Abstract
Background: The SARS-CoV-2 pandemic has resulted in a dramatic rise of the demand for medical devices and drugs. In this context, an important shortage of programmable syringe pumps, used to administrate different drugs in intensive care units, was seen. The opportunity of administrating combinations of five intensive care units selected drugs (Sufentanil, Clonidine, Loxapine, Midazolam, and Ketamine) was considered. Methods: The drug mixtures were studied in a pure form or diluted in NaCl 0.9% or G5%. Twenty-six possible combinations of the five drugs were produced in glass vials or polypropylene syringes and stored at 25 °C for 14 days. The LC method was implemented to study drugs combinations in the presence of the degradation products. The clearness and pH were also monitored. Results: All the 26 possible combinations displayed adequate physicochemical stability at 25 °C: at least 3 days and 7 days, respectively, for the dilution in 0.9% NaCl or glucose 5%, and the pure drug products mixtures. Conclusions: The study provided sufficient stability results, covering the medication administration period of at least three days. The combination of more than two drugs offers the advantage of minimizing the individual doses and reduces unwanted side-effects. Hence, this study opens up the possibility of combining the five drugs in one single syringe, which is useful especially under the current circumstances associated with an important shortage of programmable syringe pumps and pharmaceuticals.
1. Introduction
During the first wave of the 2019 SARS-CoV-2 pandemic that hit many countries worldwide, health systems were put under severe strain as hospitals experienced a significant influx of patients. The severity of clinical signs required that approximately 30% of patients remain in hospital and 5% be admitted to intensive care [], leading to the overcrowding of intensive care units (three times the average number of patients in intensive care units at Pitié-Salpêtrière hospital of Paris) and a dramatic rise in the demand for medical devices and drugs.
In this context, we faced a shortage of programmable syringe pumps used to administer different drugs in intensive care units, especially Sufentanil, Clonidine, Loxapine, Midazolam, and Ketamine, on which the study is focused. To face this shortage, the opportunity of administrating two or more combined drugs was considered, in accordance with medical staff.
The intrinsic stability of some of these drugs alone has already been described in the literature, but rarely in combination with other drugs, and drug–drug compatibility studies were often limited to two drug products.
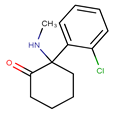
Midazolam hydrochloride is a short-acting, injectable benzodiazepine commonly used in hospitals for preoperative or conscious sedation, general anxiolysis, and induction of general anesthesia. It is administered in intensive care units as a continuous i.v. infusion to achieve long-term sedation. The drug product appears to be poorly affected by light and temperature. However, it is described to undergo hydrolysis degradation [,].
Ketamine is a non-barbiturate dissociative anesthetic, used either alone for short-term medical procedures (rapid sequence intubation, short-term procedural sedation, etc.), or in combination with other medications as a pre-anesthetic. As well as Midazolam, Ketamine solution diluted with sterile water for injection showed remarkable chemical stability after storage at room temperature with exposure to light [].
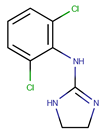
Clonidine is an imidazoline-derivative used as hypotensive agent, since it is a centrally-acting α2-adrenergic agonist; it is also an analgesic (when administered epidurally, it produces a dose-dependent analgesia), sedative, and anxiolytic drug, properties that allow its use in anesthesia and intensive care. Clonidine appeared to be sensitive to acidic and oxidative conditions but remained stable under dry heat 60 °C and basic hydrolysis conditions [].
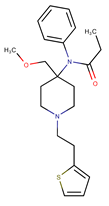
Sufentanil is a synthetic opioid with a morphinomimetic structure. It is a very potent analgesic, used in intensive care units for its sedative properties. Sufentanil seems to be susceptible to many stress conditions. A. Jappinen et al. [] observed how the increase in temperature affects the stability of Sufentanil: at 4 °C, a Sufentanil citrate solution maintained chemical stability for 23 days, but at 21 °C, it was stable for 3 days. In addition, Sufentanil was fragile under acidic and basic hydrolysis, oxidative, or light stress conditions [].
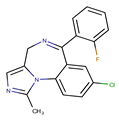
Loxapine, a dibenzoxazepine derivative, is a conventional antipsychotic (dopaminergic antagonist), mainly used in the treatment of schizophrenia and associated anxiety, agitation, and irritability. A very limited study on the drug stability was found in the literature.
