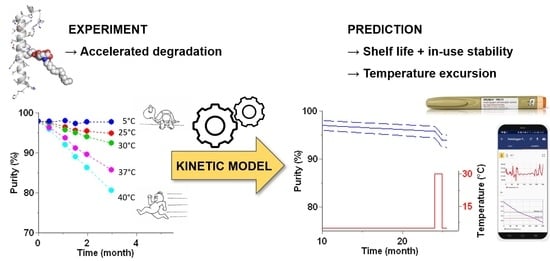
Long-Term Stability Prediction for Developability Assessment of Biopharmaceutics Using Advanced Kinetic Modeling
Abstract
1. Introduction
2. Materials and Methods
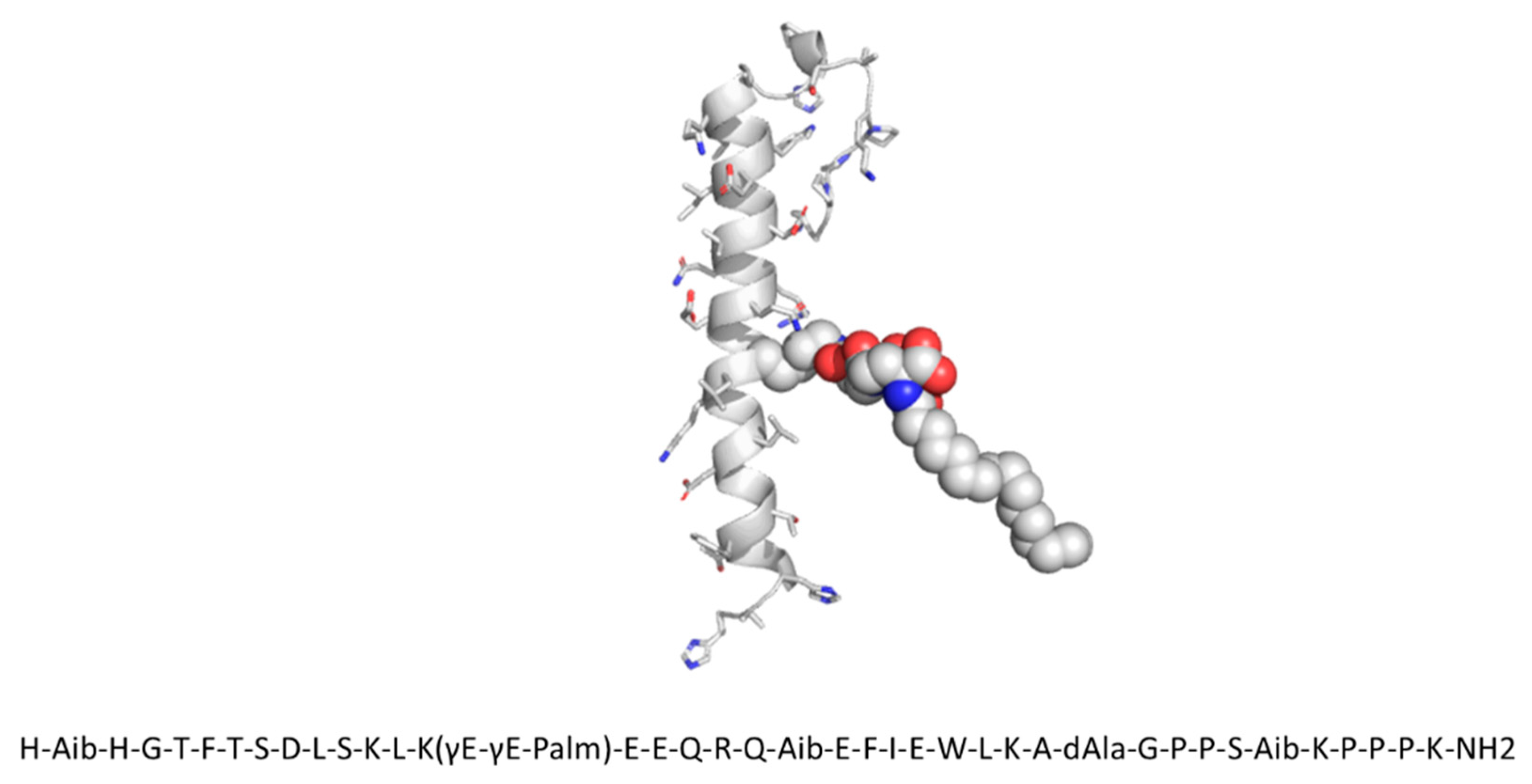
2.1. SAR441255 Sequence, Structure, and Background Information
2.2. Prototype Formulations and Primary Packaging Materials
2.3. Stability Monitoring
2.4. Advanced Kinetic Modeling and Stability Predictions
3. Results
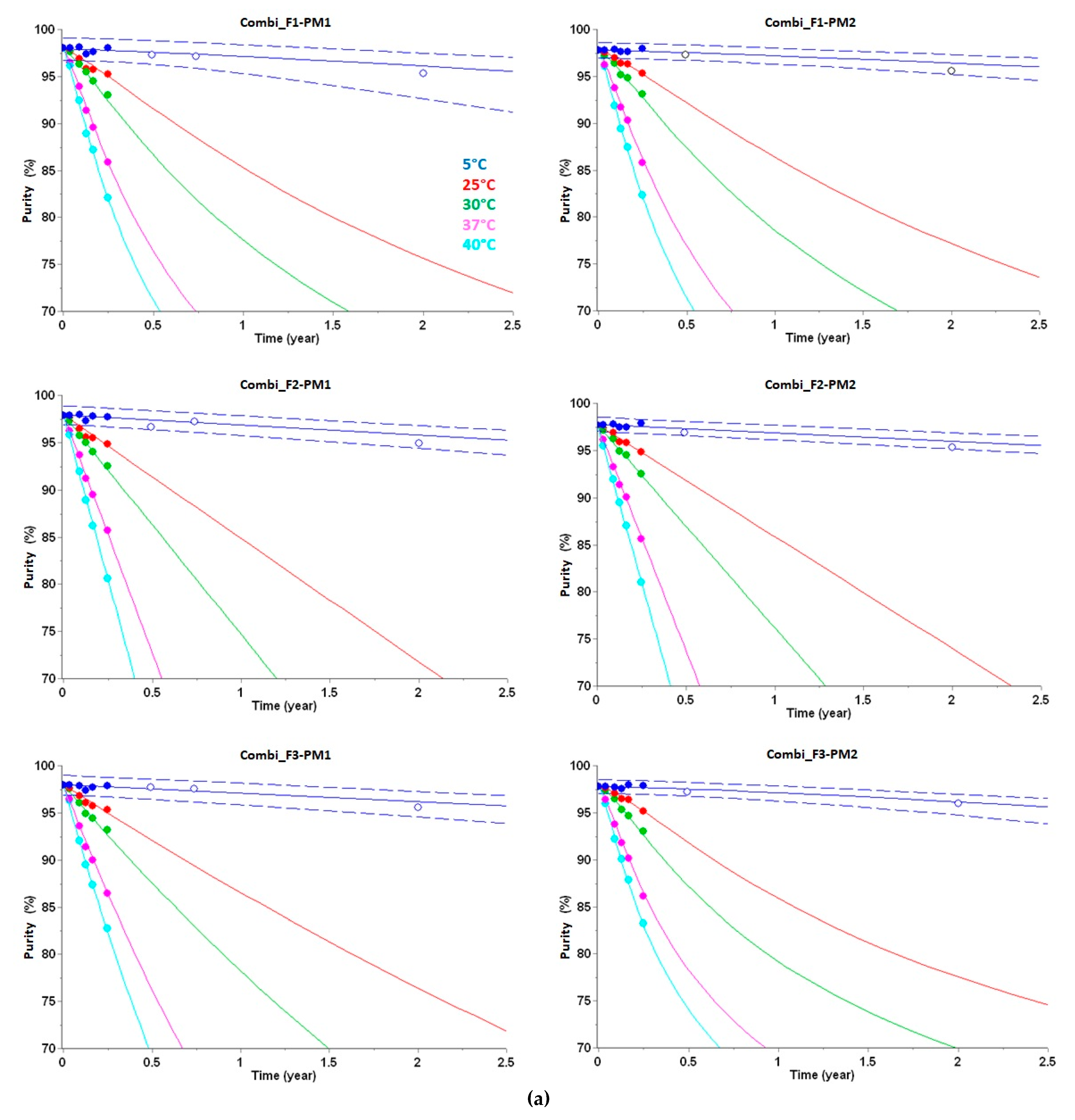
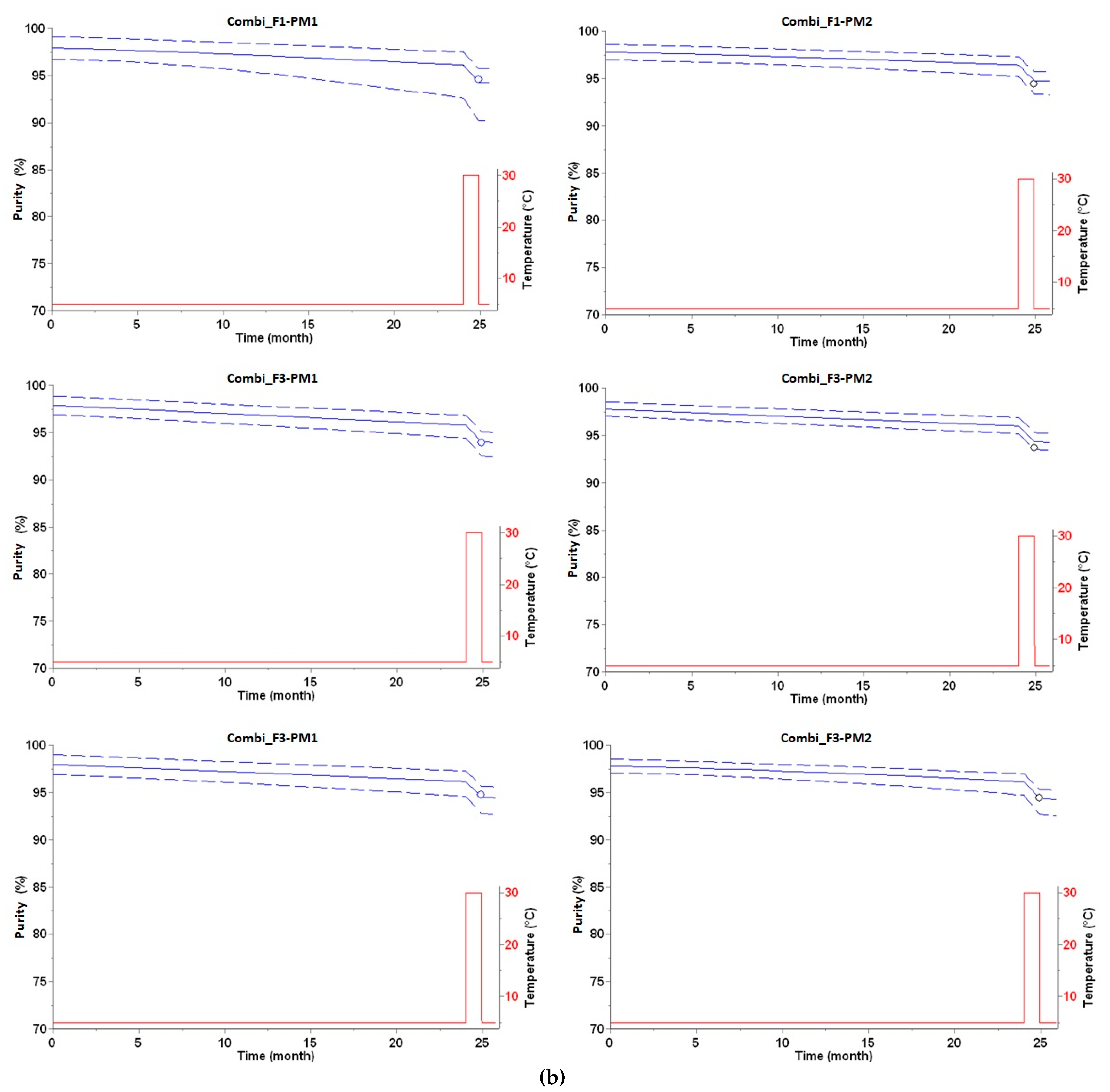
3.1. Long-Term Prediction of Purity Loss
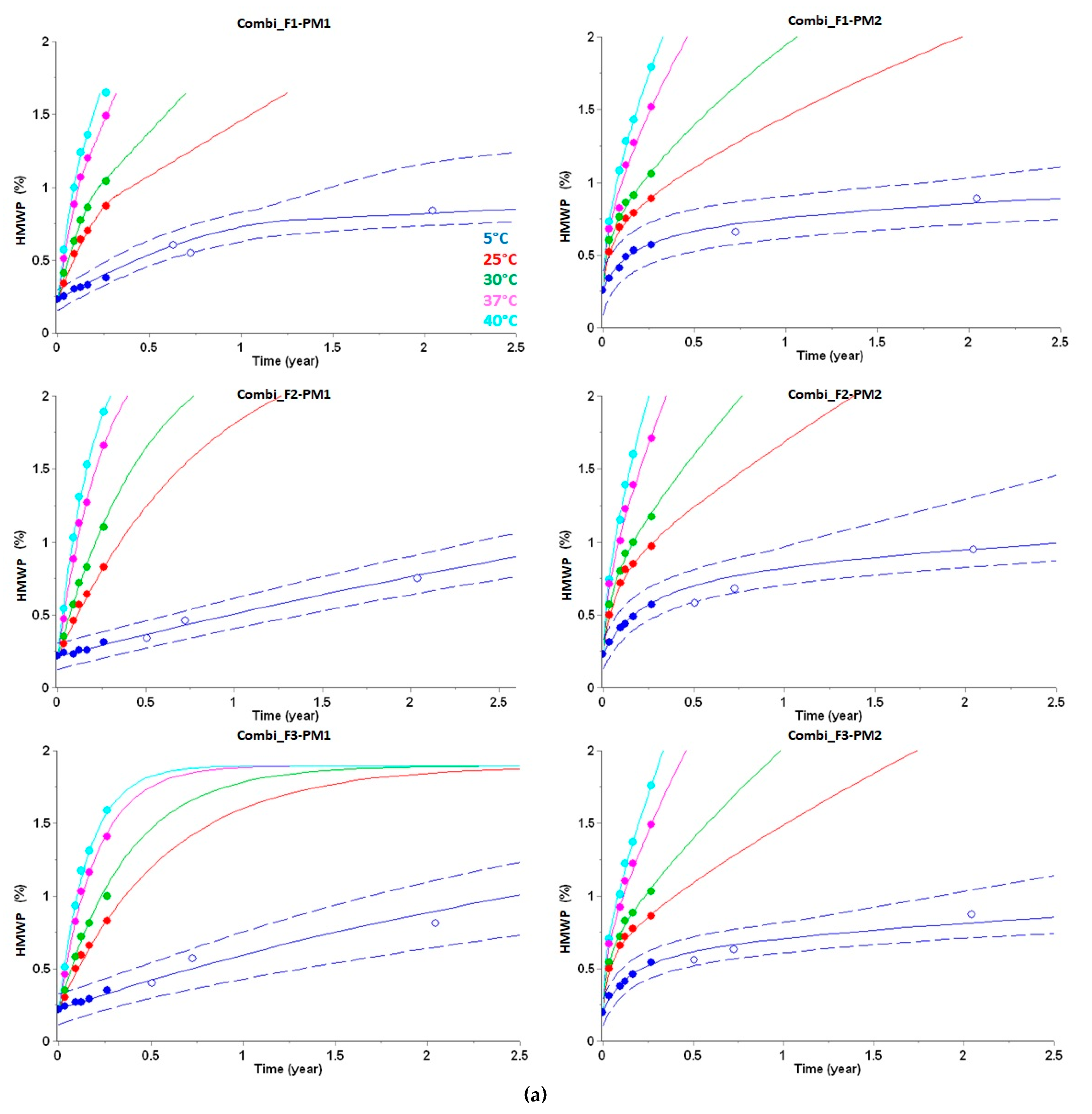
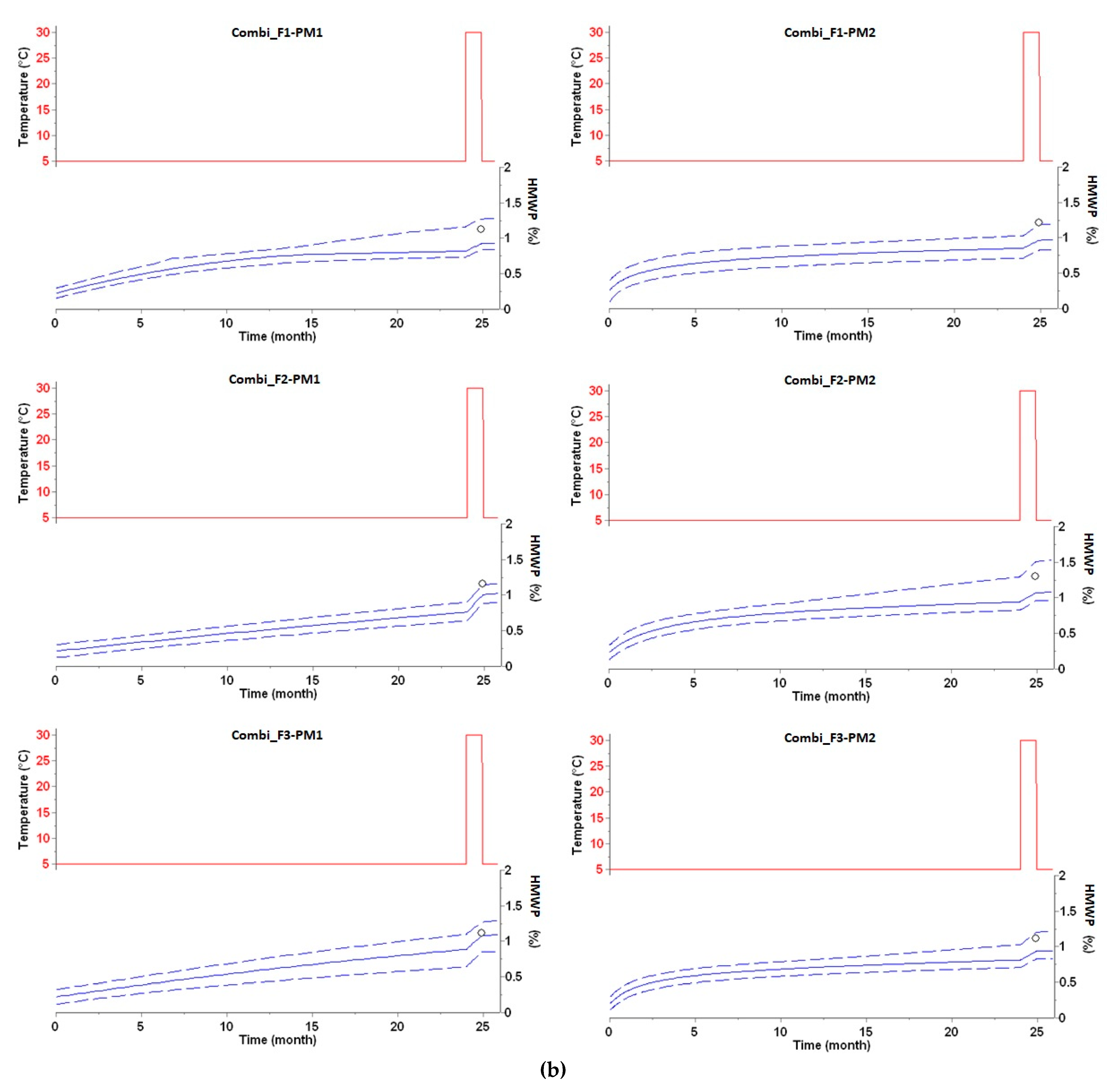
3.2. Long-Term Prediction of HMWP Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuyian, H.; Rashid, D.H.; Mohsin, M.; Tahera, K. An Overview: Stability Study Of Pharmaceutical Products And Shelf Life Prediction. Eur. J. Biomed. Pharm. Sci. 2015, 2, 30–40. [Google Scholar]
- Bott, R.F.; Oliveira, W.P. Storage Conditions for Stability Testing of Pharmaceuticals in Hot and Humid Regions. Drug Dev. Ind. Pharm. 2007, 33, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Bailly, M.; Mieczkowski, C.; Juan, V.; Metwally, E.; Tomazela, D.; Baker, J.; Uchida, M.; Kofman, E.; Raoufi, F.; Motlagh, S.; et al. Predicting Antibody Developability Profiles Through Early Stage Discovery Screening. mAbs 2020, 12, 1743053. [Google Scholar] [CrossRef]
- Bak, A.; Leung, D.; Barrett, S.E.; Forster, S.; Minnihan, E.C.; Leithead, A.W.; Cunningham, J.; Toussaint, N.; Crocker, L.S. Physicochemical and Formulation Developability Assessment for Therapeutic Peptide Delivery—A Primer. AAPS J. 2015, 17, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Pfeiffer-Marek, S.; Bossart, M.; Heubel, C.; Stock, U.; Tiwari, G.; Gebauer, B.; Elshorst, B.; Pfenninger, A.; Lukasczyk, U.; et al. Peptide Optimization