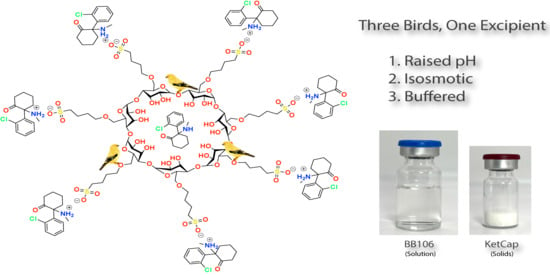
Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Chemicals and Reagents
2.3. Instrumentation
2.3.1. pH and Mass Measurements
2.3.2. Nuclear Magnetic Resonance Spectroscopy
2.3.3. GC/MS
2.3.4. Moisture Readings
2.3.5. Osmolality
2.3.6. HPLC
2.4. Fehling’s Colorimetric Assay for Stability of Captisol®
2.5. Titration Method
2.6. Ketamine Freebase Captisol® Phase Solubility
2.7. Preparation of Captisol® Acid (CapAcid)
2.8. Synthesis of Ketamine Freebase
2.9. Resolution of S-Ketamine
Resolution of R-Ketamine
2.10. Preparation of Ketamine Captisol® Formulations
2.10.1. Preparation of BB105 (96 mg/mL Ketamine Freebase)
2.10.2. Preparation of BB106 (70 mg/mL Ketamine Freebase)
2.10.3. Preparation of BB107 (70 mg/mL S-Ketamine Freebase)
2.10.4. Preparation of BB108 (70 mg/mL R-Ketamine Freebase)
2.10.5. Preparation of Ketamine-HCl-Captisol® Formulation (70 mg/mL Ketamine HCl FB Eq)
2.10.6. Preparation of Solid KetCap Stoichiometric Salt
2.10.7. Preparation of BB106 from CapAcid Solution
2.11. Analysis of Ketamine Captisol® Formulations
2.11.1. Concentration of KetCap and Commercial Ketamine HCl Samples
HPLC Sample Preparation and Analysis
2.11.2. Enantiomeric Purity of S-Ketamine and R-Ketamine Freebase
2.11.3. Density
2.11.4. Buffering Capacity: Forced Precipitation of Ketamine HCl and Formulations with 1 M NaOH
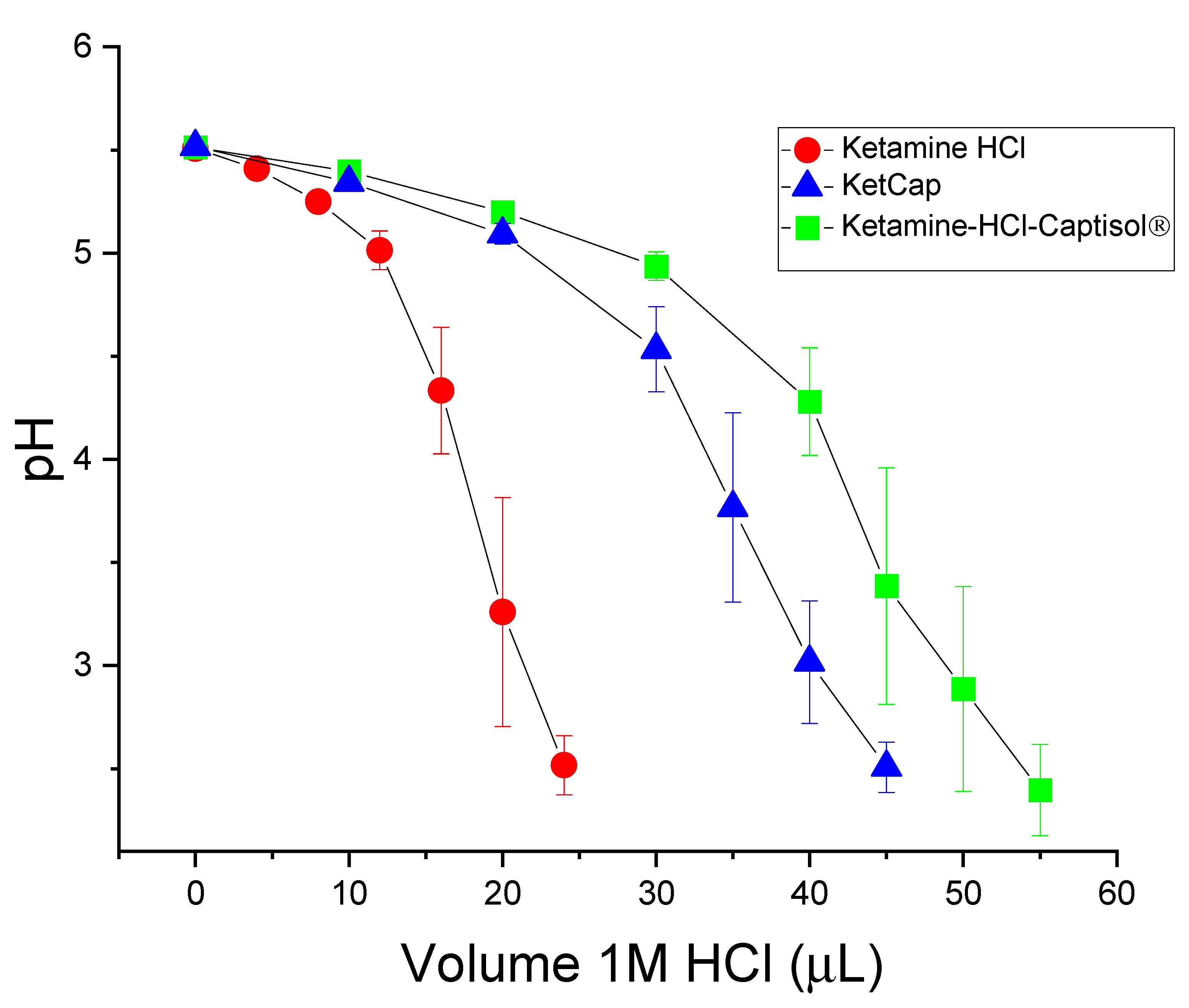
2.11.5. Buffering Capacity: Titration of Ketamine HCl and Captisol® Formulations with 1 M HCl
2.11.6. Osmolality and pH vs. dilution of Ketamine HCl and Captisol® Formulations
2.11.7. Viscosity Measurements of Captisol®, KetCap, and Ketamine HCl Formulations
3. Results and Discussion
3.1. Ketamine Freebase Phase Solubility with Captisol®
3.2. Preparation of Captisol Acid (CapAcid)
3.2.1. Preparation of Captisol Acid (CapAcid)
3.2.2. Preparation of Ketamine Captisol® Formulations
3.3. Analysis and Stability of Ketamine-Captisol® Formulations Compared to Ketamine HCl
3.3.1. Determination of Concentration of Ketamine in KetCap and Commercial Ketamine HCl Samples
3.3.2. Buffering Capacity of Ketamine Captisol® Complexes Compared to Ketamine HCl
3.4. Stability of Captisol®
Fehling’s Test for Stability of Captisol®
3.5. Viscosity Measurements of Captisol®, KetCap, and Ketamine HCl Formulations
4. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations II: Solubilization, Binding Constant, and Complexation Efficiency. Drug Discov. Today 2016, 21, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-Cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.captisol.com/technology/how-it-works#:~:text=Captisol%C2%AE%20Stabilizes&text=Interaction%20of%20the%20API%20with,reactants%20in%20the%20aqueous%20environment (accessed on 28 December 2021).
- Marinelli, A.; Capucci, A. Amiodarone (Nexterone) Injection for the Treatment and Prophylaxis of Frequently Recurring Ventricular Fibrillation. Expert Opin. Pharmacother. 2012, 13, 573–584. [Google Scholar] [CrossRef]
