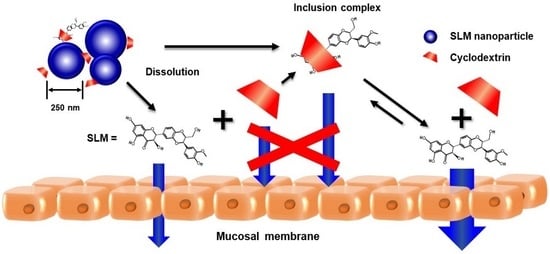
Hydroxypropyl-β-cyclodextrin Enhances Oral Absorption of Silymarin Nanoparticles Prepared Using PureNano™ Continuous Crystallizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of SLM Nanoparticles (NPs)
2.3.1. Preparation of SLM NPs Using PureNanoTM
2.3.2. Drug Content of SLM NPs
2.4. Physicochemical Properties of SLM NPs
2.4.1. Particle Size and ζ-Potential Analysis
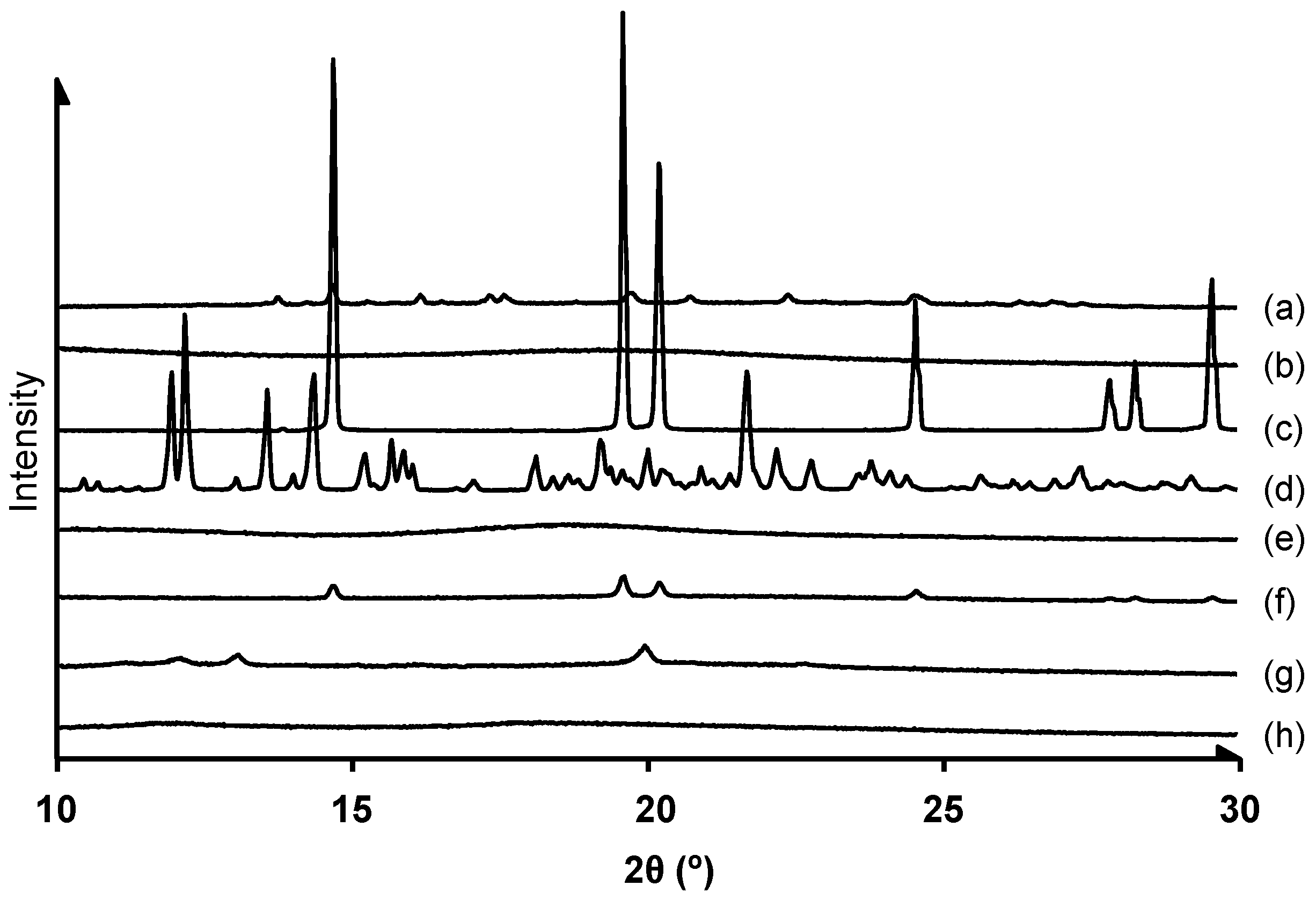
2.4.2. X-ray Diffraction Study
2.4.3. Scanning Electron Microscopy (SEM)
2.5. In Vitro Release Test
2.6. Membrane Permeability Studies
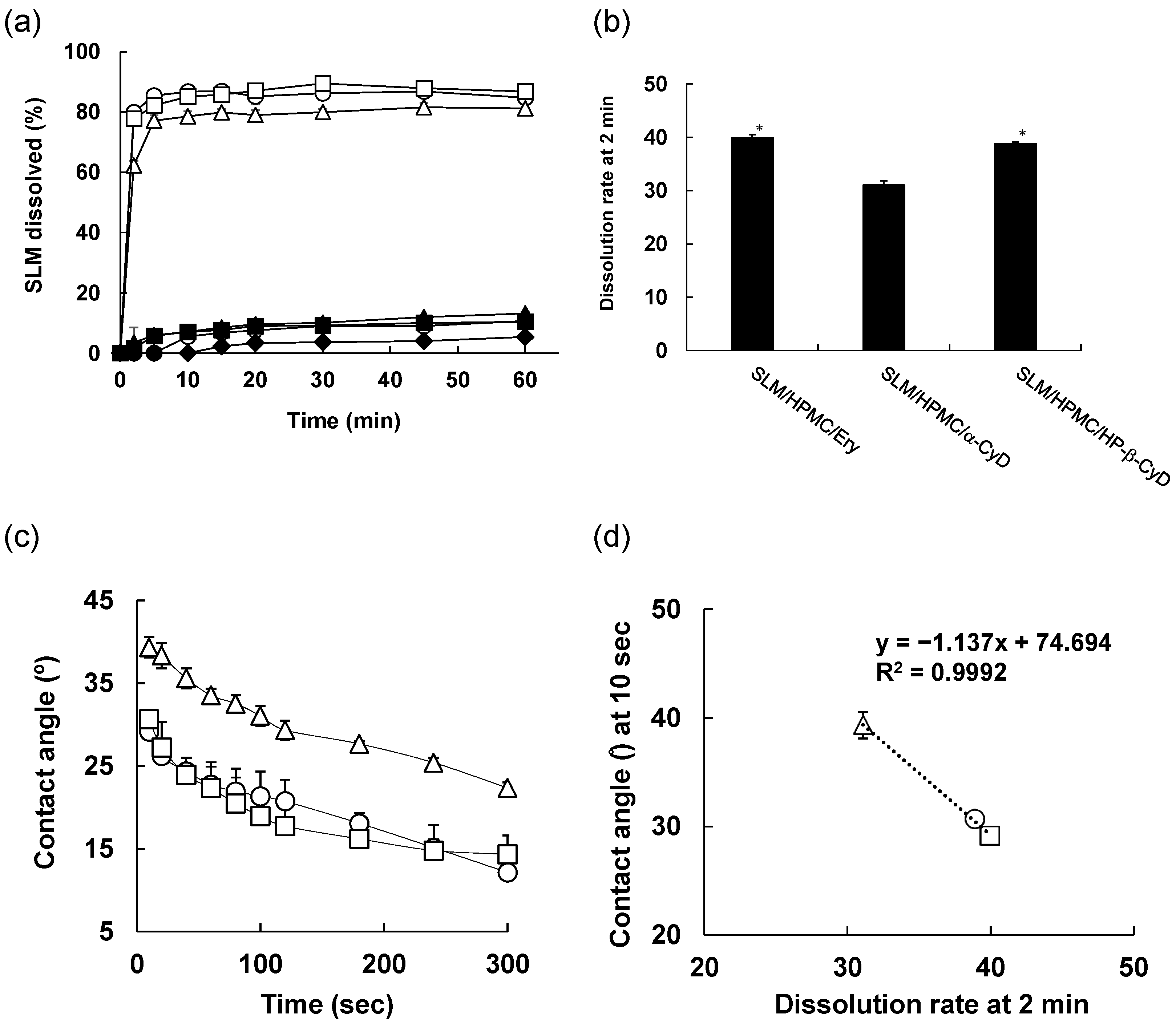
2.7. Contact Angle
2.8. Solubility
2.9. Phase Solubility
2.10. Data Analysis
3. Results and Discussion
3.1. Preparation of SLM Nanoparticles (NPs)
3.1.1. Preparation of SLM NPs Using PureNanoTM
3.1.2. Effect of Dispersion Stabilizer on Preparation of SLM NPs
3.1.3. Effect of Cryoprotectants on Preparation of SLM NPs
3.2. Physicochemical Properties of SLM NPs
3.2.1. Crystalline State Analysis
3.2.2. Scanning Electron Microscopy (SEM) of SLM NPs
3.3. In Vitro Release Test
3.4. Solubility Study of SLM NPs
3.5. Membrane Permeability Test of SLM NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cho, E.; Jung, S. Supramolecular Complexation of Carbohydrates for the Bioavailability Enhancement of Poorly Soluble Drugs. Molecules 2015, 20, 19620–19646. [Google Scholar] [CrossRef]
- Mishra, P.R.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int. J. Pharm. 2009, 371, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar] [PubMed]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Tozuka, Y.; Asamoto, F.; Takeuchi, H. Fluorescence investigation of a specific structure formed by aggregation of transglycosylated stevias: Solubilizing effect of poorly water-soluble drugs. Eur. J. Pharm. Sci. 2011, 43, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Tozuka, Y.; Imono, M.; Takeuchi, H. Transglycosylated stevia and hesperidin as pharmaceutical excipients: Dramatic improvement in drug dissolution and bioavailability. Eur. J. Pharm. Biopharm. 