Oral Formulation Based on Irbesartan Nanocrystals Improve Drug Solubility, Absorbability, and Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
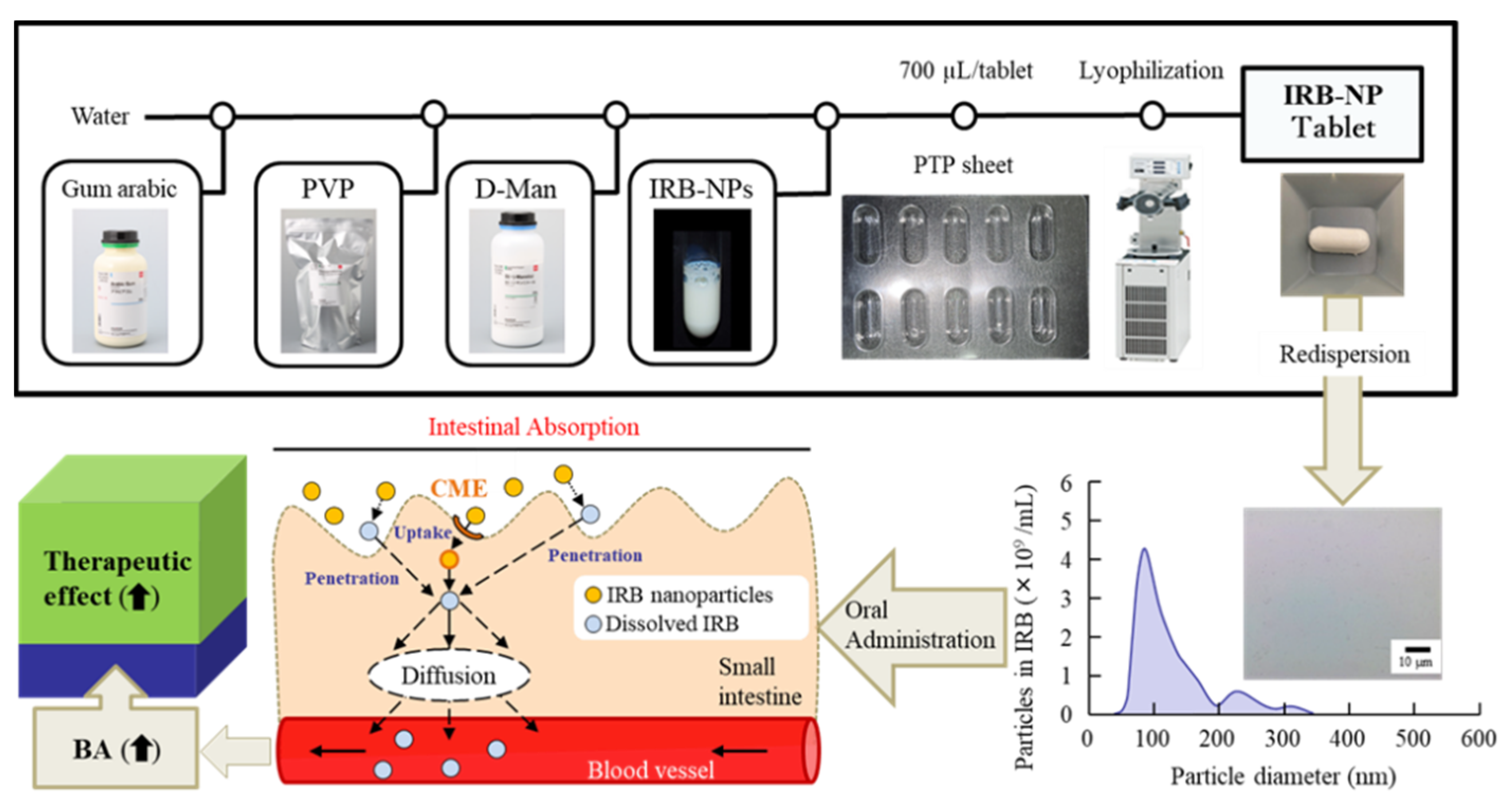
2.2. Preparation of the IRB-NP Tablet
2.3. Characterization of the IRB-NP Tablets and It’s Redispersion
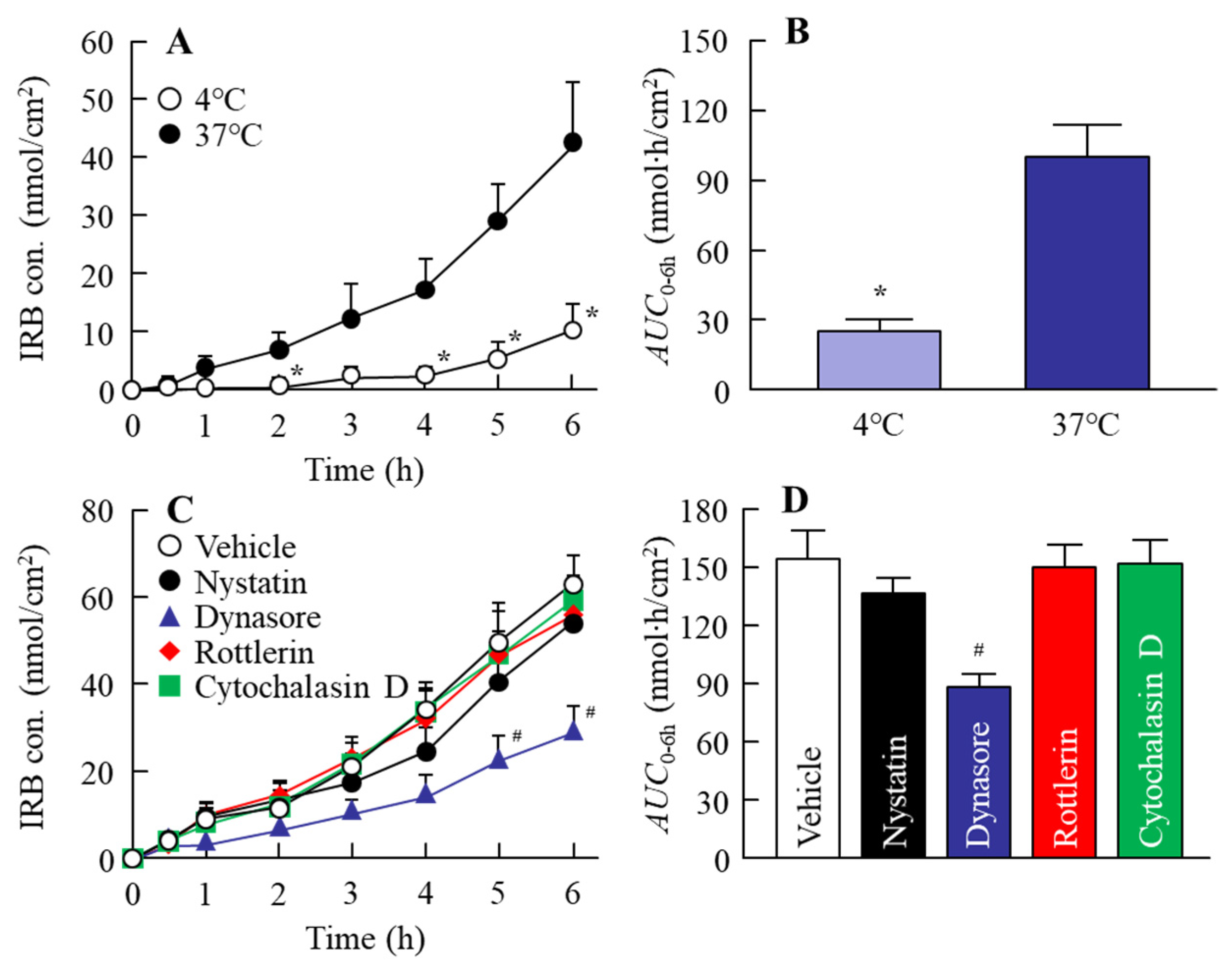
2.4. In Vitro Intestinal Penetration in the IRB Table
2.5. Measurement of the Plasma IRB Concentration
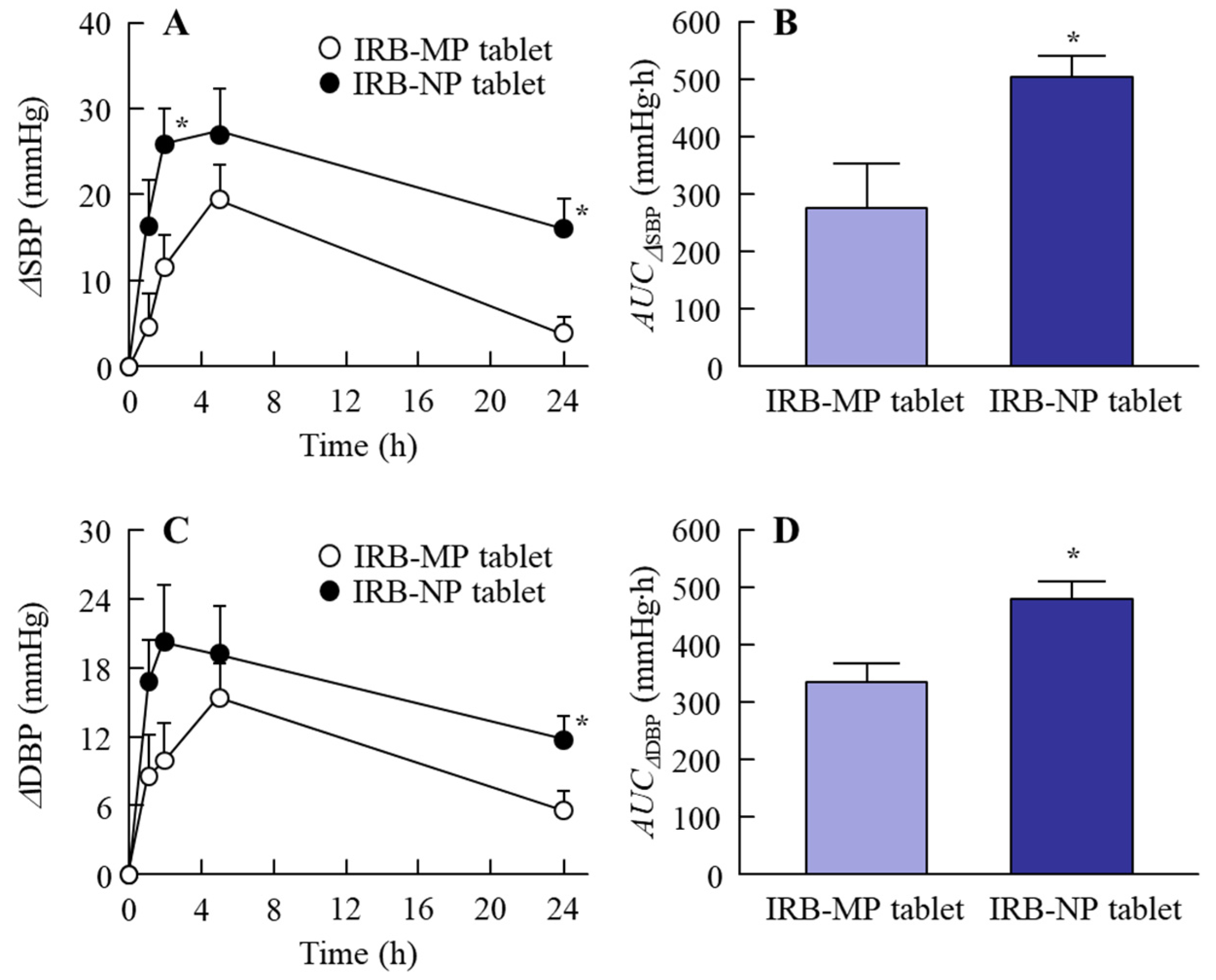
2.6. Measurement of Blood Pressure (BP)
2.7. Statistical Analysis
3. Results
3.1. Design of the IRB-NP Tablet and Evaluation of Their Characteristics
3.2. Effect of Energy-Dependent Endocytosis on the Transintestinal Penetration of the IRB-NP Tablet (Rp.8)
3.3. BP-Reducing Effect of the IRB-NP Tablet (Rp.8) in the SHR-SP Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dresser, G.K.; Nelson, S.A.E.; Mahon, J.L.; Zou, G.; Vandervoort, M.K.; Wong, C.J.; Feagan, B.G.; Feldman, R.D. Simplified therapeutic intervention to control hypertension and hypercholesterolemia: A cluster randomized controlled trial (STITCH2). J. Hypertens. 2013, 31, 1702–1713. [Google Scholar] [CrossRef]
- Zanchetti, A.; Liu, L.; Mancia, G.; Parati, G.; Grassi, G.; Stramba-Badiale, M.; Silani, V.; Bilo, G.; Corrao, G.; Zambon, A.; et al. Blood pressure and low-density lipoprotein-cholesterol lowering for prevention of strokes and cognitive decline: A review of available trial evidence. J. Hypertens. 2014, 32, 1741–1750. [Google Scholar] [CrossRef]
