Development of a Sericin Hydrogel to Deliver Anthocyanins from Purple Waxy Corn Cob (Zea mays L.) Extract and In Vitro Evaluation of Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extracts
2.2.1. Extraction of Sericin from Silkworm Cocoons
2.2.2. Extraction of Anthocyanin from Purple Waxy Corn Cobs
2.3. Preparation of Sericin-Alginate Hydrogels Containing Anthocyanin from Purple Waxy Corn Cob Extract
2.4. Characterization of Sericin-Alginate Hydrogels
2.5. In Vitro Release of Anthocyanins
2.6. Release Kinetics Study
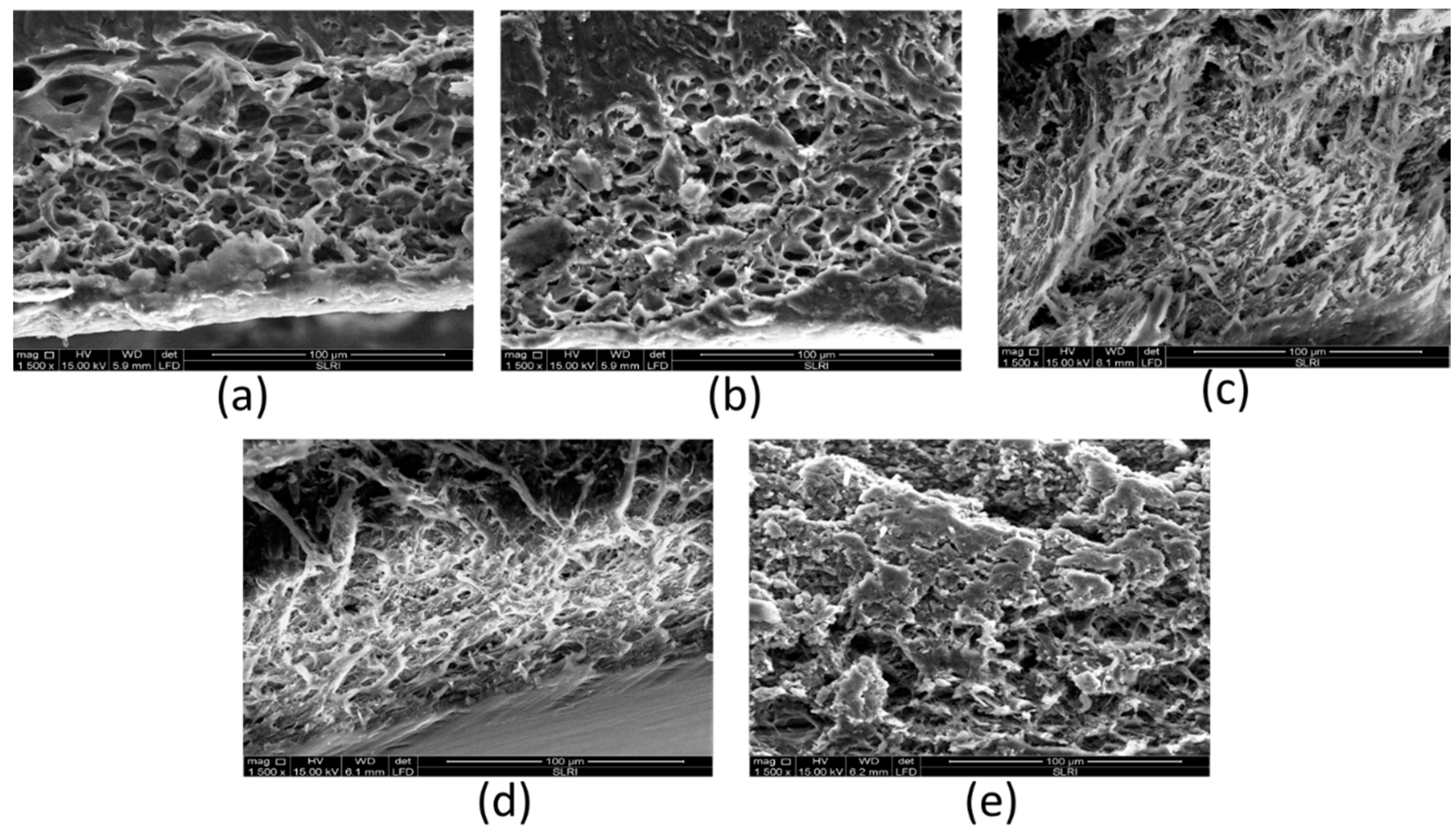
2.7. Scanning Electron Microscopy (SEM) Analyses
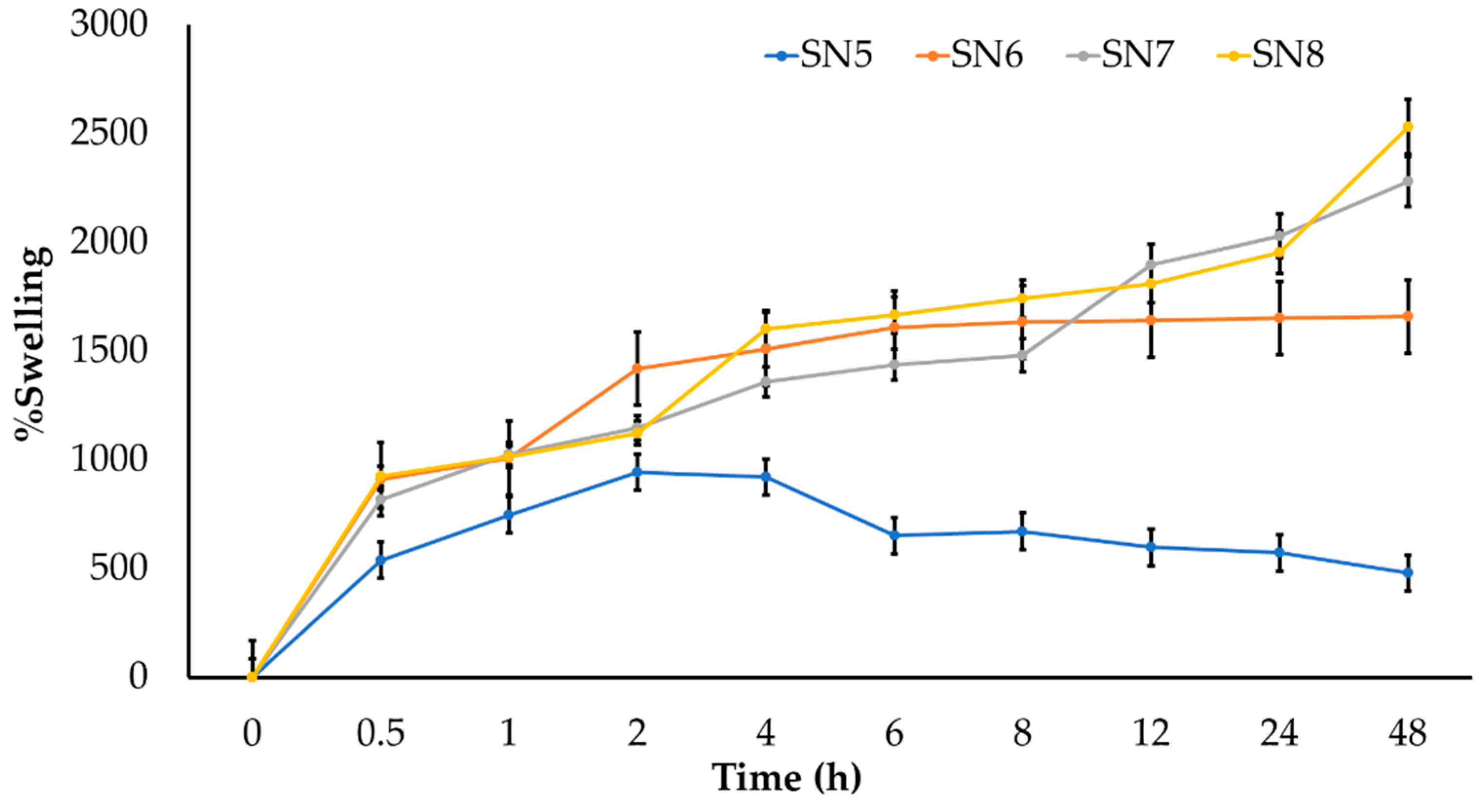
2.8. Swelling Studies
- Mw = The weight of infiltrated hydrogels
- Md = The weight of dried hydrogel
2.9. Anti-Inflammatory Study
2.9.1. Cytotoxicity Assay
2.9.2. Inhibition of Nitric Oxide (NO) Production
2.10. Real-Time Quantitative Reverse Transcription-PCR
2.11. Measurement of Inflammatory Cytokine Levels
2.12. Fourier Transform Infrared Spectroscopy FT-IR Spectroscopy
2.13. Stability Evaluation
2.14. Data Analysis
3. Results and Discussion
3.1. Extraction of Sericin by High Temperature and High Pressure (Autoclave) Technique
3.2. Extraction of Anthocyanin from Purple Waxy Corn (Zea mays L.)
3.3. Characterization of Sericin-Alginate Hydrogels
3.4. Determination of Total Anthocyanin Content in Sericin-Alginate Hydrogel Formulations
3.5. Viscosity of Hydrogel Formulations
3.6. Anti-Inflammatory Properties of Hydrogel Formulations
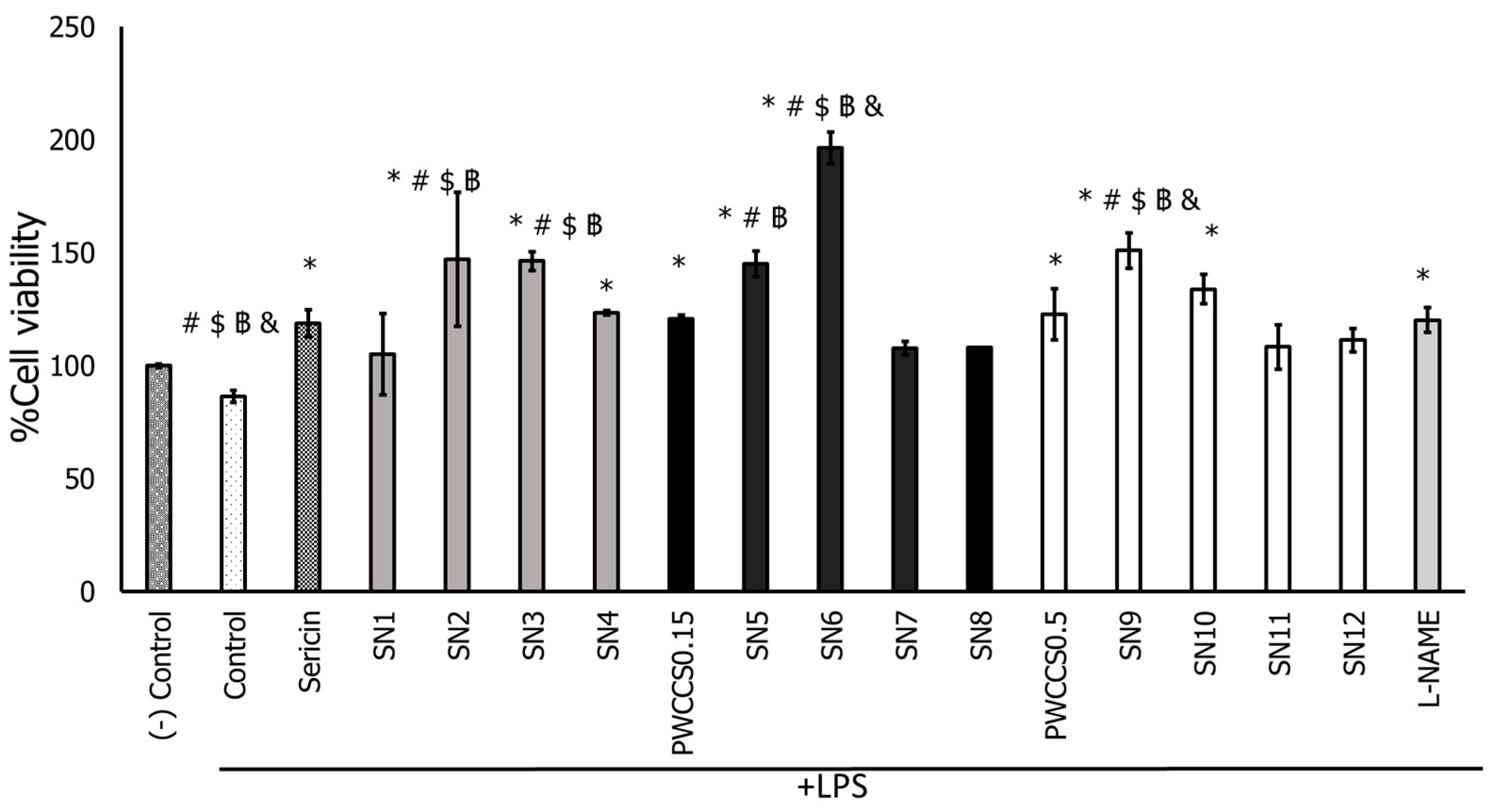
3.6.1. Cytotoxicity on RAW 264.7 Cells
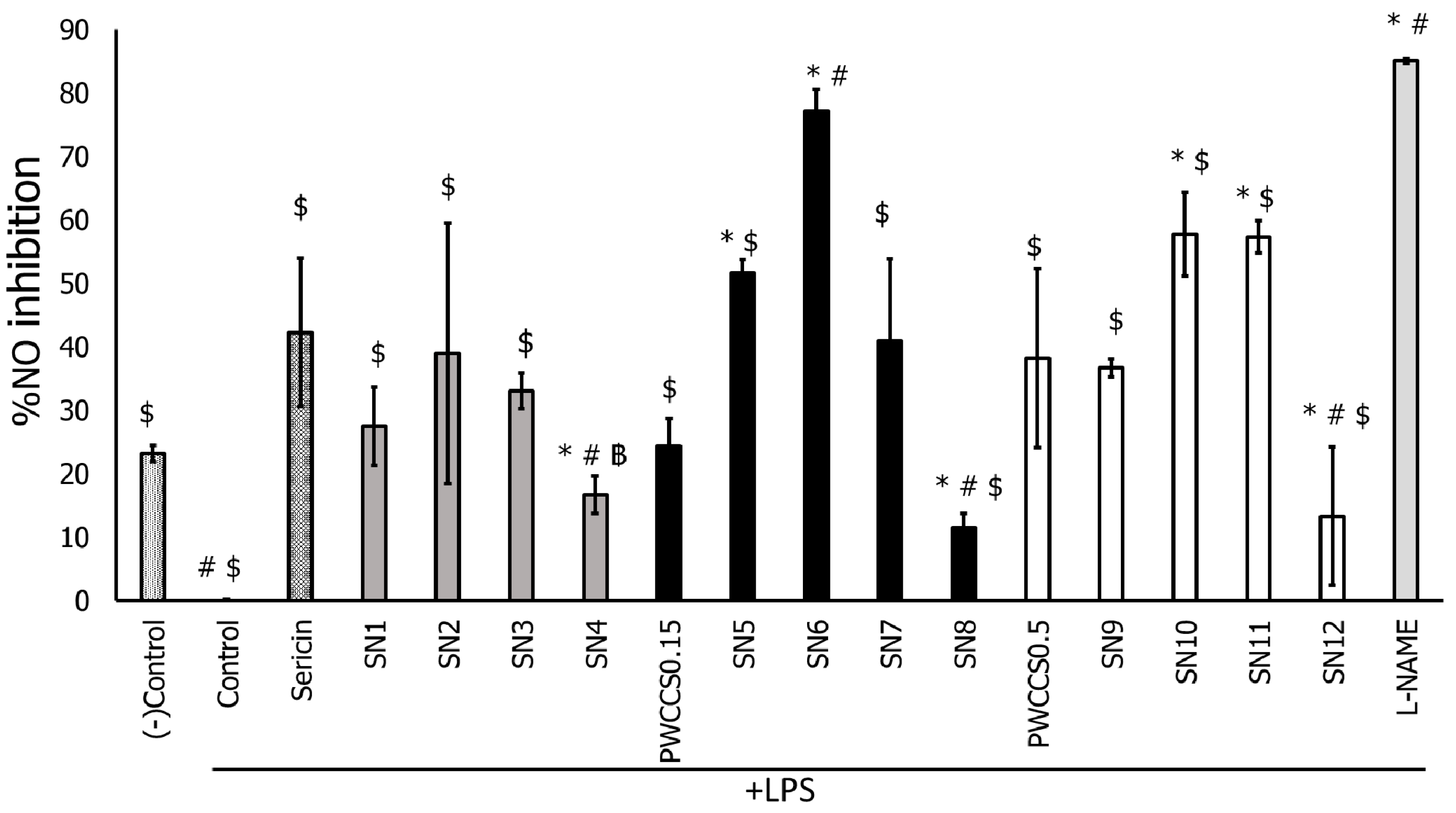
3.6.2. Inhibition of Nitric Oxide (NO) Production by LPS-Stimulated RAW 264.7 Cells
