Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics
Abstract
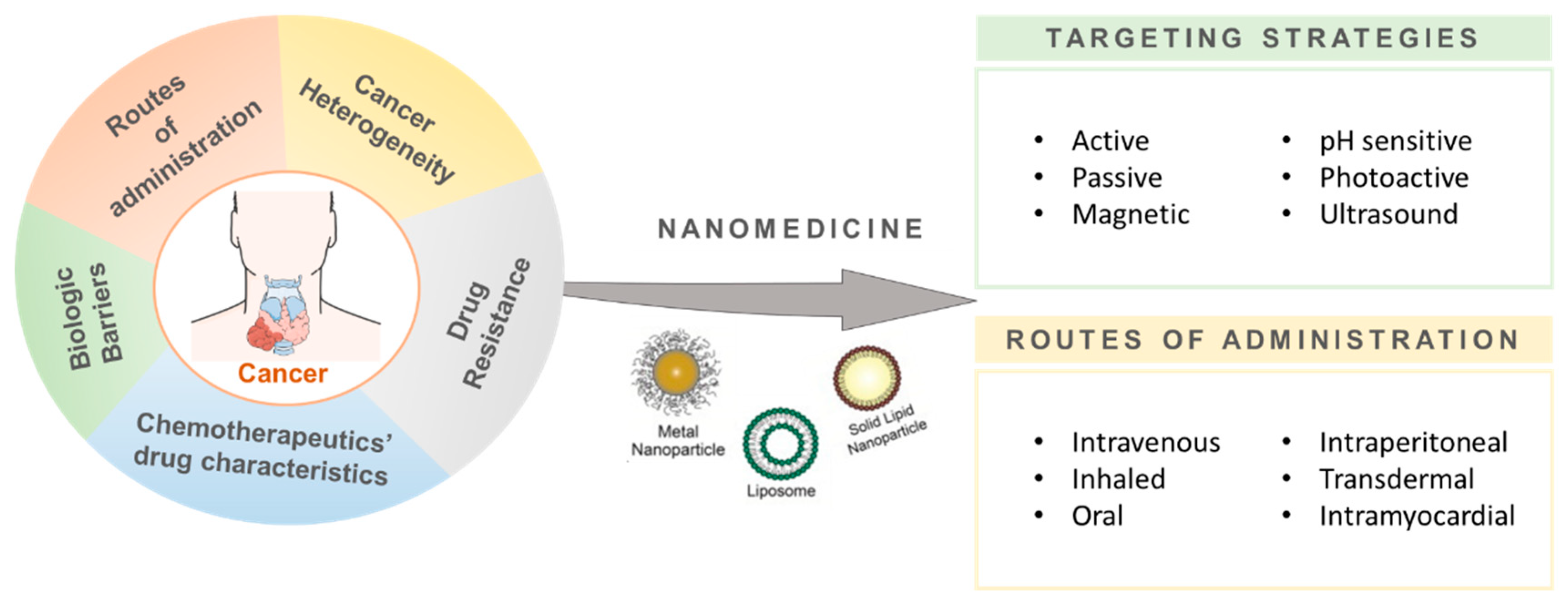
1. Introduction
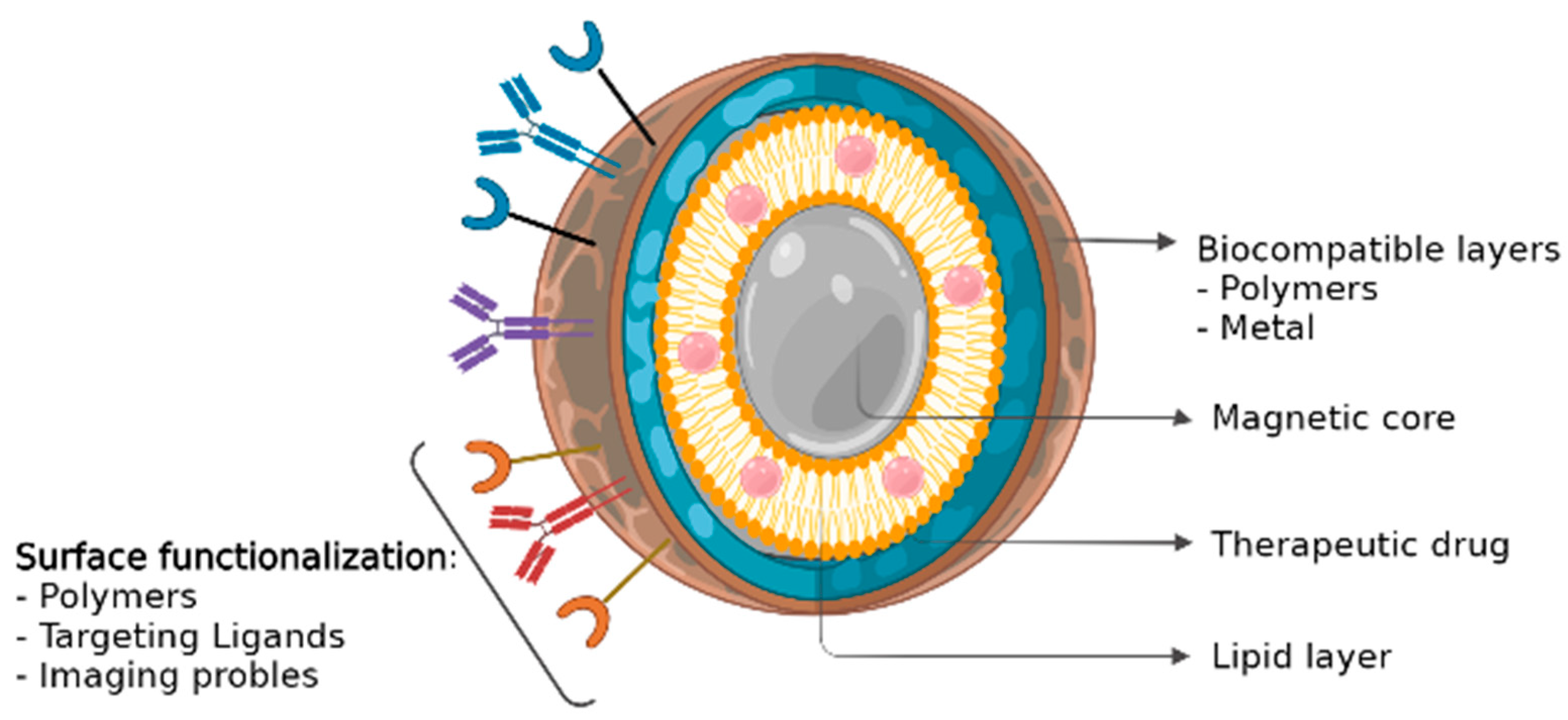
2. Magnetic Nanoparticles
2.1. Magnetic Nanoparticles as Drug Delivery Systems
2.2. Magnetic Nanoparticles in Cancer Diagnostics
2.3. Magnetic Nanoparticles for Cancer Treatment
2.4. Magnetic Nanoparticles for Theranostic Applications
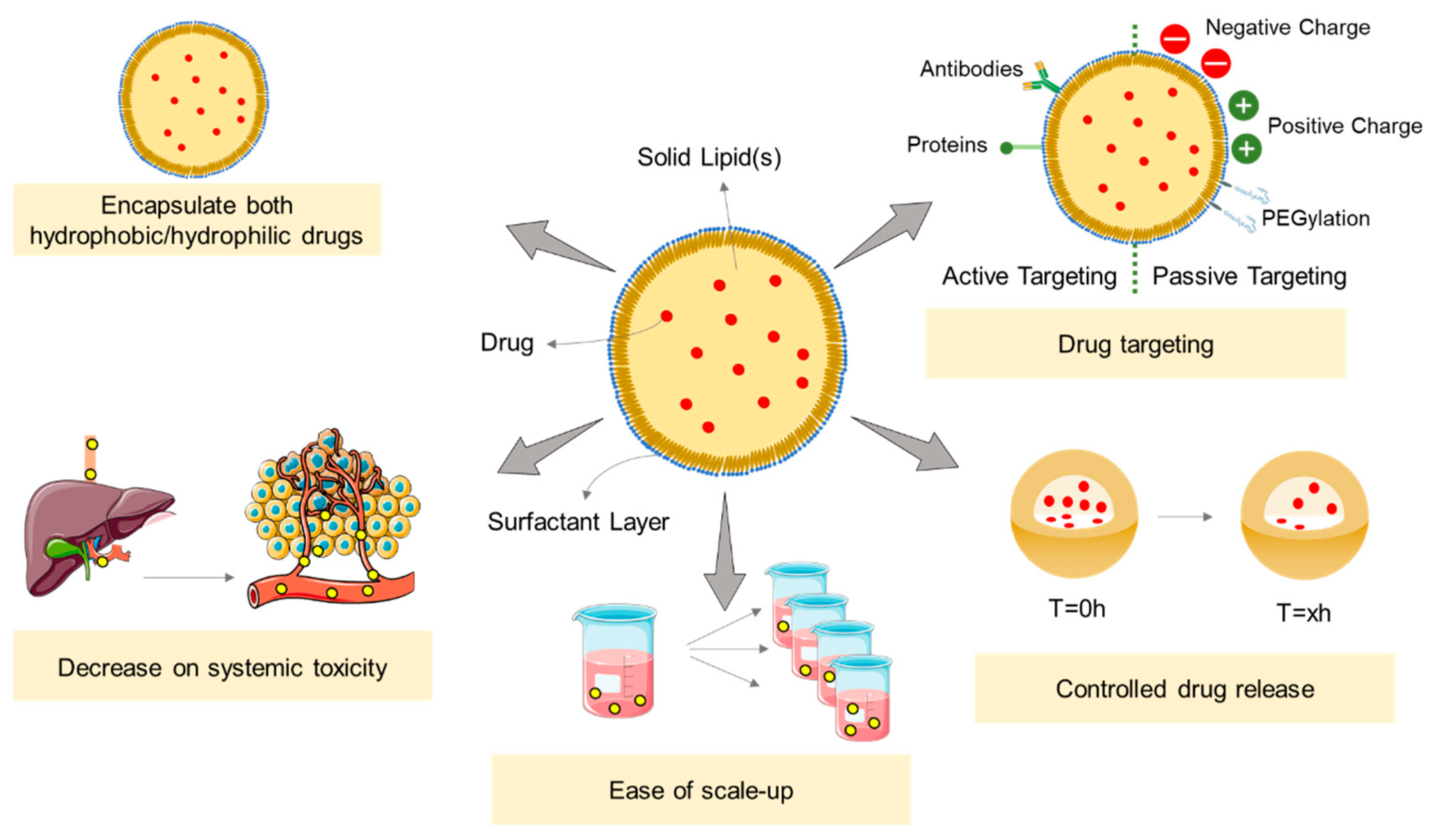
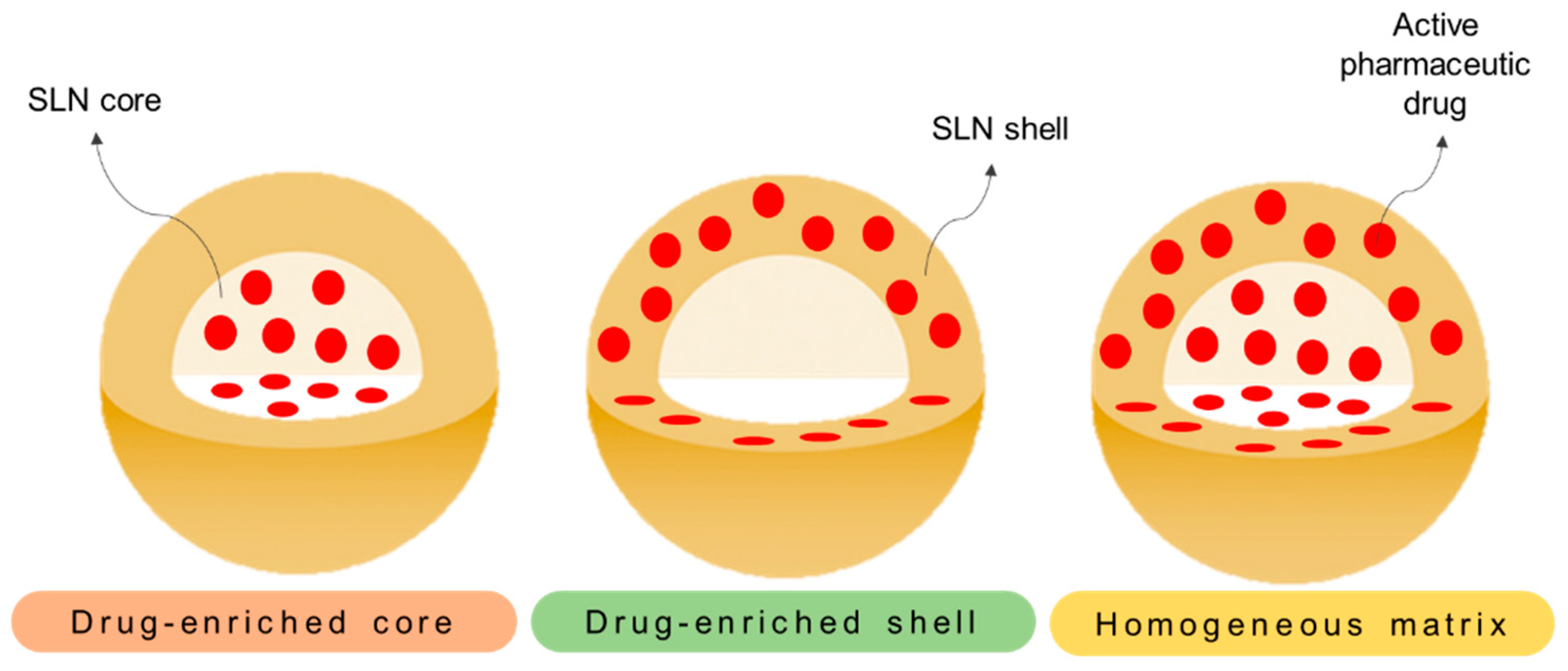
3. Solid Lipid Nanoparticles
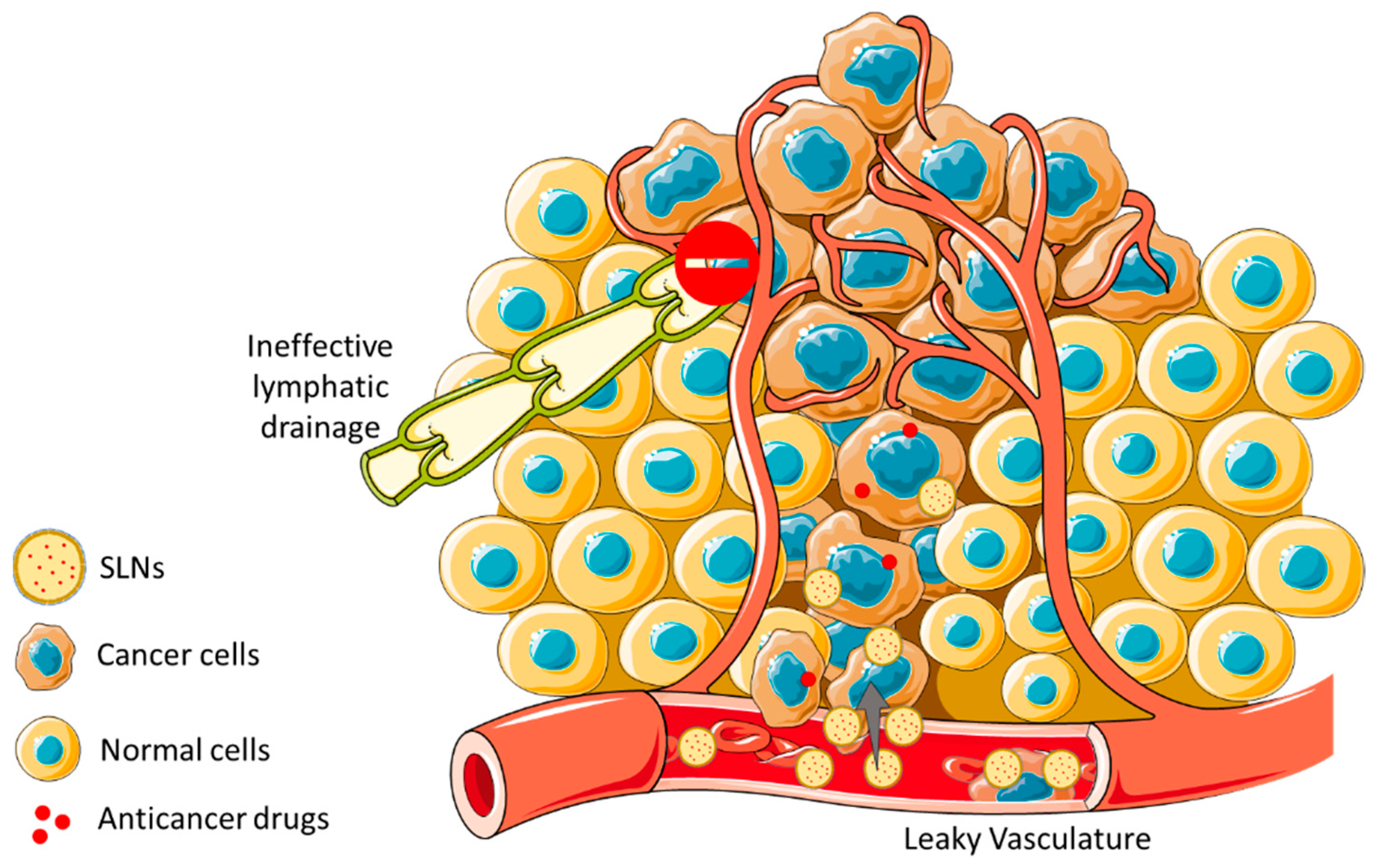
3.1. SLNs as Drug Delivery Systems
3.2. Solid Lipid Nanoparticles in Cancer Treatment
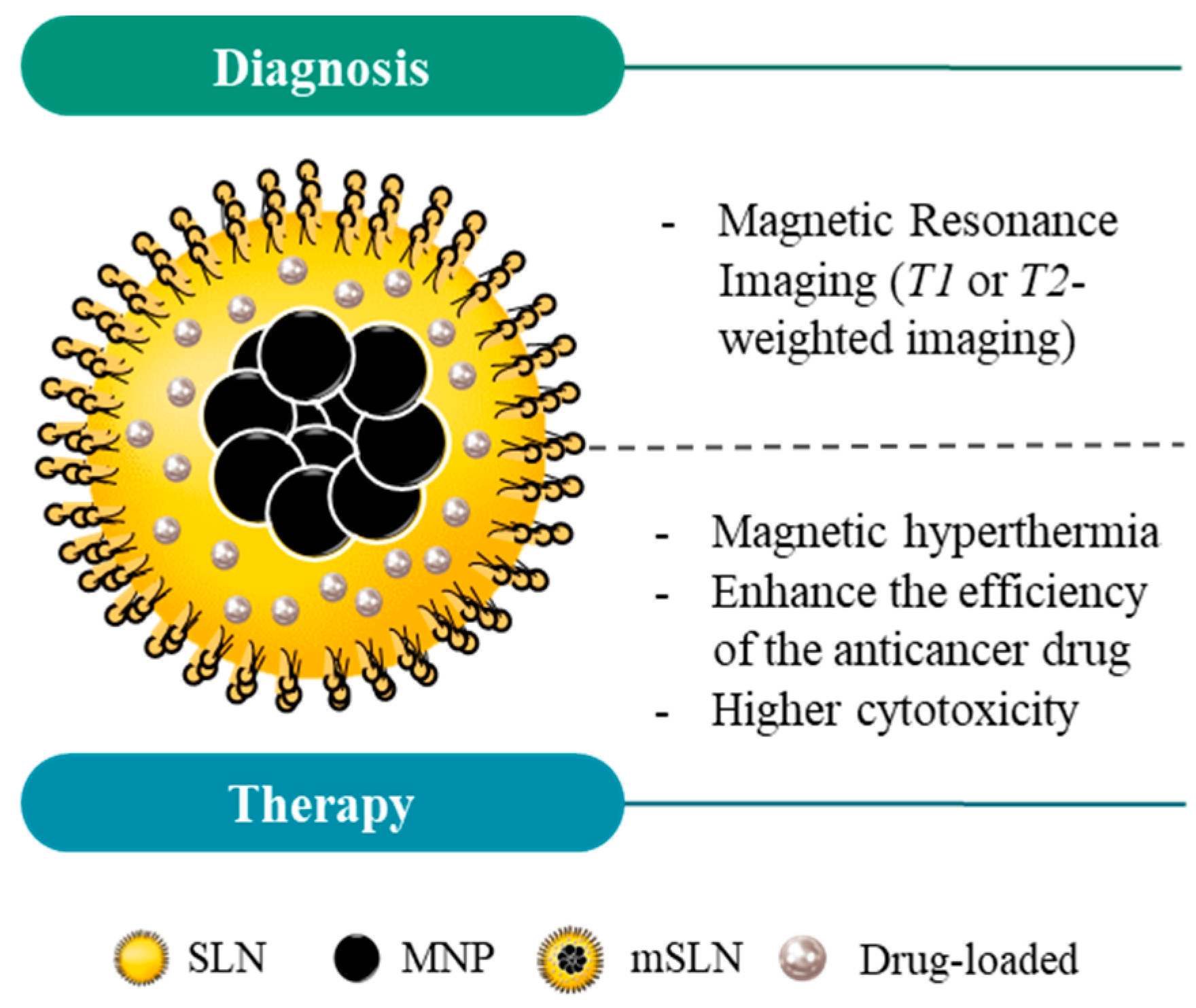
4. Magnetic Solid Lipid Nanoparticles
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global cancer data. Int. Agency Res. Cancer 2018, 263, 1–3. [Google Scholar]
- Palumbo, M.O.; Kavan, P.; Miller, W.H.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol. 2013, 4, 57. [Google Scholar] [CrossRef]
- Lee, J.J.; Saiful Yazan, L.; Che Abdullah, C.A. A review on current nanomaterials and their drug conjugate for targeted breast cancer treatment. Int. J. Nanomed. 2017, 12, 2373–2384. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Hile, E.S.; Fitzgerald, G.K.; Studenski, S.A. Persistent Mobility Disability after Neurotoxic Chemotherapy. Phys. Ther. 2010, 90, 1649–1657. [Google Scholar] [CrossRef]
- Aleman, B.M.P.; van den Belt-Dusebout, A.W.; Bruin, M.L.d.; van’t Veer, M.B.; Baaijens, M.H.A.; de Boer, J.P.; Hart, A.A.M.; Klokman, W.J.; Kuenen, M.A.; Ouwens, G.M.; et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007, 109, 1878–1886. [Google Scholar] [CrossRef]
- Grigorian, A.; O’Brien, C.B. Hepatotoxicity Secondary to Chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95–102. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to Overcome Conventional Cancer Chemotherapy Limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.J.; Leonessa, F.; Clarke, R. Multidrug Resistance in Breast Cancer: A Meta-analysis of MDR1/gp170 Expression and Its Possible Functional Significance. J. Natl. Cancer Inst. 1997, 89, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Żuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.-A.; Bouziotis, P.; Bilewicz, A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef]
- Lippacher, A.; Müller, R.; Mäder, K. Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles. Int. J. Pharm. 2001, 214, 9–12. [Google Scholar] [CrossRef]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95–99. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Abnous, K.; Mohammadi, M.; Hadizadeh, F.; Sadeghi, F.; Taghavi, S.; Jaafari, M.R.; Ramezani, M. Extensive preclinical investigation of polymersomal formulation of doxorubicin versus Doxil-mimic formulation. J. Control. Release 2017, 264, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Tietze, R.; Alexiou, C. Improving cancer imaging with magnetic nanoparticles: Where are we now? Nanomedicine 2017, 12, 167–170. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef]
- Lima-Tenório, M.K.; Pineda, E.A.G.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine. Biomedicines 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hernández, Y.; Cabal, C.; González, E.; Veintemillas-Verdaguer, S.; Martínez, E.; Morales, M.P. Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 2013, 5, 11400–11408. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Liang, L.; Veiseh, O. Recent Advancements of Magnetic Nanomaterials in Cancer Therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Muller, H.R.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef] [PubMed]
- Sanson, C.; Diou, O.; Thévenot, J.; Ibarboure, E.; Soum, A.; Brûlet, A.; Miraux, S.; Thiaudière, E.; Tan, S.; Brisson, A.; et al. Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano 2011, 5, 1122–1140. [Google Scholar] [CrossRef] [PubMed]
- Furlani, E.P. Magnetic Biotransport: Analysis and Applications. Materials 2010, 3, 2412. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef]
- Alavijeh, A.A.; Barati, M.; Barati, M.; Dehkordi, H.A. The Potential of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer Based on Body Magnetic Field and Organ-on-the-Chip. Adv. Pharm. Bull. 2019, 9, 360–373. [Google Scholar] [CrossRef]
- Sun, T.M.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.X.; Xia, Y.N. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew Chemie-Internationa. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Somoza, Á. Multifunctionalization of magnetic nanoparticles for controlled drug release: A general approach. Eur. J. Med. Chem. 2014, 82, 355–362. [Google Scholar] [CrossRef]
- Cheng, M.; Ma, D.; Zhi, K.; Liu, B.; Zhu, W. Synthesis of Biotin-Modified Galactosylated Chitosan Nanoparticles and Their Characteristics in Vitro and in Vivo. Cell. Physiol. Biochem. 2018, 50, 569–584. [Google Scholar] [CrossRef]
- Price, D.N.; Stromberg, L.; Kunda, N.K.; Muttil, P. In Vivo Pulmonary Delivery and Magnetic-Targeting of Dry Powder Nano-in-Microparticles. Mol. Pharm. 2017, 14, 4741–4750. [Google Scholar] [CrossRef]
- Khalid, M.K.; Asad, M.; Henrich-Noack, P.; Sokolov, M.; Hintz, W.; Grigartzik, L.; Zhang, E.; Dityatev, A.; Van Wachem, B.; Sabel, B.A. Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2613. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Weissleder, R. In Vivo Imaging in Cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Li, Z.; Hsu, C.-H.; Hwang, L.-P.; Lin, Y.-C.; Chou, P.-T.; Lin, Y.-Y. Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics 2018, 8, 6322–6349. [Google Scholar] [CrossRef] [PubMed]
- Guldris, N.; Argibay, B.; Kolen’Ko, Y.V.; Carbó-Argibay, E.; Sobrino, T.; Campos, F.; Salonen, L.M.; Bañobre-López, M.; Castillo, J.; Rivas, J. Influence of the separation procedure on the properties of magnetic nanoparticles: Gaining in vitro stability and T1–T2 magnetic resonance imaging performance. J. Colloid Interface Sci. 2016, 472, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Guldris, N.; Gallo, J.; García-Hevia, L.; Rivas, J.; Bañobre-López, M.; Salonen, L.M. Orthogonal Clickable Iron Oxide Nanoparticle Platform for Targeting, Imaging, and On-Demand Release. Chem. Eur. J. 2018, 24, 8624–8631. [Google Scholar] [CrossRef]
- Guldris, N.; Argibay, B.; Gallo, J.; Iglesias-Rey, R.; Carbó-Argibay, E.; Kolen’Ko, Y.V.; Campos, F.; Sobrino, T.; Salonen, L.M.; Bañobre-López, M.; et al. Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term in Vivo Tracking. Bioconjugate Chem. 2017, 28, 362–370. [Google Scholar] [CrossRef]
