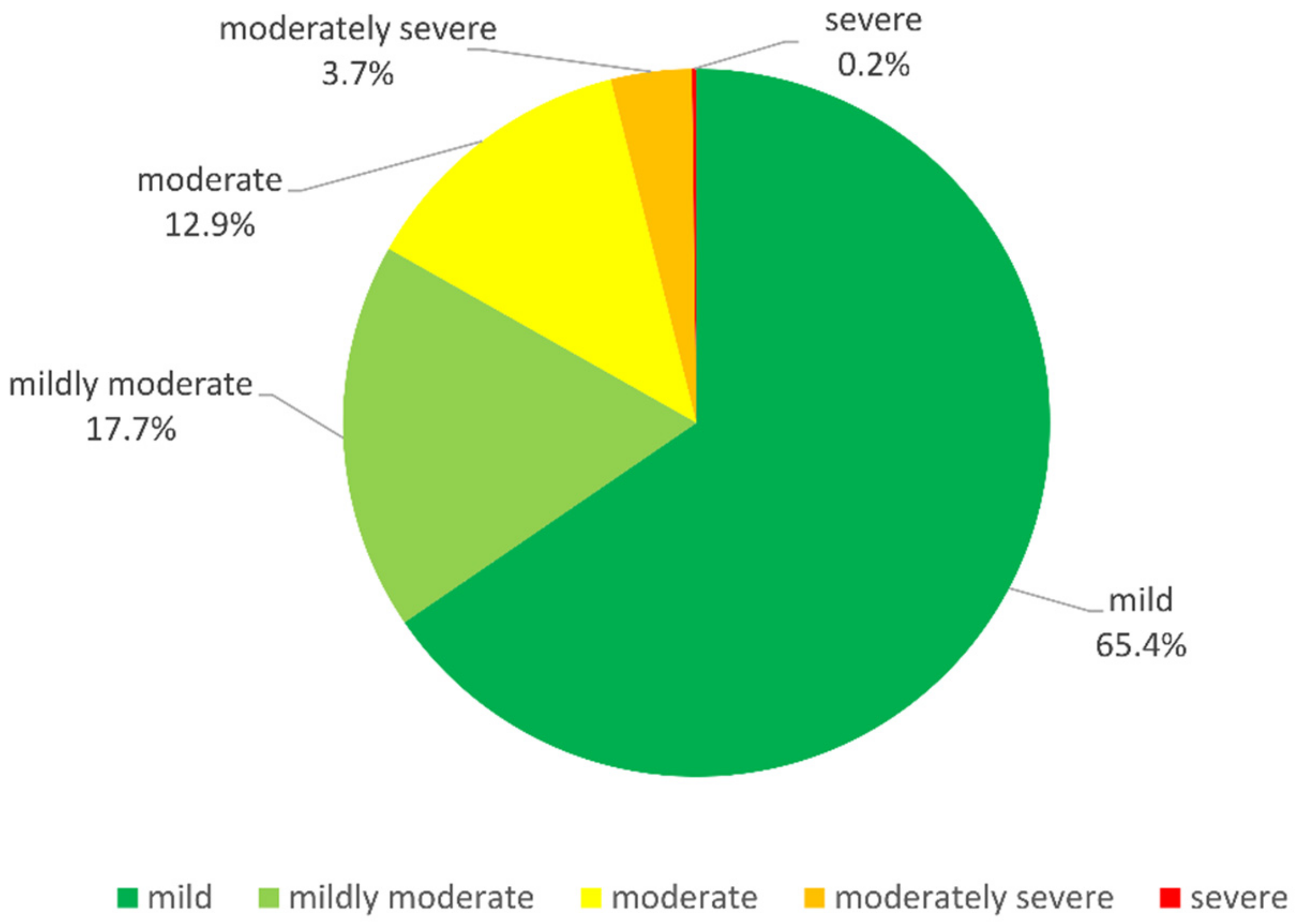
| N | 2887 | 2231 (77.3%) | 549 (19.0%) | 107 (3.7%) | |
| Severity level | <0.001 | ||||
| Mild | 1889 (65.4%) | 1469 (65.8%) | 327 (59.6%) | 93 (86.9%) | |
| Mildly moderate | 511 (17.7%) | 417 (18.7%) | 85 (15.5%) | 9 (8.4%) | |
| Moderate | 373 (12.9%) | 249 (11.2%) | 120 (21.9%) | 4 (3.7%) | |
| Moderately severe | 107 (3.7%) | 89 (4.0%) | 17 (3.1%) | 1 (0.9%) | |
| Severe | 7 (0.2%) | 7 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| Total Amount (N = 627) | Total Polypharmacy | Rx Polypharmacy | |||||
|---|---|---|---|---|---|---|---|
| Drug 1 | Drug 2 | pDDI Severity | Amount in PwP (N = 334) | Amount in Pw/oP (N = 293) | Amount in PwP (N = 242) | Amount in Pw/oP (N = 385) | |
| pDDIs of non-DMDs | |||||||
| Cholecalciferol | Magnesium | mild | 36 (5.7%) | 30 (9.0%) | 6 (2.0%) | 15 (6.2%) | 21 (5.5%) |
| Cyanocobalamin | Pantoprazole | mild | 27 (4.3%) | 25 (7.5%) | 2 (0.7%) | 23 (9.5%) | 4 (1.0%) |
| Calcium | Cholecalciferol | mild | 26 (4.1%) | 25 (7.5%) | 1 (0.3%) | 22 (9.1%) | 4 (1.0%) |
| Levothyroxine | Pantoprazole | mildly moderate | 23 (3.7%) | 22 (6.6%) | 1 (0.3%) | 22 (9.1%) | 1 (0.3%) |
| Acetylsalicylic acid | Enoxaparin | moderate | 21 (3.3%) | 20 (6.0%) | 1 (0.3%) | 19 (7.9%) | 2 (0.5%) |
| Cholecalciferol | Simvastatin | mild | 20 (3.2%) | 19 (5.7%) | 1 (0.3%) | 19 (7.9%) | 1 (0.3%) |
| Baclofen | Fampridine | mild | 20 (3.2%) | 19 (5.7%) | 1 (0.3%) | 17 (7.0%) | 3 (0.8%) |
| Cholecalciferol | Prednisolone | mild | 18 (2.9%) | 18 (5.4%) | 0 (0.0%) | 15 (6.2%) | 3 (0.8%) |
| Pantoprazole | Torasemide | mild | 18 (2.9%) | 18 (5.4%) | 0 (0.0%) | 18 (7.4%) | 0 (0.0%) |
| Cyanocobalamin | Folic acid | mild | 17 (2.7%) | 12 (3.6%) | 5 (1.7%) | 8 (3.3%) | 9 (2.3%) |
| pDDIs of DMDs incl. methylprednisolone | |||||||
| Acetylsalicylic acid | Methylprednisolone | moderate | 21 (3.3%) | 20 (6.0%) | 1 (0.3%) | 19 (7.9%) | 2 (0.5%) |
| Ibuprofen | Methylprednisolone | mildly moderate | 14 (2.2%) | 13 (3.9%) | 1 (0.3%) | 13 (5.4%) | 1 (0.3%) |
| Methylprednisolone | Ramipril | mild | 12 (1.9%) | 12 (3.6%) | 0 (0.0%) | 12 (5.0%) | 0 (0.0%) |