at the Drug Discovery-Development Interface: Tailoring of Physicochemical Properties Toward Specific Formulation Requirements. J. Pharm. Sci. 2019, 108, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.S.; Bhattachar, S.; Yazdanian, M.; Morrison, J. Commentary: Why Pharmaceutical Scientists in Early Drug Discovery Are Critical for Influencing the Design and Selection of Optimal Drug Candidates. AAPS PharmSciTech 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of Protein Pharmaceuticals: An Update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Hsu, C.C. Aggregation of Recombinant Human Growth Hormone Induced by Phenolic Compounds. Int. J. Pharm. 1996, 140, 155–168. [Google Scholar] [CrossRef]
- Bolognesi, B.; Tartaglia, G.G. Chapter Three—Physicochemical Principles of Protein Aggregation. In Oligomerization in Health and Disease; Progress in Molecular Biology and Translational Science; Giraldo, J., Ciruela, F., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 117, pp. 53–72. [Google Scholar]
- Carpenter, J.F.; Kendrick, B.S.; Chang, B.S.; Manning, M.C.; Randolph, T.W. Inhibition of Stress-Induced Aggregation of Protein Therapeutics. In Amyloid, Prions, and Other Protein Aggregates; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 309, pp. 236–255. [Google Scholar]
- Kim, Y.-S.; Cape, S.P.; Chi, E.; Raffen, R.; Wilkins-Stevens, P.; Stevens, F.J.; Manning, M.C.; Randolph, T.W.; Solomon, A.; Carpenter, J.F. Counteracting Effects of Renal Solutes on Amyloid Fibril Formation by Immunoglobulin Light Chains*. J. Biol. Chem. 2001, 276, 1626–1633. [Google Scholar] [CrossRef]
- Zbacnik, T.J.; Holcomb, R.E.; Katayama, D.S.; Murphy, B.M.; Payne, R.W.; Coccaro, R.C.; Evans, G.J.; Matsuura, J.E.; Henry, C.S.; Manning, M.C. Role of Buffers in Protein Formulations. J. Pharm. Sci. 2017, 106, 713–733. [Google Scholar] [CrossRef]
