Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis
Abstract
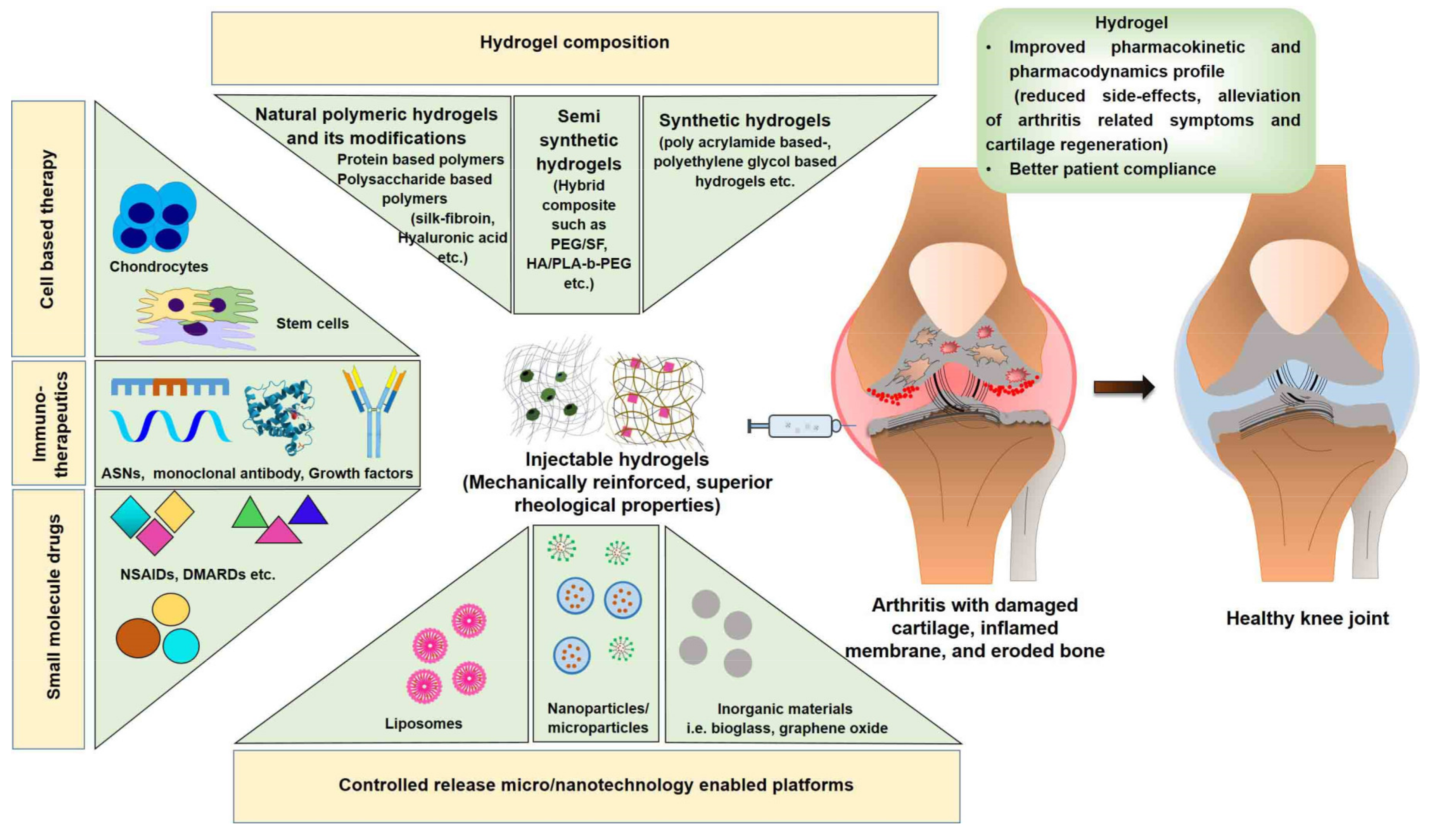
:1. Introduction
- Capacity to deliver multiple agents such as drugs, oligonucleotides, monoclonal antibodies, growth factors, and cells (chondrocytes and mesenchymal stem cells; MSCs);
- Ability of in situ-forming hydrogels to maintain a concentrated form of drug (depot) for a long period of time following intra-articular injection, thus eliminating the need for repetitive dosing (injections);
- Capacity to provide minimally invasive or non-invasive treatment options with injectable/transdermal drug delivery to treat arthritis, displaying high therapeutic efficacy and manageable toxicity at a low dose, thereby improving patient compliance;
- The ability of the hydrogel structure to mimic the extracellular matrix (ECM) microenvironment, permitting efficient tissue regeneration. Following application, hydrogels maintain hydrated structures, and are therefore suited for cell delivery applications. Moreover, hydrogels can be modified with ligands to enhance their interaction with chondrocytes and/or their progenitor cells for cartilage tissue engineering (CTE) [9].
2. Hydrogel Composition: Materials Used to Prepare Hydrogels
2.1. Hydrogels Prepared Using Naturally Derived Material
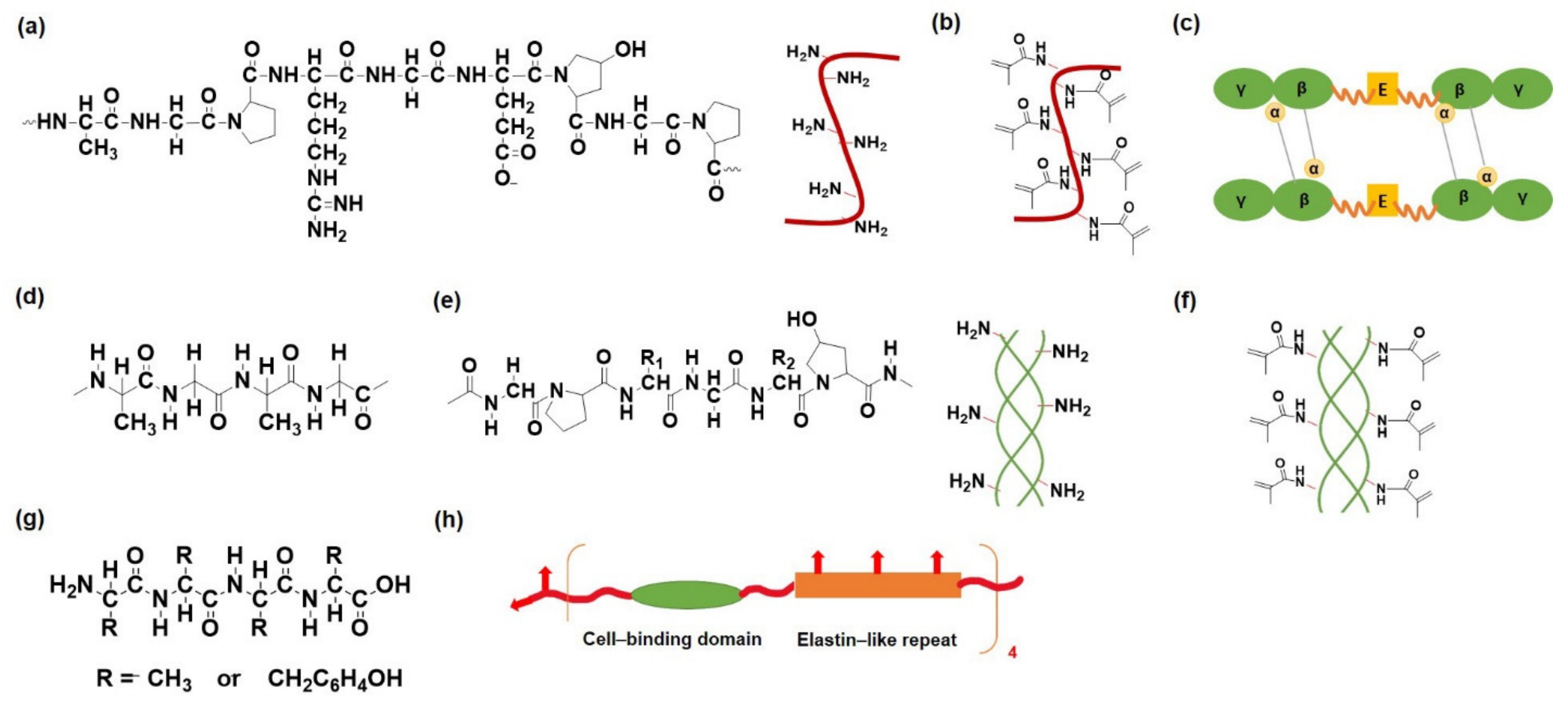
2.1.1. Protein and Peptide-Based Hydrogels
Gelatin
Fibrin
Collagen
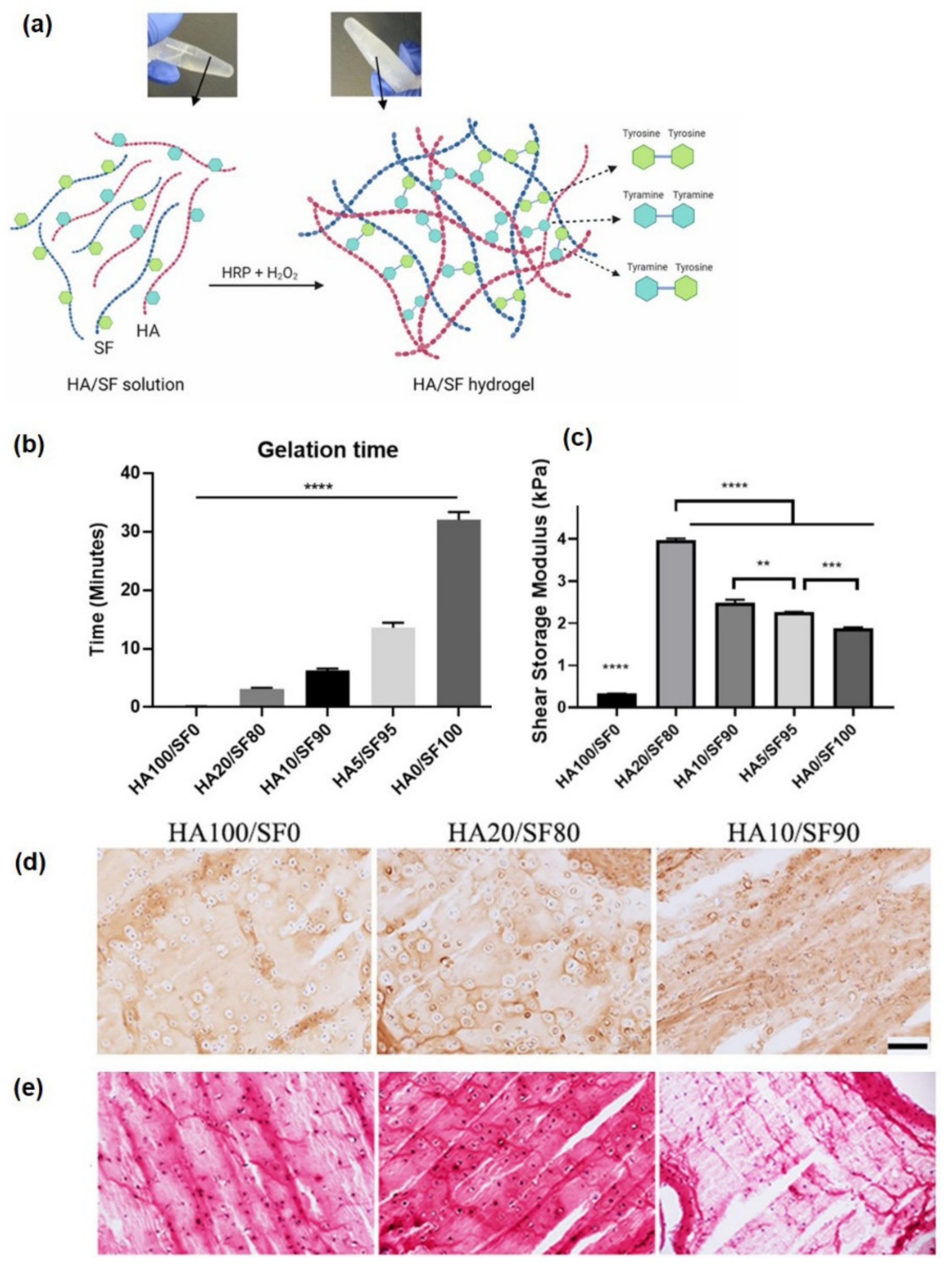
Silk Fibroin
Others
2.1.2. Polysaccharide-Based Hydrogels
Alginate
Chitosan
Hyaluronic Acid
Heparin
Dextran
Chondroitin Sulfate
Gellan Gum
2.1.3. Natural Hybrid Protein-Polysaccharide-Based Hydrogel
2.2. Hydrogels Prepared Using Synthetic Material
2.2.1. Polyacrylamide-Based Hydrogels
2.2.2. Polyethylene Glycol-Based Hydrogels
2.2.3. Poly(Vinyl Alcohol)
2.2.4. Poloxamer
2.2.5. Others
2.3. Hydrogels Prepared Using Hybrid (Semi-Synthetic) Material
3. Hydrogels as Depot Delivering System for the Treatment of Arthritis
3.1. Hydrogels Delivering Small Molecule Drugs
3.2. Hydrogels Delivering Immunotherapeutic Agents
3.3. Hydrogels Delivering Cells
4. Recent Advances in Hydrogels
4.1. Liposome-Loaded Hydrogel System
4.2. Polymeric Particulate-Loaded Hydrogel Systems
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saccomano, S.J. Osteoarthritis treatment: Decreasing pain, improving mobility. Nurse Pract. 2018, 43, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, D.; Paliwal, R.; Satapathy, T.; Paul, S.D. Rheumatiod arthritis: An updated overview of latest therapy and drug delivery. J. Pharmacopunct. 2019, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Fernandes, D.C.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. Hydrogels in the treatment of rheumatoid arthritis: Drug delivery systems and artificial matrices for dynamic in vitro models. J. Mater. Sci. Mater. Med. 2021, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, M.; Kashanian, S.; Samavati, S.S.; Derakhshankhah, H.; Jamasb, S.; McInnes, S.J. Nanotechnology application in drug delivery to osteoarthritis (OA), rheumatoid arthritis (RA), and osteoporosis (OSP). J. Drug Deliv. Sci. Technol. 2021, 61, 102011. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.; Fang, J.; Sun, X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Hiemstra, C.; van der Aa, L.J.; Zhong, Z.; Dijkstra, P.J.; Feijen, J. Rapidly in situ-forming degradable hydrogels from dextran thiols through Michael addition. Biomacromolecules 2007, 8, 1548–1556. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.W.; Lee, J.W.; Lee, K.Y. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr. Polym. 2017, 157, 1281–1287. [Google Scholar]
- Seo, J.; Park, S.H.; Kim, M.J.; Ju, H.J.; Yin, X.Y.; Min, B.H.; Kim, M.S. Injectable click-crosslinked hyaluronic acid depot to prolong therapeutic activity in articular joints affected by rheumatoid arthritis. ACS Appl. Mater. Interfaces 2019, 11, 24984–24998. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Nouri Khorasani, S.; Zare, M.; Esmaeely Neisiany, R.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef]
- Lin, X.; Tsao, C.T.; Kyomoto, M.; Zhang, M. Injectable Natural Polymer Hydrogels for Treatment of Knee Osteoarthritis. Adv. Healthc. Mater. 2021, 2101479. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Bakravi, A.; Ahamadian, Y.; Hashemi, H.; Namazi, H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv. Polym. Technol. 2018, 37, 2625–2635. [Google Scholar] [CrossRef]
- Suo, H.; Li, L.; Zhang, C.; Yin, J.; Xu, K.; Liu, J.; Fu, J. Glucosamine-grafted methacrylated gelatin hydrogels as potential biomaterials for cartilage repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 990–999. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Dolatyar, B.; Zeynali, B. In vitro study of alginate–gelatin scaffolds incorporated with silica NPs as injectable, biodegradable hydrogels. RSC Adv. 2021, 11, 16688–16697. [Google Scholar] [CrossRef]
- Han, L.; Xu, J.; Lu, X.; Gan, D.; Wang, Z.; Wang, K.; Zhang, H.; Yuan, H.; Weng, J. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B 2017, 5, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.; Stein, J.; Cai, Y.; Wexselblatt, E.; Creemers, L.; Wengel, J.; Howard, K.; Saris, D.; Yayon, A. Fibrin/hyaluronic acid hydrogel for combined delivery of gapmers and chondrocytes as a gene therapy approach for osteoarthritis. Osteoarthr. Cartil. 2018, 26, S145. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.P.; Stein, J.; Cai, Y.; Riemers, F.; Wexselblatt, E.; Wengel, J.; Tryfonidou, M.; Yayon, A.; Howard, K.A.; Creemers, L.B. Fibrin-hyaluronic acid hydrogel-based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co-delivered and resident joint cells in osteoarthritis. J. Control. Release 2019, 294, 247–258. [Google Scholar] [CrossRef]