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B.; Powers, B.; Samsa, G.P.; Gray, R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008, 148, 16–29. [Google Scholar] [CrossRef]
- Taylor, A.A.; Siragy, H.; Nesbitt, S. Angiotensin receptor blockers: Pharmacology, efficacy, and safety. J. Clin. Hypertens. 2011, 13, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Parati, G.; Bilo, G.; Gao, P.; Fagard, R.; Redon, J.; Czuriga, I.; Polák, M.; Ribeiro, J.M.; Sanchez, R.; et al. Ambulatory blood pressure values in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). Hypertension 2012, 60, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.R. The new angiotensin II receptor antagonist, irbesartan: Pharmacokinetic and pharmacodynamic considerations. Am. J. Hypertens. 1997, 10, 311S–317S. [Google Scholar] [CrossRef]
- Pouleur, H.G. Clinical overview of irbesartan: A new angiotensin II receptor antagonist. Am. J. Hypertens. 1997, 10, 318S–324S. [Google Scholar] [CrossRef][Green Version]
- Jansook, P.; Muankaew, C.; Stefánsson, E.; Loftsson, T. Development of eye drops containing antihypertensive drugs: Formulation of aqueous irbesartan/γCD eye drops. Pharm. Dev. Technol. 2015, 20, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Crull, G.; Yin, S.; Grosso, J. Low level drug product API form analysis—Avalide tablet NIR quantitative method development and robustness challenges. J. Pharm. Biomed. Anal. 2014, 89, 268–275. [Google Scholar] [CrossRef]
- Ramos, J.J.M.; Diogo, H.P. Thermal behavior and molecular mobility in the glassy state of three anti-hypertensive pharmaceutical ingredients. RSC Adv. 2017, 7, 10831–10840. [Google Scholar] [CrossRef]
- Vachharajani, N.; Chang, S.Y.; Shyu, W.C.; Greene, D.; Barbhaiya, R. Absolute bioavailability of irbesartan, an angiotensin II receptor antagonist, in man. Pharm. Res. 1995, 12, 418. [Google Scholar]
- Marino, M.R.; Langenbacher, K.M.; Ford, N.F.; Uderman, H.D. Safety, tolerability, pharmacokinetics and pharmacodynamics of irbesartan after single and multiple doses in healthy male subjects (abst). Clin. Pharmacol. Ther. 1997, 61, 207. [Google Scholar]
- Hedaya, M.A.; Helmy, S.A. Modeling of the pharmacokinetic/pharmacodynamics interaction between irbesartan and hydrochlorothiazide in normotensive subjects. Biopharm. Drug Dispos. 2015, 36, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shen, J.; Burgess, D.J. Nano-amorphous spray dried powder to improve oral bioavailability of itraconazole. J. Control. Release 2014, 192, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Müllertz, A.; Ogbonna, A.; Ren, S.; Rades, T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1622–1636. [Google Scholar] [CrossRef]