2010, 76, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Wu, Y.; Loper, A.; Landis, E.; Hettrick, L.; Novak, L.; Lynn, K.; Chen, C.; Thompson, K.; Higgins, R.; Batra, U.; et al. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: A Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int. J. Pharm. 2004, 285, 135–146. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Zhang, L.; Lin, X.; Zhang, Y.; Tang, X.; Wang, Y. A combined bottom-up/top-down approach to prepare a sterile injectable nanosuspension. Int. J. Pharm. 2014, 472, 130–139. [Google Scholar] [CrossRef]
- Tozuka, Y.; Imono, M.; Uchiyama, H.; Takeuchi, H. A novel application of α-glucosyl hesperidin for nanoparticle formation of active pharmaceutical ingredients by dry grinding. Eur. J. Pharm. Biopharm. 2011, 79, 559–565. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Le, Y.; Wang, J.X.; Chen, J.F. Preparation of stable micron-sized crystalline irbesartan particles for the enhancement of dissolution rate. Drug Dev. Ind. Pharm. 2011, 37, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Nishikawa, M.; Matsui, K.; Hisazumi, K.; Onodera, R.; Tozuka, Y.; Takeuchi, H. In Vitro and In Vivo Characterization of Drug Nanoparticles Prepared Using PureNano™ Continuous Crystallizer to Improve the Bioavailability of Poorly Water Soluble Drugs. Pharm. Res. 2016, 33, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Huey, B.D.; Burgess, D.J. Scanning probe microscopy method for nanosuspension stabilizer selection. Langmuir ACS J. Surf. Colloids 2009, 25, 12481–12487. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 239–249. [Google Scholar]
- Kren, V.; Walterová, D. Silybin and Silymarin—New Effects and Applications; Biomedical papers of the Medical Faculty of the University Palacky: Olomouc, Czech Republic, 2005; Volume 149, pp. 29–41. [Google Scholar]
- Wen, Z.; Dumas, T.E.; Schrieber, S.J.; Hawke, R.L.; Fried, M.W.; Smith, P.C. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab. Dispos. 2008, 36, 65–72. [Google Scholar] [CrossRef]