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Monteiro, M.; Grandjean, C.; Alonso, M.J. An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-Articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Jiang, T.; Zheng, L.; Zhao, J.; Zhang, X. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater. Sci. 2018, 6, 1556–1568. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Liu, Q.; Lu, Z.; Zheng, L.; Zhao, J.; Zhang, X. Therapy for cartilage defects: Functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater. Sci. 2018, 6, 1616–1626. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Pina, S.; Oliveira, J.M.; Reis, R.L. Silk Fibroin-Based Hydrogels and Scaffolds for Osteochondral Repair and Regeneration. In Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 305–325. ISBN 978-3-319-76711-6. [Google Scholar]
- Zheng, H.Y.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; da Silva Morais, A.; Maia, F.R.; Canadas, R.F.; Costa, J.B.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018, 72, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Yodmuang, S.; McNamara, S.L.; Nover, A.B.; Mandal, B.B.; Agarwal, M.; Kelly, T.-A.N.; Chao, P.-h.G.; Hung, C.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of agarose/silk fibroin blended hydrogel for in vitro cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinescu, S.; Gălăţeanu, B.; Albu, M.; Lungu, A.; Radu, E.; Hermenean, A.; Costache, M. Biocompatibility assessment of novel collagen-sericin scaffolds improved with hyaluronic acid and chondroitin sulfate for cartilage regeneration. BioMed Res. Int. 2013, 2013, 598056. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-J.; Liu, D.-C.; Yu, Y.-H.; Tang, H. Development of Gelatin-Silk Sericin Incorporated with Poly (vinyl alcohol) Hydrogel-Based Nanocomposite for Articular Cartilage Defects in Rat Knee Joint Repair. J. Biomed. Nanotechnol. 2021, 17, 242–252. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Boughton, E.A.; Ruys, A.J.; Weiss, A.S.; Dehghani, F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 2009, 30, 4550–4557. [Google Scholar] [CrossRef]
- Cipriani, F.; Kruger, M.; De Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage regeneration in preannealed silk elastin-like co-recombinamers injectable hydrogel embedded with mature chondrocytes in an ex vivo culture platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef]
- Cipriani, F.; Ariño Palao, B.; Gonzalez de Torre, I.; Vega Castrillo, A.; Aguado Hernández, H.J.; Alonso Rodrigo, M.; Àlvarez Barcia, A.J.; Sanchez, A.; García Diaz, V.; Lopez Peña, M. An elastin-like recombinamer-based bioactive hydrogel embedded with mesenchymal stromal cells as an injectable scaffold for osteochondral repair. Regen. Biomater. 2019, 6, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Ouwerx, C.; Velings, N.; Mestdagh, M.; Axelos, M.A. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Kennedy, J.F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Thein-Han, W.; Saikhun, J.; Pholpramoo, C.; Misra, R.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yang, X.; Zhao, J.; Zhao, Y.; Yuan, X. Photocrosslinked layered gelatin-chitosan hydrogel with graded compositions for osteochondral defect repair. J. Mater. Sci. Mater. Med. 2015, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Yu, Q.; Zhang, J.; Zhou, J.; Li, X.; Zhuang, W.; Yang, X. Intra-articular Injection of Chitosan-Based Supramolecular Hydrogel for Osteoarthritis Treatment. Tissue Eng. Regen. Med. 2021, 18, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Flégeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef]
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol. Rev. 2020, 9, 1059–1079. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, S.; Funakoshi, T.; Sasazawa, F.; Todoh, M.; Tadano, S.; Iwasaki, N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthr. Cartil. 2014, 22, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.-C.; Chang, Y.-H.; Liu, H.-W.; Ding, D.-C. Transplanting human umbilical cord mesenchymal stem cells and hyaluronate hydrogel repairs cartilage of osteoarthritis in the minipig model. Tzu-Chi Med. J. 2019, 31, 11. [Google Scholar]
- Cai, Y.; López-Ruiz, E.; Wengel, J.; Creemers, L.B.; Howard, K.A. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J. Control Release 2017, 253, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Tao, C.; Sun, L.; Qi, S.; Le, Y.; Wang, J.; Li, C.; Liu, X.; Zhang, J.; Zhao, J. In situ forming injectable hydrogel for encapsulation of nanoiguratimod and sustained release of therapeutics. Int. J. Nanomed. 2019, 14, 8725. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gao, C.; Liu, H.; Liu, H.; Feng, Y.; Li, Z.; Liu, H.; Wang, J.; Yang, B.; Lin, Q. Infliximab-based self-healing hydrogel composite scaffold enhances stem cell survival, engraftment, and function in rheumatoid arthritis treatment. Acta Biomater. 2021, 121, 653–664. [Google Scholar] [CrossRef]
- Casu, B. Structure and biological activity of heparin. Adv. Carbohydr. Chem. Biochem. 1985, 43, 51–134. [Google Scholar]
- Ruppert, R.; Hoffmann, E.; Sebald, W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem. 1996, 237, 295–302. [Google Scholar] [CrossRef]
- Cai, S.; Liu, Y.; Shu, X.Z.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef]
- Tanihara, M.; Suzuki, Y.; Yamamoto, E.; Noguchi, A.; Mizushima, Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 216–221. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kiick, K.L. Polysaccharide− poly (ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules 2005, 6, 1921–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar]
- Kim, S.; Won, C.; Chu, C. Synthesis and characterization of dextran-based hydrogel prepared by photocrosslinking. Carbohydr. Polym. 1999, 40, 183–190. [Google Scholar] [CrossRef]