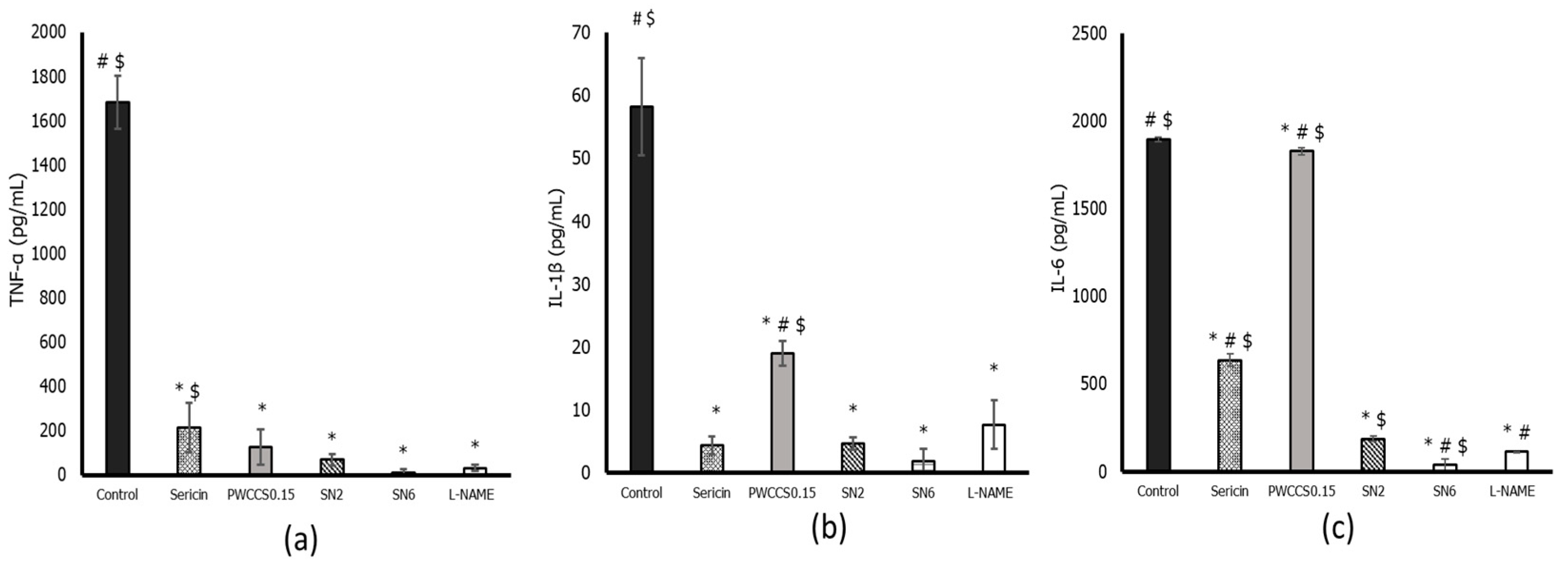
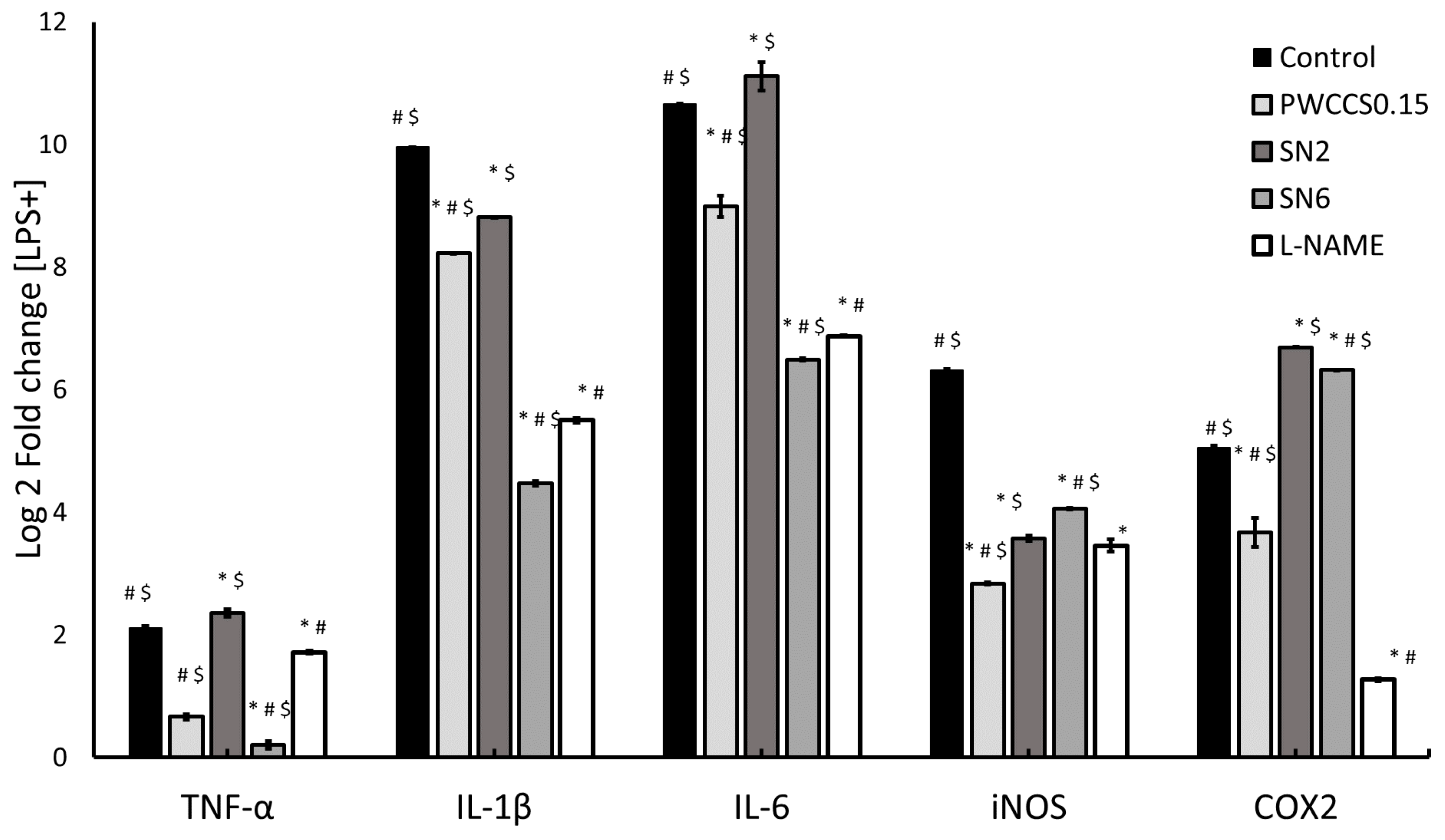
3.7. Effect of Hydrogels and Active Ingredients on Inflammatory Cytokine Gene Expression in LPS-Stimulated RAW 264.7 Cells
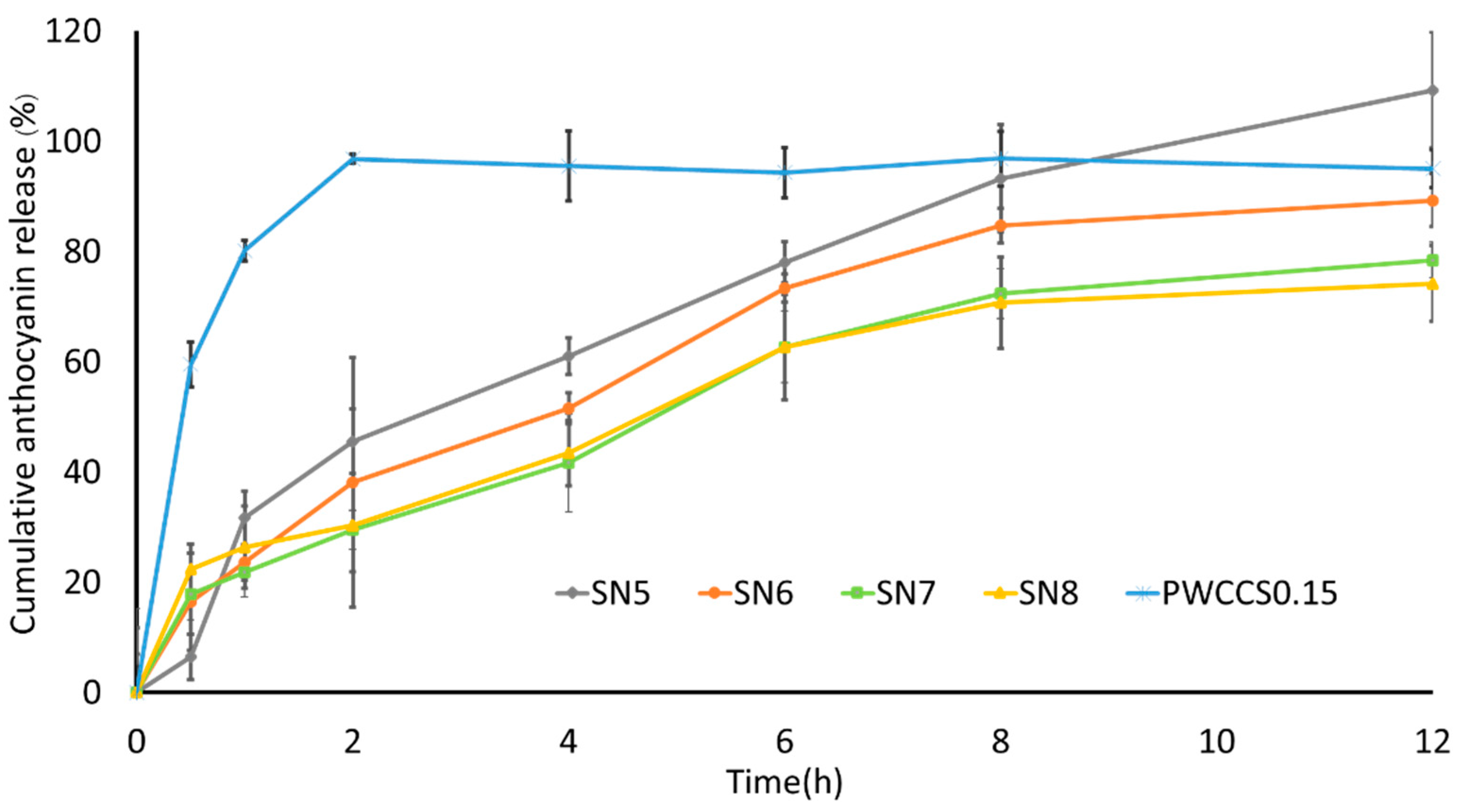
3.8. Evaluation of Releasing Anthocyanin Release from Sericin Hydrogel Formulations
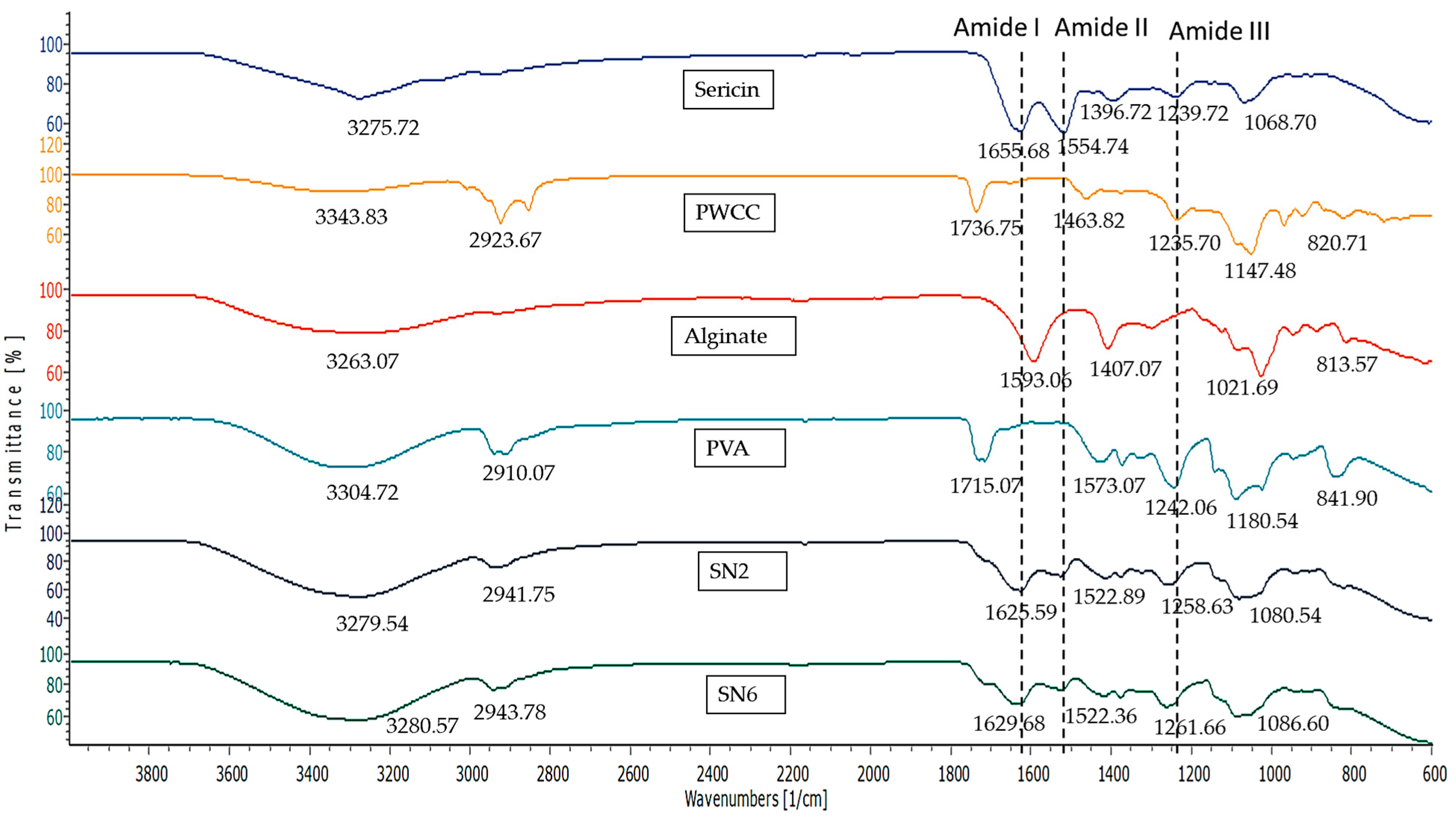
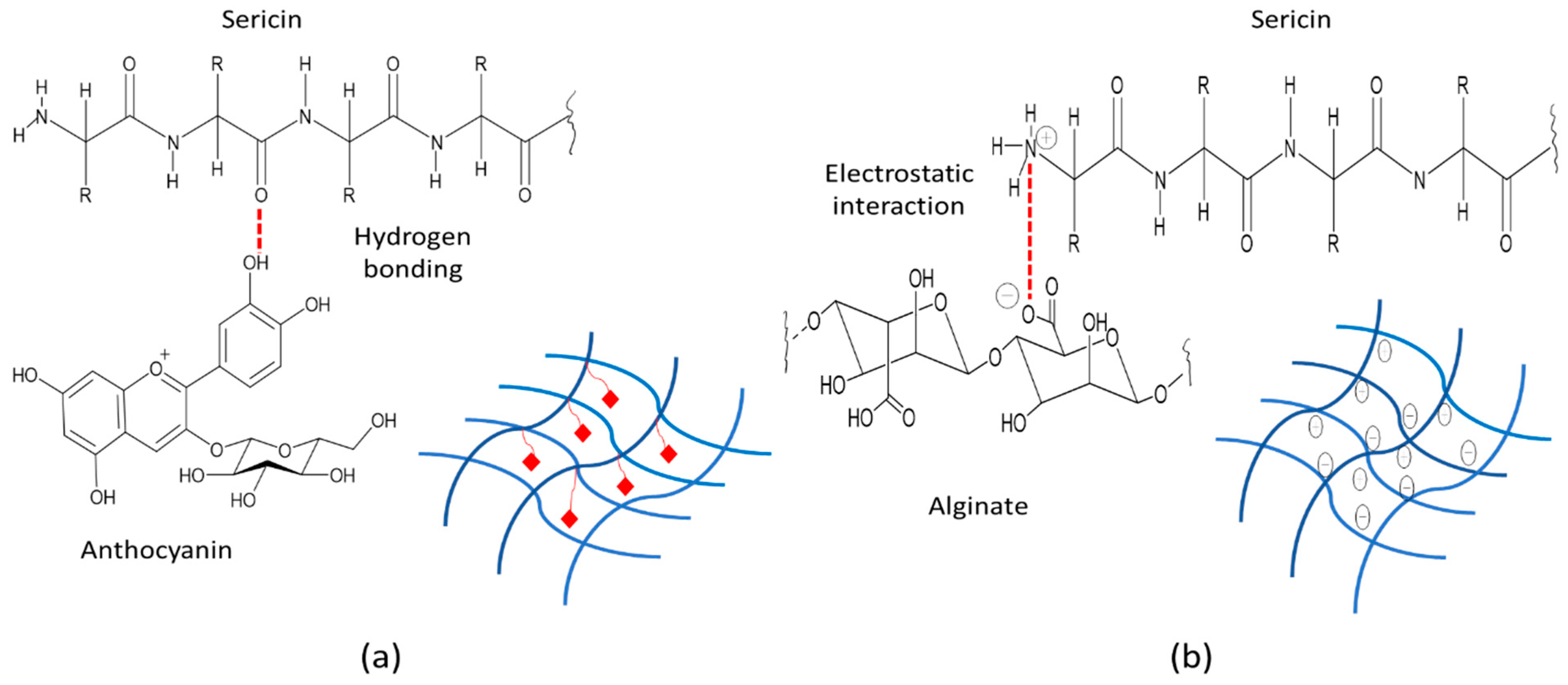
3.9. FT-IR Spectroscopy
3.10. Stability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.-C.; Zhu, L.; Wu, Z.; Yang, R.; Xu, N.; Liang, L. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 2019, 7, 99–107. [Google Scholar] [CrossRef]
- Nunes, C.D.R.; Arantes, M.B.; de Faria Pereira, S.M.; Da Cruz, L.L.; de Souza Passos, M.; De Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, Optimization and in-vitro characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Towiwat, P.; Srichana, T. Anti-inflammatory Potential of Silk Sericin. Nat. Prod. Commun. 2013, 8, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, srep12374. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, E.D.; Lima, B.M.; Rosa, P.C.P.; da Silva, M.G.C.; Vieira, M.G.A. Evaluation of proanthocyanidin-crosslinked sericin/alginate blend for ketoprofen extended release. Adv. Powder Technol. 2019, 30, 1531–1543. [Google Scholar] [CrossRef]
- Vidart, J.M.M.; Nakashima, M.; da Silva, T.L.; Rosa, P.C.P.; Gimenes, M.L.; Vieira, M.G.A.; da Silva, M.G.C. Sericin and Alginate Blend as Matrix for Incorporation of Diclofenac Sodium. Chem. Eng. Trans. 2016, 52, 343–348. [Google Scholar] [CrossRef]
