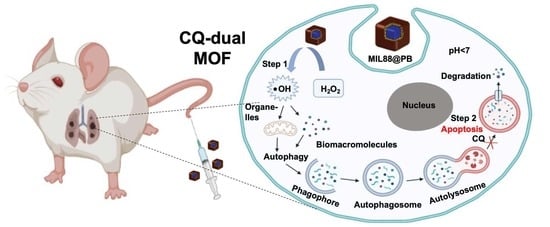
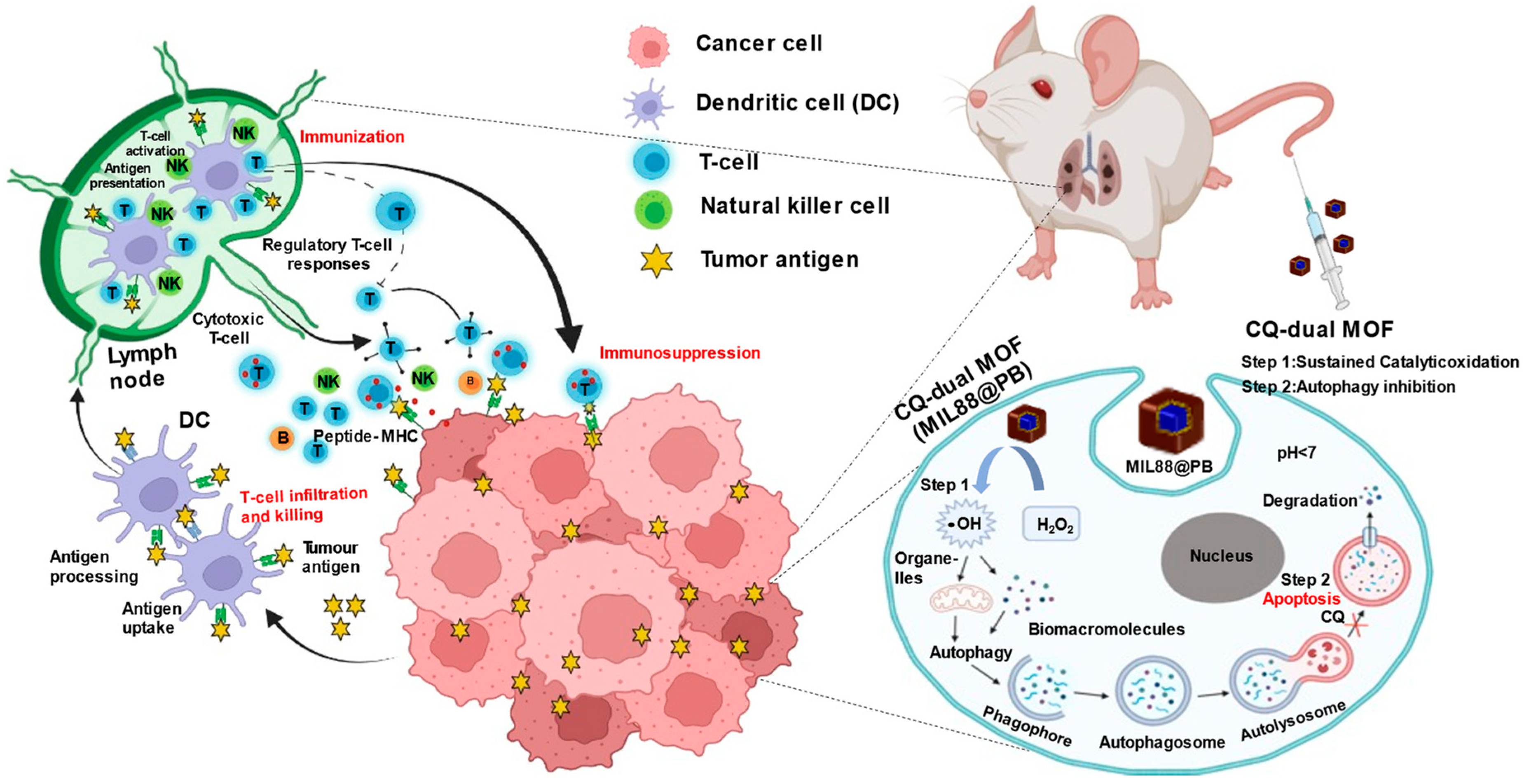
Programmed Catalytic Therapy-Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent
Abstract
:1. Introduction
2. Method
2.1. Materials
2.2. Synthesis of PB MOF
2.3. Synthesis of NH2-MIL88
2.4. Synthesis of MIL88@PB (Dual MOFs)
2.5. Characterizations
2.6. In Vitro Drug Release
2.7. In Vitro Cellular Toxicity
2.8. In Vitro Cellular Uptake
2.9. In Vitro LC3B Autophagy Protein Expression (Regulation of Autophagosomes)
2.10. In Vitro Catalytic Performance of Dual MOFs
2.11. Tissue Section Immunostaining
2.12. In Vivo Flow Cytometry Analysis
3. Results and Discussion
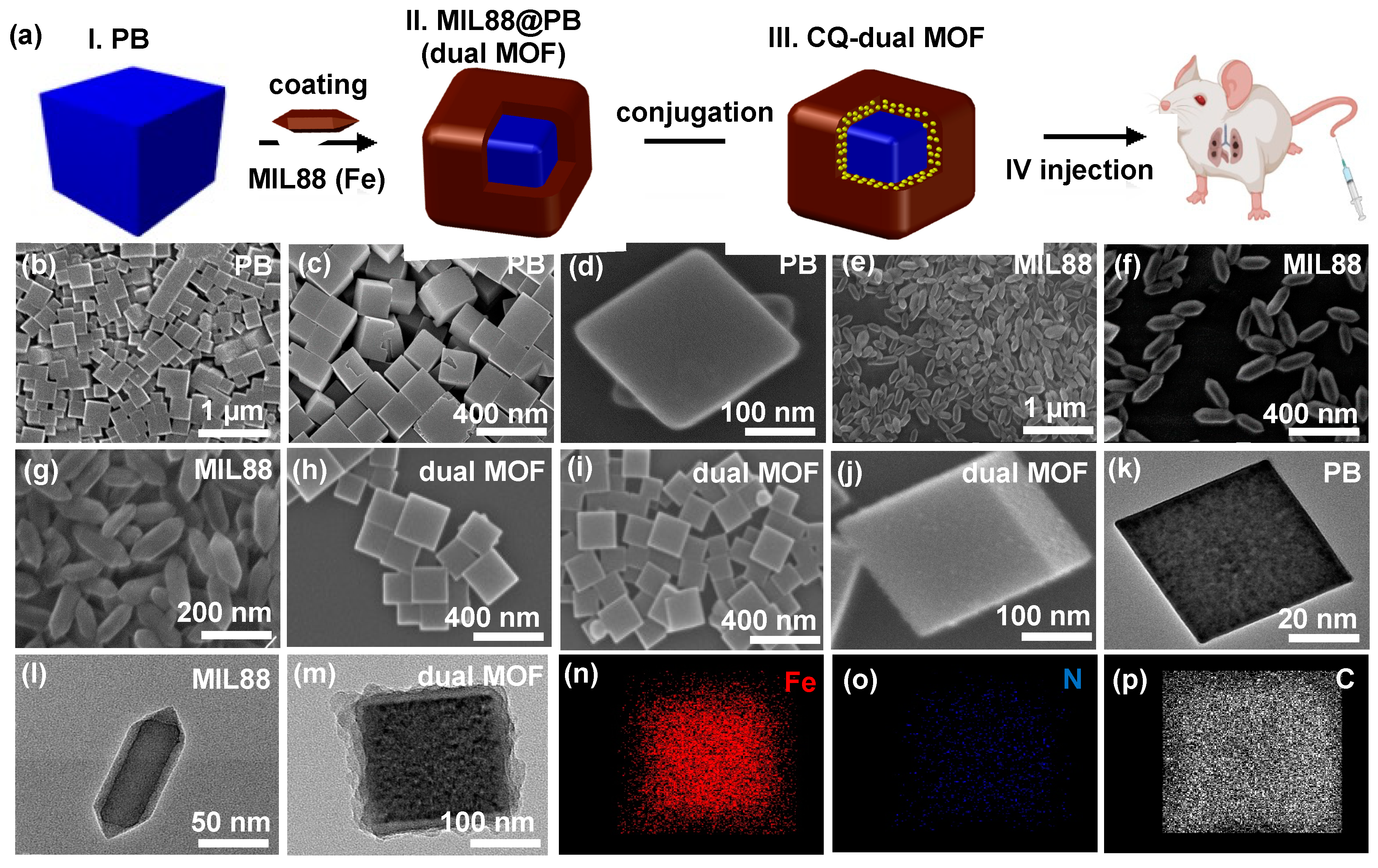
3.1. Characterization of PB, MIL88, and Dual MOF Nanoparticles
3.2. In Vitro Drug Release
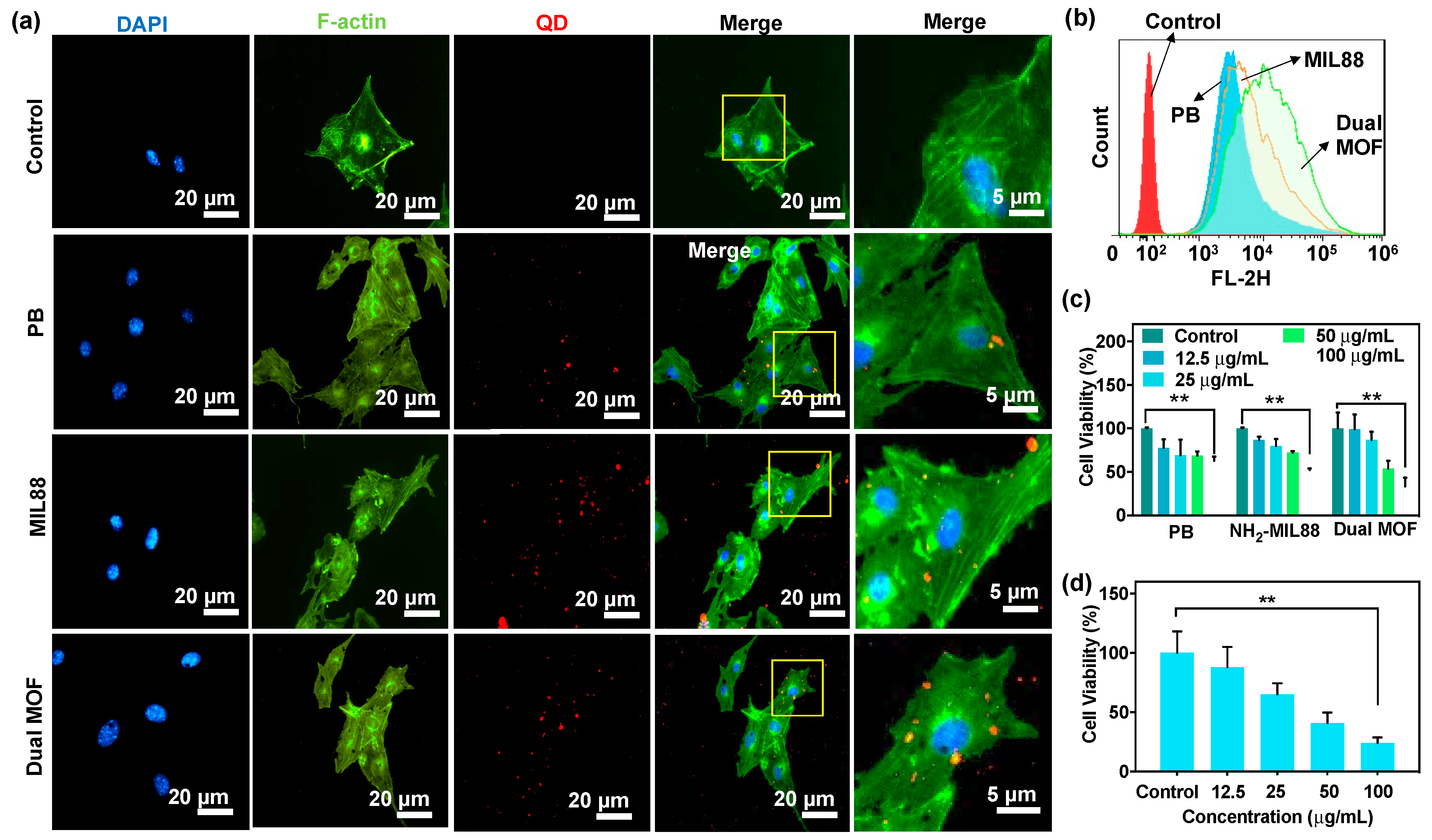
3.3. In Vitro Cellular Uptake and Cytotoxicity of PB, MIL88, and Dual MOF
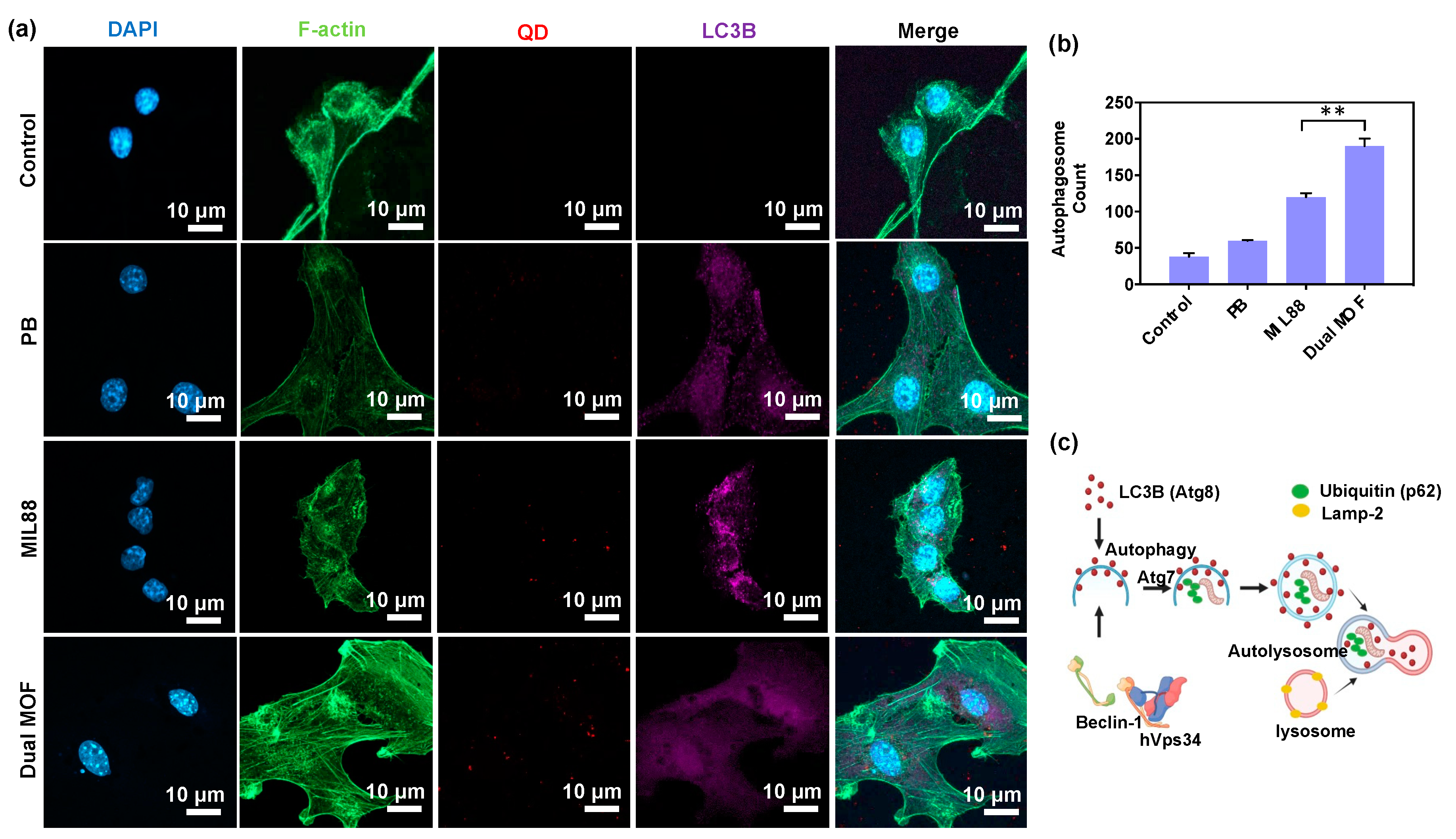
3.4. In Vitro Cellular Uptake and LC3B Protein Expression (Regulation of Autophagosomes)
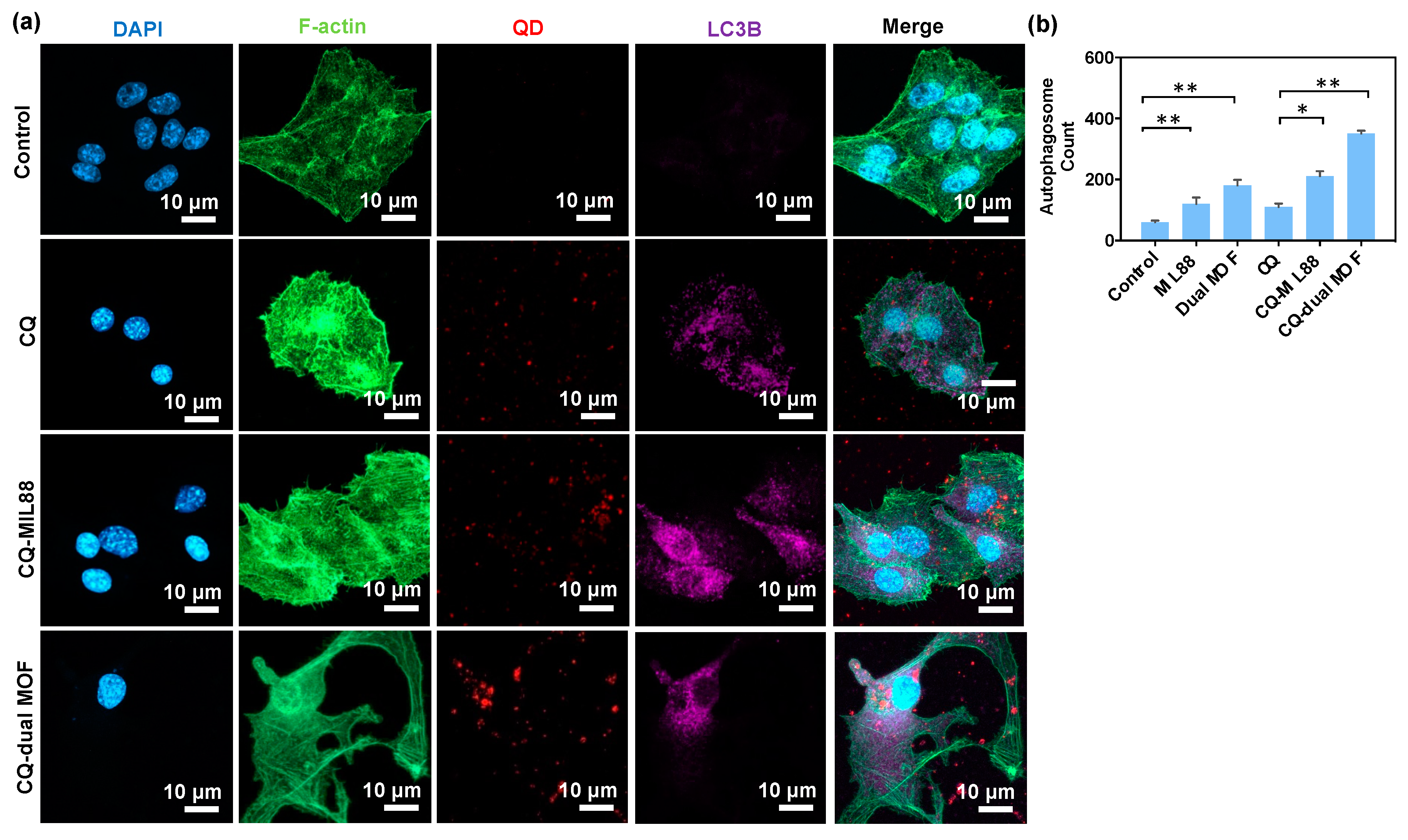
3.5. CQ Inhibition of LC3B Protein Expression
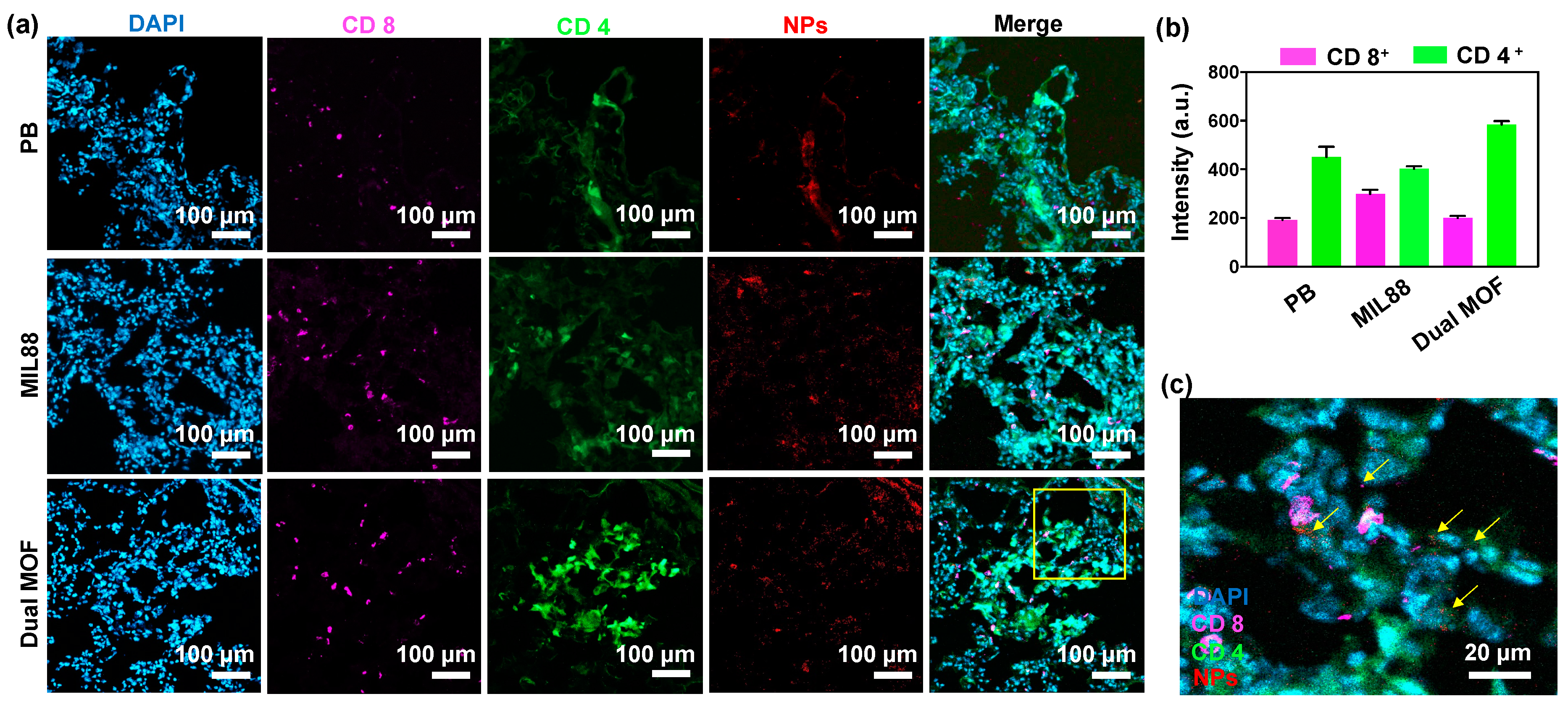
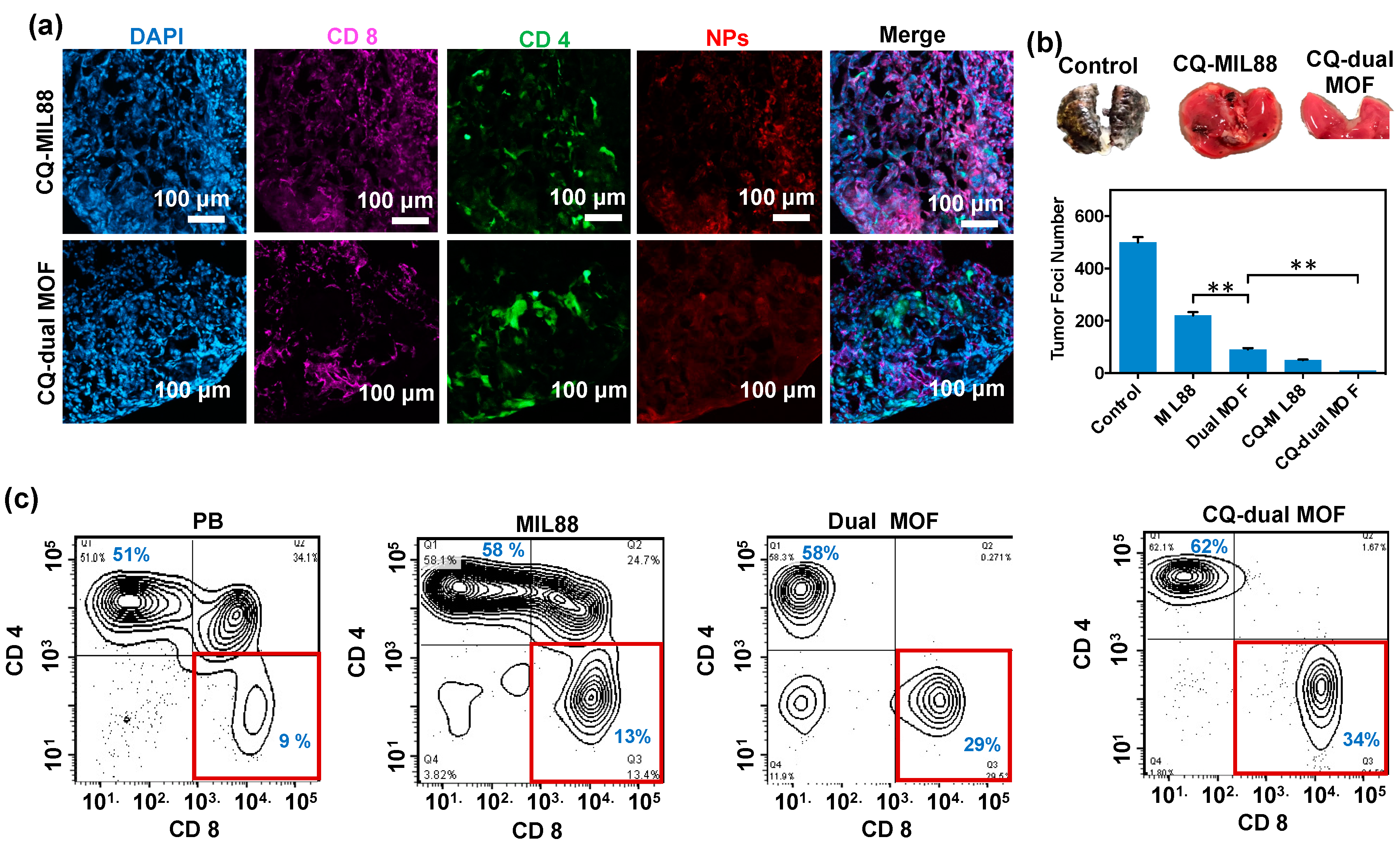