- Jin, R.; Hiemstra, C.; Zhong, Z.; Feijen, J. Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates. Biomaterials 2007, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Van Blitterswijk, C.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran–hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef]
- Jukes, J.M.; Van Der Aa, L.J.; Hiemstra, C.; Van Veen, T.; Dijkstra, P.J.; Zhong, Z.; Feijen, J.; Van Blitterswijk, C.A.; De Boer, J. A newly developed chemically crosslinked dextran–poly (ethylene glycol) hydrogel for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 565–573. [Google Scholar] [CrossRef]
- Pan, J.-f.; Yuan, L.; Guo, C.-a.; Geng, X.-h.; Fei, T.; Fan, W.-s.; Li, S.; Yuan, H.-f.; Yan, Z.-q.; Mo, X.-m. Fabrication of modified dextran–gelatin in situ forming hydrogel and application in cartilage tissue engineering. J. Mater. Chem. B 2014, 2, 8346–8360. [Google Scholar] [CrossRef]
- Fan, L.; Lin, C.; Zhao, P.; Wen, X.; Li, G. An injectable bioorthogonal dextran hydrogel for enhanced chondrogenesis of primary stem cells. Tissue Eng. Part C Methods 2018, 24, 504–513. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, T.; Zhao, P.; An, K.; Lin, C.; Liu, H. Injectable dextran hydrogels fabricated by metal-free click chemistry for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 73, 21–30. [Google Scholar] [CrossRef]
- Buma, P.; Pieper, J.S.; van Tienen, T.; van Susante, J.L.; van der Kraan, P.M.; Veerkamp, J.H.; van den Berg, W.B.; Veth, R.P.; van Kuppevelt, T.H. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects—A study in rabbits. Biomaterials 2003, 24, 3255–3263. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Sun, L.; Gong, X.; Yang, L.; Chen, C. Self-crosslinking and injectable chondroitin sulfate/pullulan hydrogel for cartilage tissue engineering. Appl. Mater. Today 2018, 10, 173–183. [Google Scholar] [CrossRef]
- Li, X.; Xu, Q.; Johnson, M.; Wang, X.; Lyu, J.; Li, Y.; McMahon, S.; Greiser, U.; Sigen, A.; Wang, W. A chondroitin sulfate based injectable hydrogel for delivery of stem cells in cartilage regeneration. Biomater. Sci. 2021, 9, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Ferris, C.J.; Gilmore, K.J.; Wallace, G.G. Modified gellan gum hydrogels for tissue engineering applications. Soft Matter 2013, 9, 3705–3711. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
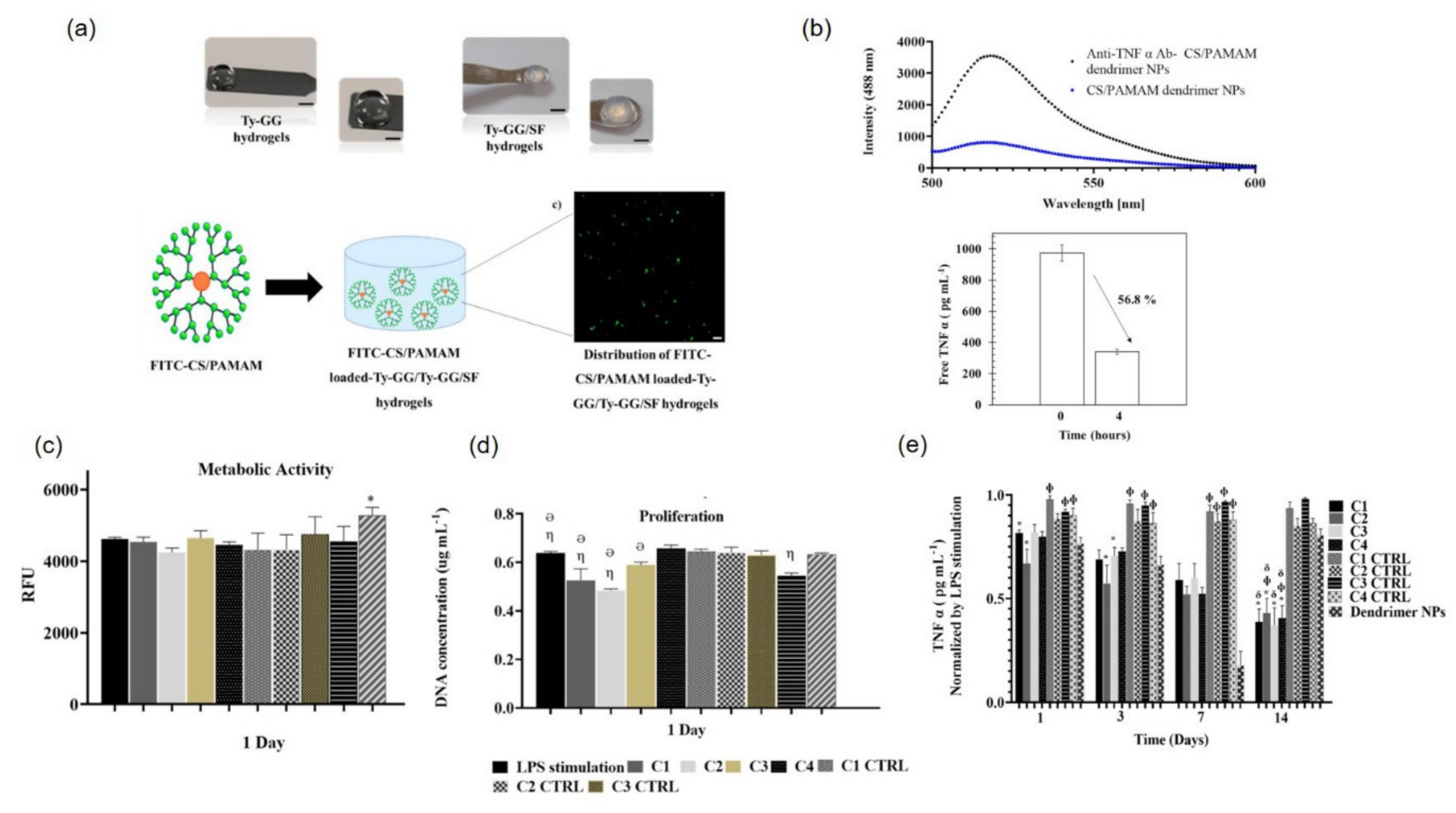
- Oliveira, I.M.; Gonçalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv. Transl. Res. 2021, 11, 1288–1300. [Google Scholar] [CrossRef]
- Oliveira, I.; Carvalho, M.; Fernandes, D.; Abreu, C.M.; Maia, F.R.; Pereira, H.; Caballero, D.; Kundu, S.C.; Reis, R.; Oliveira, J. Modulation of inflammation by anti-TNF α mAb-dendrimer nanoparticles loaded in tyramine-modified gellan gum hydrogels in a cartilage-on-a-chip model. J. Mater. Chem. B 2021, 9, 4211–4218. [Google Scholar] [CrossRef]
- Trucco, D.; Vannozzi, L.; Teblum, E.; Telkhozhayeva, M.; Nessim, G.D.; Affatato, S.; Al-Haddad, H.; Lisignoli, G.; Ricotti, L. Graphene Oxide-Doped Gellan Gum–PEGDA Bilayered Hydrogel Mimicking the Mechanical and Lubrication Properties of Articular Cartilage. Adv. Healthc. Mater. 2021, 10, 2001434. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Dastjerdi, M.B.; Ai, A.; Ahmadi, A.; Godarzi, A.; Rahimi, A.; Ai, J. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater. Sci. 2018, 6, 1286–1298. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Gonçalves, C.; Shin, M.E.; Lee, S.; Reis, R.L.; Khang, G.; Oliveira, J.M. Anti-inflammatory properties of injectable betamethasone-loaded tyramine-modified gellan gum/silk fibroin hydrogels. Biomolecules 2020, 10, 1456. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Koh, R.H.; Kim, S.-H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C 2020, 115, 111096. [Google Scholar] [CrossRef] [PubMed]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Cheng, T.H. Thermo-responsive chitosan-graft-poly (N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol. Biosci. 2006, 6, 1026–1039. [Google Scholar] [CrossRef]
- Kwon, I.K.; Matsuda, T. Photo-iniferter-based thermoresponsive block copolymers composed of poly (ethylene glycol) and poly (N-isopropylacrylamide) and chondrocyte immobilization. Biomaterials 2006, 27, 986–995. [Google Scholar] [CrossRef]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Willems, N.; Yang, H.-y.; Langelaan, M.L.; Tellegen, A.R.; Grinwis, G.C.; Kranenburg, H.-J.C.; Riemers, F.M.; Plomp, S.G.; Craenmehr, E.G.; Dhert, W.J. Biocompatibility and intradiscal application of a thermoreversible celecoxib-loaded poly-N-isopropylacrylamide MgFe-layered double hydroxide hydrogel in a canine model. Arthritis Res. Ther. 2015, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-Y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.; Huang, J.H.; Öner, F.C.; Dhert, W.J.; Kragten, A.H.; Willems, N.; Grinwis, G.C. A novel injectable thermoresponsive and cytocompatible gel of poly (N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef]
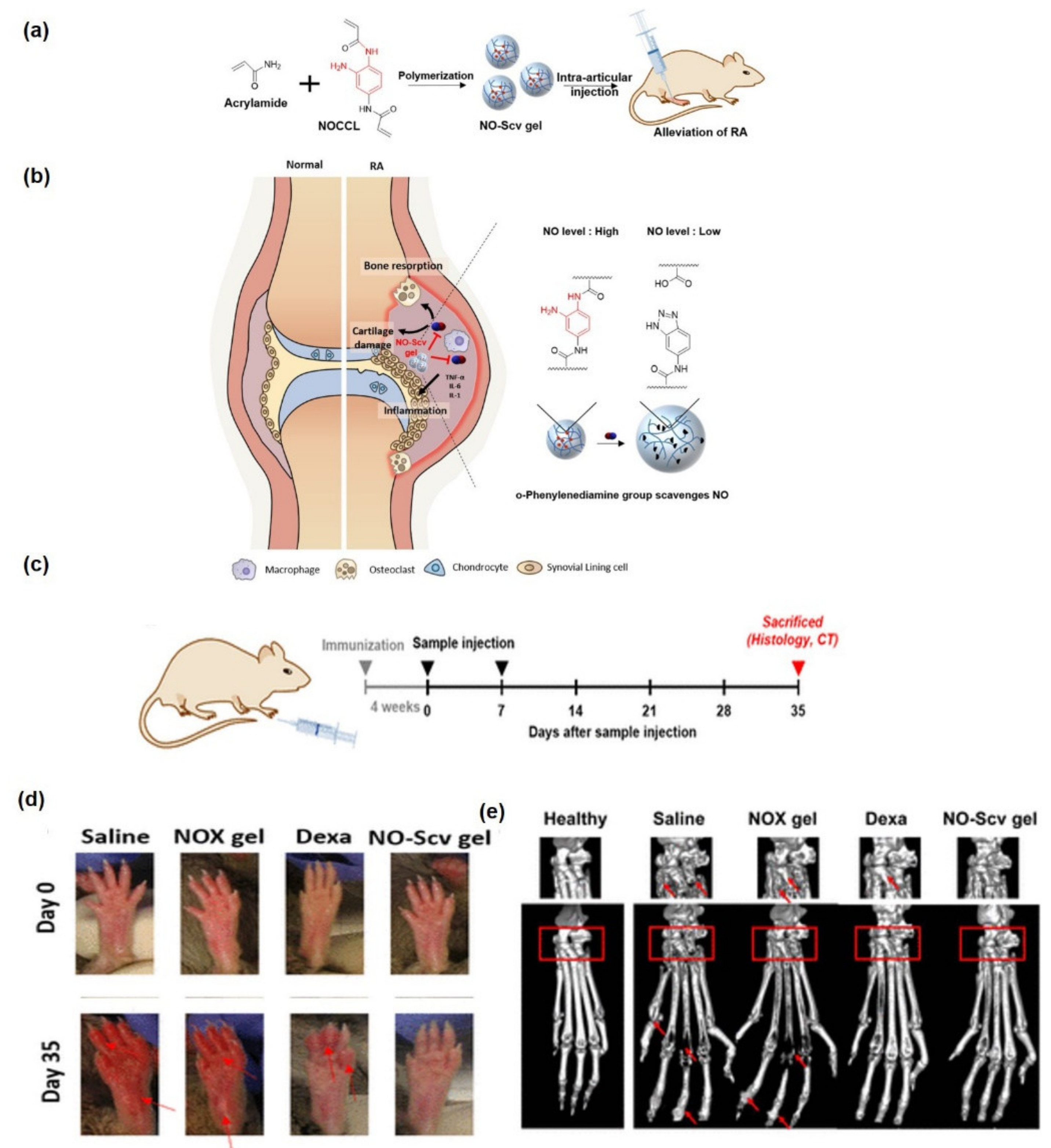
- Yeo, J.; Lee, Y.M.; Lee, J.; Park, D.; Kim, K.; Kim, J.; Park, J.; Kim, W.J. Nitric oxide-scavenging nanogel for treating rheumatoid arthritis. Nano Lett. 2019, 19, 6716–6724. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Dadsetan, M.; Szatkowski, J.P.; Yaszemski, M.J.; Lu, L. Characterization of photo-cross-linked oligo [poly (ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules 2007, 8, 1702–1709. [Google Scholar] [CrossRef]