- Keasberry, N.A.; Bañobre-López, M.; Wood, C.; Stasiuk, G.J.; Gallo, J.; Long, N.J. Tuning the relaxation rates of dual-mode T1/T2 nanoparticle contrast agents: A study into the ideal system. Nanoscale 2015, 7, 16119–16128. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New Frontiers in Molecular Imaging with Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications. Nucl. Med. Mol. Imaging 2020, 54, 65–80. [Google Scholar] [CrossRef]
- García-Hevia, L.; Bañobre-López, M.; Gallo, J. Recent Progress on Manganese-Based Nanostructures as Responsive MRI Contrast Agents. Chem. Eur. J. 2019, 25, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bañobre-López, M.; Garcia-Hevia, L.; Cerqueira, M.F.; Rivadulla, F.; Gallo, J. Tunable Performance of Manganese Oxide Nanostructures as MRI Contrast Agents. Chem. Eur. J. 2018, 24, 1295–1303. [Google Scholar] [CrossRef]
- Tse, B.W.-C.; Cowin, G.J.; Soekmadji, C.; Jovanovic, L.; Vasireddy, R.S.; Ling, M.-T.; Khatri, A.; Liu, T.; Thierry, B.; Russell, P.J. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine 2015, 10, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Sarkar, S.; Saber, R.; Delavari, H.; Alizadeh, A.M.; Mulder, H.T. Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer-coated iron-oxide nanoparticles. Cancer Nanotechnol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Gallo, J.; Vasimalai, N.; Fernandez-Arguelles, M.T.; Bañobre-López, M. Green synthesis of multimodal ‘OFF–ON’ activatable MRI/optical probes. Dalton Trans. 2016, 45, 17672–17680. [Google Scholar] [CrossRef]
- Westermark, E. A case of ureteral implantation into the bladder. J. Obstet. Women’s Dis. 1895, 9, 677–678. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrot, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Wasserman, D.D.; Healy, M. Cooling Techniques for Hyperthermia; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.-X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Müller, R.H. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Sanz, B.; Calatayud, M.P.; Torres, T.E.; Fanarraga, M.L.; Ibarra, M.R.; Goya, G.F. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials 2017, 114, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Plassat, V.; Wilhelm, C.; Marsaud, V.; Ménager, C.; Gazeau, F.; Renoir, J.-M.; Lesieur, S. Anti-Estrogen-Loaded Superparamagnetic Liposomes for Intracellular Magnetic Targeting and Treatment of Breast Cancer Tumors. Adv. Funct. Mater. 2010, 21, 83–92. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Rego, G.N.D.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Da Silva, H.R.; Gamarra, L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein 2019, 17, eAO4786. [Google Scholar] [CrossRef]
- Brezovich, I.A. Low frequency hyperthermia: Capacitive and ferromagnetic thermoseed methods. Med. Phys. Monogr. 1988, 16, 82–111. [Google Scholar]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Espiña, B.; Kolen’Ko, Y.V.; Bañobre-López, M.; Brito, M.; Martins, V.; Duarte, J.A.; Petrovykh, D.Y.; Freitas, P.; Carvalho, F. Effectiveness and Safety of a Nontargeted Boost for a CXCR4-Targeted Magnetic Hyperthermia Treatment of Cancer Cells. ACS Omega 2019, 4, 1931–1940. [Google Scholar] [CrossRef]
- Sanhaji, M.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Prina-Mello, A.; Stapf, M.; Ludwig, R.; Volkov, Y.; et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101983. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field. Int. J. Nanomed. 2019, 14, 7549–7560. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015; ISBN 9781469894553. [Google Scholar]
- Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment. Molecules 2016, 21, 1715. [Google Scholar] [CrossRef]
- Belanova, A.A.; Gavalas, N.; Makarenko, Y.M.; Belousova, M.M.; Soldatov, A.V.; Zolotukhin, P.V. Physicochemical Properties of Magnetic Nanoparticles: Implications for Biomedical Applications In Vitro and In Vivo. Oncol. Res. Treat. 2018, 41, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.T.; Karim, E.M.; Abidin, S.A.Z.; Othman, I.; Holl, M.M.B.; Chowdhury, E.H. Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells. Nanomaterials 2020, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Bianco, J.; Danhier, P.; Zhao, M.; Bastiancich, C.; Gallez, B.; Danhier, F.; Préat, V. Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int. J. Nanomed. 2018, 13, 4509–4521. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yao, J.; Zhong, Y.; Li, Y.; Lu, Q.; Zhang, Y.; Tian, X.; Guo, Z.; Bai, T. Preparation and Characterization of Fe3O4@MTX Magnetic Nanoparticles for Thermochemotherapy of Primary Central Nervous System Lymphoma in vitro and in vivo. Int. J. Nanomed. 2019, 14, 9647–9663. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Belyanina, I.; Kolovskaya, O.; Zamay, S.; Gargaun, A.; Zamay, T.; Kichkailo, A. Targeted Magnetic Nanotheranostics of Cancer. Molecules 2017, 22, 975. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, G.; Gu, X.; Wang, J.; Wang, Y.; Gao, H.; Ma, J. Magnetic and pH-sensitive nanoparticles for antitumor drug delivery. Colloids Surf. B Biointerfaces 2013, 103, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef]
- Christodoulou, E.; Nerantzaki, M.; Nanaki, S.; Barmpalexis, P.; Giannousi, K.; Dendrinou-Samara, C.; Angelakeris, M.; Gounari, E.; Anastasiou, A.D.; Bikiaris, D.N. Paclitaxel Magnetic Core⁻Shell Nanoparticles Based on Poly(lactic acid) Semitelechelic Novel Block Copolymers for Combined Hyperthermia and Chemotherapy Treatment of Cancer. Pharmaceutics 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Khafaji, M.; Zamani, M.; Vossoughi, M.; Zad, A.I. Doxorubicin/Cisplatin-Loaded Superparamagnetic Nanoparticles as A Stimuli-Responsive Co-Delivery System For Chemo-Photothermal Therapy. Int. J. Nanomed. 2019, 14, 8769–8786. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-S.; Chen, L.; Xiong, Y.-Q.; Xu, J.; Wang, J.-P.; Meng, Z.-L. Iron oxide magnetic nanoparticles combined with actein suppress non-small-cell lung cancer growth in a p53-dependent manner. Int. J. Nanomed. 2017, 12, 7627–7651. [Google Scholar] [CrossRef] [PubMed]
- Stapf, M.; Teichgräber, U.; Hilger, I. Methotrexate-coupled nanoparticles and magnetic nanochemothermia for the relapse-free treatment of T24 bladder tumors. Int. J. Nanomed. 2017, 12, 2793–2811. [Google Scholar] [CrossRef]