3.6. In Vivo Study of Mice Bearing Lung Metastasis Treated with Dual MOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, W.F.; Pepona, M.; Robertson, C.; Alvarado, J.; Dubbin, K.; Triplett, M.; Adorno, J.J.; Randles, A.; Moya, M.L. Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci. Adv. 2020, 6, eabb3308. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, A.G.; Islas, J.F.; Delgado-Gonzalez, P.; Franco-Villarreal, H.; Garza-Treviño, E.N. Therapeutic Approaches for Metastases from Colorectal Cancer and Pancreatic Ductal Carcinoma. Pharmaceutics 2021, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Ibaraki, H.; Kanazawa, T.; Owada, M.; Iwaya, K.; Takashima, Y.; Seta, Y. Anti-Metastatic Effects on Melanoma via Intravenous Administration of Anti-NF-κB siRNA Complexed with Functional Peptide-Modified Nano-Micelles. Pharmaceutics 2020, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergers, G.; Fendt, S.M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 2021, 21, 162. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34. [Google Scholar] [CrossRef]
- Joseph, R.; Soundararajan, R.; Vasaikar, S.; Yang, F.; Allton, K.L.; Tian, L.; den Hollander, P.; Isgandarova, S.; Haemmerle, M.; Mino, B.; et al. CD8+ T cells inhibit metastasis and CXCL4 regulates its function. Br. J. Cancer. 2021, 125, 176. [Google Scholar] [CrossRef]
