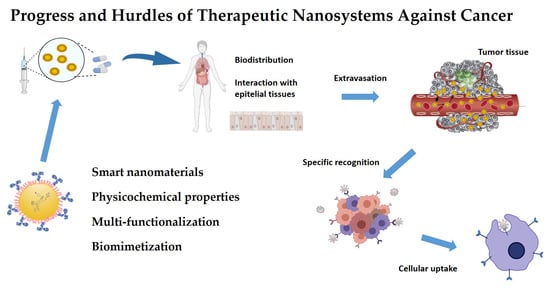
Progress and Hurdles of Therapeutic Nanosystems against Cancer
Abstract
:1. Introduction
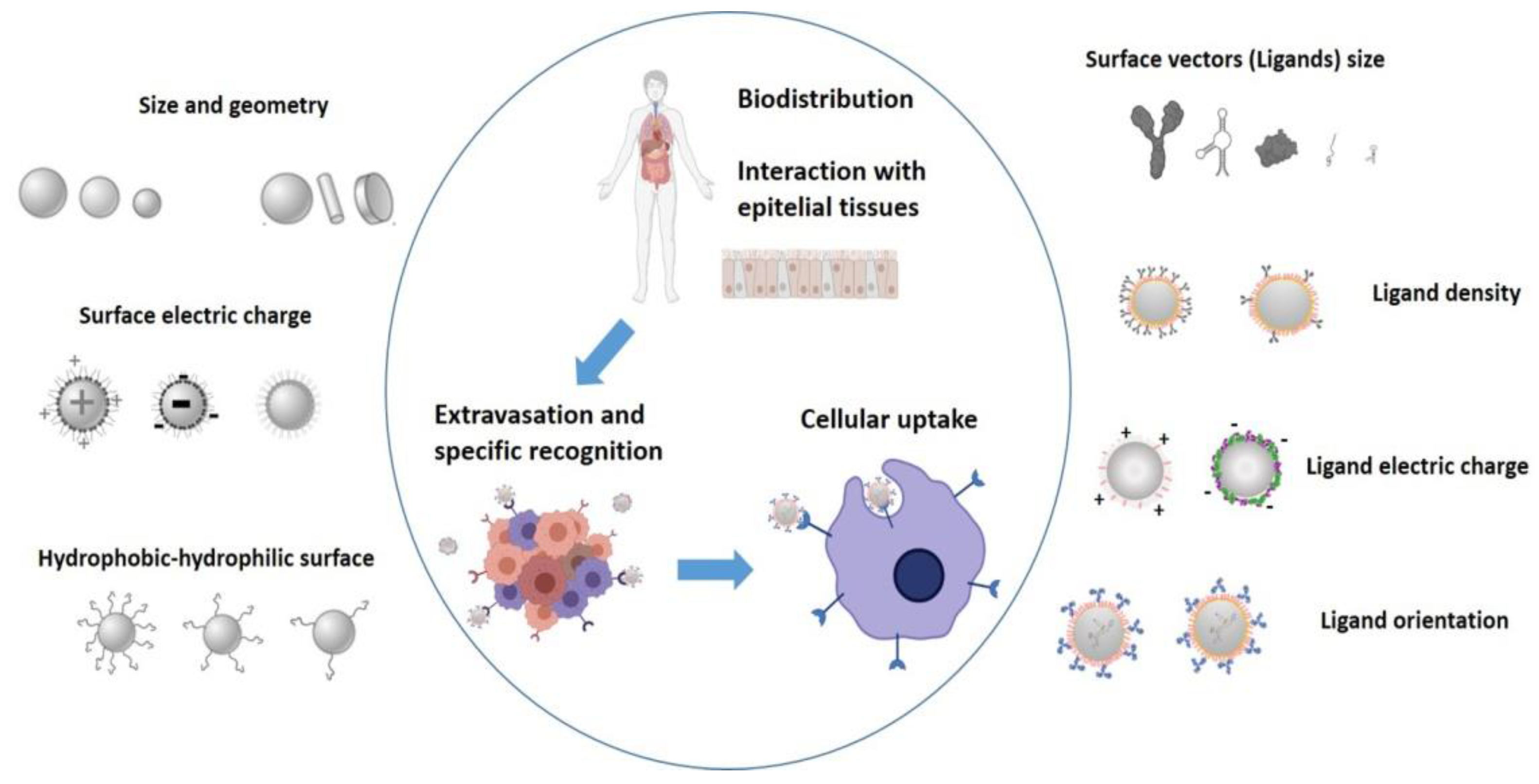
2. Factors Influencing Tumour Treatment with Nanosystems
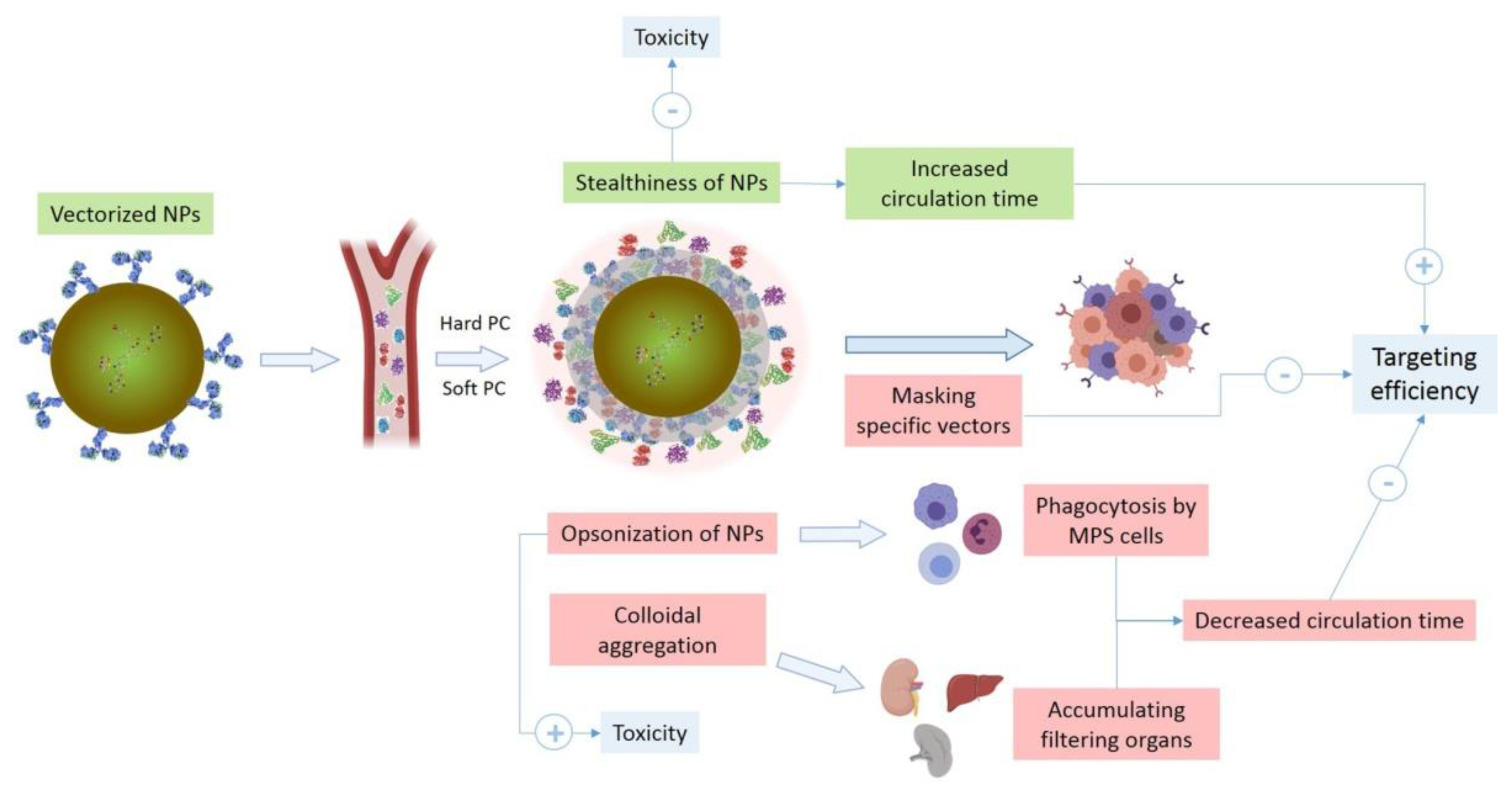
2.1. Physicochemical Properties of Nanosystems and Protein Corona Formation
2.2. Processes and Biological Barriers of the Tumour Microenvironment
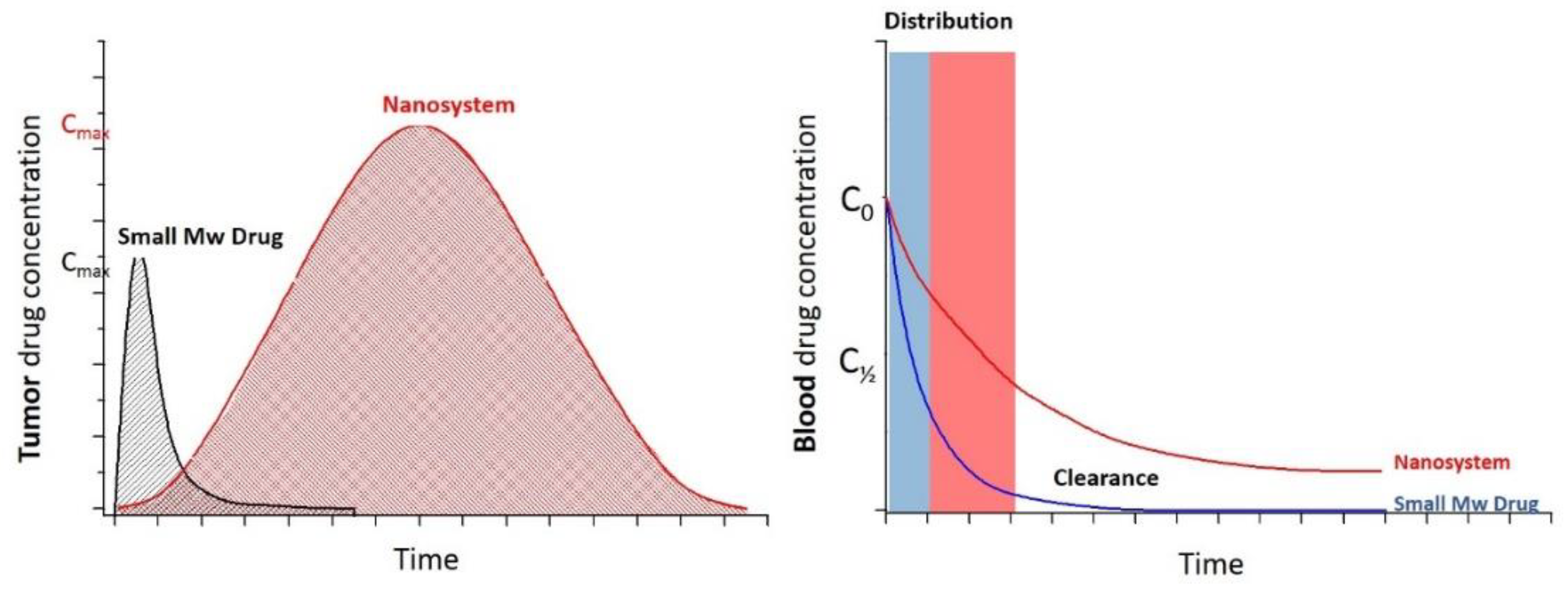
2.3. Drug Delivery: Passive and Active Targeting
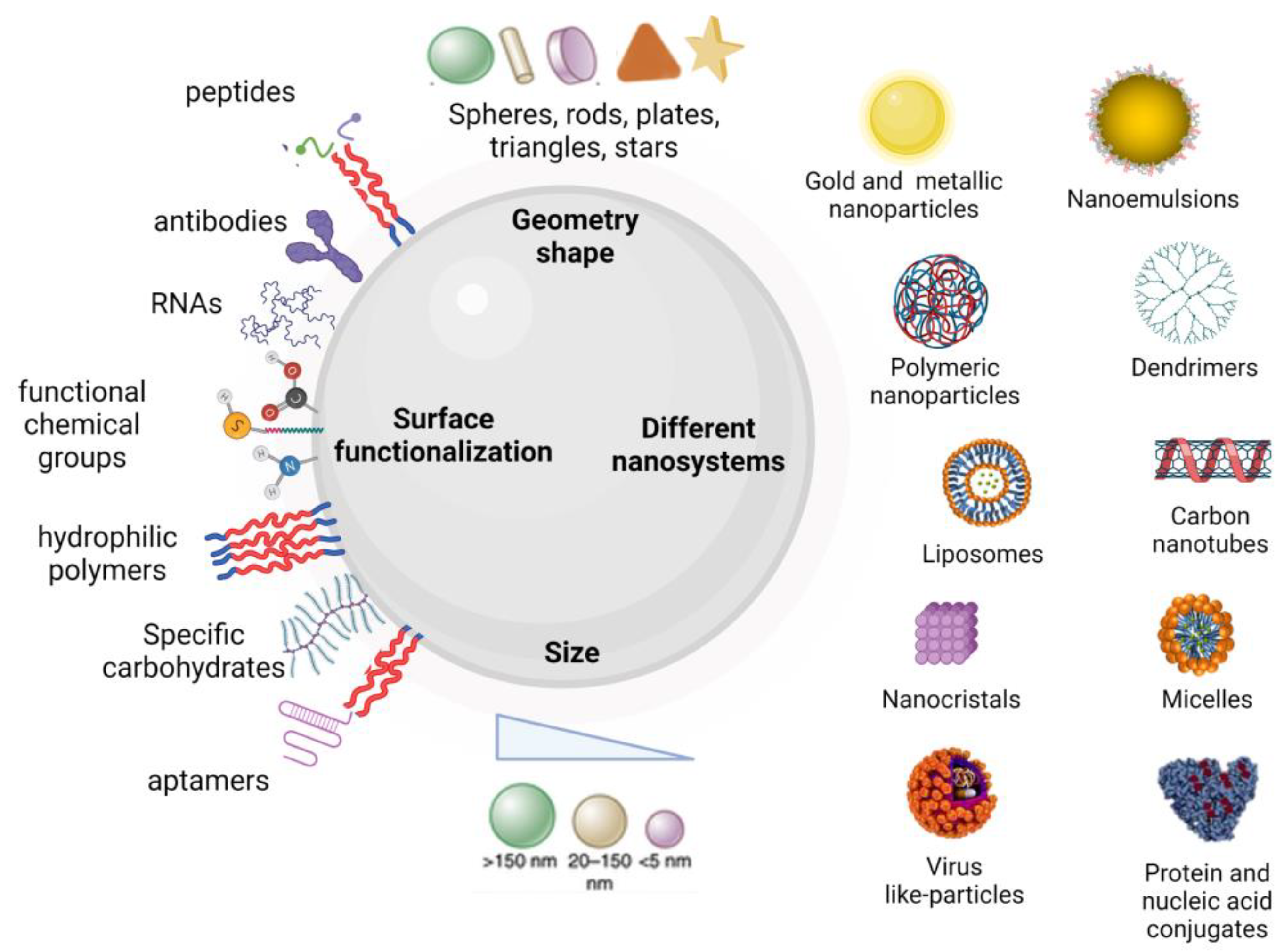
3. Nanosystem Design Strategies for Overcoming the Hurdles
3.1. Control of Nanosystem Physicochemical Properties
3.2. Biomimetism
- (1)
- Functionalization with a hydrophilic and non-ionic polymer such as polyethylene glycol (PEG). The ethylene glycol units build up a close bond with water molecules, making up a hydrophilic layer that greatly protects the NPs from aggregation, phagocytosis, opsonization and subsequent removal by MPS. This leads to an improvement in the stability of NPs in biological fluids, prolonging their circulating lives. Although nanosystems can also be functionalized with materials that have similar protective effects, such as poloxamers (Pluronic®), poloxamines (Tetronic®), polyvinyl alcohol, poly (amino