- Steinmetz, N.J.; Bryant, S.J. The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly (ethylene glycol) hydrogels. Acta Biomater. 2011, 7, 3829–3840. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.S.; Ganios, A.M.; Childers, E.P.; Weiner, S.D.; Becker, M.L. Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young’s modulus gradient. Acta Biomater. 2013, 9, 6095–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.C.; Sridhar, S.L.; Vernerey, F.J.; Bryant, S.J. Spatiotemporal neocartilage growth in matrix-metalloproteinase-sensitive poly (ethylene glycol) hydrogels under dynamic compressive loading: An experimental and computational approach. J. Mater. Chem. B 2020, 8, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Chu, S.; Randolph, M.A.; Bryant, S.J. An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly (ethylene glycol) hydrogels with localized transforming growth factor β3. Acta Biomater. 2019, 93, 97–110. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthc. Mater. 2015, 4, 702–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaalure, S.C.; Chu, S.; Bryant, S.J. An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering. Adv. Healthc. Mater. 2015, 4, 420–431. [Google Scholar] [CrossRef]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. In vitro screening of molecularly engineered polyethylene glycol hydrogels for cartilage tissue engineering using periosteum-derived and ATDC5 cells. Int. J. Mol. Sci. 2018, 19, 3341. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Richardson, B.M.; Walker, C.J.; Maples, M.M.; Randolph, M.A.; Bryant, S.J.; Anseth, K.S. Mechanobiological Interactions between Dynamic Compressive Loading and Viscoelasticity on Chondrocytes in Hydrazone Covalent Adaptable Networks for Cartilage Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2002030. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Seidi, O.; Ribeiro, N.; Colaço, R.; Serro, A.P. Tribomechanical comparison between PVA hydrogels obtained using different processing conditions and human cartilage. Materials 2019, 12, 3413. [Google Scholar] [CrossRef] [Green Version]
- Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; Caro, R.D.; Porzionato, A. Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes 2021, 9, 730. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly (vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Madry, H.; Gao, L.; Rey-Rico, A.; Venkatesan, J.K.; Müller-Brandt, K.; Cai, X.; Goebel, L.; Schmitt, G.; Speicher-Mentges, S.; Zurakowski, D. Thermosensitive hydrogel based on PEO–PPO–PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020, 32, 1906508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C 2021, 118, 111352. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Schneider, T.; Chiappisi, L.; Gradzielski, M.; Schulze-Tanzil, G.; Haag, R. Mimicking of chondrocyte microenvironment using in situ forming dendritic polyglycerol sulfate-based synthetic polyanionic hydrogels. Macromol. Biosci. 2016, 16, 580–590. [Google Scholar] [CrossRef]
- Sala, R.L.; Kwon, M.Y.; Kim, M.; Gullbrand, S.E.; Henning, E.A.; Mauck, R.L.; Camargo, E.R.; Burdick, J.A. Thermosensitive poly (N-vinylcaprolactam) injectable hydrogels for cartilage tissue engineering. Tissue Eng. Part A 2017, 23, 935–945. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Calore, A.; Wilbers, A.; Harings, J.; Moroni, L. Additive manufacturing of an elastic poly (ester) urethane for cartilage tissue engineering. Acta Biomater. 2020, 102, 192–204. [Google Scholar] [CrossRef]
- Van Midwoud, P.M.; Sandker, M.; Hennink, W.E.; de Leede, L.G.; Chan, A.; Weinans, H. In vivo pharmacokinetics of celecoxib loaded endcapped PCLA-PEG-PCLA thermogels in rats after subcutaneous administration. Eur. J. Pharm. Biopharm. 2018, 131, 170–177. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, H.; Dai, Z.; Ren, F.; Wu, Y.; Hou, R.; Zhu, Y.; Fu, J. Stiff micelle-crosslinked hyaluronate hydrogels with low swelling for potential cartilage repair. J. Mater. Chem. B 2019, 7, 5490–5501. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Dutta, N.K.; Zannettino, A.; Choudhury, N.R. Engineering DN hydrogels from regenerated silk fibroin and poly (N-vinylcaprolactam). J. Mater. Chem. B 2016, 4, 5519–5533. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Q.; Lin, H.; Yu, X.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-X.; Liu, P.; Ding, W.; Meng, Q.-B.; Su, D.-H.; Zhang, Q.-C.; Lian, R.-X.; Yu, B.-Q.; Zhao, M.-D.; Dong, J. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Agas, D.; Laus, F.; Lacava, G.; Marchegiani, A.; Deng, S.; Magnoni, F.; Silva, G.G.; Di Martino, P.; Sabbieti, M.G.; Censi, R. Thermosensitive hybrid hyaluronan/p (HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J. Cell. Physiol. 2019, 234, 20013–20027. [Google Scholar] [CrossRef]
- Kim, T.; Suh, J.; Kim, W.J. Polymeric Aggregate-Embodied Hybrid Nitric-Oxide-Scavenging and Sequential Drug-Releasing Hydrogel for Combinatorial Treatment of Rheumatoid Arthritis. Adv. Mater. 2021, 33, 2008793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Zhang, L.; Zhao, H.; Ni, T.; Liu, Y.; An, Z.; Liu, M.; Pei, R. Fabrication of an injectable BMSC-laden double network hydrogel based on silk fibroin/PEG for cartilage repair. J. Mater. Chem. B 2020, 8, 5845–5848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, Z.; Gao, R.; Yuan, J.; Zhang, J.; Li, H.; Xie, Z.; Wang, Y. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021, 128, 163–174. [Google Scholar] [CrossRef]
- Houreh, A.B.; Masaeli, E.; Nasr-Esfahani, M.H. Chitosan/polycaprolactone multilayer hydrogel: A sustained Kartogenin delivery model for cartilage regeneration. Int. J. Biol. Macromol. 2021, 177, 589–600. [Google Scholar] [CrossRef]
- Do Nascimento, M.H.M.; Ambrosio, F.N.; Ferraraz, D.C.; Windisch-Neto, H.; Querobino, S.M.; Nascimento-Sales, M.; Alberto-Silva, C.; Christoffolete, M.A.; Franco, M.K.; Kent, B. Sulforaphane-loaded hyaluronic acid-poloxamer hybrid hydrogel enhances cartilage protection in osteoarthritis models. Mater. Sci. Eng. C 2021, 128, 112345. [Google Scholar] [CrossRef]