- Maolin, Z.; Jun, L.; Min, Y.; Hongfei, H. The swelling behavior of radiation prepared semi-interpenetrating polymer networks composed of polyNIPAAm and hydrophilic polymers. Radiat. Phys. Chem. 2000, 58, 397–400. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Liu, Y.-M.; Wang, J.; Wang, X.-N.; Li, C.Y. Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, G.; Lertrat, K.; Suriharn, B. Phenolic Compound, Anthocyanin Content, and Antioxidant Activity in Some Parts of Purple Waxy Corn across Maturity Stages and Locations. Int. Food Res. J. 2017, 24, 490–497. [Google Scholar]
- Rimdusit, T.; Thapphasaraphong, S.; Puthongking, P.; Priprem, A. Effects of Anthocyanins and Melatonin from Purple Waxy Corn By-Products on Collagen Production by Cultured Human Fibroblasts. Nat. Prod. Commun. 2019, 14, 1934578–1986351. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Kwak, H.-K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide-treated RAW264.7 cells. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Series: Mater. Sci. Eng. 2017, 193, 12047. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [Green Version]
- Thapphasaraphong, S.; Rimdusit, T.; Priprem, A.; Puthongking, P. Crops of Waxy Purple Corn: A Valuable Source of Antioxidative Phytochemicals. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 74–77. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, H.S.; Park, S.N. A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritientong, T.; Kanokpanont, S.; Srichana, T. Formulation and characterization of silk sericin–PVA scaffold crosslinked with genipin. Int. J. Biol. Macromol. 2010, 47, 668–675. [Google Scholar] [CrossRef]
