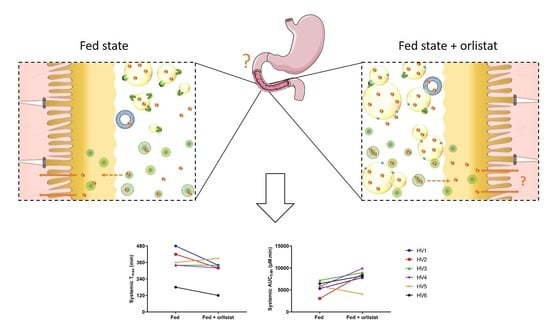
The Influence of Fed State Lipolysis Inhibition on the Intraluminal Behaviour and Absorption of Fenofibrate from a Lipid-Based Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Clinical Study
2.2.1. Study Medication
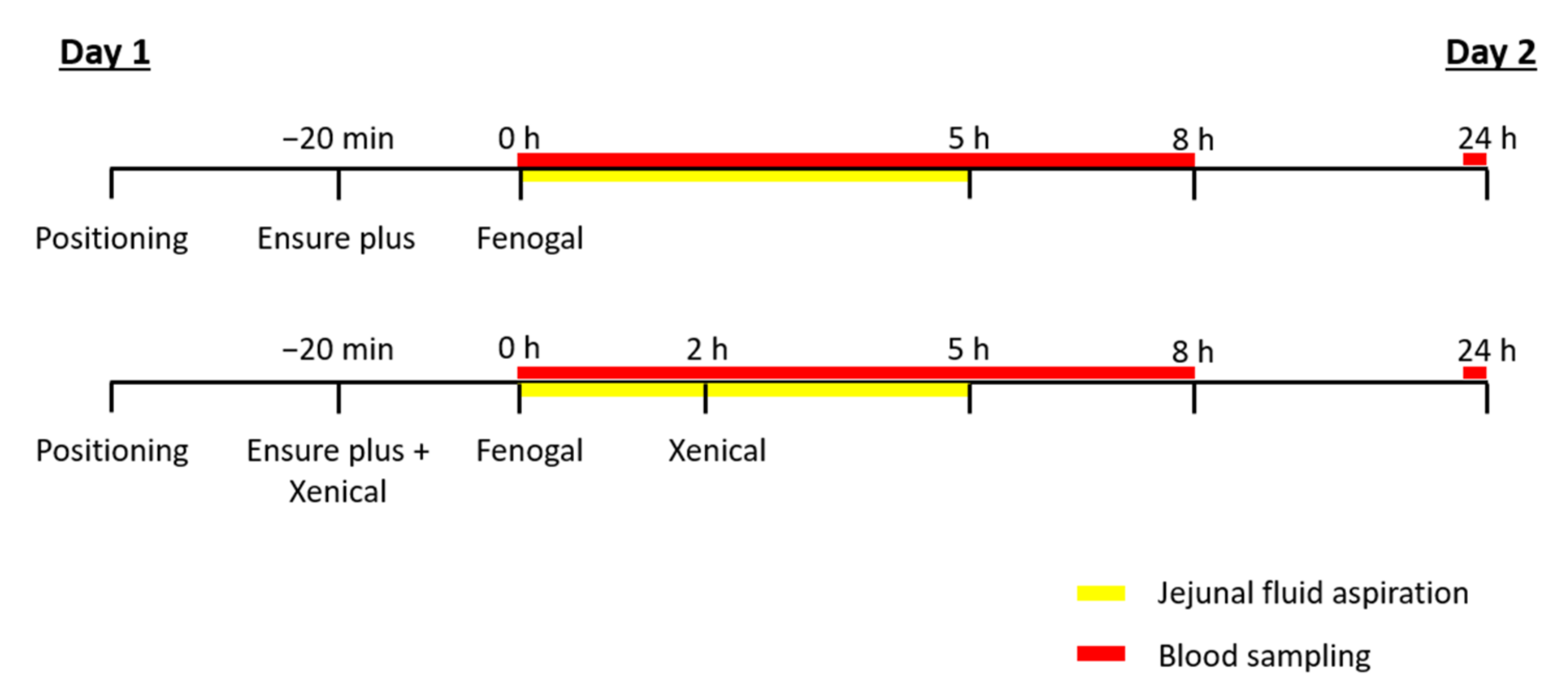
2.2.2. Study Setup
- —
- Oral administration of one capsule of Fenogal (200 mg of fenofibrate) in the fed state.