- Hayat, K.; Zaman, H.; Fang, Y.; Atif, M. Management of Rheumatoid Arthritis and the Pharmacist’s Role; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; Van Der Heijde, D.; Dougados, M.; Van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; De Wit, M. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in hydrogel-based drug sustained release systems for bone tissue engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Ong, C.; Lirk, P.; Tan, C.; Seymour, R. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cokelaere, S.M.; Plomp, S.G.; de Boef, E.; de Leeuw, M.; Bool, S.; van de Lest, C.H.; van Weeren, P.R.; Korthagen, N.M. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur. J. Pharm. Biopharm. 2018, 128, 327–336. [Google Scholar] [CrossRef]
- Ravindran, V.; Rachapalli, S.; Choy, E.H. Safety of medium-to long-term glucocorticoid therapy in rheumatoid arthritis: A meta-analysis. Rheumatology 2009, 48, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, S.; Yuan, F.; Sun, P.; Zhang, Y. GEL-MAN Hydrogel Loaded with Triamcinolone Acetonide for the Treatment of Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 872. [Google Scholar] [CrossRef]
- Agostini, S.B.N.; Malta, I.H.S.; Rodrigues, R.F.; Freitas, J.T.J.; de Sousa Lino, M.E.; Dos Santos, R.S.; Elisei, L.S.; Moraes, T.R.; dos Reis Giusto, L.A.; de Oliveira, M.K. Preclinical evaluation of methotrexate-loaded polyelectrolyte complexes and thermosensitive hydrogels as treatment for rheumatoid arthritis. Eur. J. Pharm. Sci. 2021, 163, 105856. [Google Scholar] [CrossRef]
- Klimak, M.; Nims, R.J.; Pferdehirt, L.; Collins, K.H.; Harasymowicz, N.S.; Oswald, S.J.; Setton, L.A.; Guilak, F. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 2021, 133, 74–86. [Google Scholar] [CrossRef]
- Shams, S.; Martinez, J.M.; Dawson, J.R.; Flores, J.; Gabriel, M.; Garcia, G.; Guevara, A.; Murray, K.; Pacifici, N.; Vargas, M.V. The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front. Pharmacol. 2021, 12, 1233. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Nixon, J.S.; Bottomley, K.M. Metalloproteinase inhibitors: New opportunities for the treatment of rheumatoid arthritis and osteoarthritis. Expert Opin. Investig. Drugs 2000, 9, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A. The future of osteoarthritis therapeutics: Emerging biological therapy. Curr. Rheumatol. Rep. 2013, 15, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesinghe, S.N.; Lindsay, M.A.; Jones, S.W. Oligonucleotide Therapies in the Treatment of Arthritis: A Narrative Review. Biomedicines 2021, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ali, S.A.; Kapoor, M. Antisense oligonucleotide-based therapies for the treatment of osteoarthritis: Opportunities and roadblocks. Bone 2020, 138, 115461. [Google Scholar] [CrossRef]
- Gabriel, S.E. Tumor necrosis factor inhibition: A part of the solution or a part of the problem of heart failure in rheumatoid arthritis? Arthritis Rheum. 2008, 58, 637–640. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Wang, Z.; Gao, H.; Ding, J.; He, Z. Intraarticular Injection of Infliximab-Loaded Thermosensitive Hydrogel Alleviates Pain and Protects Cartilage in Rheumatoid Arthritis. J. Pain Res. 2020, 13, 3315. [Google Scholar] [CrossRef]
- Gikas, P.; Bayliss, L.; Bentley, G.; Briggs, T. An overview of autologous chondrocyte implantation. J. Bone Jt. Surg. Br. Vol. 2009, 91, 997–1006. [Google Scholar] [CrossRef]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C 2018, 88, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Carthew, J.; Donderwinkel, I.; Shrestha, S.; Truong, V.X.; Forsythe, J.S.; Frith, J.E. In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells. Acta Biomater. 2020, 101, 249–261. [Google Scholar] [CrossRef]
- Mohan, N.; Mohanan, P.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Rahul, V.; Nair, P.D. Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 2017, 104, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tian, Q.; Hedrick, J.L.; Hui, J.H.P.; Ee, P.L.R.; Yang, Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 2010, 31, 7298–7307. [Google Scholar] [CrossRef] [PubMed]
- Stalder, E.; Zumbuehl, A. Liposome-Containing Mechanoresponsive Hydrogels. Macromol. Mater. Eng. 2017, 302, 1600549. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, O.S.; Yu, C.-H.; Akram, M.; Kim, H.-S.; Kim, M.-S.; Kang, J.-H.; Majid, A.; Chang, S.-Y.; Bae, O.-N. Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model. Int. J. Pharm. 2017, 525, 92–100. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, B.; Chaudhuri, S.; Roy, T.; Mukherjee, A.; Sengupta, S. Methotrexate aspasomes against rheumatoid arthritis: Optimized hydrogel loaded liposomal formulation with in vivo evaluation in Wistar rats. AAPS PharmSciTech 2018, 19, 1320–1336. [Google Scholar] [CrossRef]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Garakani, S.S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Motlagh, G.H. Injectable PNIPAM/Hyaluronic acid hydrogels containing multipurpose modified particles for cartilage tissue engineering: Synthesis, characterization, drug release and cell culture study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res. Ther. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Vogus, D.; Zhao, Z.; He, W.; Krishnan, V.; Kim, J.; Shi, Y.; Sarode, A.; Ukidve, A.; Mitragotri, S. Injectable hyaluronic acid hydrogels encapsulating drug nanocrystals for long-term treatment of inflammatory arthritis. Bioeng. Transl. Med. 2021, e10245. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.-E.; Song, H.-R.; Kim, Y.-M.; Song, S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium–loaded alginate microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef] [PubMed]