- Nazrul-Hakim, M.; Yong, Y.K.; Chiong, H.; Sulaiman, M.R.; Zuraini, A.; Zakaria, Z.A.; Yuen, K.H. Cytoprotective and enhanced anti-inflammatory activities of liposomal piroxicam formulation in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Nanomed. 2013, 8, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Gholijani, N.; Gharagozloo, M.; Farjadian, S.; Amirghofran, Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 2015, 13, 157–164. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and stability studies of emulsion systems containing pumice. Braz. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Agrawal, A.; Chaudhary, H.; Gulrajani, M.; Gupta, C. Cleaner process for extraction of sericin using infrared. J. Clean. Prod. 2013, 52, 488–494. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernandez, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2016, 7, 77–88. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Ski. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Saenjum, C.; Pattananandecha, T.; Nakagawa, K. Antioxidative and Anti-Inflammatory Phytochemicals and Related Stable Paramagnetic Species in Different Parts of Dragon Fruit. Molecules 2021, 26, 3565. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E. Biosynthesis of nitric oxide from l-arginine: A pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989, 38, 1709–1715. [Google Scholar] [CrossRef]
- Huang, C.; Li, W.; Zhang, Q.; Chen, L.; Chen, W.; Zhang, H.; Ni, Y. Anti-inflammatory activities of Guang-Pheretima extract in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. BMC Complement. Altern. Med. 2018, 18, 46. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, srep06234. [Google Scholar] [CrossRef] [Green Version]