- Singh, R.; Chen, J.; Miller, T.; Bergren, M.; Mallik, R. Solution Stability of Captisol-Stabilized Melphalan (Evomela) versus Propylene Glycol-Based Melphalan Hydrochloride Injection. Pharm. Dev. Technol. 2018, 23, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Tolerability of Hypertonic Injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Martinez, R.; Festini, T.; Peris, J.-E. Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv. Ther. 2019, 36, 2986–2996. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.; Schug, S.A. The Role of Ketamine in Pain Management. Biomed. Pharmacother. 2006, 60, 341–348. [Google Scholar] [CrossRef]
- Schwenk, E.S.; Viscusi, E.R.; Buvanendran, A.; Hurley, R.W.; Wasan, A.D.; Narouze, S.; Bhatia, A.; Davis, F.N.; Hooten, W.M.; Cohen, S.P. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 2018, 43, 456–466. [Google Scholar] [CrossRef]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for Chronic Pain: Risks and Benefits. Br. J. Clin. Pharmacol. 2014, 77, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chizh, B.; Headley, P. NMDA Antagonists and Neuropathic Pain—Multiple Drug Targets and Multiple Uses. Curr. Pharm. Des. 2005, 11, 2977–2994. [Google Scholar] [CrossRef] [PubMed]
- Elia, N.; Tramèr, M.R. Ketamine and Postoperative Pain—A Quantitative Systematic Review of Randomised Trials. Pain 2005, 113, 61–70. [Google Scholar] [CrossRef]
- Radvansky, B.M.; Shah, K.; Parikh, A.; Sifonios, A.N.; Le, V.; Eloy, J.D. Role of Ketamine in Acute Postoperative Pain Management: A Narrative Review. BioMed Res. Int. 2015, 2015, 749837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.P.; Bhatia, A.; Buvanendran, A.; Schwenk, E.S.; Wasan, A.D.; Hurley, R.W.; Viscusi, E.R.; Narouze, S.; Davis, F.N.; Ritchie, E.C.; et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 2018, 43, 521–546. [Google Scholar] [CrossRef]
- Peltoniemi, M.A.; Hagelberg, N.M.; Olkkola, K.T.; Saari, T.I. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin. Pharmacokinet. 2016, 55, 1059–1077. [Google Scholar] [CrossRef]
- Eide, P.K.; Stubhaug, A.; Øye, I.; Breivik, H. Continuous Subcutaneous Administration of the N -Methyl-D-Aspartic Acid (NMDA) Receptor Antagonist Ketamine in the Treatment of Post-Herpetic Neuralgia. Pain 1995, 61, 221–228. [Google Scholar] [CrossRef]
- Webster, L.R.; Walker, M.J. Safety and Efficacy of Prolonged Outpatient Ketamine Infusions for Neuropathic Pain. Am. J. Ther. 2006, 13, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhang, Y.; Zhang, J.; Li, J.; Li, H.; Jin, H. Determination and Toxicokinetics Comparison of Ketamine and S(+)-Ketamine in Dog Plasma by HPLC-MS Method. Biomed. Chromatogr. 