- Lôbo, G.C.N.B.; Paiva, K.L.R.; Silva, A.L.G.; Simões, M.M.; Radicchi, M.A.; Báo, S.N. Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy. Pharmaceutics 2021, 13, 1167. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; da Silva, C.G.; Jager, M.J.; Cruz, L.J.; Ossendorp, F. Combining Photodynamic Therapy with Immunostimulatory Nanoparticles Elicits Effective Anti-Tumor Immune Responses in Preclinical Murine Models. Pharmaceutics 2021, 13, 1470. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhao, Y.Y.; Shen, J.; Sun, X.; Liu, Y.; Liu, H.; Wang, Y.; Wang, J. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy. Nano Lett. 2019, 19, 2774. [Google Scholar] [CrossRef]
- Chiang, M.R.; Su, Y.L.; Chang, C.Y.; Chang, C.W.; Hu, S.H. Lung metastasis-targeted donut-shaped nanostructures shuttled by the margination effect for the PolyDox generation-mediated penetrative delivery into deep tumors. Mater. Horiz. 2020, 7, 1051. [Google Scholar] [CrossRef]
- Shen, W.T.; Hsu, R.S.; Fang, J.H.; Hu, P.F.; Chiang, C.S.; Hu, S.H. Marginative delivery-mediated extracellular leakiness and T cell infiltration in lung metastasis by a biomimetic nanoraspberry. Nano Lett. 2021, 21, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol. Life Sci. 2018, 75, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.