- Randolph, T.W.; Jones, L.S. Surfactant-Protein Interactions. In Rational Design of Stable Protein Formulations: Theory and Practice; (Pharmaceutical Biotechnology); Carpenter, J.F., Manning, M.C., Eds.; Springer: Boston, MA, USA, 2002; pp. 159–175. ISBN 978-1-4615-0557-0. [Google Scholar]
- Pinholt, C.; Hartvig, R.A.; Medlicott, N.J.; Jorgensen, L. The Importance of Interfaces in Protein Drug Delivery—Why Is Protein Adsorption of Interest in Pharmaceutical Formulations? Expert Opin. Drug Deliv. 2011, 8, 949–964. [Google Scholar] [CrossRef]
- Furman, J.L.; Chiu, M.; Hunter, M.J. Early Engineering Approaches to Improve Peptide Developability and Manufacturability. AAPS J. 2015, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Garripelli, V.K.; Wu, Z.; Gupta, S. Developability Assessment for Monoclonal Antibody Drug Candidates: A Case Study. Pharm. Dev. Technol. 2021, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jarasch, A.; Koll, H.; Regula, J.T.; Bader, M.; Papadimitriou, A.; Kettenberger, H. Developability Assessment during the Selection of Novel Therapeutic Antibodies. J. Pharm. Sci. 2015, 104, 1885–1898. [Google Scholar] [CrossRef]
- Kumar, S.; Plotnikov, N.V.; Rouse, J.C.; Singh, S.K. Biopharmaceutical Informatics: Supporting Biologic Drug Development via Molecular Modelling and Informatics. J. Pharm. Pharmacol. 2018, 70, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, D.; Mason, B.; Rossomando, T.; Li, N.; Liu, D.; Cheung, J.K.; Xu, W.; Raghava, S.; Katiyar, A.; et al. Structure, Heterogeneity and Developability Assessment of Therapeutic Antibodies. mAbs 2018, 11, 239–264. [Google Scholar] [CrossRef]
- Evers, A.; Pfeiffer-Marek, S.; Bossart, M.; Elvert, R.; Lorenz, K.; Heubel, C.; Garea, A.V.; Schroeter, K.; Riedel, J.; Stock, U.; et al. Multiparameter Peptide Optimization toward Stable Triple Agonists for the Treatment of Diabetes and Obesity. Adv. Ther. 2020, 3, 2000052. [Google Scholar] [CrossRef]