An extensive study of the literature revealed no data regarding the stability behaviour of at least three of the selected drugs mixed.
This research is a comprehensive physicochemical compatibility study of intensive care units selected drugs (Sufentanil, Clonidine, Loxapine, Midazolam, and Ketamine), with the aim of understanding if it is possible to encompass all the combinations of the five molecules in the same injection solution, either entirely or partially, in order to reduce the number of syringes requested. From a medical staff perspective, drugs’ combination stability must be of at least two days to cover the medication administration period in intensive care units.
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
Midazolam (5 mg mL−1) and sufentanil (5 µg mL−1) were provided by Mylan® Laboratory (Saint-Priest, France), clonidine (Catapressan® 0.15 mg mL−1) was obtained from Boehringer Ingelheim (Paris, France), and loxapine (Loxapac® 25 mg mL−1) and ketamine (50 mg mL−1) were supplied by Eisai® (Courbevoie, France) and Panpharma® (Luitré-Dompierre, France), respectively. ChemAxon prediction and calculation software (MarvinSketch version 15.11.9, Cambridge, MA, USA) was used to predict the drugs’ main physicochemical properties such as pKa and logP (Table 1). Reference standards of the five products were purchased from Sigma-Aldrich (St Quentin-Fallavier, France). Formic acid 99–100% (AnalaR Normapur®) was supplied by VWR International (Fontenay-sous-Bois, France). HCl 1 M and NaOH 10 M were obtained by VWR International. Analytical grade methanol came from Sigma–Aldrich (St Quentin-Fallavier, France). Sterile water (Versylene®), sodium chloride (0.9% NaCl), and glucose 5% (G5%) were obtained from Fresenius Kabi France SA (Sevres, France). Polypropylene syringes were purchased from Becton Dickinson® (Rungis, France). Glass vials used for stability study were provided by Interchim® (Montluçon, France).

Table 1.
Summary of the drugs products and active pharmaceutical ingredient (API) properties.
2.1.2. Instrumentation
A reversed-phase liquid chromatography (LC) method was implemented to study drug combinations in the presence of potential degradation products. The LC system (Dionex, Les Ulis, France) consists of a quaternary pump, a vacuum degasser, a photo-diode array (PDA) detector, and an autosampler, piloted by Chromeleon® software version 6.80 SR11 (Dionex, Les Ulis, France). The pH of each drug product and its mixtures was measured using a Mettler Toledo SevenCompact™ pH meter (Columbus, OH, USA).
2.2. Methodology
2.2.1. Stress Testing and High Performance LC Method Optimization and Validation
Degradation Protocol
Prior to the method validation, stress testing was performed to develop the method, according to International Council on Harmonisation (ICH) guidelines for a better understanding of the drugs’ intrinsic stability. Each drug product and mixture of 5 compounds were subjected to high temperature (60 ± 0.5 °C), acidic and basic hydrolysis (HCl 1N and NaOH 1N, respectively, stored at 22 ± 3 °C) and oxidative (0.5% H2O2 stored at 22 ± 3 °C) stress conditions. A dilution factor of 10 was applied to each drug product. Samples were analyzed at days 0, 1, 2, 5, and 7 and the chromatograms were compared with the ones of control samples. For the purpose of interpretation, a 20% degradation level was considered.
Method Optimization
The aim of the optimization was to develop a single method able to separate the 5 drugs with appropriate resolution factor and without interferences with potential degradation products. In order to achieve this goal, several chromatographic parameters such as mobile phase composition, column, pH, temperature or detection wavelength were appropriately chosen and optimized. Separation was considered as appropriate if resolutions between two peaks were all superior to 1.5. A desirable separation and resolution was obtained using a mobile phase composed of methanol (solvent A) and 0.1% formic acid in water (solvent B) set in a gradient mode (0–10 min: 80% A; 10–17 min: 80% → 30% A; 17–22 min: 30% → 80% A); the flow rate was set at 0.8 mL/min and the injection volume at 40 μL. The selected column was an Agilent Zorbax SB-C8 column, 5µm × 4.6 × 250 mm, maintained at 25 °C ± 1 °C. The vials were thermostated at 8 °C during the LC process. Due to the shortening of width of Sufentanil absorption in the UV region, the detection wavelength was set at 205 nm. The other 4 molecules were detected and quantified at 205 and 225 nm.