-S.; Yoo, C.-B.; Lee, J.-H.; Lee, H.-H.; Park, S.-E.; Han, H.-S.; Lee, S.-Y.; Kwon, B.-M.; Choi, J.-H.; Lee, K.-T. Regulation of ROS-Dependent JNK Pathway by 2′-Hydroxycinnamaldehyde Inducing Apoptosis in Human Promyelocytic HL-60 Leukemia Cells. Pharmaceutics 2021, 13, 1794. [Google Scholar] [CrossRef]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 2018, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Nanocatalytic medicine. Adv. Mater. 2019, 31, e1901778. [Google Scholar] [CrossRef]
- Nirosha Yalamandala, B.; Shen, W.T.; Min, S.H.; Chiang, W.H.; Chang, S.J.; Hu, S.H. Advances in functional metal-organic frameworks based on-demand drug delivery systems for tumor therapeutics. Adv. NanoBiomed. Res. 2021, 1, 2100014. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-Small Iron Nanoparticles Target Mitochondria Inducing Autophagy, Acting on Mitochondrial DNA and Reducing Respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Sumkhemthong, S.; Prompetchara, E.; Chanvorachote, P.; Chaotham, C. Cisplatin-induced hydroxyl radicals mediate pro-survival autophagy in human lung cancer H460 cells. Biol. Res. 2021, 54, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 2009, 335, 1. [Google Scholar]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631. [Google Scholar] [CrossRef]