- —
- Oral administration of one capsule of Fenogal (200 mg of fenofibrate) and two capsules of Xenical (2 × 120 mg of orlistat) in the fed state.
2.3. Sample Analysis
2.3.1. Jejunal Samples
2.3.2. Plasma Samples
2.4. Data Analysis
3. Results and Discussion
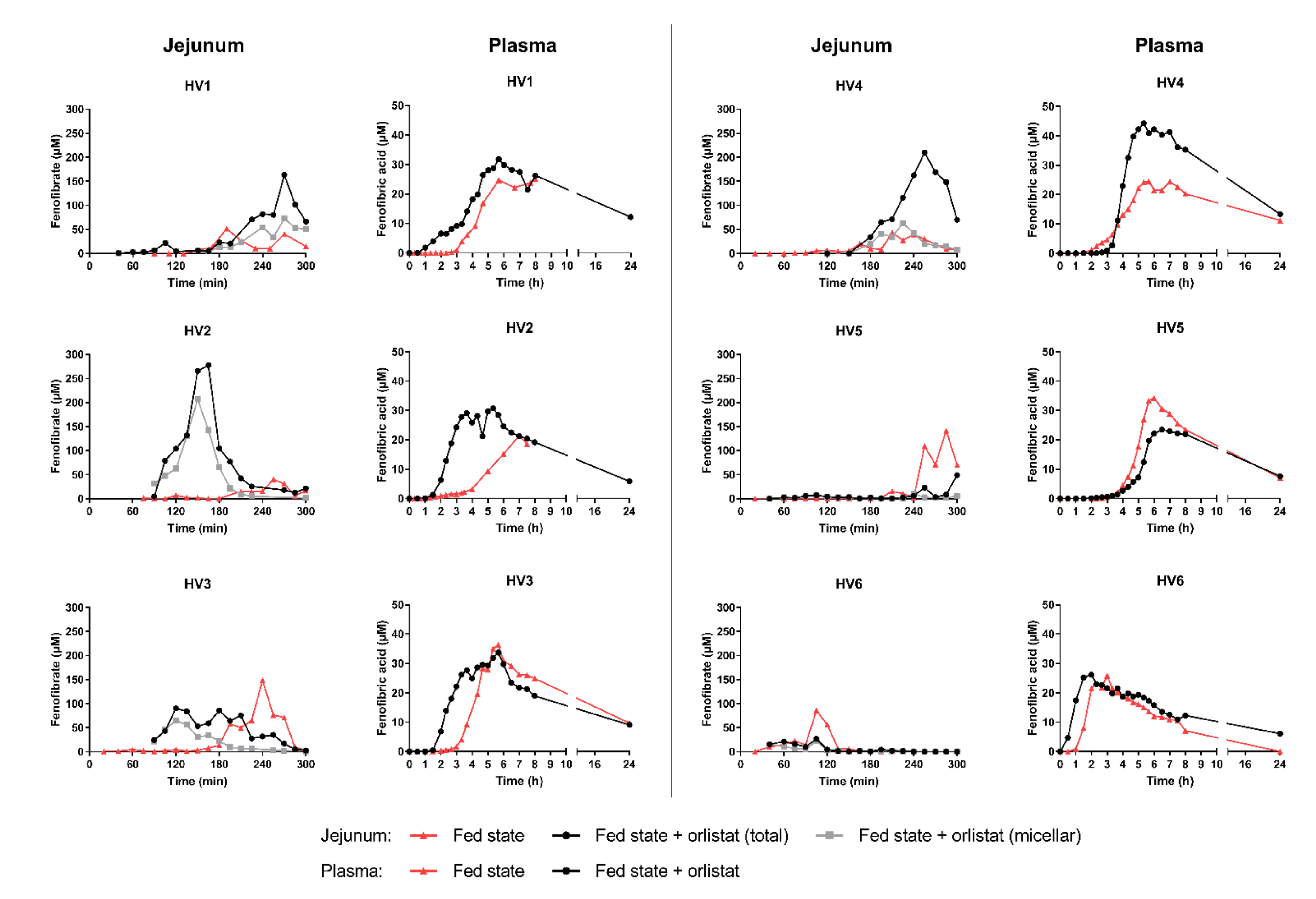
3.1. Effect on the Onset of Absorption
3.2. Effect on the Extent of Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauron, R.; Wilkins, M.; Jessent, V.; Dubois, A.; Maillot, C.; Weil, A. Absence of a food effect with a 145 mg nanoparticle fenofibrate tablet formulation. Int. J. Clin. Pharmacol. Ther. 2006, 44, 64–70. [Google Scholar] [CrossRef]
- Guichard, J.P.; Blouquin, P.; Qing, Y. A new formulation of fenofibrate: Suprabioavailable tablets. Curr. Med. Res. Opin. 2000, 16, 134–138. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef]
- Hens, B.; Brouwers, J.; Corsetti, M.; Augustijns, P. Gastrointestinal behavior of nano- and microsized fenofibrate: In vivo evaluation in man and in vitro simulation by assessment of the permeation potential. Eur. J. Pharm. Sci. 2015, 77, 40–47. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; De Niet, S.; Lebrun, S.; Tremege, M.; Rennie, T.W.; Coffiner, M.; Streel, B.; Cahay, B. The lidose hard capsule formulation of fenofibrate is suprabioavailable compared to the nanoparticle tablet formulation under high-fat fed conditions. J. Pharm. Pharm. Sci. 2015, 18, 61–67. [Google Scholar] [CrossRef][Green Version]
- Fernandez, S.; Rodier, J.D.; Ritter, N.; Mahler, B.; Demarne, F.; Carrière, F.; Jannin, V. Lipolysis of the semi-solid self-emulsifying excipient Gelucire® 44/14 by digestive lipases. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2008, 1781, 367–375. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Yeap, Y.Y.; Anby, M.U.; Pouton, C.W.; Porter, C.J.H. Lipid-based formulations and drug supersaturation: Harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm. Res. 2013, 30, 2976–2992. [Google Scholar] [CrossRef]