acids) or polysaccharides, PEG remains the most frequently used. The length and density of PEG determines the targeting ability of the NP, as these properties will mediate the adsorption of plasma proteins. The PEG coating is also frequently used to mask the net positive charge on the surface of nanomaterials, preventing their interaction with negatively charged cell membranes. However, PEG functionalization can lead to immunological problems since non-human pegylated enzymes trigger an anti-PEG antibody-mediated response. This can make it more difficult for nanosystems to be effectively delivered, as they will exhibit a shorter half-life, faster clearance, or prolonged loss of blood circulation. Aggarwal et al. [63] reported that some plasma proteins (e.g., Apolipoprotein E, IgG, etc.) can be adsorbed on PEG-coated surfaces, which shows that this coating does not totally prevent protein adsorption. Muro et al. used hybrid ion surface ligands as an alternative to PEG for reducing PC formation [64]. Strong electrostatic interactions between water molecules and bipolar ligands generate highly stable NP, while minimizing non-specific interactions with biomolecules. Furthermore, Moyano et al. [65] showed that with certain hybrid ion ligands an almost complete inhibition of PC formation occurs in some specific cases, leading to prolonged NP circulation times [38]. For all this, pegylation is still a challenge in the field of nanoformulation [6,8,12,66,67].
- (2)
- In vitro binding of specific proteins to the surface. Ja-Hyoung et al. carried out a pre-coating of NPs with the human epidermal growth factor receptor-type 2 (HER2) protein by combining it with the glutathione-s-transferase construct. They demonstrated that the formation of a protective protein layer can reduce the adsorption of serum proteins while maintaining the selective capacity of the NP [68]. Human serum albumin (HSA) is considered a dysopsonin, i.e., a molecule that prevents sequestration by MPS, promoting a prolonged period of blood circulation. Thiele et al. showed that the adsorption of HSA onto polystyrene microparticles inhibits phagocytosis by dendritic cells [69]. In addition, albumin accumulates in malignant and inflamed tissues and serves as the principal nutrient for tumour growth. Thus, its adsorption favours NP internalization in the target cells [70].
- (3)
- Binding of peptides to NP surfaces. Discher et al. computationally designed CD47 peptides from human CD47, and attached them to the surface of NPs, which prevented NP phagocytosis by macrophages [71].