- Hu, P.; Wu, T.; Fan, W.; Chen, L.; Liu, Y.; Ni, D.; Bu, W.; Shi, J. Near infrared-assisted Fenton reaction for tumor-specific and mitochondrial DNA-targeted photochemotherapy. Biomaterials 2017, 141, 86. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Liu, H.; Yuan, Q.; Ren, F.; Han, Y.; Sun, Q.; Li, Z.; Gao, M. Boosting H2O2-guided chemodynamic therapy of cancer by enhancing reaction kinetics through versatile biomimetic fenton nanocatalysts and the second near-infrared light irradiation. Adv. Funct. Mater. 2020, 30, 1906128. [Google Scholar] [CrossRef]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An ultrasound activated vesicle of janus Au-MnO nanoparticles for promoted tumor penetration and sono-chemodynamic therapy of orthotopic liver cancer. Angew. Chem. Int. Ed. 2020, 59, 1682. [Google Scholar] [CrossRef]
- Warhurst, D.; Hockley, D. Mode of Action of Chloroquine on Plasmodium berghei and P. cynomolgi. Nature 1967, 214, 935. [Google Scholar] [CrossRef]
- Wu, T.T.; Li, W.M.; Yao, Y.M. Interactions between autophagy and inhibitory cytokines. Int. J. Biol. Sci. 2016, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y.M. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Ligeon, L.A.; Pena-Francesch, M.; Vanoaica, L.D.; Núñez, N.G.; Talwar, D.; Dick, T.P.; Münz, C. Oxidation inhibits autophagy protein deconjugation from phagosomes to sustain MHC class II restricted antigen presentation. Nat. Commun. 2021, 12, 1508. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. 2012, 51, 984. [Google Scholar] [CrossRef]
- Guo, M.; Li, H. The Hydrolyzed Mil-88B(Fe) With improved surface area for high-capacity lithium ion battery. Front. Energy Res. 2021, 9, 781008. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Tian, Y.; Wu, M.; Zhou, Q.; Jiang, S.; Niu, Z. Size dependent cellular uptake of rod-like bionanoparticles with different aspect ratios. Sci. Rep. 2016, 6, 24567. [Google Scholar] [CrossRef] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Breus, V.V.; Pietuch, A.; Tarantola, M.; Basché, T.; Janshoff, A. The effect of surface charge on nonspecific uptake and cytotoxicity of. CdSe/ZnS core/shell quantum dots. J. Nanotechnol. 2015, 6, 281. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Ding, W.X.; Stolz, D.B.; Yin, X.M. Induction of macroautophagy by exogenously introduced calcium. Autophagy 2008, 4, 754. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Mater. 2020, 32, 1907152. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Maycotte, P.; Aryal, S.; Cummings, C.T.; Thorburn, J.; Morgan, M.J.; Thorburn, A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 2012, 8, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noman, M.Z.; Parpal, S.; Moer, K.V.; Xiao, M.; Yu, Y.; Vinlund, J.; Milito, A.D.; Hasmin, M.; Andersson, M.; Amaravadi, R.K.; et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunothearpy. Sci. Adv. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
 ), MIL88(
), MIL88(  ), and dual MOF. Slight disappearances reflections of 2-Theta and intensities in the dual MOF were due to the shielding effect of MIL88 on PB. (g,h) Catalytic performance of control, PB, MIL88, and dual MOF.
), and dual MOF. Slight disappearances reflections of 2-Theta and intensities in the dual MOF were due to the shielding effect of MIL88 on PB. (g,h) Catalytic performance of control, PB, MIL88, and dual MOF.
 ), MIL88(
), MIL88(  ), and dual MOF. Slight disappearances reflections of 2-Theta and intensities in the dual MOF were due to the shielding effect of MIL88 on PB. (g,h) Catalytic performance of control, PB, MIL88, and dual MOF.