- Abedi, M.; Abolmaali, S.S.; Abedanzadeh, M.; Farjadian, F.; Samani, S.M.; Tamaddon, A.M. Core–Shell Imidazoline–Functionalized Mesoporous Silica Superparamagnetic Hybrid Nanoparticles as a Potential Theranostic Agent for Controlled Delivery of Platinum(II) Compound. Int. J. Nanomed. 2020, 15, 2617–2631. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Zhang, C.; Fan, Y.; Li, D.; Cao, X.; Xia, J.; Shi, X.; Guo, R. LDH-stabilized ultrasmall iron oxide nanoparticles as a platform for hyaluronidase-promoted MR imaging and chemotherapy of tumors. Theranostics 2020, 10, 2791–2802. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Sun, W.; Fan, Y.; Yin, F.; Van Hest, J.C.M.; Wang, H.; Du, L.; et al. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics 2020, 10, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yuan, Z.; Shou, C.; Fan, G.; Wang, H.; Gao, F.; Rui, Y.; Xu, K.; Yin, P. cRGD-Conjugated Fe3O4@PDA-DOX Multifunctional Nanocomposites for MRI and Antitumor Chemo-Photothermal Therapy. Int. J. Nanomed. 2019, 14, 9631–9645. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Qian, W.; Wang, L.; Wu, H.; Zhou, H.; Wang, A.Y.; Yang, L.; Chen, H.; Huang, J. Functionalized milk-protein-coated magnetic nanoparticles for MRI-monitored targeted therapy of pancreatic cancer. Int. J. Nanomed. 2016, 11, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mei, C.; He, L.; Xiao, Z.; Chan, L.; Zhang, D.; Shi, C.; Chen, T.; Luo, L. Designing multifunctional cancer-targeted nanosystem for magnetic resonance molecular imaging-guided theranostics of lung cancer. Drug Deliv. 2018, 25, 1811–1825. [Google Scholar] [CrossRef]
- Ali, A.; Hsu, F.-T.; Hsieh, C.-L.; Shiau, C.-Y.; Chiang, C.-H.; Wei, Z.-H.; Chen, C.-Y.; Huang, H.-S. Erlotinib-Conjugated Iron Oxide Nanoparticles as a Smart Cancer-Targeted Theranostic Probe for MRI. Sci. Rep. 2016, 6, 36650. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef]
- Müller, R.H.; Maassen, S.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) as potential carrier for human use: Interaction with human granulocytes. J. Control. Release 1997, 47, 261–269. [Google Scholar] [CrossRef]
- Almeida, A.J.; Runge, S.; Müller, R.H. Peptide-loaded solid lipid nanoparticles (SLN): Influence of production parameters. Int. J. Pharm. 1997, 149, 255–265. [Google Scholar] [CrossRef]
- Westesen, K.; Siekmann, B. Investigation of the gel formation of phospholipid-stabilized solid lipid nanoparticles. Int. J. Pharm. 1997, 151, 35–45. [Google Scholar] [CrossRef]
- Kumar, M.; Kakkar, V.; Mishra, A.K.; Chuttani, K.; Kaur, I.P. Intranasal delivery of streptomycin sulfate (STRS) loaded solid lipid nanoparticles to brain and blood. Int. J. Pharm. 2014, 461, 223–233. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.-L.; Jan, J.-S.; Tang, R.-D.; Wang, J.-Y.; Taliyan, R.; Yang, C.-H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 111–121. [Google Scholar] [CrossRef]
- Shegokar, R.; Singh, K.K.; Müller, R.H. Production & stability of stavudine solid lipid nanoparticles—From lab to industrial scale. Int. J. Pharm. 2011, 416, 461–470. [Google Scholar] [CrossRef]
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid Nanoparticles for Nasal/Intranasal Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J. Biomed. Nanotechnol. 2009, 5, 84–92. [Google Scholar] [CrossRef]
- Fang, Y.-P.; Chuang, C.-H.; Wu, P.-C.; Huang, Y.-B.; Tzeng, C.-C.; Chen, Y.-L.; Gao, M.-Y.; Tsai, M.-J.; Tsai, Y.-H. Amsacrine analog-loaded solid lipid nanoparticle to resolve insolubility for injection delivery: Characterization and pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 1019–1028. [Google Scholar] [CrossRef]
- Reddy, L.H.; Sharma, R.K.; Chuttani, K.; Mishra, A.K.; Murthy, R.S.R. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J. Control. Release 2005, 105, 185–198. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Abass, H.A.; Attia, M.A.; Heikal, O.A. Formulation and evaluation of metoclopramide solid lipid nanoparticles for rectal suppository. J. Pharm. Pharmacol. 2013, 65, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.K.; Dhandayuthabani, R.; Muzammil, M.S.; Kumari, V.S. Solid lipid nanoparticles for oral drug delivery. Proc. Mater. Today 2019, 36, 313–324. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Moon, J.H.; Moxley, J.W., Jr.; Zhang, P.; Cui, H. Nanoparticle approaches to combating drug resistance. Futur. Med. Chem. 2015, 7, 1503–1510. [Google Scholar] [CrossRef]
- Brazel, C.S.; Huang, X. The Cost of Optimal Drug Delivery: Reducing and Preventing the Burst Effect in Matrix Systems. In Carrier-Based Drug Delivery; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 879, pp. 267–282. [Google Scholar]
- Arduino, I.; Depalo, N.; Re, F.; Magro, R.D.; Panniello, A.; Margiotta, N.; Fanizza, E.; Lopalco, A.; Laquintana, V.; Cutrignelli, A.; et al. PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: An in vitro study. Int. J. Pharm. 2020, 583, 119351. [Google Scholar] [CrossRef]
- Dhiman, S.; Mishra, N.; Sharma, S. Development of PEGylated solid lipid nanoparticles of pentoxifylline for their beneficial pharmacological potential in pathological cardiac hypertrophy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1901–1908. [Google Scholar] [CrossRef][Green Version]
- Stylianopoulos, T. EPR-effect: Utilizing size-dependent nanoparticle delivery to solid tumors. Ther. Deliv. 2013, 4, 421–423. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Liu, Y.; Sun, D.; Fan, Q.; Ma, Q.; Dong, Z.; Tao, W.; Tao, H.; Liu, Z.; Wang, C. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020, 13, 564–569. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In Cancer Nanotechnology; Humana Press: New York, NY, USA, 2010; Volume 624, pp. 25–37. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Cordero, L.B.; Alkorta, I.; Arana, L.; Cordero, L.B.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Natfji, A.A.; Ravishankar, D.; Osborn, H.M.I.; Greco, F. Parameters Affecting the Enhanced Permeability and Retention Effect: The Need for Patient Selection. J. Pharm. Sci. 2017, 106, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Campos, J.; Varas-Godoy, M.; Haidar, Z.S. Physicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: A proof-of-concept study in breast cancer cells. Nanomedicine 2017, 12, 473–490. [Google Scholar] [CrossRef]
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surf. B Biointerfaces 2016, 146, 114–126. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef]
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur. J. Pharm. Biopharm. 2018, 132, 168–179. [Google Scholar] [CrossRef]
- Dudhipala, N.; Puchchakayala, G. Capecitabine lipid nanoparticles for anti-colon cancer activity in 1,2-dimethylhydrazine-induced colon cancer: Preparation, cytotoxic, pharmacokinetic, and pathological evaluation. Drug Dev. Ind. Pharm. 2018, 44, 1572–1582. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109006. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bae, E.J.; Lee, M.-K. Enhanced anticancer activity and intracellular uptake of paclitaxel-containing solid lipid nanoparticles in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2018, 13, 7549–7563. [Google Scholar] [CrossRef] [PubMed]