- O’Driscoll, C.M.; Griffin, B.T. Biopharmaceutical challenges associated with drugs with low aqueous solubility-The potential impact of lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 617–624. [Google Scholar] [CrossRef]
- Christiansen, M.L.; Holm, R.; Kristensen, J.; Kreilgaard, M.; Jacobsen, J.; Abrahamsson, B.; Müllertz, A. Cinnarizine food-effects in beagle dogs can be avoided by administration in a Self Nano Emulsifying Drug Delivery System (SNEDDS). Eur. J. Pharm. Sci. 2014, 57, 164–172. [Google Scholar] [CrossRef]
- Fernandez, S.; Chevrier, S.; Ritter, N.; Mahler, B.; Demarne, F.; Carrière, F.; Jannin, V. In vitro gastrointestinal lipolysis of four formulations of piroxicam and cinnarizine with the self emulsifying excipients Labrasol® and Gelucire® 44/14. Pharm. Res. 2009, 26, 1901–1910. [Google Scholar] [CrossRef]
- Lüthi-Peng, Q.; Märki, H.P.; Hadváry, P. Identification of the active-site serine in human pancreatic lipase by chemical modification with tetrahydrolipstatin. FEBS Lett. 1992, 299, 111–115. [Google Scholar] [CrossRef]
- Gargouri, Y.; Chahinian, H.; Moreau, H.; Ransac, S.; Verger, R. Inactivation of pancreatic and gastric lipases by THL and C12:0-TNB: A kinetic study with emulsified tributyrin. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1991, 1085, 322–328. [Google Scholar] [CrossRef]
- Carrière, F.; Renou, C.; Ransac, S.; Lopez, V.; De Caro, J.; Ferrato, F.; De Caro, A.; Fleury, A.; Sanwald-Ducray, P.; Lengsfeld, H.; et al. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G16–G28. [Google Scholar] [CrossRef]
- Bénarouche, A.; Point, V.; Carrière, F.; Cavalier, J.F. Using the reversible inhibition of gastric lipase by Orlistat for investigating simultaneously lipase adsorption and substrate hydrolysis at the lipid-water interface. Biochimie 2014, 101, 221–231. [Google Scholar] [CrossRef]
- Borgström, B. Mode of action of tetrahydrolipstatin: A derivative of the naturally occurring lipase inhibitor lipstatin. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1988, 962, 308–316. [Google Scholar] [CrossRef]