Method Validation
- (a)
- Validation protocol
Relevant validation parameters (specificity, linearity, accuracy, precision, and limits of detection and quantification) as per ICH Q2R1 [] and SFSTP guidelines [] were evaluated.
(a.1) Specificity
Specificity was established by observing the separation of the 5 main peaks eventually with degradation products. Peak purity was analyzed using a PDA detector.
(a.2) Linearity and Accuracy
Linearity and accuracy were determined over three days by three operators analyzing different concentrations of each drug. Seven-point calibration curves (70–80–90–100–110–120–130%) around the target concentration were considered. For each drug, linear adjustment was validated by means of Fischer’s statistical analysis and ANOVA variance analysis. An appropriate quantification method for each drug was decided with the support of a Student’s t-test comparison of the linear regression slopes, between the drug solely and in presence of the other ones.
(a.3) Precision
For each drug, intraday and interday repeatability were assessed for a target concentration by assaying six samples solutions on three consecutive days.
(a.4) LOD and LOQ
Limits of detection (LOD) and quantification (LOQ) were established by the graphical method considering the signal–background noise ratio of 3:1 and 10:1, respectively.
- (b)
- Stability study
The finding of an optimized protocol allowed the execution of a stability study. Applying the number of combinations formula (Equation (1)), 26 possible combinations, named from M1 to M26, were obtained: 10 for mixture of 2 or 3 drugs, 5 for 4 drugs, and 1 for combination of all the 5 drugs (Table 2).
where k is the number of combinations and n the number of elements (drugs).

Table 2.
Drugs mixtures qualitative composition.
For each combination, samples were made up in triplicate and allocated in 4 mL hermetically sealed glass vials or polypropylene syringe. The study was performed considering two steps. First, a compatibility study was realized employing the five pure drugs products. Their final concentrations in each combination was related to the number of drugs contained within it, so a dilution factor of 1/2, 1/3, 1/4 and 1/5 was applied respectively for the combinations of 2, 3, 4, and 5 drugs (Table 3). The second step considered the execution of a stability study in real operating conditions; therefore, NaCl 0.9% and Glucose 5% were used as diluting solvents to recreate the real set-up of administration (Table 3). The samples were prepared in glass vials and polypropylene syringe, then stored at 25 °C.

Table 3.
Drugs mixtures quantitative composition.
Different parameters were observed to verify the stability of the preparations:
(b.1) Content variation
For the first step described above, high performance LC was performed to analyze the content of each combination at days 0, 2, 3, 7, and 14, while for the second one, the study was conducted until day 7, in order to observe any variation of the concentrations out of the range of ± 5%. For each mixture, the working solution was prepared by diluting stock solution in water in sort to obtain a final concentration of 1 μg mL−1, 5 μg mL−1, 5 μg mL−1, 25 μg mL−1, and 30 μg mL−1, respectively, for sufentanil, ketamine, midazolam, loxapine, and clonidine. These results are validated by monitoring the appearance of degradation products following the ICH Q3B [] recommendations.
(b.2) pH and visual control
The pH of each drug product taken individually and the 26 combinations of pure products (step 1) was measured to find out any change of the parameter, since a stable value is an indicator of chemico-physical stability. pH was measured at room temperature at days 0, 3, 7 and 14. Samples were inspected against a light and dark background to monitor clearness and turbidity.
(b.3) Statistical interpretation
All statistical tests were performed using GraphPad Prism V 9.3.1.471 for Windows (GraphPad Software, La Jolla, CA, USA) using a two-way ANOVA.
3. Results
3.1. Method Validation
3.1.1. Specificity
The complexity of method validation in this study was to develop a single method capable of identifying and quantifying simultaneously the five molecules in the presence of the degradation products. The developed analytical procedure reached the ability to separate not only the five molecules, but also their degradation products generated under stress conditions. Hence, the absence of interference validated the stability, indicating power and the suitability of the method to identify and quantify all the molecules in question (Figure 1). As Sufentanil, the most hydrophilic of the five products, is eluted after only 3 min of running, (Figure 1), a complementary method, as described by Lambropoulos et al. [], was developed for its content determination within the mixtures.