- Eskiler, G.G.; Cecener, G.; Dikmen, G.; Egeli, U.; Tunca, B. Solid lipid nanoparticles: Reversal of tamoxifen resistance in breast cancer. Eur. J. Pharm. Sci. 2018, 120, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Siram, K.; Liu, X.; Khandelwal, V.K.M.; Shen, X.; Wang, X.; Franklin, G. Solid Lipid Nanoparticles of Albendazole for Enhancing Cellular Uptake and Cytotoxicity against U-87 MG Glioma Cell Lines. Molecules 2017, 22, 2040. [Google Scholar] [CrossRef]
- Da Rocha, M.C.O.; Da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; De Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Valdivia, L.; García-Hevia, L.; Bañobre-López, M.; Gallo, J.; Valiente, R.; Fanarraga, M.L. Solid Lipid Particles for Lung Metastasis Treatment. Pharmaceutics 2021, 13, 93. [Google Scholar] [CrossRef]
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18. [Google Scholar] [CrossRef]
- Battaglia, L.; Muntoni, E.; Chirio, D.; Peira, E.; Annovazzi, L.; Schiffer, D.; Mellai, M.; Riganti, C.; Salaroglio, I.C.; Lanotte, M.; et al. Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: Preliminary study for glioma treatment. Nanomedicine 2017, 12, 639–656. [Google Scholar] [CrossRef]
- Liu, B.; Han, L.; Liu, J.; Han, S.; Chen, Z.; Jiang, L. Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int. J. Nanomed. 2017, 12, 955–968. [Google Scholar] [CrossRef]
- Thakkar, A.; Chenreddy, S.; Wang, J.; Prabhu, S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef]
- Jain, V.; Gupta, A.; Pawar, V.K.; Asthana, S.; Jaiswal, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Assisted Immunotherapy for Intervention of Experimental Leishmaniasis via Amphotericin B-Loaded Solid Lipid Nanoparticles. Appl. Biochem. Biotechnol. 2014, 174, 1309–1330. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Jin, J.; Bae, K.H.; Yang, H.; Lee, S.J.; Kim, H.; Kim, Y.; Joo, K.M.; Seo, S.W.; Park, T.G.; Nam, D.-H. In Vivo Specific Delivery of c-Met siRNA to Glioblastoma Using Cationic Solid Lipid Nanoparticles. Bioconjugate Chem. 2011, 22, 2568–2572. [Google Scholar] [CrossRef]
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hémadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.-E.; Kauss, T.; Gaudin, K.; et al. Solid Lipid Nanoparticles for Image-Guided Therapy of Atherosclerosis. Bioconjugate Chem. 2016, 27, 569–575. [Google Scholar] [CrossRef]
- De Escalona, M.M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic solid lipid nanoparticles in hyperthermia against colon cancer. Int. J. Pharm. 2016, 504, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, E.; Bongio, C.; Sacchetti, F.; Maretti, E.; Montanari, M.; Iannuccelli, V.; Vismara, E.; Leo, E. Self-Assembled Lipid Nanoparticles for Oral Delivery of Heparin-Coated Iron Oxide Nanoparticles for Theranostic Purposes. Molecules 2017, 22, 963. [Google Scholar] [CrossRef] [PubMed]
- Świętek, M.; Brož, A.; Tarasiuk, J.; Wroński, S.; Tokarz, W.; Kozieł, A.; Błażewicz, M.; Bačáková, L. Carbon nanotube/iron oxide hybrid particles and their PCL-based 3D composites for potential bone regeneration. Mater. Sci. Eng. C 2019, 104, 109913. [Google Scholar] [CrossRef] [PubMed]
- Świętek, M.; Panchuk, R.; Skorokhyd, N.; Černoch, P.; Finiuk, N.; Klyuchivska, O.; Hrubý, M.; Molčan, M.; Berger, W.; Trousil, J.; et al. Magnetic Temperature-Sensitive Solid-Lipid Particles for Targeting and Killing Tumor Cells. Front. Chem. 2020, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Cox, A.G.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Wydra, R.J.; Oliver, C.E.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. Accelerated generation of free radicals by iron oxide nanoparticles in the presence of an alternating magnetic field. RSC Adv. 2015, 5, 18888–18893. [Google Scholar] [CrossRef] [PubMed]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Su, Y.-C. Iron-oxide embedded solid lipid nanoparticles for magnetically controlled heating and drug delivery. Biomed. Microdevices 2008, 10, 785–793. [Google Scholar] [CrossRef]
- Igartua, M.; Saulnier, P.; Heurtault, B.; Pech, B.; Proust, J.E.; Pedraz, J.L.; Benoit, J.P. Development and characterization of solid lipid nanoparticles loaded with magnetite. Int. J. Pharm. 2001, 233, 149–157. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef]
- Müller, R.H.; Maaβen, S.; Weyhers, H.; Specht, F.; Lucks, J.S. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles. Int. J. Pharm. 1996, 138, 85–94. [Google Scholar] [CrossRef]
- García-Hevia, L.; Casafont, Í.; Oliveira, J.; Terán, N.; Fanarraga, M.L.; Gallo, J.; Bañobre-López, M. Magnetic lipid nanovehicles synergize the controlled thermal release of chemotherapeutics with magnetic ablation while enabling non-invasive monitoring by MRI for melanoma theranostics. Bioact. Mater. 2022, 8, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Babincová, M.; Čičmanec, P.; Altanerová, V.; Altaner, C.; Babinec, P. AC-magnetic field controlled drug release from magnetoliposomes: Design of a method for site-specific chemotherapy. Bioelectrochemistry 2002, 55, 17–19. [Google Scholar] [CrossRef]
- Pang, X.; Cui, F.; Tian, J.; Chen, J.; Zhou, J.; Zhou, W. Preparation and characterization of magnetic solid lipid nanoparticles loaded with ibuprofen. Asian J. Pharm. Sci. 2009, 4, 132–137. [Google Scholar]
- Oliveira, R.R.; Carrião, M.; Pacheco, M.T.; Branquinho, L.C.; de Souza, A.L.R.; Bakuzis, A.; Lima, E.M. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia. Mater. Sci. Eng. C 2018, 92, 547–553. [Google Scholar] [CrossRef]
- Ahmadifard, Z.; Ahmeda, A.; Rasekhian, M.; Moradi, S.; Arkan, E. Chitosan-coated magnetic solid lipid nanoparticles for controlled release of letrozole. J. Drug Deliv. Sci. Technol. 2020, 57, 101621. [Google Scholar] [CrossRef]
- Grillone, A.; Battaglini, M.; Moscato, S.; Mattii, L.; Fernández, C.D.J.; Scarpellini, A.; Giorgi, M.; Sinibaldi, E.; Ciofani, G. Nutlin-loaded magnetic solid lipid nanoparticles for targeted glioblastoma treatment. Nanomedicine 2019, 14, 727–752. [Google Scholar] [CrossRef]
- Andreozzi, E.; Wang, P.; Valenzuela, A.; Tu, C.; Gorin, F.; Dhenain, M.; Louie, A. Size-Stable Solid Lipid Nanoparticles Loaded with Gd-DOTA for Magnetic Resonance Imaging. Bioconjugate Chem. 2013, 24, 1455–1467. [Google Scholar] [CrossRef]