- Das, S.; Roy, P.; Auddy, R.G.; Mukherjee, A. Silymarin nanoparticle prevents paracetamol-induced hepatotoxicity. Int. J. Nanomed. 2011, 6, 1291–1301. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Rosqvist, E.; Smått, J.H.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int. J. Pharm. 2019, 563, 217–227. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, Y.; Zhang, Y.; Zu, Y.; Lian, B.; Wu, M.; Zua, C.; Wua, W. Silymarin nanoparticles through emulsion solvent evaporation method for oral delivery with high antioxidant activities, bioavailability, and absorption in the liver. RSC Adv. 2016, 6, 93137–93146. [Google Scholar] [CrossRef]
- Yang, K.Y.; Du, H.; Yousaf, A.M.; Kim, D.W.; Shin, Y.J.; Bae, O.N.; Kim, Y.I.; Kim, J.O.; Yong, C.S.; Choi, H.G. Silymarin-loaded solid nanoparticles provide excellent hepatic protection: Physicochemical characterization and in vivo evaluation. Int. J. Nanomed. 2013, 8, 3333–3343. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Uekama, K.; Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carr. Syst. 1987, 3, 1–40. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Kataoka, M.; Yamashita, S.; Yamamoto, K.; Moribe, K. Inhibition mechanism of hydroxypropyl methylcellulose acetate succinate on drug crystallization in gastrointestinal fluid and drug permeability from a supersaturated solution. Eur. J. Pharm. Sci. 2014, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Reverchon, E. Supercritical antisolvent micronization of cyclodextrins. Powder Technol. 2008, 183, 239–246. [Google Scholar] [CrossRef]
- Wongmekiat, A.; Tozuka, Y.; Oguchi, T.; Yamamoto, K. Formation of fine drug particles by cogrinding with cyclodextrins. I. The use of beta-cyclodextrin anhydrate and hydrate. Pharm. Res. 2002, 19, 1867–1872. [Google Scholar] [CrossRef]
- Onodera, R.; Hayashi, T.; Nakamura, T.; Aibe, K.; Tahara, K.; Takeuchi, H. Preparation of silymarin nanocrystals using a novel high pressure crystallization technique and evaluation of its dissolution and absorption properties. Asian J. Pharm. 2016, 11, 211–212. [Google Scholar] [CrossRef][Green Version]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Kayaert, P.; Van den Mooter, G. Is the amorphous fraction of a dried nanosuspension caused by milling or by drying? A case study with Naproxen and Cinnarizine. Eur. J. Pharm. Biopharm. 