- Sogo, T.; Terahara, N.; Hisanaga, A.; Kumamoto, T.; Yamashiro, T.; Wu, S.; Sakao, K.; Hou, D.-X. Anti-inflammatory activity and molecular mechanism of delphinidin 3-sambubioside, a Hibiscus anthocyanin. BioFactors 2015, 41, 58–65. [Google Scholar] [CrossRef]
- Gil Lee, S.; Brownmiller, C.R.; Lee, S.-O.; Kang, H.W. Anti-Inflammatory and Antioxidant Effects of Anthocyanins of Trifolium pratense (Red Clover) in Lipopolysaccharide-Stimulated RAW-267.4 Macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure–activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Lima, S.C.; Costa, P.; Celia, C.; Reis, S. Design and Characterization of Sodium Alginate and Poly(vinyl) Alcohol Hydrogels for Enhanced Skin Delivery of Quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.-M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)-bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Mandal, B.B.; Ghosh, B.; Kundu, S.C. Non-mulberry silk sericin/poly (vinyl alcohol) hydrogel matrices for potential biotechnological applications. Int. J. Biol. Macromol. 2011, 49, 125–133. [Google Scholar] [CrossRef]
- Santinon, C.; Borges, D.; da Silva, M.G.C.; Vieira, M.G.A. Evaluation of different covalent crosslinking agents into valsartan-loaded sericin and alginate particles for modified release. Powder Technol. 2021, 390, 240–255. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot studyin vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Aljar, M.A.A.; Rashdan, S.; El-Fattah, A.A. Environmentally Friendly Polyvinyl Alcohol−alginate/Bentonite Nanocomposite Hydrogel Beads as Efficient Adsorbents for Removal of Toxic Methylene Blue from Aqueous Solution. Polymers 2021, 13, 4000. [Google Scholar] [CrossRef]