- Abidi, H.; Ghaedi, M.; Rafiei, A.; Jelowdar, A.; Salimi, A.; Asfaram, A.; Ostovan, A. Magnetic solid lipid nanoparticles co-loaded with albendazole as an anti-parasitic drug: Sonochemical preparation, characterization, and in vitro drug release. J. Mol. Liq. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Ghiani, S.; Capozza, M.; Cabella, C.; Coppo, A.; Miragoli, L.; Brioschi, C.; Bonafè, R.; Maiocchi, A. In vivo tumor targeting and biodistribution evaluation of paramagnetic solid lipid nanoparticles for magnetic resonance imaging. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 693–700. [Google Scholar] [CrossRef]
- Rocha, C.V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. (Para)magnetic hybrid nanocomposites for dual MRI detection and treatment of solid tumours. Chem. Commun. 2020, 56, 8695–8698. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, J.; García-Hevia, L.; Melguizo, C.; Prados, J.; Bañobre-López, M.; Gallo, J. Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents. Pharmaceutics 2020, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- De Moura, C.L.; Gallo, J.; García-Hevia, L.; Pessoa, O.D.L.; Ricardo, N.M.P.S.; López, M.B. Magnetic Hybrid Wax Nanocomposites as Externally Controlled Theranostic Vehicles: High MRI Enhancement and Synergistic Magnetically Assisted Thermo/Chemo Therapy. Chem. Eur. J. 2020, 26, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Grillone, A.; Riva, E.R.; Mondini, A.; Forte, C.; Calucci, L.; Innocenti, C.; Fernandez, C.D.J.; Cappello, V.; Gemmi, M.; Moscato, S.; et al. Active Targeting of Sorafenib: Preparation, Characterization, and In Vitro Testing of Drug-Loaded Magnetic Solid Lipid Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1681–1690. [Google Scholar] [CrossRef]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; Fernández, C.D.J.; Prato, M.; Drago, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2019, 11, 72–88. [Google Scholar] [CrossRef]
- Grillone, A.; Riva, E.R.; Moscato, S.; Sacco, R.; Mattoli, V.; Ciofani, G. Targeted delivery of anti-cancer drug sorafenib through magnetic solid lipid nanoparticles. In 10th Annual TechConnect World Innovation Conference and Expo, Held Jointly with the 18th Annual Nanotech Conference and Expo, and the 2015 National SBIR/STTR Conference; Taylor and Francis Inc.: Oxfordshire, UK, 2015. [Google Scholar]

| MNP (Particle Size) + Surface Modification | Treatment + Cancer Model | Results | Ref |
|---|---|---|---|
| SPIONs (250 nm) were coated with targeted CXCR4. | Treatment: 869 kHz and 20 kA·m−1 for the first 30 min of the experiment, followed by another 30 min at 554 kHz, and 24 kA·m−1. Cancer model: glioblastoma (LN229) and normal kidney cells (HK-2). | In vitro, the targeted treatment conjugated with MH strategy showed a lethal outcome of, approximately, 100% for LN229 cancer cells after 72 h of treatment. The safety profile of NPs was confirmed by the minimal cytotoxicity observed in control group (JK cells—HK-2 cell line). | [82] |
| IONPs (not specified) were coated with DMSA and conjugated with Gem and the pseudo-peptide NucAnt (N6L). | Treatment: H = 15.4 kA m−1; f = 435 kHz. Cancer model: pancreatic cancer model (BxPC-3 and PANC-1 cancer cell lines). Athymic nude mice were subcutaneously injected with 2 × 106 BxPC-3 cells. | Combined chemotherapy and treatment with NPs-based MH showed increased cytotoxicity and cell death in vitro (~90% of viable cells compared to approximately 10% when no MH was applied). In vivo, Gem MNPs and the hyperthermia therapy managed to cause an almost complete tumor remission in mice xenografts (at day 28) when compared to the groups receiving only the mono-modal MNP therapy or just the hyperthermia. | [83] |
| IONPs (46 nm). Fe3O4@Au MNPs were prepared and loaded with C225. | Treatment: I = 30 A; f = 230 kHz. Cancer model: glioblastoma cancer model (U251 cancer cell line). Male and female Balb/c nu/nu nude mice were subcutaneously injected with 2 × 106 U251 cells. | The combined triple therapy decreased, in vitro, cell viability with a high rate of apoptosis via caspase-3, caspase-8, and caspase-9 expression upregulation. In vivo, a significant tumor growth inhibition (approximately 95% of tumor remission) was measured compared to the control groups. | [84] |
| SPIONs (100 nm) were modified with anti-CD44 antibody. | Treatment: I = 50 A; f = 237 kHz. Cancer model: head and neck squamous cell carcinoma stem cells model (Cal-27 cancer cell line). Male Balb/c nude mice were subcutaneously injected with 5 × 107 Cal-27 cells. | CD44-SPIONPs exhibited good biocompatibility and a programmed cell death in cancer stem cells after an AMF application. In vivo, 33.43% of tumor growth inhibition was observed on the treated group. | [85] |
| 225Ac SPIONs (10 nm) were attached the attachment of CEPA and transtuzumab to the surface. | Treatment: magnetic flux density from 100 to 300 G and frequency range of 386–633 kHz. Cancer model: ovarian cancer model (SKOV-3 cancer cell line). | 225Ac@Fe3O4-CEPA-trastuzumab showed a high cytotoxic effect towards SKOV-3 ovarian cancer cells expressing the HER2 receptor, in vitro. | [17] |
| MNP (Particle Size) + Surface Modification | Treatment + Cancer Model | Results | Ref |
|---|---|---|---|
| SPIONs (12 nm). SPIONs were coated with a DMSA, MF66, and covalently functionalized with (i) DOX (MF66-DOX), (ii) pseudopeptide NuCant (MF66-N6L), and (iii) with both (MF66-DOX-N6L). | Treatment: DOX + AMF (H = 15.4 kA/m; f = 435 kHz). Cancer model: breast cancer model (BT474 cell line). Female athymic nude mice were subcutaneously injected (on rear backside) with 2.0 × 106 BT474 cells. | The thermo-chemotherapeutic treatment favors the tumor regression in 50% comparatively to control group in vivo (between day 6 and day 17). MF66-DOX-N6L plus hyperthermia application increased their internalization in cancer cells and enhanced in 90% the cytotoxic effect in vitro, comparatively to control group. | [95] |
| IONPs (112 nm). MnFe2O4 MNPs were synthesized and were encapsulated in PTX loaded thioether-containing ω-hydroxyacid-co-poly(d,l-lactic acid) (TEHA-co-PDLLA). | Treatment: PTX + AMF (25 mT; f = 765 kHz). Cancer model: colorectal cancer model (Caco-2 cell line) + human mesenchymal stem cells derived from adipose tissue. | In vitro experiments showed that NPs were able to sustain PTX release for up 18 days. Moreover, NPs showed great anticancer activity in a dose-dependent manner with low toxicity toward the primary human stem cells derived from adipose tissue. | [96] |
| IONPs (122 nm). IONPs were modified with a layer of di-carboxylate polyethylene glycol and carboxylate-methoxy polyethylene glycol. Then, IONPs were coated with silica, obtaining PEGylated silica-coated IONs (PS-IONs). | Treatment: DOX + CDDP. Cancer model: breast cancer model (MCF7 cell line); mouse fibroblast cell line (L929). | NPs showed a dual stimuli-triggered release behavior. A release rate of 69% and 84%, for DOX and CDDP, respectively, was measured during the first 30 h in an acidic environment under photothermal conditions. PS-IONs demonstrated potent antitumor activity in vitro, which was significatively enhanced when exposed to low-power near-IR laser irradiation. | [97] |
