Tailoring Chlorthalidone Aqueous Solubility by Cocrystallization: Stability and Dissolution Behavior of a Novel Chlorthalidone-Caffeine Cocrystal
Abstract
1. Introduction
2. Materials, Preparative Methods, and Characterization Techniques
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cocrystal CTD-CAF
2.2.2. Solid-State Stability Tests
2.2.3. Solubility Studies
2.2.4. Polymer Selection
2.2.5. Powder Dissolution under Non-Sink Conditions and Phase Stability
2.2.6. Induced Precipitation Experiments
2.2.7. Preparation of Capsule Formulations
2.2.8. Dissolution Experiments under Sink Conditions
2.3. Characterization Techniques
2.3.1. Powder X-ray Diffraction Analysis (PXRD)
2.3.2. Single-Crystal X-ray Diffraction Analysis (SCXRD)
2.3.3. Infrared Spectroscopy Analysis (IR)
2.3.4. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA-DSC)
2.3.5. High-Performance Liquid Chromatography (HPLC)
2.4. Statistical Analysis
3. Results
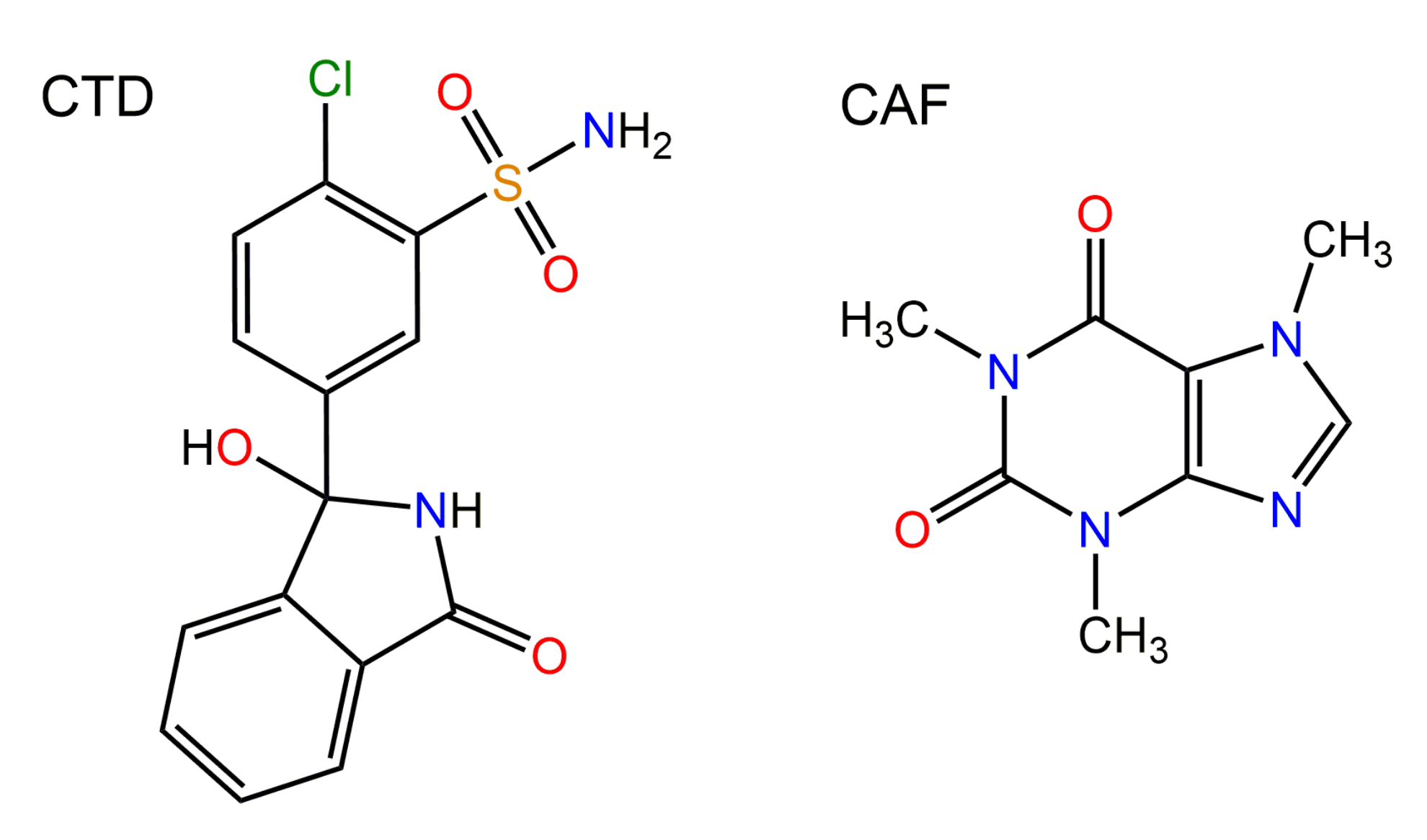