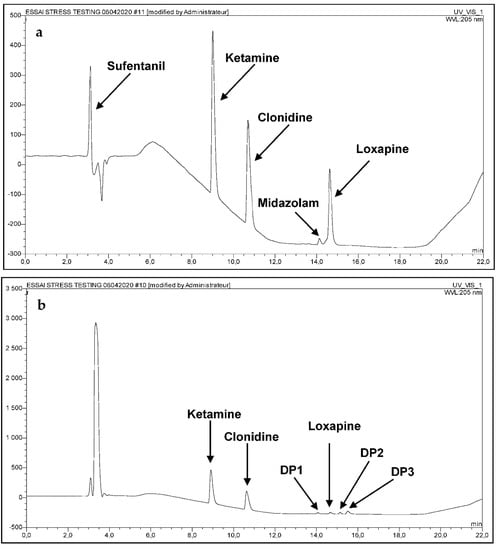
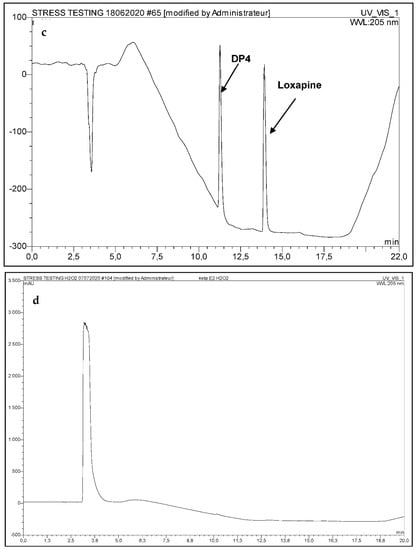
Figure 1.
(a) Chromatogram of the five drugs in water (b) chromatogram of the five drugs under oxidative conditions (c) chromatogram of Loxapine under acidic and thermal conditions (60 °C) after 24 h exposure (d) Oxidative media blank sample.
3.1.2. Linearity, Accuracy, and Precision
Validation results are summarized in the tables below (Table 4). The ANOVA non-parametric statistical test was used to verify homogeneity of variance for linearity, accuracy, and precision.

Table 4.
LC method validation parameters.
For all the calibration curves, a good linear relationship was found between the signal and the concentration (Fcalc > 4.38 = Fcritical, 5%; (1;19)) and the Fischer’s statistical analysis demonstrated the absence of deviation from linearity (Fcalc < Fcritical, 5%; (5, 14)).
Aside from the curve of Clonidine, the intercept value of the other drugs was statistically different from zero (tcalc > 2.093 = tcritical 5%, 19 degrees of freedom), making it difficult to use a single-point calibration for routine analysis. Methods were found to be precise and accurate (Table 4 and Supplementary Materials Figure S1A–E).
For each drug, the Student’s t-test based on the standard error of regression was used to compare the slopes and the y-intercepts of the regression lines of the drug solely, and in the presence of the other drugs. A matrix effect associated with the presence of the other drugs was highlighted with Ketamine and Midazolam (tcalc > 2.042 = tcritical 5%, 38 degrees of freedom). As a result, a single calibration curve incorporating the five molecules was performed for the stability study.
3.2. Impact of Stress Conditions on the Drugs’ Stability
For better understanding of the physicochemical behavior of the drugs in the mixture, upstream stress testing provided a major contribution in terms of extrapolating and/or mitigating the impact of such stress parameters on the mixture stability.
3.2.1. Physical Stability
Under alkaline conditions, Loxapine, Midazolam, and Ketamine generated an immediate precipitation, which in the case of Ketamine gave rise to the formation of crystals since the first day of exposure. The acidification visibly affected only Loxapine, causing a precipitation.
3.2.2. Chemical Degradation
Acidic and Basic Hydrolysis
Approximately 90% of the initial Ketamine concentration was lost after 24 h under alkaline conditions, due to precipitation and crystallization, but no degradation product was observed. However, the acidic catalysis had no impact on the Ketamine concentration over the study period (Supplementary Materials Figure S2B).
Despite the precipitation observed with the Loxapine drug product under acidic condition, the content of the API was barely degraded, generating a degradation product at room temperature (25 °C) after 24 h exposure. The degradation was potentiated at 60 °C, ending up by completely converting Loxapine to its degradation product after only five days of exposure (Supplementary Materials Figure S2C).