- Dauer, K.; Kamm, W.; Wagner, K.G.; Pfeiffer-Marek, S. High-Throughput Screening for Colloidal Stability of Peptide Formulations Using Dynamic and Static Light Scattering. Mol. Pharm. 2021, 18, 1939–1955. [Google Scholar] [CrossRef]
- Qiu, F.; Scrivens, G. Accelerated Predictive Stability: Fundamentals and Pharmaceutical Industry; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-802786-8. [Google Scholar]
- Faya, P.; Seaman, J.W.; Stamey, J.D. Using Accelerated Drug Stability Results to Inform Long-Term Studies in Shelf Life Determination. Stat. Med. 2018, 37, 2599–2615. [Google Scholar] [CrossRef]
- Freed, A.L.; Colgan, S.T.; Kochling, J.D.; Alasandro, M.S. AAPS Workshop: Accelerating Pharmaceutical Development through Predictive Stability Approaches, April 4–5, 2016. AAPS Open 2017, 3, 8. [Google Scholar] [CrossRef][Green Version]
- Kuzman, D.; Bunc, M.; Ravnik, M.; Reiter, F.; Žagar, L.; Bončina, M. Long-Term Stability Predictions of Therapeutic Monoclonal Antibodies in Solution Using Arrhenius-Based Kinetics. Sci. Rep. 2021, 11, 20534. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Williams, H.; Debie, E.; Fu, M.; Bujalski, R.; Qiu, F.; Wu, Y.; Li, H.; Wang, J.; Hoaglund-Hyzer, C.; et al. Utilization of Risk-Based Predictive Stability within Regulatory Submissions; Industry’s Experience. AAPS Open 2020, 6, 1. [Google Scholar] [CrossRef]
- Oliva, A.; Fariña, J.B.; Llabrés, M. An Improved Methodology for Data Analysis in Accelerated Stability Studies of Peptide Drugs: Practical Considerations. Talanta 2012, 94, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Carella, A.J.; Gumkowski, M.J.; Lukulay, P.; MacDonald, B.C.; Roy, M.C.; Shamblin, S.L. Improved Protocol and Data Analysis for Accelerated Shelf-Life Estimation of Solid Dosage Forms. Pharm. Res. 2007, 24, 780–790. [Google Scholar] [CrossRef]