- Liudvinaviciute, D.; Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Dagys, L. Formation and characteristics of alginate and anthocyanin complexes. Int. J. Biol. Macromol. 2020, 164, 726–734. [Google Scholar] [CrossRef]
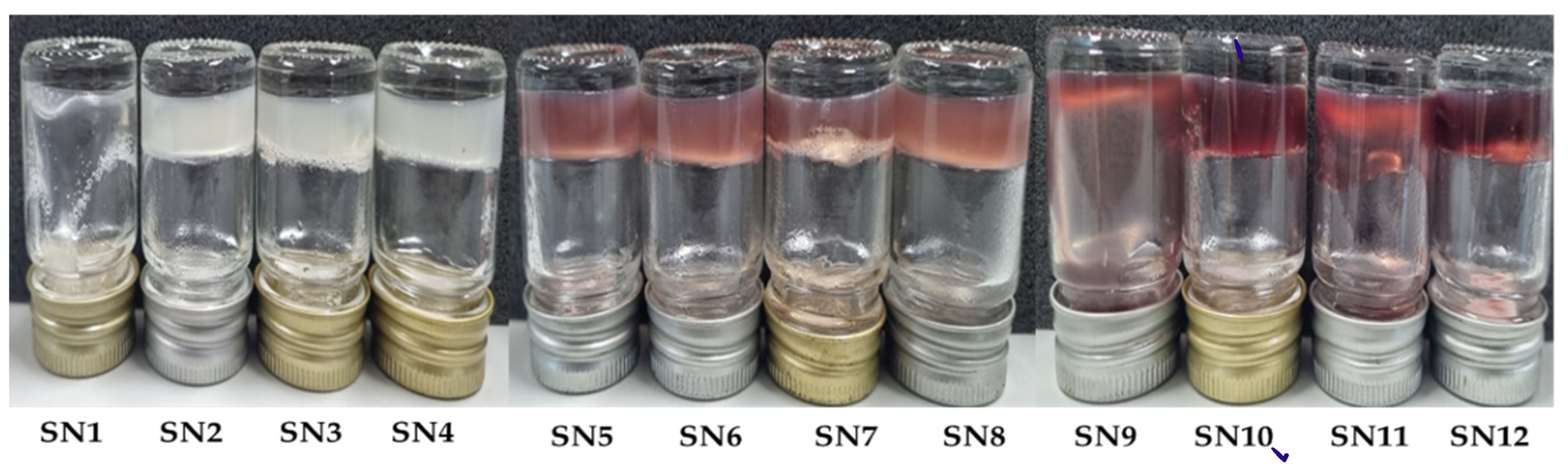

| Formulation | 5% w/v Sericin (mL) | 10% w/v PVA (mL) | 5% w/v Alginate (mL) | 10% w/v PWCCS (mL) | Final Volume (mL) | Final Conc. of Alginate (%) | Final Conc. of PWCC (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SN1 | 2.00 | 2.00 | - | - | - | - | - | - | 5.00 | 0.00 | 0.00 |
| SN2 | 2.00 | 2.00 | 0.20 | - | - | - | - | - | 5.00 | 0.20 | 0.00 |
| SN3 | 2.00 | 2.00 | - | 0.30 | - | - | - | - | 5.00 | 0.30 | 0.00 |
| SN4 | 2.00 | 2.00 | - | - | 0.40 | - | - | - | 5.00 | 0.40 | 0.00 |
| SN5 | 2.00 | 2.00 | - | - | - | - | 0.075 | - | 5.00 | 0.00 | 0.15 |
| SN6 | 2.00 | 2.00 | 0.20 | - | - | - | 0.075 | - | 5.00 | 0.20 | 0.15 |
| SN7 | 2.00 | 2.00 | - | 0.30 | - | - | 0.075 | - | 5.00 | 0.30 | 0.15 |
| SN8 | 2.00 | 2.00 | - | - | 0.40 | - | 0.075 | - | 5.00 | 0.40 | 0.15 |
| SN9 | 2.00 | 2.00 | - | - | - | - | - | 0.25 | 5.00 | 0.00 | 0.50 |
| SN10 | 2.00 | 2.00 | 0.20 | - | - | - | - | 0.25 | 5.00 | 0.20 | 0.50 |
| SN11 | 2.00 | 2.00 | - | 0.30 | - | - | - | 0.25 | 5.00 | 0.30 | 0.50 |
| SN12 | 2.00 | 2.00 | - | - | 0.40 | - | - | 0.25 | 5.00 | 0.40 | 0.50 |
| Code | Conc. of PWCC (%) | pH | Viscosity (Pa·s) | Total Anthocyanin (mg C3GE/L) | Appearance |
|---|---|---|---|---|---|
| PWCCS | 0.15 | - | - | 43.75 ± 1.44 a | |
| PWCCS | 0.50 | - | - | 101.93 ± 19.54 b | |