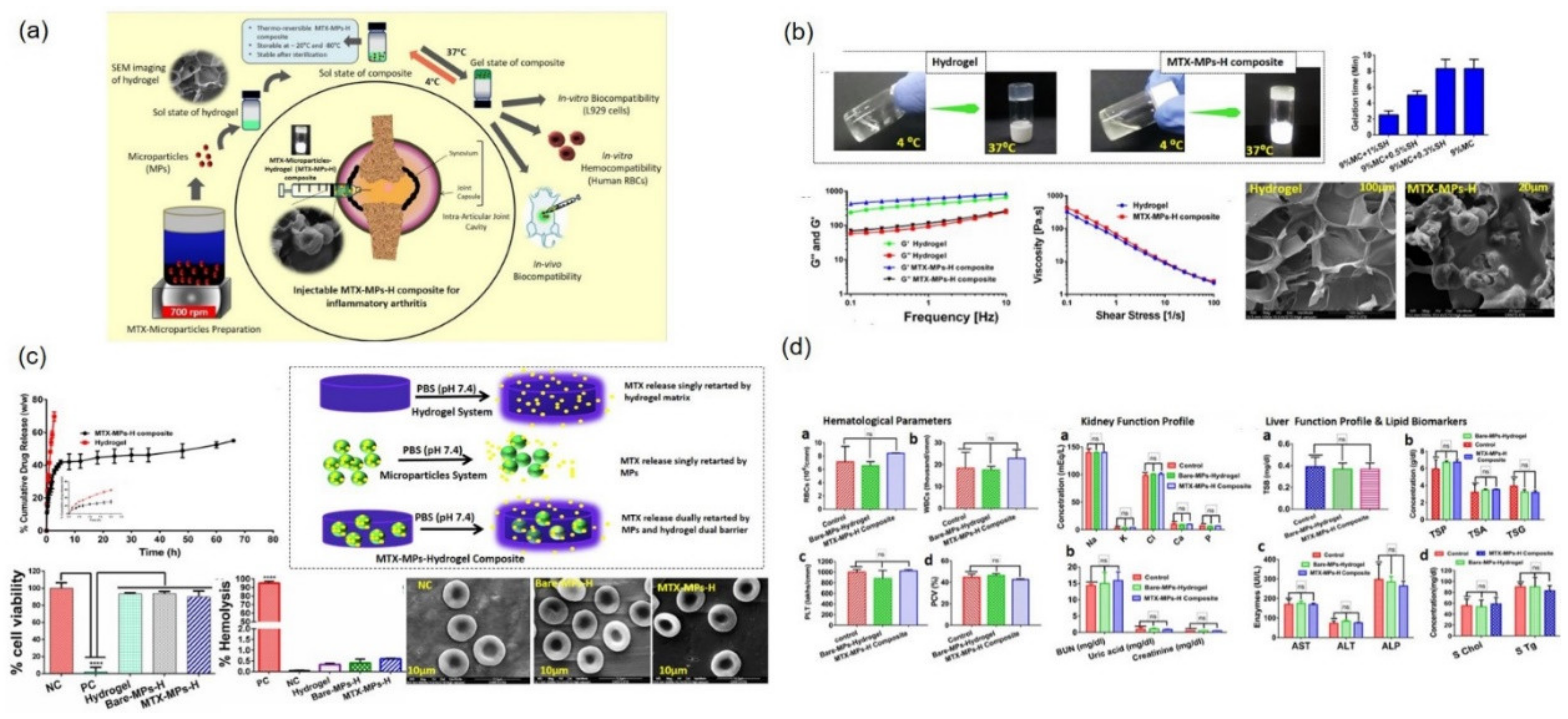
- Dhanka, M.; Pawar, V.; Chauhan, D.S.; Jain, N.K.; Prabhuraj, R.; Shetty, C.; Kumawat, M.K.; Prasad, R.; Srivastava, R. Synthesis and characterization of an injectable microparticles integrated hydrogel composite biomaterial: In-vivo biocompatibility and inflammatory arthritis treatment. Colloids Surf. B Biointerfaces 2021, 201, 111597. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef]
- Abou-ElNour, M.; Soliman, M.E.; Skouras, A.; Casettari, L.; Geneidi, A.S.; Ishak, R.A. Microparticles-in-thermoresponsive/bioadhesive hydrogels as a novel integrated platform for effective intra-articular delivery of triamcinolone acetonide. Mol. Pharm. 2020, 17, 1963–1978. [Google Scholar] [CrossRef]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The effect of triptolide in rheumatoid arthritis: From basic research towards clinical translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.H.; Cake, M.A.; Spoelstra, G.; Read, R.A. Biodistribution and clearance of intra-articular liposomes in a large animal model using a radiographic marker. J. Liposome Res. 2007, 17, 249–261. [Google Scholar] [CrossRef]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Ho, M.J.; Lee, E.; Lee, J.W.; Choi, Y.W.; Kang, M.J. Cationic PLGA/Eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint. Int. J. Nanomed. 2015, 10, 5263. [Google Scholar]
- Küçüktürkmen, B.; Umut Can, Ö.; Bozkir, A. In situ hydrogel formulation for intra-articular application of diclofenac sodium-loaded polymeric nanoparticles. Turk. J. Pharm. Sci. 2017, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Fernandes, D.C.; Maia, F.R.; Canadas, R.F.; Reis, R.L.; Oliveira, J.M. Bioengineered nanoparticles loaded-hydrogels to target TNF Alpha in inflammatory diseases. Pharmaceutics 2021, 13, 1111. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and scaffolds: A winning combination for tissue engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Sethu, S.N.; Namashivayam, S.; Devendran, S.; Nagarajan, S.; Tsai, W.-B.; Narashiman, S.; Ramachandran, M.; Ambigapathi, M. Nanoceramics on osteoblast proliferation and differentiation in bone tissue engineering. Int. J. Biol. Macromol. 2017, 98, 67–74. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A review of bioactive glass/natural polymer composites: State of the art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Y.; Zhu, T.; Gao, S.; Ye, K.; Zhou, F.; Qiu, D.; Wang, X.; Tian, Y.; Qu, X. Biphasic double-network hydrogel with compartmentalized loading of bioactive glass for osteochondral defect repair. Front. Bioeng. Biotechnol. 2020, 8, 752. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Zhang, F.-M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.-A.; Tu, L.-J.; Zhou, Z.-N.; Li, X.-J. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; Grodzinsky, A.J. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat. Rev. Rheumatol. 2017, 13, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. Dual Delivery of TGF-β3 and Ghrelin in Microsphere/Hydrogel Systems for Cartilage Regeneration. Molecules 2021, 26, 5732. [Google Scholar] [CrossRef]
- Martin, A.R.; Patel, J.M.; Locke, R.C.; Eby, M.R.; Saleh, K.S.; Davidson, M.D.; Sennett, M.L.; Zlotnick, H.M.; Chang, A.H.; Carey, J.L. Nanofibrous hyaluronic acid scaffolds delivering TGF-β3 and SDF-1α for articular cartilage repair in a large animal model. Acta Biomater. 2021, 126, 170–182. [Google Scholar] [CrossRef] [PubMed]

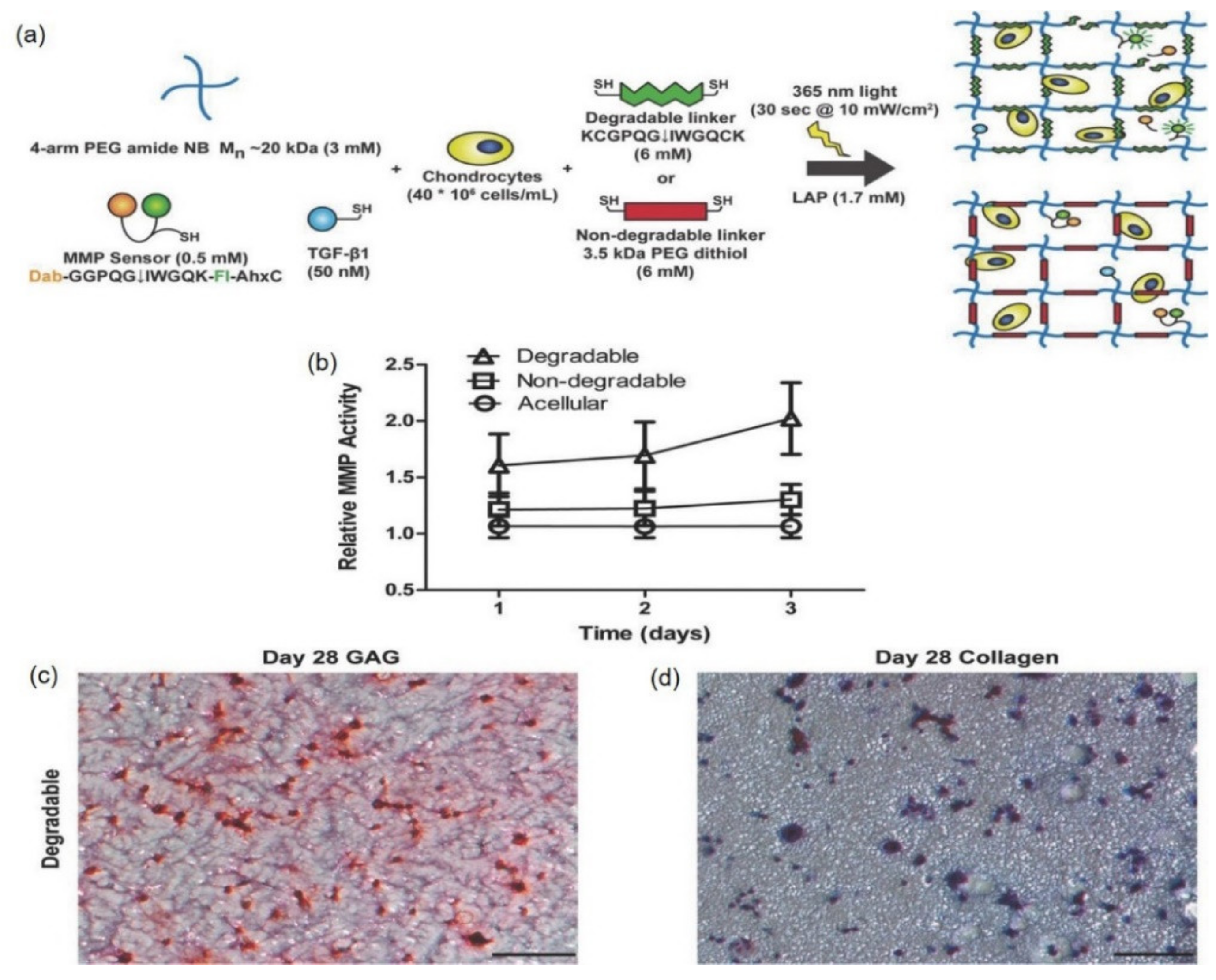
| Hybrid Hydrogel Composite | Hybrid Semi-Synthetic Hydrogel Composition | Cross-Linking Mechanism | Improved Hydrogel Properties | Hydrogel-Encapsulated Agent | Therapeutic Outcome/Disease Model | Refs. | |
|---|---|---|---|---|---|---|---|
| Natural Polymer and/or Its Modification | Synthetic Polymer and/or Its Modification | ||||||
| GC/poly(EO-co-Gly)-CHO | Glycol chitosan (GC) | Poly(ethylene oxide-co-glycidol)-CHO(poly(EO-co-Gly)-CHO) (as cross-linker) | Schiff’s base formation | Hybrid hydrogel showed 3.1 times decrease in gelation time from 204 sec to 64 s and a consequently 2.4- and 7-fold increase in degradation time and strength modulus, respectively, with an increase in cross-linker concentration from 0.25 to 2.0 (wt.%). | Chondrocytes | Both in vitro and in vivo studies (ICR mice) confirmed slow degradation of hydrogel with a lifetime of more than 3 months, maintained cell morphology and chondrogenic ability. | [112] |
| MeHA/F127DA | Methacrylated HA (HAMA) | Pluronic F127 diacrylate (F127DA) nano-micelle | Photo-cross-linking | The optimized hybrid NMgel showed a low swelling ratio. The G′ of hybrid NMgel increased from 2 kPa to 10 kPa and a maximum of 20 kPa with increasing HAMA content from 0.25% to 0.75% and 1.5%. | Stem cells | Hybrid hydrogel efficiently supported the cartilage regeneration following 8-week post-implantation in thyroid cartilage defects in rabbits. | [113] |
| SF/PVCL | Silk fibroin (SF) | Poly(N-vinylcaprolactam) (PVCL) | Photo-cross-linking | Tsol–gel 32–35 °C, increased water uptake ability and higher elastic response. | Mouse pre-chondrocyte (ATDC5) cells | In vitro studies with ATDC5 cells reported enhanced chondrogenic response. | [114] |
| ALG-POL/SF | SF | Synthesized alginate- poloxamer 407 copolymer | Chemical cross-linking (HRP and H2O2) | The hybrid hydrogel (5.6% ALG-POL + 8% SF) exhibited a large swelling index, thermoresponsive, highly porous, and strong mechanical characteristics. Gelation time 7.5 min and G′ value ~ 5 kPa. | Chondrocyte | The hydrogel facilitated in vitro chondrocyte growth without affecting their chondrogenic phenotype. | [115] |