- Zhi, J.; Melia, A.T.; Eggers, H.; Joly, R.; Patel, I.H. Review of Limited Systemic Absorption of Orlistat, a Lipase Inhibitor, in Healthy Human Volunteers. J. Clin. Pharmacol. 1995, 35, 1103–1108. [Google Scholar] [CrossRef]
- Riethorst, D.; Baatsen, P.; Remijn, C.; Mitra, A.; Tack, J.; Brouwers, J.; Augustijns, P. An In-Depth View into Human Intestinal Fluid Colloids: Intersubject Variability in Relation to Composition. Mol. Pharm. 2016, 13, 3484–3493. [Google Scholar] [CrossRef]
- Schwizer, W.; Asal, K.; Kreiss, C.; Mettraux, C.; Borovicka, J.; Rémy, B.; Güzelhan, C.; Hartmann, D.; Fried, M. Role of lipase in the regulation of upper gastrointestinal function in humans. Am J. Physiol. 1997, 273, G612–620. [Google Scholar] [CrossRef]
- Chaikomin, R.; Russo, A.; Rayner, C.K.; Feinle-Bisset, C.; O’Donovan, D.G.; Horowitz, M.; Jones, K.L. Effects of lipase inhibition on gastric emptying and alcohol absorption in healthy subjects. Br. J. Nutr. 2006, 96, 883–887. [Google Scholar] [CrossRef][Green Version]
- O’Donovan, D.; Horowitz, M.; Russo, A.; Feinle-Bisset, C.; Murolo, N.; Gentilcore, D.; Wishart, J.M.; Morris, H.A.; Jones, K.L. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia 2004, 47, 2208–2214. [Google Scholar] [CrossRef]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef]
- Feinle, C.; O’Donovan, D.; Doran, S.; Andrews, J.M.; Wishart, J.; Chapman, I.; Horowitz, M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am. J. Physiol.—Gastrointest. Liver Physiol. 2003, 284, 798–807. [Google Scholar] [CrossRef]
- Riethorst, D.; Mitra, A.; Kesisoglou, F.; Xu, W.; Tack, J.; Brouwers, J.; Augustijns, P. Human intestinal fluid layer separation: The effect on colloidal structures & solubility of lipophilic compounds. Eur. J. Pharm. Biopharm. 2018, 129, 104–110. [Google Scholar] [CrossRef]
- Yeap, Y.Y.; Trevaskis, N.L.; Quach, T.; Tso, P.; Charman, W.N.; Porter, C.J.H. Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol. Pharm. 2013, 10, 1874–1889. [Google Scholar] [CrossRef]
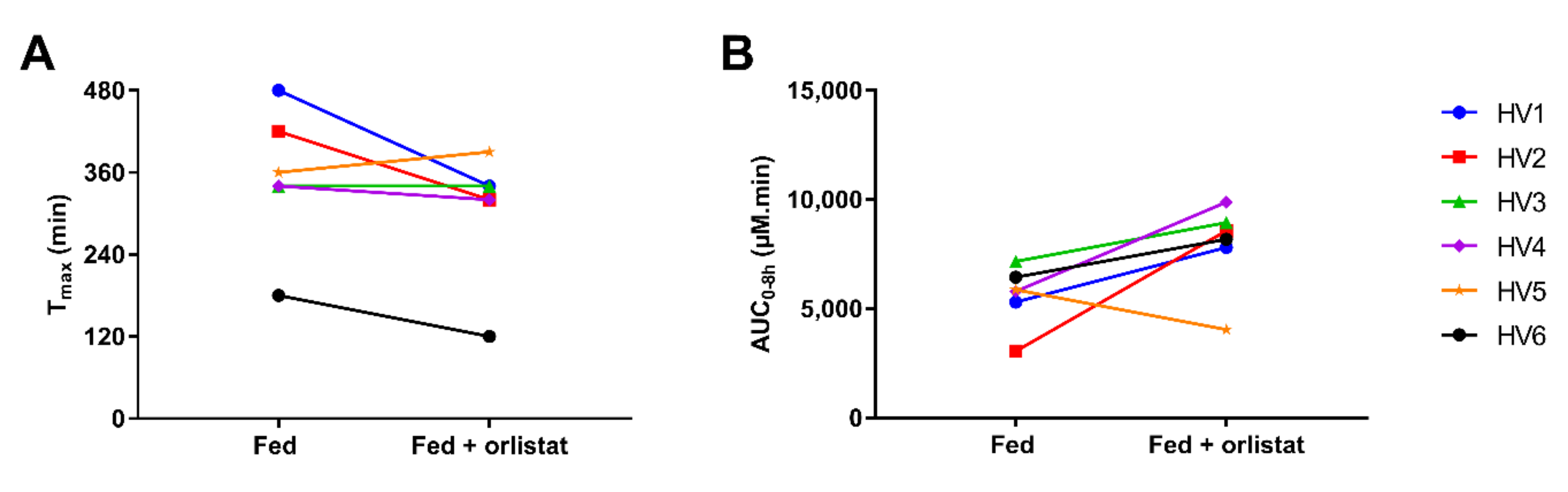