| SN1 | - | 6.55 ± 0.00 | 35.52 ± 0.00 e | - | White and translucent |
| SN2 | - | 6.62 ± 0.10 | 37.51 ± 0.00 g | - | White and translucent |
| SN3 | - | 6.51 ± 0.01 | 38.52 ± 0.00 i | - | White and translucent |
| SN4 | - | 6.48 ± 0.09 | 39.55 ± 0.00 j | - | White and translucent |
| SN5 | 0.15 | 6.57 ± 0.00 | 35.39 ± 0.00 c | 41.48 ± 6.62 a | Light purple and homogenous |
| SN6 | 0.15 | 6.71 ± 0.28 | 35.50 ± 0.00 d | 39.21 ± 3.26 a | Light purple and homogenous |
| SN7 | 0.15 | 6.58 ± 0.00 | 36.52 ± 0.00 f | 41.81 ± 4.06 a | Light purple and homogenous |
| SN8 | 0.15 | 6.65 ± 0.33 | 37.55 ± 0.00 h | 40.08 ± 8.01 a | Light purple and homogenous |
| SN9 | 0.50 | 6.47 ± 0.00 | 34.27 ± 0.00 a | 107.61 ± 11.98 b | Dark purple |
| SN10 | 0.50 | 6.45 ± 0.05 | 34.49 ± 0.00 b | 102.87 ± 1.53 b | Dark purple |
| SN11 | 0.50 | 6.49 ± 0.05 | 35.50 ± 0.00 d | 100.19 ± 19.22 b | Dark purple |
| SN12 | 0.50 | 6.61 ± 0.18 | 36.52 ± 0.01 f | 100.13 ± 2.52 b | Dark purple |
| Sample | Conditions | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | R2 | n | KP | ||
| PWCCS0.15 | pH5.5, 37 °C | 0.94 | 23.71 | 0.90 | 0.13 | 0.74 | 26.64 | 0.84 | 0.23 | 75.20 |
| SN5 | 0.97 | 7.05 | 0.89 | 0.05 | 1.00 | 32.09 | 1.00 | 0.50 | 31.67 | |
| SN6 | 0.89 | 6.13 | 0.81 | 0.05 | 0.96 | 28.53 | 0.98 | 0.56 | 24.72 | |
| SN7 | 0.96 | 5.16 | 0.94 | 0.06 | 0.96 | 25.22 | 0.98 | 0.56 | 21.11 | |
| SN8 | 0.91 | 3.34 | 0.88 | 0.05 | 0.96 | 15.37 | 0.96 | 0.49 | 15.78 | |
| Sample | Conc. of PWCCS (%) | pH | Viscosity (Pa·s) | Total Anthocyanin (mg C3G/L) | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| PWCCS | 0.15 | - | - | - | - | 43.75 ± 1.44 * | 13.15 ± 0.28 * |