- Williams, H.E.; Bright, J.; Roddy, E.; Poulton, A.; Cosgrove, S.D.; Turner, F.; Harrison, P.; Brookes, A.; MacDougall, E.; Abbott, A.; et al. A Comparison of Drug Substance Predicted Chemical Stability with ICH Compliant Stability Studies. Drug Dev. Ind. Pharm. 2019, 45, 379–386. [Google Scholar] [CrossRef]
- Wu, Y.; Freed, A.; Lavrich, D.; Raghavachari, R.; Huynh-Ba, K.; Shah, K.; Alasandro, M. Strategies of Bringing Drug Product Marketing Applications to Meet Current Regulatory Standards. AAPS PharmSciTech 2015, 16, 986–991. [Google Scholar] [CrossRef][Green Version]
- International Council for Harmonisation. Stability Testing of New Drug Substances and Products; Step 5; Guideline Q1A(R2); ICH: Geneva, Switzerland, 2003. [Google Scholar]
- International Council for Harmonisation. Evaluation for Stability Data; Step 4; Guideline Q1E; ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Capen, R.; Christopher, D.; Forenzo, P.; Huynh-Ba, K.; LeBlond, D.; Liu, O.; O’Neill, J.; Patterson, N.; Quinlan, M.; Rajagopalan, R.; et al. Evaluating Current Practices in Shelf Life Estimation. AAPS PharmSciTech 2018, 19, 668–680. [Google Scholar] [CrossRef]
- Roduit, B.; Hartmann, M.; Folly, P.; Sarbach, A.; Baltensperger, R. Prediction of Thermal Stability of Materials by Modified Kinetic and Model Selection Approaches Based on Limited Amount of Experimental Points. Thermochim. Acta 2014, 579, 31–39. [Google Scholar] [CrossRef]
- Clénet, D. Accurate Prediction of Vaccine Stability under Real Storage Conditions and during Temperature Excursions. Eur. J. Pharm. Biopharm. 2018, 125, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Clénet, D.; Hourquet, V.; Woinet, B.; Ponceblanc, H.; Vangelisti, M. A Spray Freeze Dried Micropellet Based Formulation Proof-of-Concept for a Yellow Fever Vaccine Candidate. Eur. J. Pharm. Biopharm. 2019, 142, 334–343. [Google Scholar] [CrossRef]