- Jog, R.; Burgess, D.J. Pharmaceutical amorphous nanoparticles. J. Pharm. Sci. 2017, 106, 39–65. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Deguchi, S.; Ogata, F.; Watanabe, M.; Otake, H.; Yamamoto, N.; Kawasaki, N.; Nagai, N. Nanocrystalline Suspensions of Irbesartan Enhance Oral Bioavailability by Improving Drug Solubility and Leading Endocytosis Uptake into the Intestine. Pharmaceutics 2021, 13, 1404. [Google Scholar] [CrossRef]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef]
- Kumar, S.; Burgess, D.J. Nanosuspensions. In Long Acting Injections and Implants; Wright, J.C., Burgess, D.J., Eds.; Springer: Boston, MA, USA, 2012; pp. 239–261. [Google Scholar]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Pini, E.; Angioni, G.; Manca, M.L.; Perricci, J.; Sinico, C.; Fadda, A.M. Nanocrystals as tool to improve piroxicam dissolution rate in novel orally disintegrating tablets. Eur. J. Pharm. Biopharm. 2011, 79, 552–558. [Google Scholar] [CrossRef]
- Kumar, S.; Burgess, D.J. Wet milling induced physical and chemical instabilities of naproxen nano-crystalline suspensions. Int. J. Pharm. 2014, 466, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, A.; Kristl, J.; Baumgartner, S.; Planinšek, O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int. J. Pharm. 