| IONPs (non-mentioned). Surface modification is not mentioned. | Treatment: ferumoxytol. Cancer model: mouse mammary tumor virus—polyoma middle T antigen—MMTV-PyMT; MDA-MB-468). Human fibrosarcoma cells (HT1080); murine macrophages (RAW264.7); human dermal fibroblasts (PCS-201-012); human umbilical vein endothelial cells (HUVECs). Female FVB/N were injected with 2.3 × 106 MMTV-PyMT cancer cells. | Ferumoxytil NPs caused tumor growth inhibition by increasing caspase-3 activity. Moreover, macrophages exposed to the NPs enhanced mRNA transcription associated with pro-inflammatory Th1-type responses. In vivo, IONs significantly inhibited the growth of subcutaneous adenocarcinomas compared to controls (tumor size reduction of 53% at day 21), as well as the development of liver metastasis. Additionally, NPs allowed its use as T2-weighted image for tumor imaging. | [98] |
| IONPs (20 nm). Surface modification is not mentioned. | Treatment: AT. Cancer model: lung cancer model (A549 and H1975) and human normal lung epithelial cells (BEAS2B); mouse normal liver cells (AML12); rat normal liver cells (BRL3A). Male athymic nude mice were subcutaneously injected with 5 × 105 A549 and H1975 into the dorsal flanks. | AT-MNPs demonstrated inhibition in cancer viability (less than 50% viable cells), whilst displaying no toxicity in vivo. AT-MNP treatment intensified the non-small-cell lung cancer apoptosis, activating the caspase-3 route and downregulating the anti-apoptotic proteins Bcl2 and BclXL, in addition to upregulating the proapoptotic Bax and Bad signals. | [99] |
| SPIONs (165 nm). Surface modification is not mentioned. | Treatment: MTX + AMF (H023.9 kA/m, f = 410 kHz). Cancer model: human bladder cancer cell line (T24). Male SCID (BALB/cJHanHsd-Prkdc) were subcutaneously injected with 2 × 106 T24 cancer cells dorsally between the hindlegs. | The results revealed that the relapse-free destruction of tumors was superior when the combination of chemotherapy and magnetic hyperthermia was used (13 days post-treatment versus 15 days post-treatment under monotherapy). The authors also observed an impairment of proapoptotic signaling, cell survival, and cell cycle pathways. | [100] |
| MNP (Particle Size) + Composition | Treatment + Cancer Model | Results | Ref |
|---|---|---|---|
| MnO2 NPs (107 nm) loaded with poly(N-vinylcaprolactam) nanogels (PVCL NGs) (DOX/MnO2@PVCL NG). | Treatment: DOX Cancer model: melanoma cancer model (B16 cancer cell line). In vivo: mouse model of subcutaneous B16 melanoma. | NPs showed interesting biocompatibility properties in addition to redox responsiveness in tumoral tissues. In an in vivo tumor model (with relatively high concentration of GSH), a release of Mn+2 from DOX/MnO2@PVCL NG occurred that enhanced T1-weighted MRI. In parallel, the DOX release from the NPs inhibited the tumor growth (1 versus 14 relative tumor growth for dual-treatment and control, respectively). | [103] |
| Fe3O4 IONPs (200–300 nm) were synthesized and functionalized with PDA, PEG, and cRGD (Fe3O4@PDA-PEG-cRGD). | Treatment: DOX + photothermal effect (1 W/cm2). Cancer model: colon cancer model (HCT-116 cancer cell line). Male nude mice were subcutaneously injected with HCT-116 cells (5 × 106/mL). | In vitro and in vivo, NPs were capable of targeting tumor cells and promoting the drug internalization. The cytotoxic effect was also significant (survival rate of 25.6% comparatively to control group) whilst the nanocarriers displayed good thermal stability and photothermal conversion efficiency, pH responsiveness, and an enhancement of T2-MRI contrast. In vivo, the authors observed a decrease in tumor growth around 67% when compared the dual-treatment with the control. | [104] |
| IONPs (26 nm) were coated with casein (CION) and functionalized with the tumor-targeting ATF of urokinase plasminogen activator and the antitumor drug CDDP (ATF-CNIO-CDDP). | Treatment: CDDP. Cancer model: pancreatic cancer model (MIA PaCa-2 cancer cell line). Female nu/nu mice were injected with 1 × 106 MIA PaCa-2 cells (orthotopic pancreatic tumor model). | NPs promote a T2-MRI contrast, combined with an improvement of therapeutic effectiveness (0.75 g versus 1.5 g of tumor weight for treated group and control, respectively) and a decrease on harmful side effects in comparison to the free drug. | [105] |
| SPIONs (260 nm) were coated with FA and ACPP (F/A-PLGA@DOX/SPIO). | Treatment: DOX. Cancer model: human non-small cell lung cancer model (A549 cell line). Normal liver cell (L02 cell line). Male BALB/c nude mice were subcutaneously injected with 3 × 107 A549 cells into the right-rear leg. | F/A-PLGA@DOX/SPIO induced apoptosis in the cancer cells, accelerating the overproduction of ROS. MRI was used to track the NPs in cancer cells (T2-weighted MRI). In vivo, a reduction in tumor growth was observed (around 67% comparatively to control group), NPs showed a good biocompatibility and long plasma stability, with a capability to induce tumor necrosis, whilst no significant damage or inflammation was detected in healthy organs. | [106] |
| SPIONs (6 nm) were coated with dextran (FeDC-E NPs). | Treatment: erlotinib. Cancer model: lung cancer model (CL1-5-F4 cancer cell line). Male BALB/c nude mice were subcutaneously injected with 2.5 × 106 of CL1-5-F4 cells. | Theranostic NPs showed a significant therapeutic effect with targeting properties against invasive and migrative cancer cells. These NPs enabled their localization using T2-weighted MRI. EGFR–ERK–NF-κB signaling pathways were suppressed when after tumors treatment. | [107] |
| SLN (Particle Size) + Surface Modification/Loading | Drug + Cancer Model | Results | Ref |
|---|---|---|---|
| SLNs (200 nm). Surface modification is not mentioned. | Drug: DOX. Cancer model: murine malignant melanoma (B16F10 cells). C57BL/6 mice (12–16 weeks old) were intravenously injected with 1 × 105 B16F10 cells. | In vivo, mice treated with SLNs-DOX, obtained, approximately, a 60% reduction of tumor area when compared to mice treated with free DOX. No significant differences were found in the survival rates or body weight between different treatment groups, indicating no detectable SLPs-DOX in vivo toxicity during the timeframe of these tests. | [151] |