2010, 24, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Dybek, M.; Wallach, J.; Kavanagh, P.V.; Colestock, T.; Filemban, N.; Dowling, G.; Westphal, F.; Elliott, S.P.; Adejare, A.; Brandt, S.D. Syntheses and Analytical Characterizations of the Research Chemical 1-[1-(2-fluorophenyl)-2-phenylethyl]Pyrrolidine (Fluorolintane) and Five of Its Isomers. Drug Test. Anal. 2019, 11, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Hofstetter, R.; Fassauer, G.M.; Link, A.; Siegmund, W.; Oswald, S. Quantitative Chiral and Achiral Determination of Ketamine and Its Metabolites by LC–MS/MS in Human Serum, Urine and Fecal Samples. J. Pharm. Biomed. Anal. 2017, 139, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Fehling, H. The Quantitative Determination of Sugar and Starch by Means of Copper Sulfate. Ann. Chem. Pharm. 1849, 72, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Ligand. How to Solubalize a Drug with Captisol. Available online: https://www.captisol.com/faqs/category/656/how-to-solubilize-a-drug-with-captisol (accessed on 28 December 2021).
- Stevens, C.L. Aminoketones and Methods for Their Production. US3254124A, 31 May 1966. [Google Scholar]
- Steiner, K.; Gangkofner, S.; Grunenwald, J.M. Process for the Resolution of Racemic Ketamine. CA2253575C, 29 November 2005. [Google Scholar]
- Chen, C.; Lu, X. Enantioselective Syntheses of (S)-Ketamine and (S)-Norketamine. Org. Lett. 2019, 21, 6575–6578. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of Cyclodextrin Solubilization of Drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Sotthivirat, S.; Haslam, J.L.; Stella, V.J. Evaluation of Various Properties of Alternative Salt Forms of Sulfobutylether-β-Cyclodextrin, (SBE)7M-β-CD. Int. J. Pharm. 2007, 330, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Klimscha, W.; Horvath, G.; Szikszay, M.; Dobos, I.; Benedek, G. Antinociceptive Effect of the S(+)-Enantiomer of Ketamine on Carrageenan Hyperalgesia after Intrathecal Administration in Rats. Anesth. Analg. 1998, 86, 561–565. [Google Scholar] [CrossRef]
- Sigtermans, M.J.; van Hilten, J.J.; Bauer, M.C.R.; Arbous, S.M.; Marinus, J.; Sarton, E.Y.; Dahan, A. Ketamine Produces Effective and Long-Term Pain Relief in Patients with Complex Regional Pain Syndrome Type 1. Pain 2009, 145, 304–311. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Wang, D. The Effect of Ketamine Infusion in the Treatment of Complex Regional Pain Syndrome: A Systemic Review and Meta-Analysis. Curr. Pain Headache Rep. 2018, 22, 12. [Google Scholar] [CrossRef]
- Fukumoto, K.; Toki, H.; Iijima, M.; Hashihayata, T.; Yamaguchi, J.; Hashimoto, K.; Chaki, S. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. J. Pharmacol. Exp. Ther. 2017, 361, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Qu, Y.; Abe, M.; Nozawa, D.; Chaki, S.; Hashimoto, K. (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol. Psychiatry 2017, 82, e43–e44. [Google Scholar] [CrossRef]
- Kimura, H.; Hirayama, M.; Yoshida, K.; Uosaki, Y.; Nakahara, M. Effect of Water on Hydrolytic Cleavage of Non-Terminal α-Glycosidic Bonds in Cyclodextrins To Generate Monosaccharides and Their Derivatives in a Dimethyl Sulfoxide–Water Mixture. J. Phys. Chem. A 2014, 118, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, F.; Berteau, C.; Filipe-Santos, O.; Wang, T.; Rojas, H.; Granger, C. Evaluation of the Impact of Viscosity, Injection Volume, and Injection Flow Rate on Subcutaneous Injection Tolerance. Med. Devices: Evid. Res. 2015, 8, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, R.P.; Khatri, H.; Dibble, A.R.G. Injectability as a Function of Viscosity and Dosing Materials for Subcutaneous Administration. Int. J. Pharm. 2019, 554, 376–386. [Google Scholar] [CrossRef] [PubMed]