), and dual MOF. Slight disappearances reflections of 2-Theta and intensities in the dual MOF were due to the shielding effect of MIL88 on PB. (g,h) Catalytic performance of control, PB, MIL88, and dual MOF.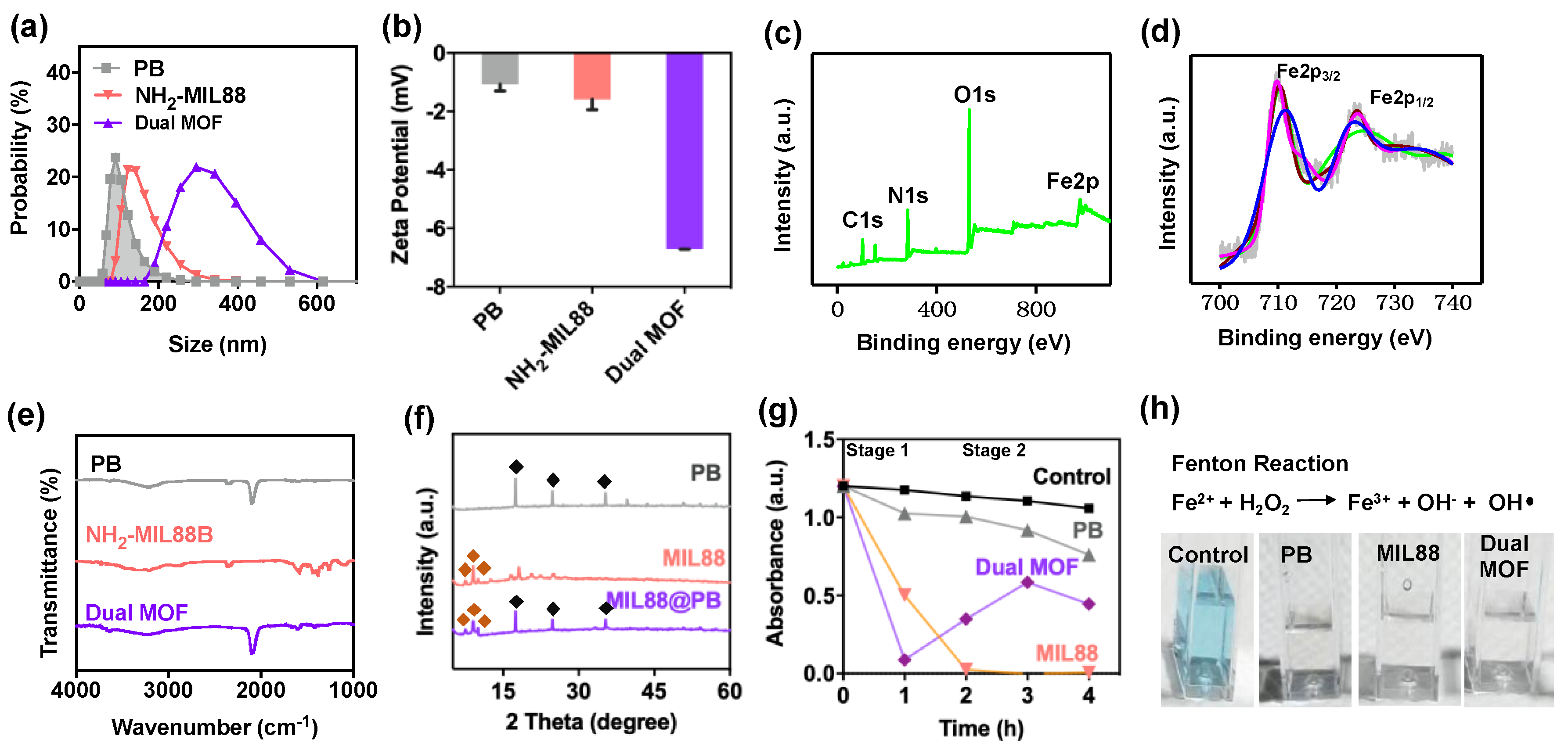
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirosha Yalamandala, B.; Chen, P.-H.; Moorthy, T.; Huynh, T.M.H.; Chiang, W.-H.; Hu, S.-H. Programmed Catalytic Therapy-Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent. Pharmaceutics 2022, 14, 527. https://doi.org/10.3390/pharmaceutics14030527
Nirosha Yalamandala B, Chen P-H, Moorthy T, Huynh TMH, Chiang W-H, Hu S-H. Programmed Catalytic Therapy-Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent. Pharmaceutics. 2022; 14(3):527. https://doi.org/10.3390/pharmaceutics14030527
Chicago/Turabian StyleNirosha Yalamandala, Bhanu, Pin-Hua Chen, Thrinayan Moorthy, Thi My Hue Huynh, Wen-Hsuan Chiang, and Shang-Hsiu Hu. 2022. "Programmed Catalytic Therapy-Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent" Pharmaceutics 14, no. 3: 527. https://doi.org/10.3390/pharmaceutics14030527
APA StyleNirosha Yalamandala, B., Chen, P.-H., Moorthy, T., Huynh, T. M. H., Chiang, W.-H., & Hu, S.-H. (2022). Programmed Catalytic Therapy-Mediated ROS Generation and T-Cell Infiltration in Lung Metastasis by a Dual Metal-Organic Framework (MOF) Nanoagent. Pharmaceutics, 14(3), 527. https://doi.org/10.3390/pharmaceutics14030527