Under the basic condition, the concentration of Midazolam decreased down to 50% after only 24 h of exposure. The acidic stress had a low impact on the drug (85% of the concentration was found), generating a degradation product less lipophilic than Midazolam (RT at 10.1 min) (Supplementary Materials Figure S2D).
Neither acidic nor basic catalysis generated loss of Clonidine and Sufentanil, where concentrations remained greater than 97% of their initial concentrations after seven days of exposure (Supplementary Materials Figure S2A–E).
Thermal Degradation
Thermal stress did not generate significant loss in any of the molecules over the seven-day study period (Supplementary Materials Figure S2A–E).
Oxidative Degradation
The five molecules reacted differently to the oxidative stress. Clonidine and Sufentanil showed less sensitivity, as the half-life (t1/2) was 138 and 468 h, respectively. Ketamine was more degraded, as the concentration decreased down to 50%, after 64 h exposure (kinetics constant (k) of 1.5 × 10−3 µmol L−1 h−1). Loxapine and Midazolam was revealed to be extremely fragile towards oxidative conditions, giving a half-life (t1/2) of 17 and 28 h, respectively, and a kinetics constant (k) of 2.2 × 10−3 and 2.8 × 10−4 µmol L−1 h−1, respectively. Loxapine generated three more lipophilic degradation products. All the degradations followed zero-order kinetics models (Figure 2).
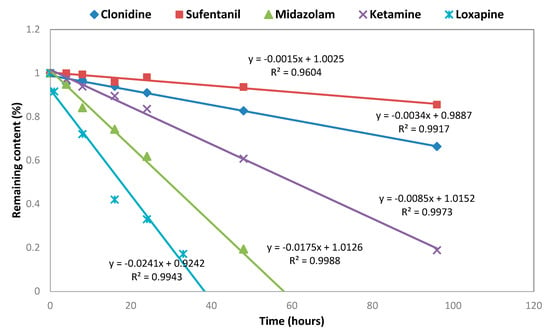
Figure 2.
Degradation kinetic under oxidative conditions.
3.3. Compatibility Study
3.3.1. Content Variation
The initial concentration of each drug was indicated as 100% and all the following concentrations were expressed as a percentage of the initial concentration. With the acceptance criteria of ±5% from the initial concentration, all of the 26 possible combinations of the five drugs displayed adequate stability at 25 °C: at least 3 days and 7 days, respectively, for the dilution in 0.9% NaCl or G5%, and the pure drug products’ mixtures (Figure 3 and Supplementary Materials Figure S3A,B). Furthermore, no degradation product was observed over the study period. The same applies for the containers, where no interaction with polypropylene or glass vials was established.
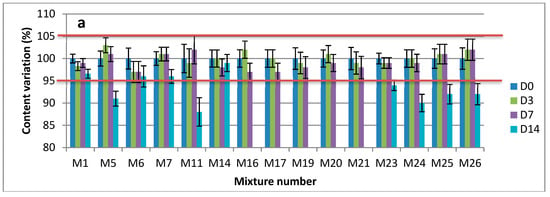
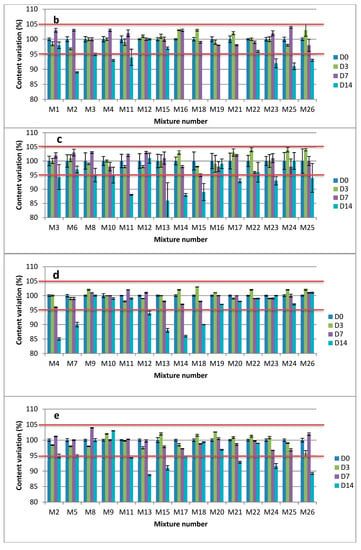
Figure 3.
Observed concentrations of pure drugs (as mean percentage of initial concentration ± SD) in mixtures at 22 ± 3 °C: (a) Clonidine; (b) Sufentanil; (c) Loxapine; (d) Midazolam; (e) Ketamine.
3.3.2. pH and Visual Control
All the solutions remained clear, colorless, and showed no visible particles during the whole study. The variation of the pH of all the mixtures between D0 and D14 was lower than 0.4 pH unit.