| PTX-SLN (<200 nm). Surface modification is not mentioned. | Drug: PTX. Cancer model: breast cancer model (MCF-7 cancer cell line). | Xu et al. observed an enhanced anticancer activity of PTX-SLNs, which significantly increased the intracellular uptake (almost 10 ng more of PTX per mg of protein comparatively to control) of the drug when compared to the free drug. The results demonstrated that the use of SLNs could efficiently avoid the multidrug resistance mechanisms observed in breast cancer cells. | [147] |
| SLN-TMZ (279 nm). Surface modification is not mentioned. | Drug: TMZ. Cancer model: melanoma cancer model (JR8 and A2058 cell lines; B16-F10 mouse melanoma cell line). Female C57BL6/J mice were subcutaneously injected with 1 × 106 B16-F10 cells. | NPs showed in vitro and in vivo their ability to target tumor cells and promote drug internalization, reducing the therapeutic dosage needed to be administered in the in vivo model. Here, SLN-TMZ also displayed a higher mice survival rate compared to that obtained using the free drug (increasing from 50 to 100%). Moreover, the in vitro tumor angiogenesis was found to be inhibited (HUVEC method). | [152] |
| Chol-CUR-SLN (170 nm). Surface modification is not mentioned. | Drug: CUR. Cancer model: breast cancer model (MDA-MB-231 cell line). | In vitro results showed that Chol-CUR-SLN efficiently targeted and accumulated in cancer cells. It also exhibited a higher inhibitory effect on cell viability (20% of higher cytotoxicity in comparison to free drug) and proliferation when compared to free CUR. Chol-CUR-SLN significantly improved the induction of apoptosis (63.87% versus 55.4%) in MDA-MB-231 cells, compared to free CUR. | [153] |
| SLN-MTX (300 nm) loaded with an ApoE mimicking chimera peptide to actively target the brain. | Drug: MTX. Cancer model: glioblastoma cancer model (F98/Fischer glioblastoma human primary culture). | A reduction of tumor growth (relative tumor growth of approximately 4 versus 10 for treated and control groups, respectively) was observed with SLN-MTX. Moreover, an increase of apoptosis was noted, demonstrating that the developed SLN could be an alternative to conventional therapy. | [154] |
| TAT PTX/TOS-CDDP SLNs (100 nm) modified with DSPE-PEG and TAT for co-delivery of PTX and TOS-CDDP. | Drug: PTX + TOS-CDDP. Cancer model: cervical cancer model (HeLa cancer cell line). BALB/c nude mice were subcutaneously injected with 1 × 106 of HeLa cells. | TAT PTX/TOS-CDDP SLNs had a slower drug release in comparison with PTX/TOS-CDDP SLNs. Here, the drug release was greatly affected by a lower pH. The in vitro cellular uptake study also showed that tumor cells could uptake more efficiently the TAT PTX/TOS-CDDP SLNs when compared with other SLNs. Moreover, these nanoparticles showed a synergistic effect in the suppression of tumor growth in vivo (inhibition rate of 72.2%) with lower toxicity (calculated by the bodyweight loss during the experiment). Moreover, the formulation increased the drug accumulation in tumor tissue in comparison to the administration of the free drug. | [155] |
| c-SLN (200 nm). Surface modification is not mentioned. | Drug: FA+ ASP. Cancer model: pancreatic cancer model (PaCa-2 and Panc-1 cell lines). Male SCID mice were subcutaneously injected with 1 × 106 PaCa-2 cells. | In vitro studies demonstrated that NPs with the conjugated treatment effectively inhibited cell growth, inducing apoptosis. The use of the dual treatment loaded in the SLNs presented significantly better results in cell viability assays when compared to the cells treated with the free drugs. The in vivo studies presented a tumor growth suppression of 45% compared to the control group. However, this result was not statistically significant. By performing the immunohistochemistry analysis, an increased expression of pro-apoptotic proteins was detected. | [156] |
| mSLN (Particle Size) + Surface Modification | Drug + Cancer Model | Results | Ref |
|---|---|---|---|
| Wax-mSLNs (200 nm). Surface modification is not mentioned. | Drug: DOX. Cancer model: murine melanoma B16f10, Hs578t, and Dox-resistance cell lines (t84 and HCT-15). | Efficacy studies showed that DOX delivery in combination with 1 h of MH promoted a significant cytotoxic effect in vitro in melanoma cell lines compared to a treatment in which no MH was supplied (~5% vs. ~50%, respectively, when using 1 µg DOX/mL of DOX-mSLNs). Similar results were obtained in 3D in vitro using melanoma spheroids. The same dual treatment approach was applied to DOX-resistant cell lines obtaining approximately 40% of cell viability reduction. | [186] |
| Wax-mSLNs (250–300 nm). Surface modification is not mentioned. | Drug: OncoA. Cancer model: human lung carcinoma cell line (A549 cell line). | mSLNs showed an outstanding performance as a T2-contrast agent in MRI (r2 > 800 mm−1 s−1). In vitro, the combination of co-loaded MNPs and OncoA with MH greatly decreased the cell viability (virtually 0% vs. 53% when performed without MH application) at the same 40 µg OncoA/mL and 25 µg Fe/mL doses). | [187] |
| Wax-mSLNs (200 nm). Surface modification is not mentioned. | Drug: DOX. MH: 224 kHz, 13 A, 27.6 W for 1 h for in vitro 174.5 kHz, 23 mT for 1 h for in vivo. Cancer model: murine malignant melanoma cells (B16F10 cell line); C57BL/6 mice (8–10 weeks old) were subcutaneously injected in interscapular region of mice with 5 × 105 B16F10 cells. | mSLNs-DOX showed higher cytotoxicity activity than free DOX in the whole range of DOX concentration tested both in vitro and in vivo. In vitro, a remarkable enhanced cytotoxicity was obtained when cells were exposed to the combination of chemotherapy (0.5 µ/mL) and 1 h MH (40% of viable cells vs. 85% without MH). Under a higher incubation concentration of mLNVs-DOX (1 μg DOX/mL), the results showed a cytotoxicity virtually to 100% under a combination of mLNVs-DOX with MH. In vivo, the dual treatment promoted the slowest tumor growth and smallest tumor volume, which was on average 3 and 2.1-fold smaller than the saline and free-DOX groups. Regarding imaging capability, T2-MRI relaxation times of animal tumors treated with mSLNs were on average over 15% shorter than those of control animals injected only with saline. | [176] |
| Sor-mag-SLN (250 nm). Surface modification is not mentioned. | Drug: Sor. Cancer model: liver cancer model (HepG2 cell line). | The nanocarriers showed a loading efficiency of 90% and stability in an aqueous environment. Moreover, the developed nanoparticles presented a good cytocompatibility with a high antiproliferative effect against the cancer cells (40% higher in comparison to control group). This effect was associated with the capability of these nanocarriers to be specifically accumulated in the tumor region and the application of a local AMF. | [188] |