2009, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, S.; Zhu, Z.; Lyu, C.; Bai, C.; Ge, H.; Yang, X.; Pan, W. Controlled delivery of carvedilol nanosuspension from osmotic pump capsule: In Vitro and in vivo evaluation. Int. J. Pharm. 2014, 475, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.A.; Churchill, P.C.; Picken, M.; Webb, R.C.; Kurtz, T.W.; Bidani, A.K. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am. J. Hypertens. 2001, 14, 311–320. [Google Scholar] [CrossRef]
- Nagai, R.; Nagata, S.; Fukuya, F.; Higaki, J.; Rakugi, H.; Ogihara, T. Changes in autonomic activity and baroreflex sensitivity with the hypertension process and age in rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of endocytosis in the transdermal penetration mechanism of ketoprofen nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef]
- The Eighteenth Edition of the Japanese Pharmacopoeia (JP). Available online: https://www.mhlw.go.jp/content/11120000/000788359.pdf (accessed on 4 February 2022).
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Baracia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamindependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Jog, R.; Burgess, D.J. Comprehensive quality by design approach for stable nanocrystalline drug products. Int. J. Pharm. 2019, 564, 426–460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jog, R.; Shen, J.; Zolnik, B.; Sadrieh, N.; Burgess, D.J. In Vitro and in vivo performance of different sized spray-dried crystalline itraconazole. J. Pharm. Sci. 2015, 104, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Jinno, J.-I.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Ghosh, T.; Ghosh, A.; Prasad, D. A review on new generation orodispersible tablets and its future prospective. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–7. [Google Scholar]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Fukuda, S.; Tsuchikura, S.; Iida, H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp. Anim. 2004, 53, 67–72. [Google Scholar] [CrossRef]
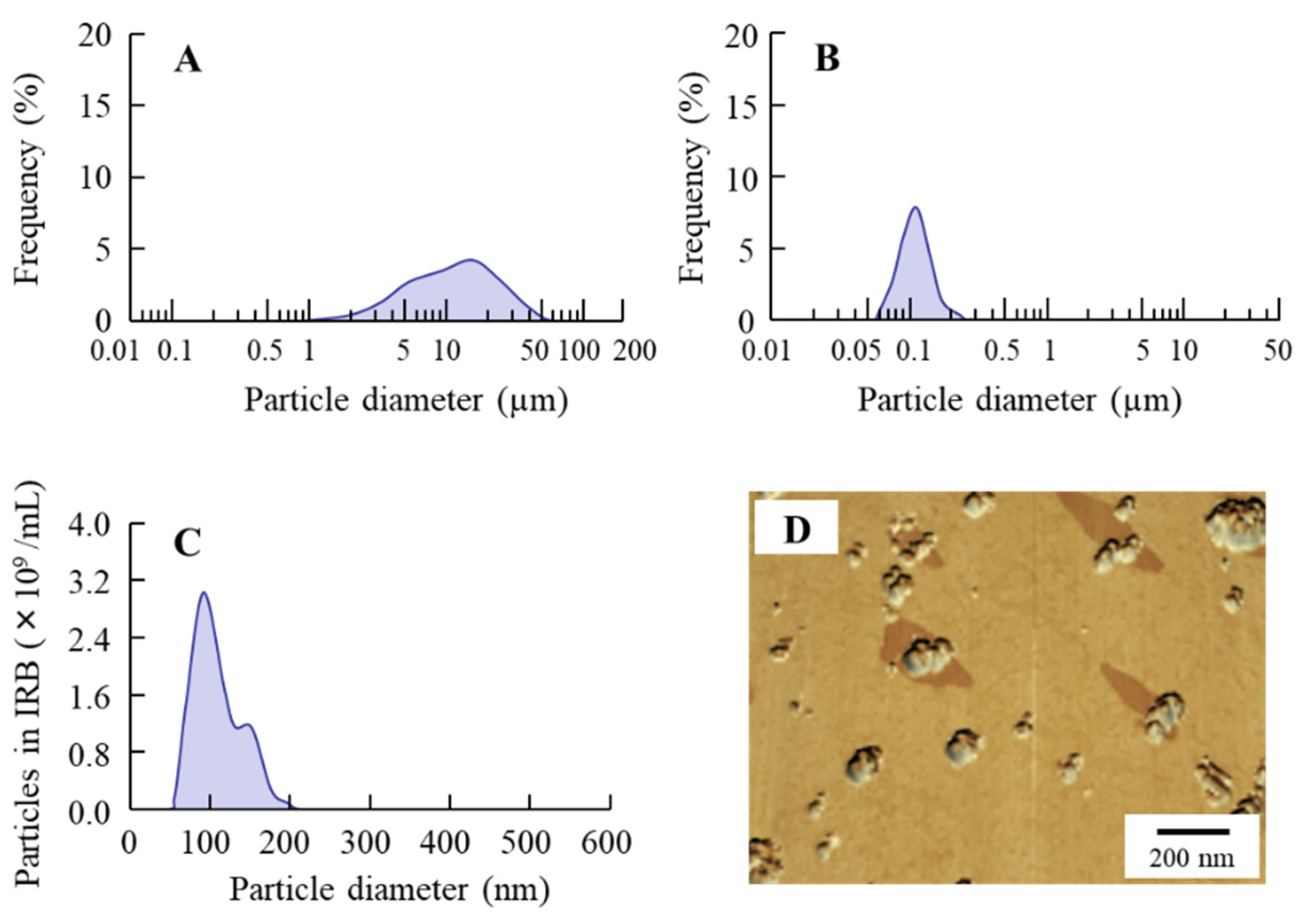
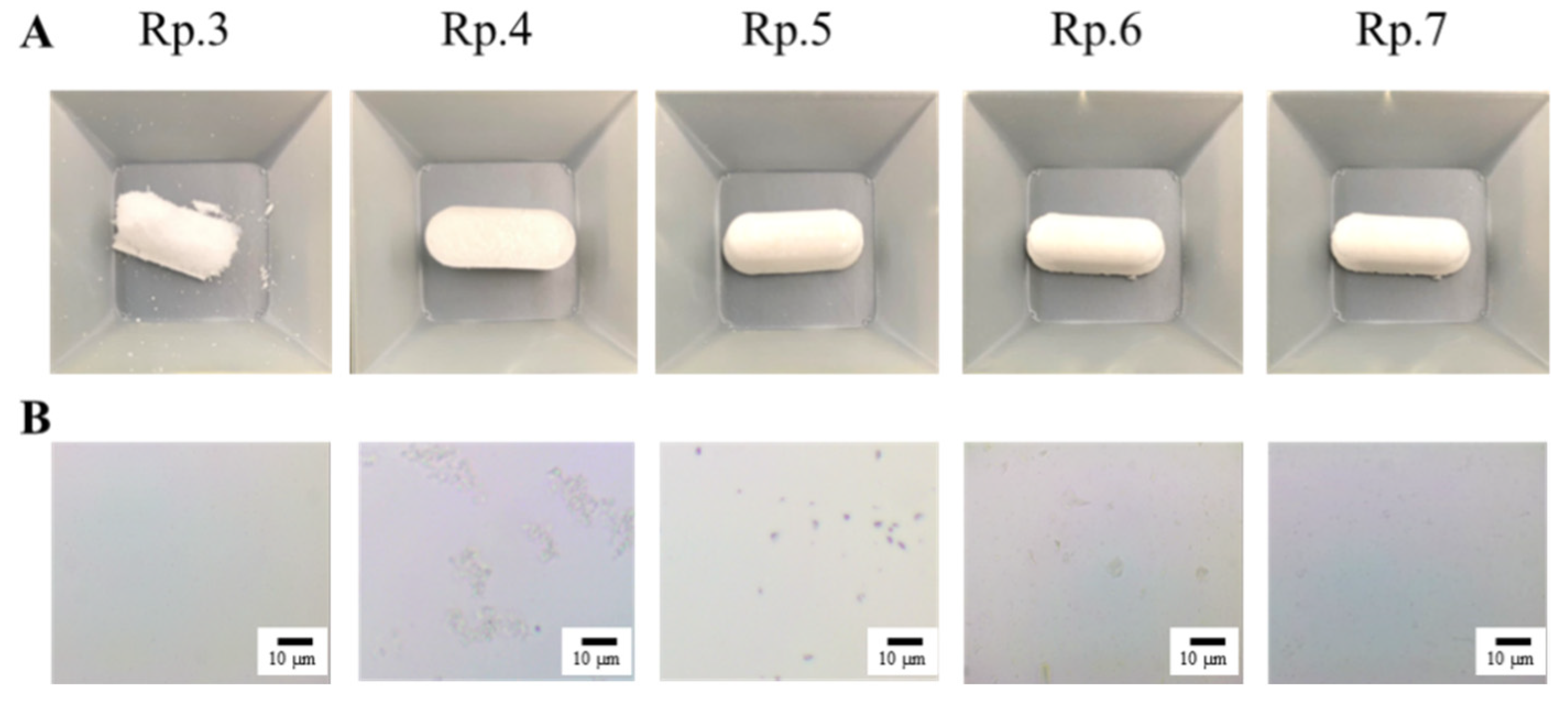
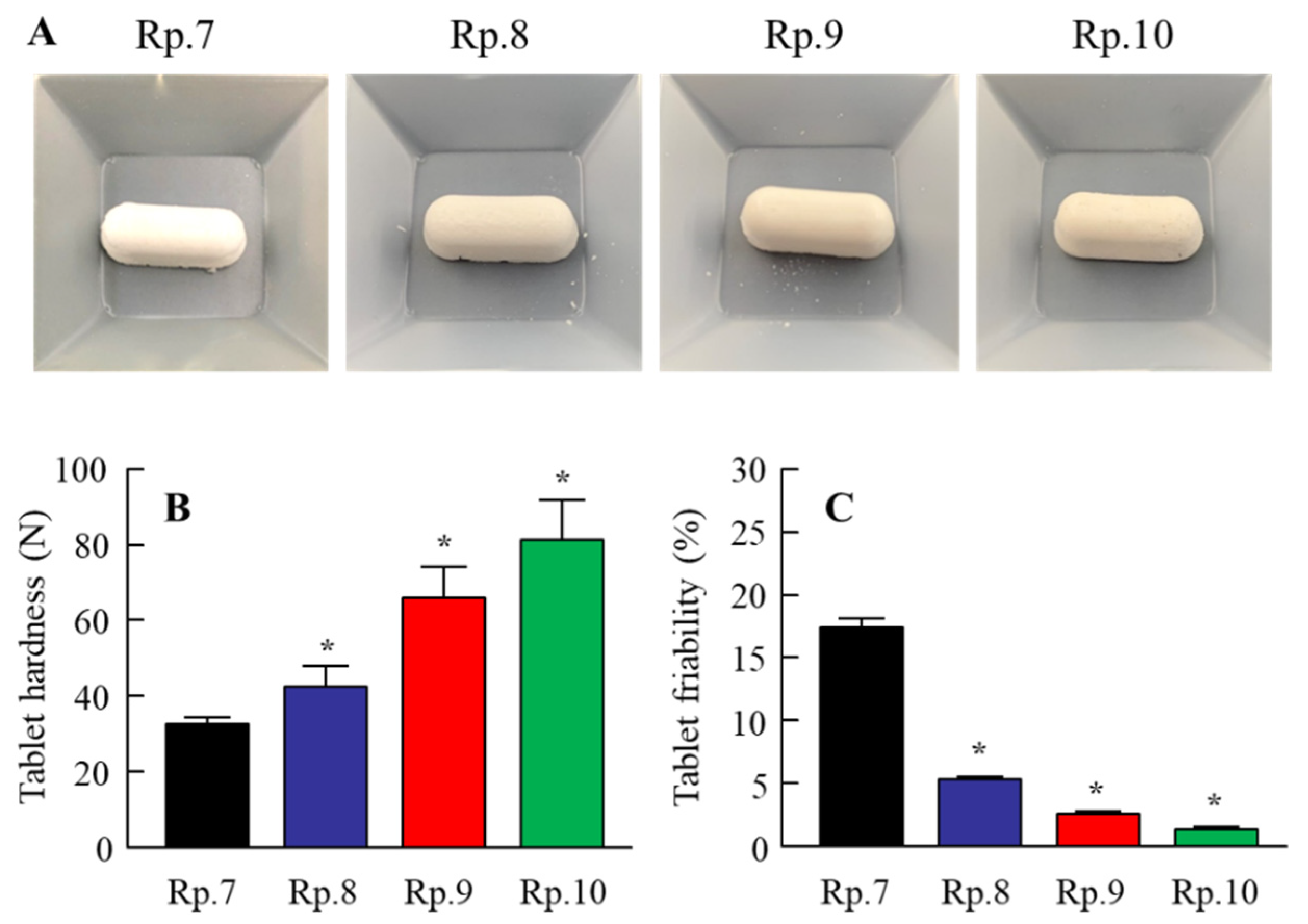

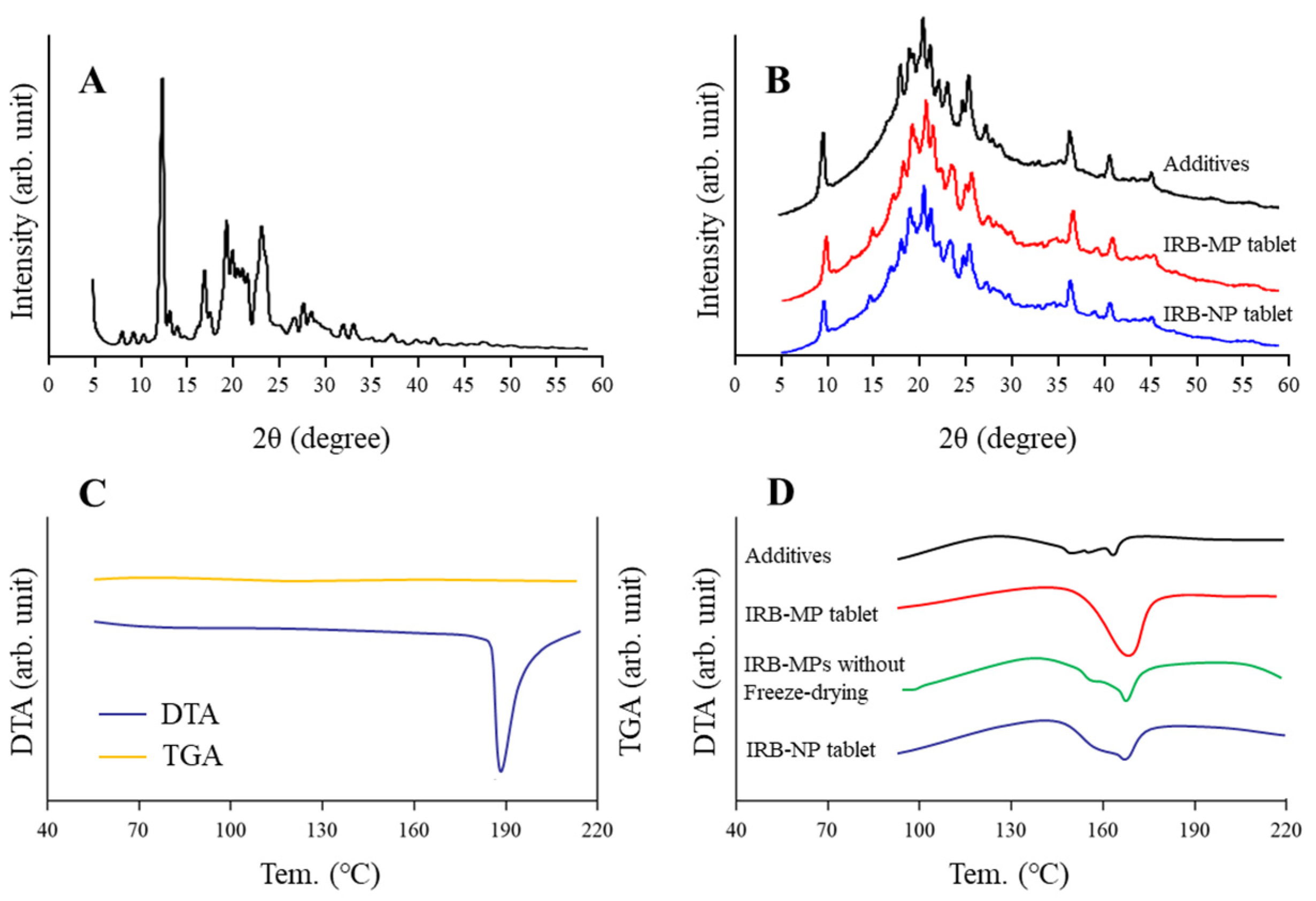

| Formulation | Content (w/w%) | Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| IRB | MC | HPβCD | Mannitol | PVP | Gum Arabic | |||
| Rp.1 (IRB-MP suspensions) | 1 | 0.5 | 5 | ― | ||||
| IRB-MP tablet | 1 | 0.5 | 5 | 4 | 0.4 | 10 | Freeze-drying | |
| Rp.2 (IRB-NP suspensions) | 1 | 0.5 | 5 | Bead mill | ||||
| IRB-NP tablet | Rp.3 | 1 | 0.5 | 5 | 4 | Bead mill, Freeze-drying | ||
| Rp.4 | 1 | 0.5 | 5 | 0.4 | Bead mill, Freeze-drying | |||
| Rp.5 | 1 | 0.5 | 5 | 6 | Bead mill, Freeze-drying | |||
| Rp.6 | 1 | 0.5 | 5 | 4 | 6 | Bead mill, Freeze-drying | ||