| Sample | [Ketamine] (mg/mL FB eq) | Average Osmolality (mOsm/kg) ± SEM |
|---|---|---|
| 0.9% Saline | - | 290.8 ± 1.1 |
| Ketamine HCl | 70 | 527.0 ± 2.7 |
| Ketamine HCl | 96 | 704.4 ± 9.9 |
| Ketamine HCl | 100 | 740.1 ± 6.8 |
| BB105 (KetCap) | 96 | 448.0 ± 4.4 |
| BB106 (KetCap) | 70 | 291.4 ± 4.0 |
| BB107 (EsKetCap) | 70 | 298.2 ± 9.5 + |
| BB108 (ArKetCap) | 70 | 289.3 * |
| Ketamine-HCl-10.7%-Captisol® | 70 | 938.5 ± 2.2 |
| Ketamine-HCl-15%-Captisol® | 96 | 1443.4 ± 28.0 |
| Ketamine-HCl-10%-HPβCD | 70 | 618.0 ± 5.7 |
| Ketamine-HCl-10%-Propylene glycol 1 | 70 | 1899.0 ± 49 |
| Sample | Theoretical [Ketamine] (mg/mL FB eq) | [Ketamine] (mg/mL FB eq) ± SEM | Measured pH (Mean ± SEM) |
|---|---|---|---|
| BB106 | 70 | 73.1 ± 1.3 | 5.51 |
| BB106 10-month stability | 70 | 72.4 ± 0.4 | - |
| Ketalar Ketamine HCl | 50 | 52.8 ± 0.2 | 4.09 ± 0.01 |
| Mylan Ketamine HCl | 50 | 51.7 ± 1.1 | 4.18 ± 0.03 |
| Hospira Ketamine HCl | 100 | 103.8 ± 2.0 | 3.88 ± 0.03 |
| West-Ward Ketamine HCl | 100 | 105.9 ± 3.0 | 3.94 ± 0.02 |
| Sample | Mean Volume NaOH to Precipitate (µL) ± SEM |
|---|---|
| Ketamine HCl (70 mg/mL FB equiv) | 5.33 ± 0.33 |
| Ketamine-HCl-10%-HPβCD (70 mg/mL Ketamine FB equiv) | 7.67 ± 0.33 |
| Ketamine-HCl-10%-Propylene glycol (70 mg/mL FB equiv) | 5.33 ± 0.33 |
| Ketamine-HCl-10%-Captisol® (70 mg/mL Ketamine FB equiv) | 8.33 ± 0.67 |
| BB106 (70 mg/mL Ketamine FB equiv) | 9.67 ± 0.67 |
| Sample | Mean Viscosity (cP) ± SEM |
|---|---|
| H2O | 1.01 ± 0.03 |
| Ketamine HCl (70 mg/mL FB eq) | 1.19 ± 0.04 |
| BB106 (70 mg/mL Ketamine) | 1.86 ± 0.04 |
| 10% Captisol® | 1.58 ± 0.02 |
| 12% Captisol® | 1.63 ± 0.03 |
| 15% Captisol® | 1.75 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallach, J.; Gamrat, J.; Jauhola-Straight, R.; Becker, J.; Eckrich, T. Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection. Pharmaceutics 2022, 14, 556. https://doi.org/10.3390/pharmaceutics14030556
Wallach J, Gamrat J, Jauhola-Straight R, Becker J, Eckrich T. Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection. Pharmaceutics. 2022; 14(3):556. https://doi.org/10.3390/pharmaceutics14030556
Chicago/Turabian StyleWallach, Jason, James Gamrat, Rebekah Jauhola-Straight, Jeffrey Becker, and Thomas Eckrich. 2022. "Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection" Pharmaceutics 14, no. 3: 556. https://doi.org/10.3390/pharmaceutics14030556
APA StyleWallach, J., Gamrat, J., Jauhola-Straight, R., Becker, J., & Eckrich, T. (2022). Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection. Pharmaceutics, 14(3), 556. https://doi.org/10.3390/pharmaceutics14030556