| Citalopram | Methylprednisolone | moderately severe | 10 (1.6%) | 10 (3.0%) | 0 (0.0%) | 10 (4.1%) | 0 (0.0%) |
| Methylprednisolone | Torasemide | mild | 10 (1.6%) | 10 (3.0%) | 0 (0.0%) | 10 (4.1%) | 0 (0.0%) |
| Dipyrone | Methylprednisolone | moderate | 9 (1.4%) | 9 (2.7%) | 0 (0.0%) | 9 (3.7%) | 0 (0.0%) |
| Methylprednisolone | Solifenacin | mildly moderate | 9 (1.4%) | 9 (2.7%) | 0 (0.0%) | 8 (3.3%) | 1 (0.3%) |
| Citalopram | Fingolimod | moderately severe | 7 (1.1%) | 5 (1.5%) | 2 (0.7%) | 3 (1.2%) | 4 (1.0%) |
| Mitoxantrone | Ondansetron | mildly moderate | 7 (1.1%) | 4 (1.2%) | 3 (1.0%) | 3 (1.2%) | 4 (1.0%) |
| Interferon beta-1a | Ramipril | mildly moderate | 7 (1.1%) | 5 (1.5%) | 2 (0.7%) | 4 (1.7%) | 3 (0.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, P.; Frahm, N.; Debus, J.L.; Mashhadiakbar, P.; Langhorst, S.E.; Streckenbach, B.; Baldt, J.; Heidler, F.; Hecker, M.; Zettl, U.K. Prevalence and Severity of Potential Drug–Drug Interactions in Patients with Multiple Sclerosis with and without Polypharmacy. Pharmaceutics 2022, 14, 592. https://doi.org/10.3390/pharmaceutics14030592
Bachmann P, Frahm N, Debus JL, Mashhadiakbar P, Langhorst SE, Streckenbach B, Baldt J, Heidler F, Hecker M, Zettl UK. Prevalence and Severity of Potential Drug–Drug Interactions in Patients with Multiple Sclerosis with and without Polypharmacy. Pharmaceutics. 2022; 14(3):592. https://doi.org/10.3390/pharmaceutics14030592
Chicago/Turabian StyleBachmann, Paula, Niklas Frahm, Jane Louisa Debus, Pegah Mashhadiakbar, Silvan Elias Langhorst, Barbara Streckenbach, Julia Baldt, Felicita Heidler, Michael Hecker, and Uwe Klaus Zettl. 2022. "Prevalence and Severity of Potential Drug–Drug Interactions in Patients with Multiple Sclerosis with and without Polypharmacy" Pharmaceutics 14, no. 3: 592. https://doi.org/10.3390/pharmaceutics14030592
APA StyleBachmann, P., Frahm, N., Debus, J. L., Mashhadiakbar, P., Langhorst, S. E., Streckenbach, B., Baldt, J., Heidler, F., Hecker, M., & Zettl, U. K. (2022). Prevalence and Severity of Potential Drug–Drug Interactions in Patients with Multiple Sclerosis with and without Polypharmacy. Pharmaceutics, 14(3), 592. https://doi.org/10.3390/pharmaceutics14030592