- (4)
- Coating of NPs with cell membranes. Membranes extracted from autologous leukocytes and red blood cells (RBCs) provide the NP surface with a biomimetic character that substantially prolongs its circulation in vivo [72,73,74]. Tasciotti et al. covered the NPs with leukocyte membranes, achieving approximately a ten-fold decrease in IgG and albumin adsorption onto the NP surface [8,75].
3.3. Active Targeting
- (1)
- Using several ECM components: Arg-Gly-Asp peptide (RGD), which recognizes the integrin αvβ3 (transmembrane receptor that works as a tumour-specific marker of angiogenic activity) has been attached to NPs to specifically target angiogenic vessels, increasing their specific accumulation at the tumour site, and thus reducing toxicity [79]. Functionalization with ligands that target EGFR is carried out with monoclonal antibodies, fragments (cetuximab, trastuzumab or panitumumab) or endogenous ligands such as epidermal growth factor (EGF). This potentially increases the EPR effect in different tumour types, as demonstrated by Zalba et al. [80]. They developed EGF-conjugated liposomes targeted against EGFR, causing a significant decrease in IC50 oxaliplatin in EGFR-positive colorectal cancer cell lines [29]. Other nanocarriers have been designed to inhibit the expression of matrix metalloproteinases (MMPs), considered as tumour biomarkers. They integrate MMP substrates (collagen, gelatin, fibrinogen, etc.). Synthetic substrates sensitive to MMP are easy to incorporate and, at the same time, offer selectivity and sensitivity. However, the responsiveness of NPs to MMP varies with the peptides used [32,81]. Functionalization with HA (the main component of ECM) leads to the targeting of the residual sugar of CD44 receptors [82], which has an increased expression in a large number of tumours. Liu et al. demonstrated that HA-protected NPs of 200 nm showed an optimal EPR effect in mice with 4T1 mouse breast tumours [83]. This provided a dramatic suppression of primary tumour growth (95%), and an important inhibition of tumour metastasis (90%). Furthermore, multifunctional dual-orientation nanosystems with EGFR and CD44 have been recently developed for the improvement of the EPR effect. Although the dual combination of EGFR and HA has not been widely studied yet, it has appeared as an effective anti-tumoural nanotherapy to reduce the uncertainty of the single target [84,85].
- (2)
- Using tumour-specific pathophysiological conditions, such as the hypoxia. Small interfering RNA (siRNA) has been administered and vectored against HIF-1α [86]. In another study, NPs were functionalized with Saposin C, a lysosomal protein that binds to phosphatidyl serine (a specific molecular marker of hypoxia and apoptosis) of hypoxic TME. The resulting NPs showed remarkable therapeutic efficacy in the tumour model, crossing the blood–brain barrier, exhibiting specific retention in tumour tissue, and sensitizing hypoxic cells [87].
3.4. Adaptive Nanomedicine
- (1)
- Vascular agents such as bradykinin, serotonin, histamine, prostaglandins, nitrous oxide or tumour necrosis factor α (TNFα) induce a state similar to inflammation. This improves the vascular permeability of the therapeutic NP. All these vasomodulators are toxic if they are administered freely. However, such a toxicity is drastically reduced when they are encapsulated in nanosystems. Another strategy is the remodelling of the tumour vasculature. Jain et al. investigated the administration of antiangiogenic or angiogenic agents such as VEGF [88,89] and the functionalization of nanosystems with anti-VEGF receptor antibody [90]. This improved the transvascular delivery of NPs in murine tumour models [8,28,31].
- (2)
- Enzymatic agents, such as collagenase [91] and hyaluronidase [92,93], digest the physical structure of the ECM, reducing IFP in tumours, while direct induction of the apoptosis of TME cells can reduce pressure on the microvasculature in order to improve the depth of penetration of the nanosystems in the tumours [31]. Jain et al. used losartan, an angiotensin II receptor antagonist and anti-fibrotic agent that decompresses tumour vessels, increases tumour perfusion, and inhibits the synthesis of collagen I by fibroblasts associated with carcinoma. This improved the accumulation and efficiency of liposomes containing doxorubicin [94]. On the other hand, Chen et al. investigated a synergistic enzyme therapy that altered the tumour vasculature. Nanocarriers containing MMP-9-activatable doxorubicin (DOX) prodrug were combined with nanocarriers loaded with combetastatin A4 (CA4, carbonic anhydrase 4). CA4-loaded nanosystems altered tumour blood vasculature and selectively enhanced MMP-9 expression in tumours to promote doxorubicin accumulation, leading to the effective treatment of 4T1 and C26 tumours [95].
- (3)
- The use of external stimuli to physically modify a delivery site (by reconfiguring TME, increasing vessel leakage or destroying physical barriers in the TME) before or simultaneously with the delivery of nanosystems enhances the EPR effect. This includes radiation, ultrasound, hyperthermia and photodynamic therapy [10,29,31,96].