| AD/CS/SF | SF | Alginate-dopamine (AD), chondroitin sulfate-NHS (CS-NHS) | Chemical cross-linking (HRP and H2O2) | The AD/CS/SF hydrogel showed a lap shear strength of 120 kPa, with a comparable gelation time and adhesive strength (121 kPa) with that of commercial Enbucrilate tissue adhesive. Slow degradation with retention of 60% of mass even after 20 days in phosphate-buffer saline (PBS). | Exosomes (EXO) isolated from BMSCs | Hybrid AD/CS/SF/EXO hydrogel promoted the cartilage defect regeneration in situ, and ECM remodeling. The exosomes secreted by the hydrogels could induce the migration of BMSCs to the hydrogel and neocartilage via the chemokine signaling pathway in osteochondral defect model rats. | [116] |
| HA-SH/p(HPMAm-lac)-PEG | Thiolated HA (HA-SH) | Vinyl sulfonated triblock polymer: methacrylated poly[N-(2-hydroxypropyl)methacrylamide mono/dilactate]/polyethylene glycol (p(HPMAm-lac)-PEG) | Michael-type addition | Gelation temperature 20–22 °C, longer residence time with complete hydrogel degradation in 40–70 days in PBS. | -None | In vivo efficacy studies in OA mouse model exhibited reversion of inflammation-related symptoms with downregulation of TNF-α, NF-kB, and RANKL and induction of MSC maturation in to chondroblasts and cartilage formation. | [117] |
| HA/PLA-b-PEG (with NO-cleavable cross-linker) (DA-NOCCL) | Azide-HA (HA-N3) | Azide-PLA-b-PEG | Click cycloaddition (azide-alkyne reaction) | Hydrogel possessed self-healing behavior, providing visco-supplementation with dual drug (both hydrophilic and hydrophobic)-releasing features, in response to different NO concentrations. | Dexamethasone (Dex) | Intra-articular injection of dex-encapsulated, NO-scavenging hybrid hydrogel remarkably suppresses NO-mediated pro-inflammatory cytokine levels and showed superior therapeutic effects in CIA mice models. | [118] |
| PEG/SF hydrogel | SF | 6-amino-2-cyanobenzothiazole (CBT)/bocethylmercapto-L-cysteine-functionalized 4-armed PEG (PEG-CBT/PEG-d-Cys) | Thiol based bio-orthogonal reaction and ultrasonication | Porous DN hydrogel (150 µm pore size), short gelation time of 10 sec and superior mechanical properties. PEG-SF (50:50) showed highest G′ value of 15 kPa and compressive stress 0.37 MPa compared to 2–3 kPa (G’) and 0.08 MPa with pure PEG hydrogel. | BMSCs | Hydrogel maintained in vitro BMSC viability and increased differentiation. Moreover, PEG/SF hydrogel promoted the regeneration of cartilage defects in vivo in cartilage defect SD rat model. | [119] |
| HA/PEG (namely DAHP) | Furan-HA (FHA) | Maleimide- PEG | Diels–Alder cross-linking | Hybrid DAHP (FHA: Mal-PEG-Mal, 1:5) showed the fastest gelation time of ~1800 s, while FHA:Mal-PEG-Mal (1:1.25 and 1:2.5) displayed gelation time of more than 1 h at 37 ℃. Hydrogel exhibited slow-release kinetics for MSC-sEVs. | Mesenchymal stem cell-derived small extracellular vesicles (MSC-sEVs) | HA/PEG Hydrogel retained the therapeutic functions of sEVs, and an in vivo test unveiled that the hydrogel could enhance the therapeutic efficacy of MSC-sEVs for OA improvement in traumatic OA rat model. | [120] |
| Graphene oxide (GO) doped GG/PEGDA bilayered hydrogel | Gellan gum (GG) | Poly(ethylene glycol) diacrylate (PEGDA) | Photo cross-linked and Ionic cross-linked (MgCl2) | Bilayered GG/PEGDA hydrogel mimicked the mechanical and lubrication features of articular superficial and deep cartilage zones with a Young’s modulus of ~300 and 700 kPa, respectively. | None- | Bilayered doped hydrogel demonstrated antiwear properties and was non-cytotoxic to human chondrocytes. | [80] |
| CS/PCL/KGN | Chitosan (CS) | Polycaprolactone (PCL) | Thermosensitive | Multi-layered CS/PCL/KGN hydrogel scaffold showed lesser swelling extent and greater compressive modulus, with sustained KGN release. | Kartogenin (KGN) | The scaffold promoted the proliferation and chondrogenic differentiation of laden MSCs, with increased production of type II collagen and Sox9. | [121] |
| PL407-PL338/HA | HA | Poloxamer 407 and 338(PL407, PL338) | Thermosensitive | Poloxamer/HA hybrid hydrogel exerted viscoelastic behavior and cubic phase organization. Gelation temperature and G′ value was determined as 32 °C and 6.6 kPa, respectively. | Sulforaphane (SFN) | The hydrogel showed non-cytotoxicity to both osteoblast and chondrosarcoma cell lines. In vitro/ex vivo experiments exhibited an increased expression of type II collagen, and proteoglycan accumulation. | [122] |
| Formulation (Liposome/Nanoparticles/Microspheres) | Therapeutic Agent | Hydrogel Characteristics | Characteristics of Colloidal Drug Carrier | Therapeutic Outcome | Refs. |
|---|---|---|---|---|---|
| Methotrexate entrapped ultradeformable liposomal carbopol gel | Methotrexate (MTX) | Transdermal delivery, viscosity 11847 mPa.s, gel formulation revealed a non-Newtonian pseudoplastic (shear-thinning) flow pattern | Liposome size 100 nm, high drug content 98%, skin permeation studies demonstrated permeability coefficient Kp values as 9.6 × 10−3 cm/h. | Improved anti-rheumatic activity was observed in AIA rat model, reduced expression of TNF-α and IL-1β in paw tissues, reduced edema volume improved tissue architecture and body weight gain (23%). | [150] |
| Carbopol hydrogel loaded with methotrexate aspasomes | Methotrexate (MTX) | Transdermal delivery, Tsol–gel 37 °C | Aspasome 386 nm, drug loading 19.41% and in vitro drug release for more than 24 h. | Reduced TNF-α, IL-β production, cartilage damage, inflammation, and bone resorption in AIA rat model. | [151] |
| Poly(N-isopropylacrylamide)(PNIPAM)/HA (HA)hydrogel containing nano/microparticles | Chondrogenic small molecule melatonin | Injectable, PNIPAm/HA hydrogel showed 50% shrinkage at equilibrium state, in vitro degradation with only 43% degradation after 40 days, compression module 109.04 kPa | Chitosan-g acrylic acid coated PLGA MPs were of size 2.1–2.2 µm, with loading content 3.4% and encapsulation efficiency 16%; NPs were 130 nm with loading content 2%, encapsulation efficiency 8%, and controlled release up to 15 days. | High chondrogenic differentiation potential (in vitro studies) for CTE. | [152] |