2012, 81, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kayaert, P.; Van den Mooter, G. An investigation of the adsorption of hydroxypropylmethyl cellulose 2910 5 mPa s and polyvinylpyrrolidone K90 around Naproxen nanocrystals. J. Pharm. Sci. 2012, 101, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Soares, S.; Costa, A.; Andrade, J.C.; Seabra, V.; Reis, S.; Sarmento, B. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2012, 2, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Haddadi, A. Investigation and optimization of formulation parameters on preparation of targeted anti-CD205 tailored PLGA nanoparticles. Int. J. Nanomed. 2015, 10, 7371–7384. [Google Scholar] [CrossRef][Green Version]
- Hara, T.; Hirayama, F.; Arima, H.; Yamaguchi, Y.; Uekama, K. Improvement of solubility and oral bioavailability of 2-(N-cyanoimino)-5-[(E)-4-styrylbenzylidene]-4-oxothiazolidine (FPFS-410) with antidiabetic and lipid-lowering activities in dogs by 2-hydroxypropyl-beta-cyclodextrin. Chem. Pharm. Bull. 2006, 54, 344–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bajaj, A.; Rao, M.R.; Pardeshi, A.; Sali, D. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS PharmSciTech 2012, 13, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kristiansen, H. Binding specificity between small organic solutes in aqueous solution: Classification of some solutes into two groups according to binding tendencies. J. Pharm. Sci. 1970, 59, 1601–1608. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. In situ molecular elucidation of drug supersaturation achieved by nano-sizing and amorphization of poorly water-soluble drug. Eur. J. Pharm. Sci. 2015, 77, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef]



| Condition | Particle Size (nm) | PDI |
|---|---|---|
| PureNanoTM | 188.6 | 0.138 |
| Evaporation | 224.8 | 0.166 |
| Freeze Dry (FD) | >1000 | Not detected |
| Compound | Stabilizer | Condition | Particle Size (nm) | PDI |
|---|---|---|---|---|
| PureNanoTM | 337.6 | 0.045 | ||
| HPMC | Evaporation | 339.0 | 0.165 | |
| FD | 677.7 | 0.463 | ||