- Clénet, D.; Imbert, F.; Probeck, P.; Rahman, N.; Ausar, S.F. Advanced Kinetic Analysis as a Tool for Formulation Development and Prediction of Vaccine Stability. J. Pharm. Sci. 2014, 103, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Neyra, C.; Clénet, D.; Bright, M.; Kensinger, R.; Hauser, S. Predictive Modeling for Assessing the Long-Term Thermal Stability of a New Fully-Liquid Quadrivalent Meningococcal Tetanus Toxoid Conjugated Vaccine. Int. J. Pharm. 2021, 609, 121143. [Google Scholar] [CrossRef] [PubMed]
- Roque, C.; Ausar, S.F.; Rahman, N.; Clénet, D. Stability Modeling in QbD: Accelerating Formulation Development and Predicting Shelf Life of Products. In Quality by Design—An Indispensable Approach to Accelerate Biopharmaceutical Product Development (Single User Digital Version); Parenteral Drug Association, Inc.: Bethesda, MD, USA, 2021; ISBN 978-1-945584-22-0. [Google Scholar]
- Roduit, B.; Luyet, C.A.; Hartmann, M.; Folly, P.; Sarbach, A.; Dejeaifve, A.; Dobson, R.; Schroeter, N.; Vorlet, O.; Dabros, M.; et al. Continuous Monitoring of Shelf Lives of Materials by Application of Data Loggers with Implemented Kinetic Parameters. Molecules 2019, 24, 2217. [Google Scholar] [CrossRef] [PubMed]
- Bossart, M.; Wagner, M.; Elvert, R.; Evers, A.; Hübschle, T.; Kloeckener, T.; Lorenz, K.; Moessinger, C.; Eriksson, O.; Velikyan, I.; et al. Effects on Weight Loss and Glycemic Control with SAR441255, a Potent Unimolecular Peptide GLP-1/GIP/GCG Receptor Triagonist. Cell Metab. 2022, 34, 59.e10–74.e10. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Haack, T.; Lorenz, M.; Bossart, M.; Elvert, R.; Henkel, B.; Stengelin, S.; Kurz, M.; Glien, M.; Dudda, A.; et al. Design of Novel Exendin-Based Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists. J. Med. Chem. 2017, 60, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Bossart, M.; Pfeiffer-Marek, S.; Elvert, R.; Schreuder, H.; Kurz, M.; Stengelin, S.; Lorenz, M.; Herling, A.; Konkar, A.; et al. Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists Specifically Optimized for Multidose Formulations. J. Med. Chem. 2018, 61, 5580–5593. [Google Scholar] [CrossRef]
- Hinnen, D.; Strong, J. IGlarLixi: A New Once-Daily Fixed-Ratio Combination of Basal Insulin Glargine and Lixisenatide for the Management of Type 2 Diabetes. Diabetes Spectr. 2018, 31, 145–154. [Google Scholar] [CrossRef][Green Version]
- Dauer, K.; Pfeiffer-Marek, S.; Kamm, W.; Wagner, K.G. Microwell Plate-Based Dynamic Light Scattering as a High-Throughput Characterization Tool in Biopharmaceutical Development. Pharmaceutics 2021, 13, 172. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Hägglund, P.; López-Alarcón, C.; Davies, M.J. Oxidative Crosslinking of Peptides and Proteins: Mechanisms of Formation, Detection, Characterization and Quantification. Molecules 2022, 27, 15. [Google Scholar] [CrossRef]
- Meyer, B.K.; Ni, A.; Hu, B.; Shi, L. Antimicrobial Preservative Use in Parenteral Products: Past and Present. J. Pharm. Sci. 2007, 96, 3155–3167. [Google Scholar] [CrossRef]
- Home Page—Advanced Kinetics and Technology Solutions. Available online: https://www.akts.com/ (accessed on 25 March 2021).
- Campa, C.; Pronce, T.; Paludi, M.; Weusten, J.; Conway, L.; Savery, J.; Richards, C.; Clénet, D. Use of Stability Modeling to Support Accelerated Vaccine Development and Supply. Vaccines 2021, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Clénet, D. Accelerated Stability Predictions for Vaccines. S.T.P. Pharma Prat. J. 2020, 36–38. Available online: https://www.sfstp.org/ (accessed on 20 December 2021).