| Silk fibroin hydrogel containing chitosan NPs | Transforming growth factor-β1 (TGF-β1) and bone morphogenic protein-2 (BMP-2) | Water absorption capacity 20%, in vitro hydrogel degradation of 40% in 32 days | Chitosan NPs size 343.7 ± 20.48 nm, 75–80% TGF-β1 and 80% BMP-2 release in 15 days. | Enhanced chondrogenic ability both in vitro and in vivo (New Zealand white rabbit articular cartilage defect model). | [153] |
| HA hydrogel encapsulating nanocrystals | Camptothecin (CPT) | Intra-articular injection, gelation time 5 min | CPT nanocrystal size 160–560 nm, in vitro drug release up to 1 month. | Decreased cytokine level (IL-1β and IL-6) in joint homogenate. Histological and micro-CT analysis at 60 days showed joint recovery with CPT-hydrogel compared with disease control (severe joint destruction) in CIA rat model. | [154] |
| HA-fibrin hydrogel encapsulating nanocapsules | Dexamethasone (Dex) and galectin-3 inhibitor (GI) | Intra-articular injection, T with 29G needle, gelation time less than 30 s, hydrogel viscosity following gelation 81.3 mPa.s, and elastic behavior with G′ > G″ | Dex nanocapsules size 135 ± 9 nm and GI nanocapsules were 122 ± 11 nm, 100% drug release in 24 h from nanocapsules while it took 72 h to show 100% drug release from hydrogel. | Acute synovitis CIA rat model studies reported significant increase (~40%) in knee diameter with reduction in swelling and inflammation. | [27] |
| Polymeric NPs hydrogel system, [carboxylic acid termini-functionalized poly(organophosphazene)CP] | Triamcinolone acetonide (TCA) | Intra-articular injection, gelation temperature 32–37 °C, exhibited high viscosity of 518.75 Pa.s at 37 °C, rheology study: G′ and G″ values of 1284 and 765 Pa, respectively, at 37 °C indicated a gel state. | Self-assembled organophosphazene NPs size 140 nm, sustained release profile up to 35 days. | Long-term anti-inflammatory effect and prevention of cartilage degeneration by inactivating MMPs were observed in MIA-induced OA rat model. | [155] |
| Chitosan hydrogel loaded with diclofenac-sodium-loaded alginate microspheres | Diclofenac sodium (DS) | Intra-articular administration, injectable thermosensitive hydrogel, exhibited Tsol–gel at 31.72 ± 0.42 °C, gelation time 5 min. | Alginate microspheres, in vitro drug release up to 5 days. | Improved anti-inflammatory efficacy in New Zealand rabbits with experimental arthritis. | [156] |
| Hydrogel containing methotrexate-loaded alginate microspheres | Methotrexate (MTX) | Injectable thermosensitive hydrogel, Tsol–gel 37 °C, gelation time 5 min, swelling degree 5.8%, G′ and G″ at 37 °C was 500 and 100 Pa, respectively, at oscillatory frequency of 1 Hz. Viscosity of sample was decreased with increased shear rate. | Non-cross-linked and cross-linked alginate MPs size 5–6 and 8 µm, respectively, high encapsulation efficiency. Fast drug release (95–98% release in 8 h) with non-cross-linked MPs while cross-linked alginate MPs showed sustained release, 75% MTX release in 66 h. | Significant decrease in swelling and paw edema following treatment with cross-linked MTX-MP-loaded-hydrogel composite with no signs of toxicity in AIA rat model. | [157] |
| DMA-MPC coated GelMA hydrogel microspheres | DS | Intra-articular administration, modified hydrogel microspheres | GelMA microspheres 150 µm, porous structure, loading efficiency (10–15%), DMA-MPC polymer coated microspheres demonstrated 76% degradation at 28 days. | Intra-articular injection at rat knee joint (osteoarthritic rat model) showed improved lubrication and anti-inflammatory effects with reduced expression of matrix metalloproteinase-13 and ADAMTS5. | [158] |
| TCA-loaded MPs in poly(polyethylene glycol methacrylate) poly(PEGMA)hydrogel | TCA | Intra-articular injection, gelation temperature 33–37 °C, viscosity 12,426 cP at shear rate of 0.5 rpm, and detachment force 6063 dyne/cm2 | PLA/methoxy-PEG-poly(δ-decalactone) (mPEG-PDL) MPs, entrapment efficiency 84%, loading content 7.6%, and 90% TCA release in 160 h. | Percent inhibition of inflammation vs. time profile demonstrated AUC values with 450–514%/day and significantly reduced adjuvant-induced joint inflammation in rats. | [159] |
| MTX-loaded polyelectrolyte complexes/Poloxamer 407 and 188 hydrogels | Methotrexate (MTX) | Intra-articular injection, gelation behavior close to physiological temperature at 36.7 °C | Oligochitosan NPs (PEC) spherical in shape, size 470 nm, 50% drug release in 1 h. | Hybrid hydrogel composite exhibited reduced plasmatic IL-1β compared to free MTX group, reduced systemic exposure of MTX. | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Lee, J.; Ghosh, T.; Nguyen, V.Q.; Dey, A.; Yoon, B.; Um, W.; Park, J.H. Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis. Pharmaceutics 2022, 14, 540. https://doi.org/10.3390/pharmaceutics14030540
Gupta A, Lee J, Ghosh T, Nguyen VQ, Dey A, Yoon B, Um W, Park JH. Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis. Pharmaceutics. 2022; 14(3):540. https://doi.org/10.3390/pharmaceutics14030540
Chicago/Turabian StyleGupta, Anuradha, Jungmi Lee, Torsha Ghosh, Van Quy Nguyen, Anup Dey, Been Yoon, Wooram Um, and Jae Hyung Park. 2022. "Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis" Pharmaceutics 14, no. 3: 540. https://doi.org/10.3390/pharmaceutics14030540
APA StyleGupta, A., Lee, J., Ghosh, T., Nguyen, V. Q., Dey, A., Yoon, B., Um, W., & Park, J. H. (2022). Polymeric Hydrogels for Controlled Drug Delivery to Treat Arthritis. Pharmaceutics, 14(3), 540. https://doi.org/10.3390/pharmaceutics14030540