| Mag-SLN (150 nm). Surface modification is not mentioned. | Cancer model: myeloid leukemia cancer model (HL-60/wt cell lines; L-60/adr with MRP1 = ABCC1 over-expression; HL-60/vinc with P-glycoprotein = ABCB1 over-expression), leukemia cancer model (Jurkat T-cells), and glioblastoma cancer model (U251 cell line). | The developed nanoparticles showed promising results in the context of cancer therapy, in particular against drug-resistant cell lines. The mag-SLN revealed higher cytotoxicity against resistance cell lines in comparison to DOX alone when under an AMF. Moreover, the data showed that the cells treated with a dual treatment presented an increase of nuclei fragmentation and condensed chromatin. The mag-SLNs plus MH presented apoptotic and necrotic activities. The authors proposed that the production of ROS was the cause of the higher cytotoxicity observed in the cells treated with the particles. | [168] |
| LMNV (100 nm). Surface modification is not mentioned. | Drug: TMZ. Cancer model: glioblastoma cancer model (U-87 cell line) and brain-endothelial cell model (bEnd.3 cell lines, an immortalized mouse BEC line). | In vitro results showed that lipid-based magnetic nanovectors presented a good loading capacity with a sustained release profile of the encapsulated chemotherapeutic drug. Moreover, a complete drug release was observed after the exposure to (i) low pH (4.5), (ii) increased concentration of hydrogen peroxide (50 µM), and (iii) increased temperature achieved through the application of an AMF. The authors noted that these nanovectors could be used as a potential hyperthermia agent, since they managed to increase apoptotic levels and decrease proliferative rates when a magnetic field of 20 mT and 750 kHz was applied, increasing the temperature to 43 °C. During in vitro tests, the capacity of LMNVs to cross the BBB was observed, where after 24 h of exposure, 40% of LMNVs were able to translocate inside the glioblastoma cells. | [189] |
| Gd(III)-loaded pSLNs were modified with with cellular receptors, DSPE-PEG2000-folate. | Cancer model: murine macrophage model (Raw 264.7 cell line), lymphoma cancer model (U937 cell line), and human ovarian adenocarcinoma (IGROV-1 cell line). Female Balb/C nu/nu were subcutaneously injected with 1 × 107 of IGROV-1 cells. | The data showed that pSLNs could effectively internalize in in vitro and in vivo models. Moreover, the authors detected the nanoparticles’ T1-MRI signal, at least after 30 min post-injection. The cytotoxic studies showed a decrease in cell viability when the loaded Gd(III) concentration increased within the pSLN (below 50% of viable cells). The results also demonstrated that Gd(III)-loaded pSLNs could efficiently target the cancer cells and due to the EPR effect in conjunction with its targeting properties allowed a higher internalization capacity. Moreover, they could be used as a molecular imaging tool. A macrophage uptake experiment in vivo showed that the nanoparticles could avoid the macrophage internalization and circulate for at least 6 h, increasing altogether the tumor uptake. However, the authors noted an excessive accumulation in the liver with slow elimination rates after performing the biodistribution study. | [184] |
| Sor-Mag-SLNs (300 nm). Surface modification is not mentioned. | Drug: Sor. Cancer model: liver cancer model (HepG2 cell line). | The results showed an increase of the cytotoxic effects of sorafenib. Using an external magnetic field, it was possible to guide and improve the drug effect in the desired area. Quantitative evaluation of cell mortality indicated 95% of cell death compared to the control (5%). Moreover, the authors mentioned that the nanocarriers could be an effective approach to reduce the undesired side effects of chemotherapeutic drugs and improve their pharmacokinetic properties. | [190] |
| Nut-Mag-SLNs (180 nm) were loaded with fluorescenin-PEG-DSPE (FITC-PEG-DSPE). | Drug: Nut. Cancer model: glioblastoma cancer model (U-87 cancer cell line) and brain endothelial cell model (bEnd.3 cell lines, an immortalized mouse BEC line). | Nut-Mag-SLNs presented a good colloidal stability and could efficiently cross an in vitro blood–brain barrier model. The authors observed that the nanovectors were magnetically activated, enabling their pass through the BBB, and could also deliver the drug loads to glioblastoma cells. Moreover, they observed an enhanced antitumor activity as they obtained a 50% reduction in the metabolic activity with lower drug concentrations. Increased pro-apoptotic activity was also noted. These nanocarriers presented several advantages compared to the free drug in overcoming several limitations in glioblastoma treatments, for instance, (i) Nut-Mag-SLNs could cross the BBB, (ii) Nut-Mag-SLNs had the ability to be magnetically guided to the tumor region, and (iii) the nanoparticles showed a powerful inhibition of cancer cell proliferation while increasing the pro-apoptotic activity. | [181] |
| mSLNs (180 nm). Surface modification is not mentioned. | Cancer model: colon cancer model (HT-29 cell line). | By applying magnetic hyperthermia, results showed that mSLNs could constantly maintain the maximum temperature achieved (46 °C, in 40 min) during 1 h of exposure to a magnetic field (250 kHz and 4 kA/m). These results translated into a decrease in cell viability after magnetic treatment (up to 52% comparatively to 100% of control group). Interestingly, no cytotoxic effect was observed if only one (but not both) of the components was used alone for treatment. | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, M.; Belmonte-Reche, E.; Gallo, J.; Baltazar, F.; Bañobre-López, M. Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics. Pharmaceutics 2022, 14, 506. https://doi.org/10.3390/pharmaceutics14030506
Cerqueira M, Belmonte-Reche E, Gallo J, Baltazar F, Bañobre-López M. Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics. Pharmaceutics. 2022; 14(3):506. https://doi.org/10.3390/pharmaceutics14030506
Chicago/Turabian StyleCerqueira, Mónica, Efres Belmonte-Reche, Juan Gallo, Fátima Baltazar, and Manuel Bañobre-López. 2022. "Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics" Pharmaceutics 14, no. 3: 506. https://doi.org/10.3390/pharmaceutics14030506
APA StyleCerqueira, M., Belmonte-Reche, E., Gallo, J., Baltazar, F., & Bañobre-López, M. (2022). Magnetic Solid Nanoparticles and Their Counterparts: Recent Advances towards Cancer Theranostics. Pharmaceutics, 14(3), 506. https://doi.org/10.3390/pharmaceutics14030506