| SN2 | 0.00 | 6.62 ± 0.10 | 6.54 ± 0.00 | 37.51 ± 0.00 * | 36.55 ± 0.01 * | - | - |
| SN5 | 0.15 | 6.57 ± 0.00 | 6.57 ± 0.06 | 35.39 ± 0.00 * | 37.52 ± 0.01 * | 41.48 ± 6.62 | 36.03 ± 0.06 |
| SN6 | 0.15 | 6.71 ± 0.28 | 6.49 ± 0.05 | 35.57 ± 0.00 | 35.57 ± 0.00 | 39.20 ± 3.25 | 37.26 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanpipit, N.; Nualkaew, N.; Kiatponglarp, W.; Priprem, A.; Thapphasaraphong, S. Development of a Sericin Hydrogel to Deliver Anthocyanins from Purple Waxy Corn Cob (Zea mays L.) Extract and In Vitro Evaluation of Anti-Inflammatory Effects. Pharmaceutics 2022, 14, 577. https://doi.org/10.3390/pharmaceutics14030577
Kanpipit N, Nualkaew N, Kiatponglarp W, Priprem A, Thapphasaraphong S. Development of a Sericin Hydrogel to Deliver Anthocyanins from Purple Waxy Corn Cob (Zea mays L.) Extract and In Vitro Evaluation of Anti-Inflammatory Effects. Pharmaceutics. 2022; 14(3):577. https://doi.org/10.3390/pharmaceutics14030577
Chicago/Turabian StyleKanpipit, Nattawadee, Natsajee Nualkaew, Worawikunya Kiatponglarp, Aroonsri Priprem, and Suthasinee Thapphasaraphong. 2022. "Development of a Sericin Hydrogel to Deliver Anthocyanins from Purple Waxy Corn Cob (Zea mays L.) Extract and In Vitro Evaluation of Anti-Inflammatory Effects" Pharmaceutics 14, no. 3: 577. https://doi.org/10.3390/pharmaceutics14030577
APA StyleKanpipit, N., Nualkaew, N., Kiatponglarp, W., Priprem, A., & Thapphasaraphong, S. (2022). Development of a Sericin Hydrogel to Deliver Anthocyanins from Purple Waxy Corn Cob (Zea mays L.) Extract and In Vitro Evaluation of Anti-Inflammatory Effects. Pharmaceutics, 14(3), 577. https://doi.org/10.3390/pharmaceutics14030577