3.5. Complementary Diagnosis of the EPR Effect
4. Novel Multifunctional Nanotherapeutics
5. Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2019, 5, 100035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Fazal, S.; Miyako, E.; Matsumura, K.; Rajan, R. Avengers against cancer: A new era of nano-biomaterial-based therapeutics. Mater. Today 2021, 51, 317–349. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl Med. 2017, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, G. Nanomedicines against Cancer. Bachelor’s Thesis, Pharmacy Faculty, Complutense University, Madrid, Spain, 2018. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumours: Poor tumour-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumour targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.A. Development and Evaluation of Smart Polymeric and Lipidic Nanoparticles for Theranosis of Breast and Pancreatic Cancer. Ph.D. Thesis, University of Granada, Granada, Spain, 2020; p. 57. [Google Scholar]
- Digiacomo, L.; Pozzi, D.; Palchetti, S.; Zingoni, A.; Caracciolo, G. Impact of the protein corona on nanomaterial inmune response and targeting ability. WIREs Nanomed. Nanobiotechnol. 2020, 12, 1615. [Google Scholar] [CrossRef] [PubMed]
- Miclăuş, T.; Bochenkov, V.E.; Ogaki, R.; Howard, K.A.; Sutherland, D.S. Spatial Mapping and Quantification of Soft and Hard Protein Coronas at Silver Nanocubes. Nano Lett. 2014, 14, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Pisani, C.; Gaillard, J.C.; Odorico, M.; Nyalosaso, J.L.; Charnay, C.; Guari, Y.; Chopineau, J.; Devoisselle, M.; Armengaud, J.; Prat, O. The timeline of corona formation around silica nanocarriers highlights the role of the protein interactome. Nanoscale 2017, 9, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Neagu, M.; Piperigkou, Z.; Karamanou, K.; Engin, A.B.; Docea, A.O.; Constantin, C.; Negrei, C.; Nikitovic, D.; Tsatsakis, A. Protein bio-corona: Critical issue in immune nanotoxicology. Arch. Toxicol. 2017, 91, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Bewersdorff, T.; Glitscher, E.A.; Bergueiro, J.; Eravci, M.; Miceli, E.; Haase, A.; Calderón, M. The influence of shape and charge on protein corona composition in common gold nanostructures. Mater. Sci. Eng. C 2020, 117, 111270. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Obst, K.; Yealland, G.; Balzus, B.; Miceli, E.; Dimde, M.; Weise, C.; Evarci, M.; Bodmeier, R.; Haag, R.; Calderón, M.; et al. Protein Corona Formation on Colloidal Polymeric Nanoparticles and Polymeric Nanogels: Impact on Cellular Uptake, Toxicity, Immunogenicity, and Drug Release Properties. Biomacromolecules 2017, 18, 1762–1771. [Google Scholar] [CrossRef]
- Peigneux, A.; Glitscher, E.A.; Charbaji, R.; Weise, C.; Wedepohl, S.; Calderón, M.; Jimenez-Lopez, C.; Hedtrich, S. Protein corona formation and its influence on biomimetic magnetite nanoparticles. J. Mater. Chem. B 2020, 8, 4870–4882. [Google Scholar] [CrossRef]
- Park, S.J. Protein-Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles. Int. J. Nanomed. 2020, 15, 5783–5802. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gao, J.; Liu, Y.; Gao, J.; Yao, L.; Yang, X.; Liu, X.; He, B.; Hu, L.; Shi, J.; et al. Role of protein corona in the biological effect of nanomaterials: Investigating methods. TrAC Trends Anal. Chem. 2019, 118, 303–314. [Google Scholar] [CrossRef]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumour Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Romero, A. Transactivation Capacity from Gen pptg1 and Its Involvment in Tumourigenesis. Ph.D. Thesis, Seville University, Seville, Spain, 2015; p. 28. [Google Scholar]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumours. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumour Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumour phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaka, T.; Nakanishi, T.; Shanmugam, S.; Takahama, S.; Zhang, H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf. B Biointerfaces 2009, 71, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Serda, R.E.; Gu, J.; Bhavane, R.C.; Liu, X.; Chiappini, C.; Decuzzi, P.; Ferrari, M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials 2009, 30, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumour. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef]
- Srivastava, I.; Misra, S.K.; Ostadhossein, F.; Daza, E.; Singh, J.; Pan, D. Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells. Nano Res. 2017, 10, 3269–3284. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.I. Nanomedicines for Breast Cancer. Bachelor’s Thesis, Pharmacy Faculty, Complutense University, Madrid, Spain, 2015. [Google Scholar]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Falahati, M.; Attar, F.; Sharifi, M.; Haertlé, T.; Berret, J.F.; Khan, R.H.; Saboury, A.A. A health concern regarding the protein corona, aggregation and disaggregation. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 971–991. [Google Scholar] [CrossRef]
- Mosquera, J.; García, I.; Henriksen-Lacey, M.; Martínez-Calvo, M.; Dhanjani, M.; Mascareñas, J.L.; Liz-Marzán, L.M. Reversible Control of Protein Corona Formation on Gold Nanoparticles Using Host-Guest Interactions. ACS Nano 2020, 14, 5382–5391. [Google Scholar] [CrossRef]
- Xu, F.; Reiser, M.; Yu, X.; Gummuluru, S.; Wetzler, L.; Reinhard, B.M. Lipid-mediated targeting with membrane-wrapped nanoparticles in the presence of corona formation. ACS Nano 2016, 10, 1189–1200. [Google Scholar] [CrossRef] [Green Version]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de Almeida Roger, J.; Chemello, G.; Pasquaroli, S.; Mancini, L.; Olivotto, I.; Zoppellaro, G.; Ugolotti, L.; et al. Stealth iron oxide nanoparticles for organotropic drug targeting. Biomacromolecules 2019, 20, 1375–1384. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef] [PubMed]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.U.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein. J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.X.; Zhu, G.Y.; Lu, B.Y.; Zhang, C.L.; Peng, Q. Concentration-dependent protein adsorption at the nano-bio interfaces of polymeric nanoparticles and serum proteins. Nanomedicine 2017, 12, 2757–2769. [Google Scholar] [CrossRef]