| Rp.7 | 1 | 0.5 | 5 | 4 | 0.4 | 6 | Bead mill, Freeze-drying | |
| Rp.8 | 1 | 0.5 | 5 | 4 | 0.4 | 10 | Bead mill, Freeze-drying | |
| Rp.9 | 1 | 0.5 | 5 | 4 | 0.4 | 12 | Bead mill, Freeze-drying | |
| Rp.10 | 1 | 0.5 | 5 | 4 | 0.4 | 16 | Bead mill, Freeze-drying | |
| Formulation | Particle Size | Solubility | Viscosity | Zeta Potentials | ||
|---|---|---|---|---|---|---|
| Mean | Range | µm | Pa∙s | mV | ||
| Rp.1 (IRB-MP suspensions) | 4.89 µm | 0.51–48 µm | 145 ± 5.6 | 2.7 ± 0.3 * | −45.8 ± 1.0 | |
| IRB-MP tablet | 5.61 µm | 0.50–57 µm | 142 ± 5.7 | 12.5 ± 0.9 | −44.1 ± 2.5 | |
| Rp.2 (IRB-NP suspensions) | 87 nm * | 50–202 nm | 281 ± 4.9 * | 2.9 ± 0.2 * | −45.8 ± 1.0 | |
| IRB-NP tablet | Rp.3 | 153 nm * | 58–380 nm | 338 ± 6.8 * | 1.3 ± 0.2 * | −45.2 ± 0.6 |
| Rp.4 | 122 nm * | 53–392 nm | 337 ± 6.5 * | 1.1 ± 0.1 * | −44.3 ± 0.7 | |
| Rp.5 | 143 nm * | 55–426 nm | 327 ± 7.1 * | 5.4 ± 0.6 * | −44.2 ± 0.6 | |
| Rp.6 | 147 nm * | 55–353 nm | 338 ± 6.2 * | 7.2 ± 0.6 * | −43.8 ± 1.2 | |
| Rp.7 | 112 nm * | 59–333 nm | 336 ± 5.9 * | 8.3 ± 0.7 * | −44.4 ± 0.5 | |
| Rp.8 | 118 nm * | 55–345 nm | 338 ± 5.1 * | 12.0 ± 0.8 | −40.6 ± 0.6 * | |
| Rp.9 | 119 nm * | 53–346 nm | 340 ± 6.3 * | 18.9 ± 1.2 * | −39.7 ± 0.5 * | |
| Rp.10 | 117 nm * | 52–345 nm | 325 ± 6.5 * | 36.7 ± 2.3 * | −38.4 ± 0.9 * | |
| Formulation | IRB-MP Tablet | Rp.2 | Rp.7 | Rp.8 | Rp.9 | Rp.10 |
|---|---|---|---|---|---|---|