| SLM | FD after 1 week | 697.1 | 0.478 | |
| PureNanoTM | 281.5 | 0.115 | ||
| S-1670 | Evaporation | 329.5 | 0.221 | |
| FD | 912.1 | 0.496 | ||
| FD after 1 week | >1000 | Not detected |
| Compound | Condition | Ratio (w/w) | Particle Size (nm) | PDI |
|---|---|---|---|---|
| 1/0.5 | 227.5 | 0.045 | ||
| PureNanoTM | 1/1 | 387.6 | 0.165 | |
| SLM/HPMC | 1/3 | 1240.9 | 0.463 | |
| 1/0.5 | >1000 | 0.115 | ||
| FD | 1/1 | 617.0 | 0.221 | |
| 1/3 | >1000 | 0.496 |
| Compound | Cryoprotectant | Ratio (w/w/w) | Particle Size (nm) | PDI |
|---|---|---|---|---|
| Erythritol | 1/1/1 | 253.8 | 0.108 | |
| Mannitol | 1/1/1 | 271.8 | 0.170 | |
| SLM/HPMC | Trehalose | 1/1/1 | 265.2 | 0.160 |
| α-CyD | 1/1/1 | 249.1 | 0.149 | |
| HP-β-CyD | 1/1/1 | 254.8 | 0.225 |
| Sample | Content (%) | Theoretical Content (%) |
|---|---|---|
| SLM/HPMC/Ery FD | 32.25 ± 0.01 | |
| SLM/HPMC/α-CyD FD | 31.30 ± 0.64 | 33.33 |
| SLM/HPMC/HP-β-CyD FD | 31.85 ± 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, R.; Hayashi, T.; Motoyama, K.; Tahara, K.; Takeuchi, H. Hydroxypropyl-β-cyclodextrin Enhances Oral Absorption of Silymarin Nanoparticles Prepared Using PureNano™ Continuous Crystallizer. Pharmaceutics 2022, 14, 394. https://doi.org/10.3390/pharmaceutics14020394
Onodera R, Hayashi T, Motoyama K, Tahara K, Takeuchi H. Hydroxypropyl-β-cyclodextrin Enhances Oral Absorption of Silymarin Nanoparticles Prepared Using PureNano™ Continuous Crystallizer. Pharmaceutics. 2022; 14(2):394. https://doi.org/10.3390/pharmaceutics14020394
Chicago/Turabian StyleOnodera, Risako, Tomohiro Hayashi, Keiichi Motoyama, Kohei Tahara, and Hirofumi Takeuchi. 2022. "Hydroxypropyl-β-cyclodextrin Enhances Oral Absorption of Silymarin Nanoparticles Prepared Using PureNano™ Continuous Crystallizer" Pharmaceutics 14, no. 2: 394. https://doi.org/10.3390/pharmaceutics14020394
APA StyleOnodera, R., Hayashi, T., Motoyama, K., Tahara, K., & Takeuchi, H. (2022). Hydroxypropyl-β-cyclodextrin Enhances Oral Absorption of Silymarin Nanoparticles Prepared Using PureNano™ Continuous Crystallizer. Pharmaceutics, 14(2), 394. https://doi.org/10.3390/pharmaceutics14020394