- Clénet, D.; Vinit, T.; Soulet, D.; Maillet, C.; Guinet-Morlot, F.; Saulnier, A. Biophysical Virus Particle Specific Characterization to Sharpen the Definition of Virus Stability. Eur. J. Pharm. Biopharm. 2018, 132, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Bansal, R.; Joshi, V.; Rathore, A.S. Aggregation Kinetics for IgG1-Based Monoclonal Antibody Therapeutics. AAPS J. 2016, 18, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, C.F.; Hubálek, F.; Andersson, J.; Poulsen, C.; Otzen, D.; Naver, H. Purification and Identification of High Molecular Weight Products Formed During Storage of Neutral Formulation of Human Insulin. Pharm. Res. 2015, 32, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, L.; Arosio, P.; Sozo, M.; Yates, A.; Norrant, E.; Morbidelli, M. Kinetic Analysis of the Multistep Aggregation Mechanism of Monoclonal Antibodies. J. Phys. Chem. B 2014, 118, 10595–10606. [Google Scholar] [CrossRef]
| Ingredient | F1 (mg/g) | F2 (mg/g) | F3 (mg/g) |
|---|---|---|---|
| acetic acid | 0.88 | 0.88 | 0.88 |
| sodium acetate trihydrate | 0.51 | 0.51 | 0.51 |
| peptide | 2.5 | 2.5 | 2.5 |
| glycerol | 20.4 | 20.4 | |
| NaCl | 6.47 | ||
| L-methionine | 3 | 3 | 3 |
| metacresol | 2.7 | 2.7 | 2.7 |
| polysorbate 20 | 0.01 | ||
| NaOH | Ad pH 4.5 | Ad pH 4.5 | Ad pH 4.5 |
| water for injection | Ad 1 g | Ad 1 g | Ad 1 g |
| Identifier | Container | Injection Stopper/Cap | Plunger Stopper | Siliconized Surfaces |
|---|---|---|---|---|
| PM1 | 2R iso vial, clear glass | 13 mm grey bromobutyl rubber with Flurotec® coating + 13 mm flip-off cap | - | none |
| PM2 | 1.5 mL cartridge, clear glass, baked in silicon | 7.5 mm aluminum flanged cap with laminated sealing disc | 6 mm bromo-butyl siliconized rubber | container + plunger |
| Name | Formulation | Packaging Material (PM) | Corresponding Kinetic Equations for Long-Term Prediction |
|---|---|---|---|
| Combi_F1-PM1 | F1 | PM1 | (2), (8) |
| Combi_F2-PM1 | F2 | PM1 | (3), (9) |
| Combi_F3-PM1 | F3 | PM1 | (4), (10) |
| Combi_F1-PM2 | F1 | PM2 | (5), (11) |
| Combi_F2-PM2 | F2 | PM2 | (6), (12) |
| Combi_F3-PM2 | F3 | PM2 | (7), (13) |
| Purity (%) after Two Years at 5 °C | Purity (%) after Two Years at 5 °C Plus 28 Days at 30 °C | |||||
|---|---|---|---|---|---|---|
| Drug Product | Experimental | Predicted | Δ | Experimental | Predicted | Δ |
| Combi_F1-PM1 | 95.3 | 96.1 | 0.8 | 94.6 | 94.3 | −0.3 |
| Combi_F2-PM1 | 94.9 | 95.8 | 0.9 | 93.9 | 94.0 | 0.1 |
| Combi_F3-PM1 | 95.6 | 96.2 | 0.6 | 94.7 | 94.5 | −0.2 |
| Combi_F1-PM2 | 95.6 | 96.4 | 0.8 | 94.4 | 94.8 | 0.4 |
| Combi_F2-PM2 | 95.3 | 96.0 | 0.7 | 93.7 | 94.3 | 0.6 |
| Combi_F3-PM2 | 96.0 | 96.1 | 0.1 | 94.4 | 94.4 | 0.0 |
| HMWP Increase (%) after Two Years at 5 °C | HMWP Increase (%) after Two Years at 5 °C Plus 28 Days at 30 °C | |||||
|---|---|---|---|---|---|---|
| Drug Product | Experimental | Predicted | Δ | Experimental | Predicted | Δ |
| Combi_F1-PM1 | 0.84 | 0.82 | −0.02 | 1.13 | 0.93 | −0.10 |
| Combi_F2-PM1 | 0.75 | 0.77 | 0.02 | 1.16 | 1.00 | −0.16 |
| Combi_F3-PM1 | 0.81 | 0.90 | 0.09 | 1.12 | 1.08 | −0.04 |
| Combi_F1-PM2 | 0.89 | 0.86 | −0.03 | 1.21 | 0.97 | −0.24 |
| Combi_F2-PM2 | 0.95 | 0.95 | 0.00 | 1.30 | 1.07 | −0.23 |
| Combi_F3-PM2 | 0.87 | 0.81 | −0.06 | 1.12 | 0.94 | −0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evers, A.; Clénet, D.; Pfeiffer-Marek, S. Long-Term Stability Prediction for Developability Assessment of Biopharmaceutics Using Advanced Kinetic Modeling. Pharmaceutics 2022, 14, 375. https://doi.org/10.3390/pharmaceutics14020375
Evers A, Clénet D, Pfeiffer-Marek S. Long-Term Stability Prediction for Developability Assessment of Biopharmaceutics Using Advanced Kinetic Modeling. Pharmaceutics. 2022; 14(2):375. https://doi.org/10.3390/pharmaceutics14020375
Chicago/Turabian StyleEvers, Andreas, Didier Clénet, and Stefania Pfeiffer-Marek. 2022. "Long-Term Stability Prediction for Developability Assessment of Biopharmaceutics Using Advanced Kinetic Modeling" Pharmaceutics 14, no. 2: 375. https://doi.org/10.3390/pharmaceutics14020375
APA StyleEvers, A., Clénet, D., & Pfeiffer-Marek, S. (2022). Long-Term Stability Prediction for Developability Assessment of Biopharmaceutics Using Advanced Kinetic Modeling. Pharmaceutics, 14(2), 375. https://doi.org/10.3390/pharmaceutics14020375