- Bonvin, D.; Aschauer, U.; Alexander, D.; Chiappe, D.; Moniatte, M.; Hofmann, H.; Mionić Ebersold, M. Protein Corona: Impact of Lymph Versus Blood in a Complex In Vitro Environment. Small 2017, 13, 1700409. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Andreozzi, P.; Dal Magro, R.; Fiordaliso, F.; Corbelli, A.; Talamini, L.; Chinello, C.; Raimondo, F.; Magni, F.; Tringali, M.; et al. Evolution of Nanoparticle Protein Corona across the Blood-Brain Barrier. ACS Nano 2018, 12, 7292–7300. [Google Scholar] [CrossRef]
- Ho, Y.T.; Azman, N.; Loh, F.; Ong, G.; Engudar, G.; Kriz, S.A.; Kah, J. Protein Corona Formed from Different Blood Plasma Proteins Affects the Colloidal Stability of Nanoparticles Differently. Bioconjug. Chem. 2018, 29, 3923–3934. [Google Scholar] [CrossRef]
- Berardi, A.; Baldelli Bombelli, F. Oral delivery of nanoparticles—Let’s not forget about the protein corona. Expert Opin. Drug Deliv. 2019, 16, 563–566. [Google Scholar] [CrossRef] [Green Version]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Hartman, K.A.; Sherman, M.B.; De Rosa, E.; Kirui, D.K.; Salvatore, F.; Tasciotti, E. Unveiling the in Vivo Protein Corona of Circulating Leukocyte-like Carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef]
- Solorio-Rodríguez, A.; Escamilla-Rivera, V.; Uribe-Ramírez, M.; Chagolla, A.; Winkler, R.; García-Cuellar, C.M.; De Vizcaya-Ruiz, A. A comparison of the human and mouse protein corona profiles of functionalized SiO2 nanocarriers. Nanoscale 2017, 9, 13651–13660. [Google Scholar] [CrossRef]
- Raoufi, M.; Hajipour, M.J.; Kamali Shahri, S.M.; Schoen, I.; Linn, U.; Mahmoudi, M. Probing fibronectin conformation on a protein corona layer around nanoparticles. Nanoscale 2018, 10, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, V.; Bubis, J.A.; Solovyeva, E.M.; Gorshkov, M.V.; Kjeldsen, F. Protein corona formed on silver nanoparticles in blood plasma is highly selective and resistant to physicochemical changes of the solution. Environ. Sci. Nano 2019, 6, 1089–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Kostarelos, K. Time-evolution of in vivo protein corona onto blood-circulating PEGylated liposomal doxorubicin (DOXIL) nanoparticles. Nanoscale 2016, 8, 6948–6957. [Google Scholar] [CrossRef] [Green Version]
- Hadjidemetriou, M.; Al-Ahmady, Z.; Mazza, M.; Collins, R.F.; Dawson, K.; Kostarelos, K. In Vivo Biomolecule Corona around Blood-Circulating, Clinically Used and Antibody-Targeted Lipid Bilayer Nanoscale Vesicles. ACS Nano 2015, 9, 8142–8156. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; McAdam, S.; Garner, G.; Thackeray, C.; Knight, D.; Smith, D.; Al-Ahmady, Z.; Mazza, M.; Rogan, J.; Clamp, A.; et al. The Human In Vivo Biomolecule Corona onto PEGylated Liposomes: A Proof-of-Concept Clinical Study. Adv. Mater. 2019, 31, 1803335. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, D.; Caracciolo, G.; Digiacomo, L.; Colapicchioni, V.; Palchetti, S.; Capriotti, A.L.; Cavaliere, C.; Zenezini Chiozzi, R.; Puglisi, A.; Laganà, A. The biomolecular corona of nanoparticles in circulating biological media. Nanoscale 2015, 7, 13958–13966. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Muro, E.; Pons, T.; Lequeux, N.; Fragola, A.; Sanson, N.; Lenkei, Z.; Dubertret, B. Small and stable sulfobetaine zwitterionic quantum dots for functional live-cell imaging. J. Am. Chem Soc. 2010, 132, 4556–4557. [Google Scholar] [CrossRef]
- Moyano, D.F.; Saha, K.; Prakash, G.; Yan, B.; Kong, H.; Yazdani, M.; Rotello, V.M. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano 2014, 8, 6748–6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Moreno, P.; Buzón, P.; Boulaiz, H.; Peula-García, J.M.; Ortega-Vinuesa, J.L.; Luque, I.; Salvati, A.; Marchal, J.A. Balancing the effect of corona on therapeutic efficacy and macrophage uptake of lipid nanocapsules. Biomaterials 2015, 61, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Day, C.M.; Hickey, S.M.; Song, Y.; Plush, S.E.; Garg, S. Novel Tamoxifen Nanoformulations for Improving Breast Cancer Treatment: Old Wine in New Bottles. Molecules 2020, 25, 1182. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [Green Version]
- Thiele, L.; Diederichs, J.E.; Reszka, R.; Merkle, H.P.; Walter, E. Competitive Adsorption of Serum Proteins at Microparticles Affects Phagocytosis by Dendritic Cells. Biomaterials 2003, 24, 1409–1418. [Google Scholar] [CrossRef]
- Galisteo-González, F.; Molina-Bolívar, J.A.; Navarro, S.A.; Boulaiz, H.; Aguilera-Garrido, A.; Ramírez, A.; Marchal, J.A. Albumin-covered lipid nanocapsules exhibit enhanced uptake performance by breast-tumour cells. Colloid. Surf. B Biointerfaces 2018, 165, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal "Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Gnanasammandhan, M.K.; Xie, C.; Huang, K.; Cui, M.Y.; Chan, J.M. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale 2016, 8, 6981–6985. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajipour, M.J.; Raheb, J.; Akhavan, O.; Arjmand, S.; Mashinchian, O.; Rahman, M.; Abdolahad, M.; Serpooshan, V.; Laurent, S.; Mahmoudi, M. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale 2015, 7, 8978–8994. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, G.; Caputo, D.; Pozzi, D.; Colapicchioni, V.; Coppola, R. Size and charge of nanoparticles following incubation with human plasma of healthy and pancreatic cancer patients. Colloids Surf. B Biointerfaces 2014, 123, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Spitler, R.; Erfanzadeh, M.; Alkilany, A.M.; Mahmoudi, M. Protein corona: Opportunities and challenges. Int. J. Biochem. Cell Biol. 2016, 75, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Z.; Li, T.; Chen, L.; Lyu, J.; Li, C.; Lin, Y.; Hao, N.; Zhou, M.; Zhong, Z. Enhanced Therapeutic Effect of RGD-Modified Polymeric Micelles Loaded with Low-Dose Methotrexate and Nimesulide on Rheumatoid Arthritis. Theranostics 2019, 9, 708–722. [Google Scholar] [CrossRef]