4. Discussion
No previous LC method separating and quantifying simultaneously the five molecules in the presence of their degradation products has been described. Despite the differences in the chemical and physical properties and concentration levels between the analytes, the gradient mode provided satisfactory results in terms of linearity, accuracy (SD < 3%), and precision (interday and intraday variation < 3%), demonstrating the stability indicating capability of the method.
All the 26 combinations prepared in glass vials or polypropylene syringes, whether as a result of the mixture of pure drugs solutions or diluted in a 0.9% NaCl or G5%, maintained at least 95% of the initial concentration for each molecule for 7 and 3 days, respectively, at 25 °C. When gathering all the results of assays (expressed in recovery, %) obtained at day 7 as a function of drug substance and the vehicle (NaCl 0.9% or G5%), two-way ANOVA showed that both the drug substance (p < 0.0001) and the choice of vehicle (p = 0.0179) had effects on the assay value and interaction was found to be significant (p < 0.0001). The lowest assay value (mean = 98.7%; SD = 1.54%) was obtained when the considered drug and vehicle were Midazolam and G5%.
Overall, apart from Loxapine for which little information was available, all the molecules were described to be compatible with polypropylene materials [,,]. However, as described by Roos et al. [], the interaction between Sufentanil and the plasticizer (DEHP) of polyvinyl chloride must be avoided.
Regarding pH, since the drug products present a similar pH, between 4 and 6, their mixtures do not show a considerable variation in pH value to one of single molecules. In view of pKa, in the five molecules (Table 1), at pH 6, calculations form ChemAxon showed that 90% to 99% of species are almost exclusively in the ionized form (Supplementary Materials Figure S4), thus substantially reducing the risk of interaction with the containers.
The strong acidity of the Midazolam drug product (pH at 3.7) increases its stability as it prevents the diazepine ring from opening, which protects the solution against photo-decomposition []. However, the significant increase in pH of its mixtures (pH values between 4 and 6) is not accompanied by any degradation of Midazolam. The stability indicating method prevented analytical problems of content determination of Midazolam related to pH variation (Supplementary Materials Figure S4).
In basic conditions, phenomena of precipitation/crystallization were observed with Loxapine, Midazolam, and Ketamine, which indicate the need to avoid mixtures with basic molecules or other drugs not evaluated in the study or similar conditions. Furthermore, under alkaline conditions, most of the drug products were in their uncharged forms, which can magnify the risk of interactions.
After three days of exposure, Loxapine in 0.9% NaCl or G5% exhibited a shorter stability period, along with a content variation of ±15% at day 7. The absence of degradation products associated with content fluctuation seems to indicate that Loxapine presents a low physical stability after D3, probably due to its lipophilic properties. Conversely, Ketamine, Clonidine, Sufentanil, and Midazolam remained stable in the same conditions until day 7.
All our experiments were conducted without any protection from daylight. However, in view of the sensitivity of the five molecules to oxidation, it is thus advocated to protect the mixtures from light, so as to avoid indirect photolysis in the presence of oxygen [].
Although the initial design of the study target of almost two-day mixture stability was achieved, the clinical use of such drug combinations requires upstream preparation under validated aseptic conditions, within hospital pharmacy sterile suites.
5. Conclusions
This study provided extensive information regarding the compatibility and stability behavior of Midazolam, Loxapine, Clonidine, Sufentanil, and Ketamine drugs products. The single high performance LC method developed and optimized demonstrated its stability-indicating capability to analyze accurately the five molecules in the presence of their degradation products without interferences. All the 26 possible combinations of the five drugs displayed satisfactory stability at 25 °C: at least 3 days and 7 days, respectively, for dilution in 0.9% NaCl or G5%, and pure drug products’ mixtures. In view of the increased consumption of pharmaceuticals related to the COVID pandemic, a combination of more than two drugs offers the advantage of minimizing the individual doses and reducing unwanted side-effects [,]. Hence, it opens up the possibility of combining the give drugs in one single syringe, especially under the current circumstances associated with an important shortage of programmable syringe pumps, syringes, and pharmaceuticals. The finding that most of the five molecules underwent physicochemical degradation under oxidation and/or alkaline is important to understand the optimal operative conditions that guarantee the quality of the preparations. This data will also be useful for the management of critically ill patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14030550/s1, Figure S1: Method validation (A) Clonidine; (B) Ketamine; (C) Loxapine; (D) Midazolam; (E) Sufentanil; Figure S2: Observed content variation under stress conditions (as mean percentage of initial concentration±SD) for each drug: (A) Clonidine; (B) Ketamine; (C) Loxapine; (D) Midazolam; (E) Sufentanil; Figure S3: (A) Drugs mixture diluted in NaCl: (a) Clonidine; (b) Sufentanil; (c) Loxapine; (d) Midazolam; (e) Ketamine, (B) Drugs mixture diluted in G5%: (a) Sufentanil; (b) Clonidine; (c) Midazolam; (d) Ketamine; (e) Loxapine; Figure S4: Microspecies distribution of the 5 drugs individually according to the pH.