| Mean particle size (range) | 5.11 µm | 136 nm | 131 nm | 138 nm | 139 nm | 137 nm |
| (0.61–57 µm) | (57–228 nm) | (65–359 nm) | (61–348 nm) | (59–341 nm) | (60–338 nm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Ogata, F.; Ike, A.; Shimomae, Y.; Osako, H.; Nakazawa, Y.; Yamamoto, N.; Kawasaki, N. Oral Formulation Based on Irbesartan Nanocrystals Improve Drug Solubility, Absorbability, and Efficacy. Pharmaceutics 2022, 14, 387. https://doi.org/10.3390/pharmaceutics14020387
Nagai N, Ogata F, Ike A, Shimomae Y, Osako H, Nakazawa Y, Yamamoto N, Kawasaki N. Oral Formulation Based on Irbesartan Nanocrystals Improve Drug Solubility, Absorbability, and Efficacy. Pharmaceutics. 2022; 14(2):387. https://doi.org/10.3390/pharmaceutics14020387
Chicago/Turabian StyleNagai, Noriaki, Fumihiko Ogata, Ayari Ike, Yurisa Shimomae, Hanano Osako, Yosuke Nakazawa, Naoki Yamamoto, and Naohito Kawasaki. 2022. "Oral Formulation Based on Irbesartan Nanocrystals Improve Drug Solubility, Absorbability, and Efficacy" Pharmaceutics 14, no. 2: 387. https://doi.org/10.3390/pharmaceutics14020387
APA StyleNagai, N., Ogata, F., Ike, A., Shimomae, Y., Osako, H., Nakazawa, Y., Yamamoto, N., & Kawasaki, N. (2022). Oral Formulation Based on Irbesartan Nanocrystals Improve Drug Solubility, Absorbability, and Efficacy. Pharmaceutics, 14(2), 387. https://doi.org/10.3390/pharmaceutics14020387