- Zalba, S.; Contreras, A.M.; Merino, M.; Navarro, I.; de Ilarduya, C.T.; Trocóniz, I.F.; Koning, G.; Garrido, M.J. EGF-liposomes promote efficient EGFR targeting in xenograft colocarcinoma model. Nanomedicine 2016, 11, 465–477. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, L. Matrix metalloproteinase-sensitive Nanocarriers. Smart Pharm. Nanocarriers 2016, 3, 83–116. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Ad. Mater. 2019, 31, 1803549. [Google Scholar] [CrossRef]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control Release 2018, 278, 127–139. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 Dual-Targeted Multifunctional Hyaluronic Acid Nanogels Boost Protein Delivery to Ovarian and Breast Cancers In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Liang, Y.; Peng, J.; Li, N.; Yu-Wai-Man, C.; Wang, Q.; Xu, Y.; Wang, H.; Tagalakis, A.D.; Du, Z. Smart nanoparticles assembled by endogenous molecules for siRNA delivery and cancer therapy via CD44 and EGFR dual-targeting. Nanomedicine 2019, 15, 208–217. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.M.; Chu, Z.; Vallabhapurapu, S.D.; Sulaiman, M.K.; Kendler, A.; Rixe, O.; Warnick, R.E.; Franco, R.S.; Qi, X. Phosphatidylserine-selective targeting and anticancer effects of SapC-DOPS nanovesicles on brain tumours. Oncotarget 2014, 5, 7105–7118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K. Normalizing tumour microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumours. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsky, W.L.; Fukumura, D.; Gohongi, T.; Ancukiewcz, M.; Weich, H.A.; Torchilin, V.P.; Yuan, F.; Jain, R.K. Augmentation of transvascular transport of macromolecules and nanoparticles in tumours using vascular endothelial growth factor. Cancer Res. 1999, 59, 4129–4135. [Google Scholar] [PubMed]
- Choi, J.; Credit, K.; Henderson, K.; Deverkadra, R.; He, Z.; Wiig, H.; Vanpelt, H.; Flessner, M.F. Intraperitoneal immunotherapy for metastatic ovarian carcinoma: Resistance of intratumoural collagen to antibody penetration. Clin. Cancer Res. 2006, 12, 1906–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Han, X.; Xu, J.; Wang, J.; Liu, Z. Hyaluronidase with pH-responsive Dextran Modification as an Adjuvant Nanomedicine for Enhanced Photodynamic-Immunotherapy of Cancer. Adv. Funct. Mater. 2019, 29, 1902440. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumour Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumours. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Shen, N.; Ci, T.; Tang, Z.; Gu, Z.; Li, G.; Chen, X. Combretastatin A4 Nanodrug-Induced MMP9 Amplification Boosts Tumour-Selective Release of Doxorubicin Prodrug. Adv. Mater. 2019, 31, 1904278. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumour imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Tanei, T.; Godin, B.; van de Ven, A.L.; Hanibuchi, M.; Matsunoki, A.; Alexander, J.; Ferrari, M. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumour and organ microenvironments. Cancer Lett. 2014, 345, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; An, L.; Lin, J.; Tian, Q.; Yang, S. Smart nanomedicine agents for cancer, triggered by pH, glutathione, H2O2, or H2S. Int. J. Nanomed. 2019, 14, 5729–5749. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Huang, Y.; Bo, R.; Jia, B.; Wu, H.; Yuan, Y.; Wang, Z.; Ma, Z.; Jing, D.; Xu, X.; et al. Trojan Horse nanotheranostics with dual transformability and multifunctionality for highly effective cancer treatment. Nat. Commun. 2018, 9, 3653. [Google Scholar] [CrossRef]
- Cano-Cortes, M.V.; Navarro-Marchal, S.A.; Ruiz-Blas, M.P.; Diaz-Mochon, J.J.; Marchal, J.A.; Sanchez-Martin, R.M. A versatile theranostic nanodevice based on an orthogonal bioconjugation strategy for efficient targeted treatment and monitoring of triple negative breast cancer. Nanomedicine 2020, 24, 102120. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, Y.; Wang, W.; Wang, G.; Zhang, S.; Cheng, H. Emerging Nano-Based Strategies Against Drug Resistance in Tumor Chemotherapy. Front. Bioeng. Biotechnol. 2021, 9, 798882. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Serrano, F.; Mut-Salud, N.; Cruz-Bustos, T.; Gomez-Samblas, M.; Carrasco, E.; Garrido, J.M.; López-Jaramillo, F.J.; Santoyo-Gonzalez, F.; Osuna, A. Functionalized immunostimulating complexes with protein A via lipid vinyl sulfones to deliver cancer drugs to trastuzumab-resistant HER2-overexpressing breast cancer cells. Int. J. Nanomedicine 2016, 11, 4777–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Rolski, J.; Chan, A.; Mackey, J.; Liu, M.; Pinter, T.; et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009, 69, 62. [Google Scholar] [CrossRef]
- Chiang, C.S.; Hu, S.H.; Liao, B.J.; Chang, Y.C.; Chen, S.Y. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine 2014, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Patnala, K.; Malla, R.R.; Vishwas, S. Nanotechnology advances in ovarian cancer. In A Theranostic and Precision Medicine Approach for Female-Specific Cancers; Academic Press: Cambridge, MA, USA, 2021; pp. 105–128. ISBN 9780128220092. [Google Scholar] [CrossRef]
- Wang, R.; Billone, P.S.; Mullet, W.M. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater. 2013, 2013, 629681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Tiwari, M. Nano cancer therapy strategies. J. Cancer Res. Ther. 2012, 8, 19–22. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [Green Version]

| NP Property | Effect on the Protein Corona | References |
|---|---|---|
| Size | Well-ordered hard corona and thin soft corona in larger NPs. | [43] |
| Less perfect packing of hard corona and random soft corona in smaller NPs. In small NPs, proteins do not undergo drastic structural modifications. | [44] | |
| Shape | Shapes exhibiting higher surface area absorb more protein (stars more than rods) | [45] |