| Tmax (min) | Cmax (µM) | Lag Time to Reach 5% of Cmax (min) | AUC0–8h (µM.min) | |||||
|---|---|---|---|---|---|---|---|---|
| Healthy Volunteer | − Orlistat | + Orlistat | − Orlistat | + Orlistat | − Orlistat | + Orlistat | − Orlistat | + Orlistat |
| HV1 | 480 | 340 | 25.0 | 31.8 | 200 | 60 | 5306 | 7809 |
| HV2 | 420 | 320 | 21.4 | 30.7 | 140 | 120 | 3051 | 8570 |
| HV3 | 340 | 340 | 36.4 | 33.7 | 200 | 120 | 7167 | 8940 |
| HV4 | 340 | 320 | 24.6 | 44.3 | 140 | 200 | 5798 | 9882 |
| HV5 | 360 | 390 | 34.3 | 23.5 | 220 | 220 | 5866 | 4043 |
| HV6 | 180 | 120 | 25.9 | 26.2 | 90 | 30 | 6448 | 8184 |
| Median (range) | 350 (180–480) | 330 (120–390) | 25.5 (21.4–36.4) | 31.3 (23.5–44.3) | 170 (90–220) | 120 (30–220) | 5832 (3051–7167) | 8377 (4043–9882) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braeckmans, M.; Brouwers, J.; Riethorst, D.; Servais, C.; Tack, J.; Augustijns, P. The Influence of Fed State Lipolysis Inhibition on the Intraluminal Behaviour and Absorption of Fenofibrate from a Lipid-Based Formulation. Pharmaceutics 2022, 14, 119. https://doi.org/10.3390/pharmaceutics14010119
Braeckmans M, Brouwers J, Riethorst D, Servais C, Tack J, Augustijns P. The Influence of Fed State Lipolysis Inhibition on the Intraluminal Behaviour and Absorption of Fenofibrate from a Lipid-Based Formulation. Pharmaceutics. 2022; 14(1):119. https://doi.org/10.3390/pharmaceutics14010119
Chicago/Turabian StyleBraeckmans, Marlies, Joachim Brouwers, Danny Riethorst, Cécile Servais, Jan Tack, and Patrick Augustijns. 2022. "The Influence of Fed State Lipolysis Inhibition on the Intraluminal Behaviour and Absorption of Fenofibrate from a Lipid-Based Formulation" Pharmaceutics 14, no. 1: 119. https://doi.org/10.3390/pharmaceutics14010119
APA StyleBraeckmans, M., Brouwers, J., Riethorst, D., Servais, C., Tack, J., & Augustijns, P. (2022). The Influence of Fed State Lipolysis Inhibition on the Intraluminal Behaviour and Absorption of Fenofibrate from a Lipid-Based Formulation. Pharmaceutics, 14(1), 119. https://doi.org/10.3390/pharmaceutics14010119