Author Contributions
Conceptualization, J.M., F.E.K. and H.S.Y.; methodology, M.G.T., S.R., P.T. and H.S.Y.; software, P.-H.S.; validation, H.S.Y.; formal analysis, H.S.Y.; data curation, P.-H.S.; writing—original draft preparation, M.G.T. and S.R.; writing—review and editing, P.-H.S., L.H., A.B. and H.S.Y.; visualization, L.G.; supervision, H.S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Public Health (France). Epidemiological Update—Situation on March 15, 2020 at Midnight. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-15-mars-2020 (accessed on 1 October 2020).
- Karlage, K.; Earhart, Z.; Green-Boesen, K.; Myrdal, P.B. Stability of midazolam hydrochloride injection 1-mg/mL solutions in polyvinyl chloride and polyolefin bags. Am. J. Health-Syst. Pharm. 2011, 68, 1537–1540. [Google Scholar] [CrossRef]
- de Diego, M.; Godoy, G.; Mennickent, S. Chemical stability of midazolam injection by high performance liquid chromatography. J. Sep. Sci. 2007, 30, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F. Stability of diluted ketamine packaged in glass vials. Can. J. Hosp. Pharm. 2013, 66, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potier, A.; Voyat, J.; Nicolas, A. Stability study of a clonidine oral solution in a novel vehicle designed for pediatric patients. Pharm. Dev. Technol. 2018, 23, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Jäppinen, A.; Turpeinen, M.; Kokki, H.; Rasi, A.; Ojanen, T.; Pelkonen, O.; Naaranlahti, T. Stability of sufentanil and levobupivacaine solutions and a mixture in a 0.9% sodium chloride infusion stored in polypropylene syringes. Eur. J. Pharm. Sci. 2003, 19, 31–36. [Google Scholar] [CrossRef]
- ICH. Stability Testing of New Drug Substances and Products Q1A (R2). International Conference on Harmonisation of Technical Requirement for Registration of Pharmaceuticals for Human Use. March 2003. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 9 September 2021).
- Hubert, P.; Nguyen-Huu, J.J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Quantative analytical procedures: Harmonization of the approaches: Part II—Statistics. STP Pharma Prat. 2006, 16, 30–60. [Google Scholar]
- ICH Q3B (R2). Impurities in New Drug Products. June 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-3-b-r2-impurities-new-drug-products-step-5_en.pdf (accessed on 9 September 2021).
- Lambropoulos, J.; Spanos, G.A.; Lazaridis, N.V. Development and validation of an HPLC assay for fentanyl, alfentanil, and sufentanil in swab samples. J. Pharm. Biomed. Anal. 2000, 23, 421–428. [Google Scholar] [CrossRef]
- Ma, C.; Decarie, D.; Ensom, H.H.M. Stability of Clonidine suspension in oral plastic syringes. Am. J. Health-Syst. Pharm. 2014, 71, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Roos, P.J.; Glerum, J.H.; Meilink, J.W. Stability of Sufentanil citrate in a portable pump reservoir, a glass container and a polyethylene container. Pharm. Weekblad 1992, 14, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Good, P.D.; Schneider, J.J.; Ravenscroft, P.J. The compatibility and stability of midazolam and dexamethasone in infusion solutions. J. Pain Symptom Manag. 2004, 27, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Sadou Yayé, H.; Secrétan, P.H.; Henriet, T.; Bernard, M.; Amrani, F.; Akrout, W.; Tilleul, P.; Yagoubi, N.; Do, B. Identification of the major degradation pathways of ticagrelor. J. Pharm. Biomed. Anal. 2015, 105, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).