| Hydrophilicity/ hydrophobicity | Hydrophobic NPs attract hydrophobic proteins by hydrophobic interaction. | [46] |
| Hydrophobic NPs attract hydrophobic domains of proteins, favouring protein denaturation/conformational changes. | ||
| Hydrophilic NPs interact with more polar and charged proteins through electrostatic interactions. | ||
| Surface charge | NPs with high density of charge tend to form thicker and denser PCs. | [47] |
| Highly positively charged NPs interact very quickly and very strongly with proteins with IP (immunoprecipitation) <5.5. Highly negatively charged NPs interact mostly with proteins with IP >5.5. Slightly negatively charged NPs presentfewer interactions with proteins. | [48] |
| Medium/Experimental Conditions | Effect on the Protein Corona | References | |
|---|---|---|---|
| Medium | Protein amount | The protein amount forming the PC depends on the serum type and concentration. | [48,49] |
| Composition | PC composition depends on the biofluid origin (e.g., interstitial fluid, blood, plasma, serum). The change in extracellular medium during circulation affects PC composition. | [50,51,52,53] | |
| Source | In human samples, inter-individual variability (age, diet and health state) has been shown to influence PCs. | [54,55] | |
| Temperature and pH | Temperature and pH influences the protein diffusivity and the electrostatic interaction NP-protein. | [56,57] | |
| Protein structural stability is affected by plasma temperature and pH resulting in an exchange of proteins from PC. Temperature and pH modifications do not significantly modify the PC abundance of proteins with high affinity towards NPs. | |||
| Time | PC is formed rapidly around NPs (<0.5 min), and over time, although the total amount and composition of the PC do not change significantly, the abundance of each protein can fluctuate. | [58,59] | |
| Fluidics | Dynamic conditions drive to an in vivo PC molecularly richer in comparison to its counterpart ex vivo PC, although the total amount of protein attached to NPs is similar to that from in vitro conditions. | [60,61,62] | |
| PC conformation is more heterogeneous upon dynamic conditions, leaving uncoated NP moieties free to interact with cells. | |||
| Composition | Trade Name | Company | Indication | Administration * | |
|---|---|---|---|---|---|
| Liposomal platforms | Liposomal doxorubicin | Myocet | Zeneus, England, UK | Combination therapy with cyclophosphamide in metastatic breast cancer | i.v. |
| Liposome-PEG doxorubicin | Doxil/ Caelyx | Ortho Biotech, Schering-Plough, NJ, USA | HIV-related Kaposi’s sarcoma, metastatic breast cancer, and metastatic ovarian cancer | i.m. | |
| Polymeric platforms | Methoxy-PEG-poly(D,L-lactide) Taxol | Genexol-PM | Samyang, Seoul, Korea | Metastatic breast cancer | i.v. |
| PEG–L-asparaginase | Oncaspar | Enzon, NJ, USA | Acute lymphoblastic leukaemia | i.v. i.m. | |
| Other platforms | Albumin-bound paclitaxel | Abraxane | Abraxis BioScience, AstraZeneca, LA, USA | Metastatic breast cancer | i.v. |
| Composition | Trade Name | Company | Indication | Administration * | Status | |
|---|---|---|---|---|---|---|
| Liposomal platforms | Liposomal annamycin | L-Annamycin | Callisto, NY, USA | Acute lymphocytic leukaemia, acute myeloid leukaemia | i.v. | Phase I |
| Liposomal cisplatin | SLIT Cisplatin | Transave, NJ, USA | Progressive osteogenic sarcoma metastatic to the lung | Aerosol | Phase II | |
| Liposomal doxorubicin | Sarcodoxome | GP-Pharm, Barcelona, Spain | Soft tissue sarcoma | i.v. | Phase I/II | |
| Liposomal lurtotecan | OSI-211 | OSI Pharmaceuticals, NY, USA | Ovarian cancer | i.v. | Phase II | |
| Liposomal vincristine | Onco TCS | Inex, Enzon, NJ, USA | Non-Hodgkin’s lymphoma | i.v. | Phase II/III | |
| Polymeric platforms | HPMA copolymer–DACHPlatinate | ProLindac | Access Pharmaceuticals, TX, USA | Ovarian cancers | i.v. | Phase II |
| PEG–arginine deaminase | Hepacid | Phoenix, Mannheim, Germany | Hepatocellular carcinoma | i.v. | Phase I/II | |
| PEG–camptothecin | Prothecan | Enzon, NJ, USA | Various cancers | i.v | Phase I/II | |
| Pluronic block-copolymer Doxorubicin | SP1049C | Supratek Pharma, QC, Canada | Oesophageal carcinoma | i.v. | Phase II | |
| Polycyclodextrin camptothecin | IT-101 | Insert Therapeutics | Metastatic solid tumours | i.v. | Phase I | |
| Polyglutamate camptothecin | CT-2106 | Cell Therapeutics, LA, USA | Colorectal and ovarian Cancers | i.v. | Phase I/II | |
| Polyglutamate paclitaxel | Xyotax | Cell Therapeutics, WS, USA | Non-small-cell lung cancer, ovarian cancer | i.v. | Phase III | |
| Poly(iso-hexyl-cyanoacrylate) Doxorubicin | Transdrug | BioAlliance Pharma, Paris, France | Hepatocellular carcinoma | i.a. | Phase I/II | |
| Other platforms | Nanocrystalline 2-methoxyestradiol | Panzem NCD | Elan, EntreMed, NY, USA | Various cancers | Oral | Phase II |
| Paclitaxel nanoparticles in porous, hydrophilic matrix | AI-850 | Acusphere, MA, USA | Solid tumours | i.v. | Phase I | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Contreras, M.; Navarro-Marchal, S.A.; Peula-García, J.M.; Jódar-Reyes, A.B. Progress and Hurdles of Therapeutic Nanosystems against Cancer. Pharmaceutics 2022, 14, 388. https://doi.org/10.3390/pharmaceutics14020388
Martín-Contreras M, Navarro-Marchal SA, Peula-García JM, Jódar-Reyes AB. Progress and Hurdles of Therapeutic Nanosystems against Cancer. Pharmaceutics. 2022; 14(2):388. https://doi.org/10.3390/pharmaceutics14020388
Chicago/Turabian StyleMartín-Contreras, Marina, Saúl A. Navarro-Marchal, José Manuel Peula-García, and Ana Belén Jódar-Reyes. 2022. "Progress and Hurdles of Therapeutic Nanosystems against Cancer" Pharmaceutics 14, no. 2: 388. https://doi.org/10.3390/pharmaceutics14020388
APA StyleMartín-Contreras, M., Navarro-Marchal, S. A., Peula-García, J. M., & Jódar-Reyes, A. B. (2022). Progress and Hurdles of Therapeutic Nanosystems against Cancer. Pharmaceutics, 14(2), 388. https://doi.org/10.3390/pharmaceutics14020388