3.1. Preparation of Cocrystal CTD-CAF
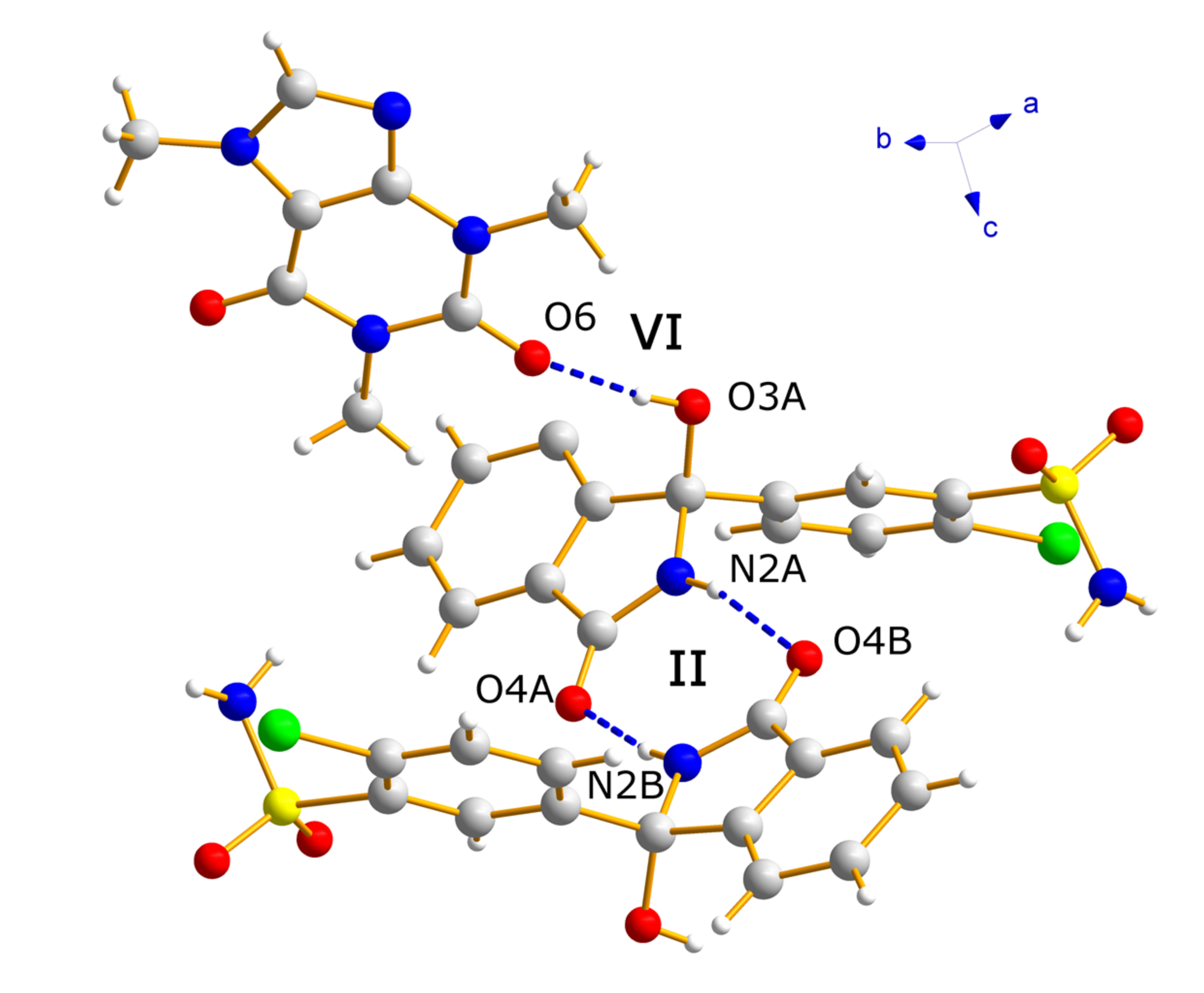
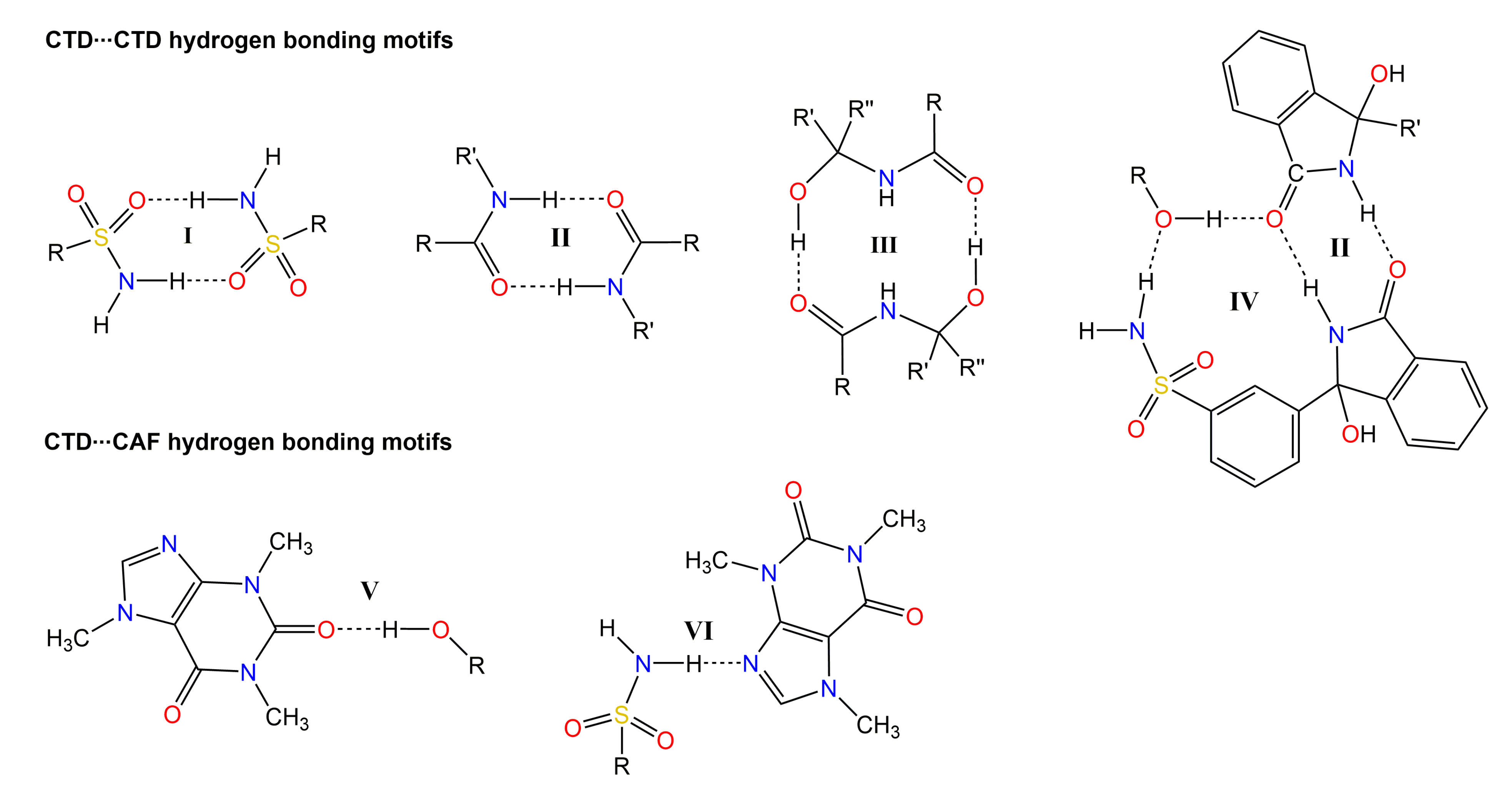
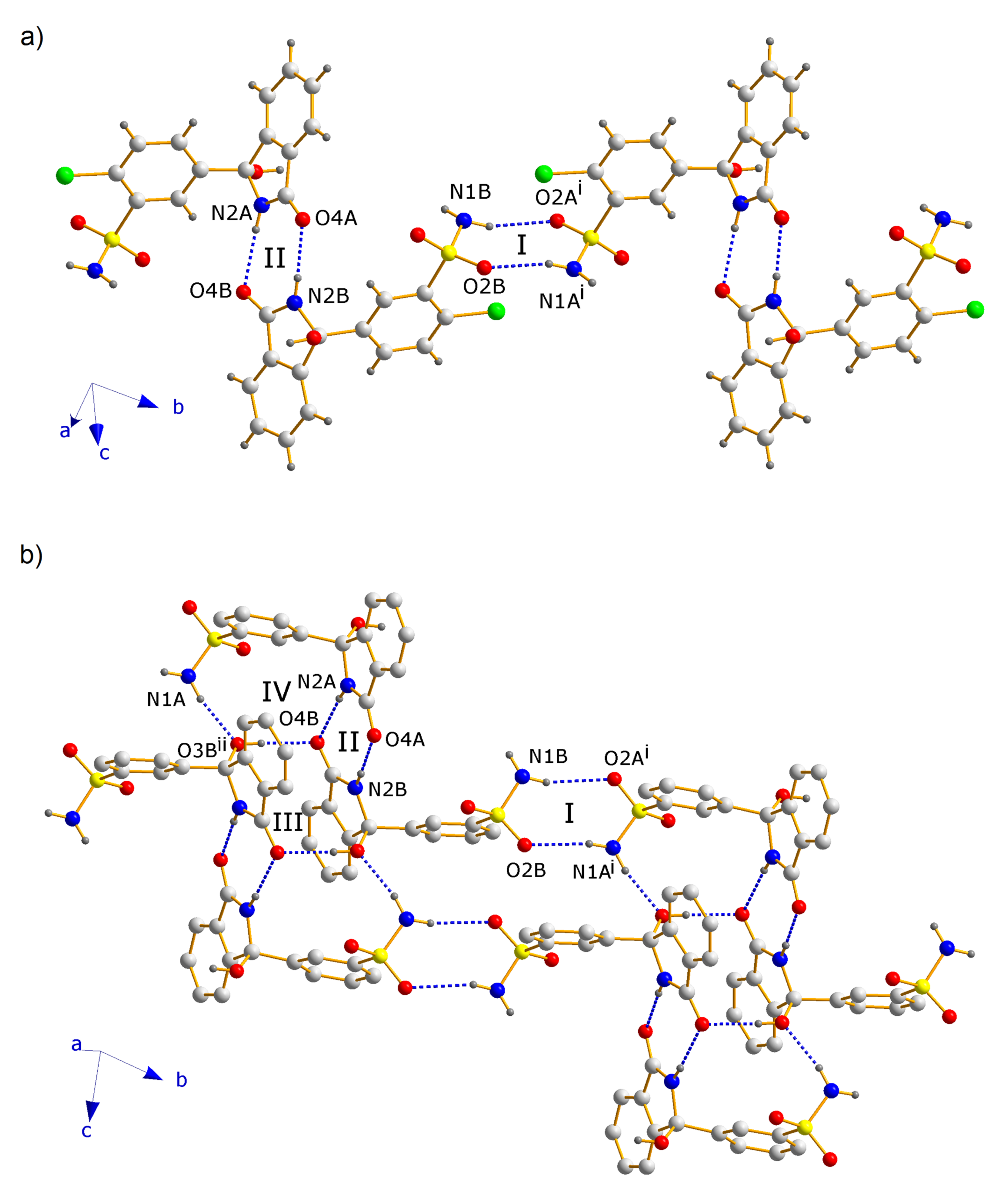
3.2. Crystallographic Analysis
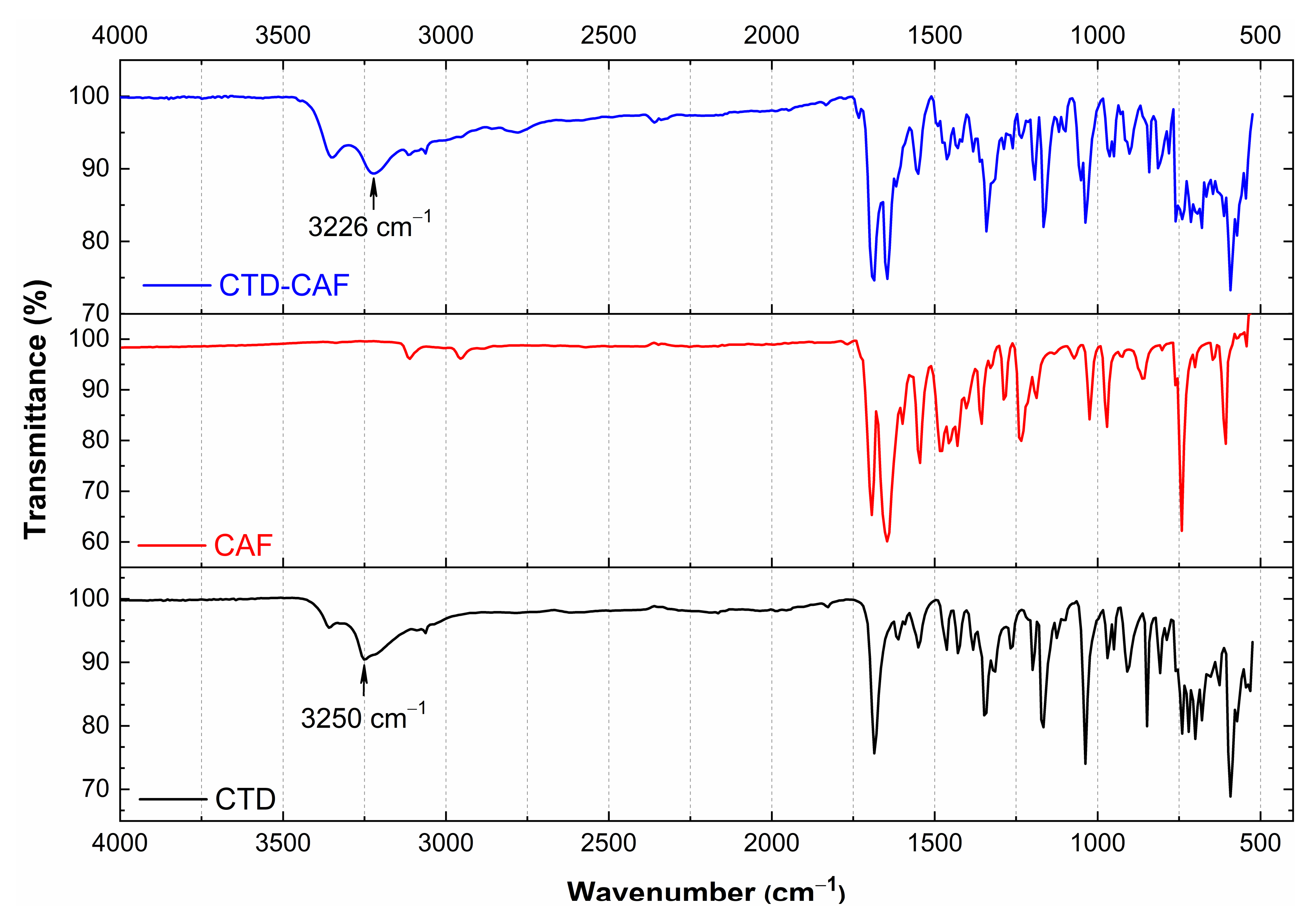
3.3. Analysis by IR Spectroscopy
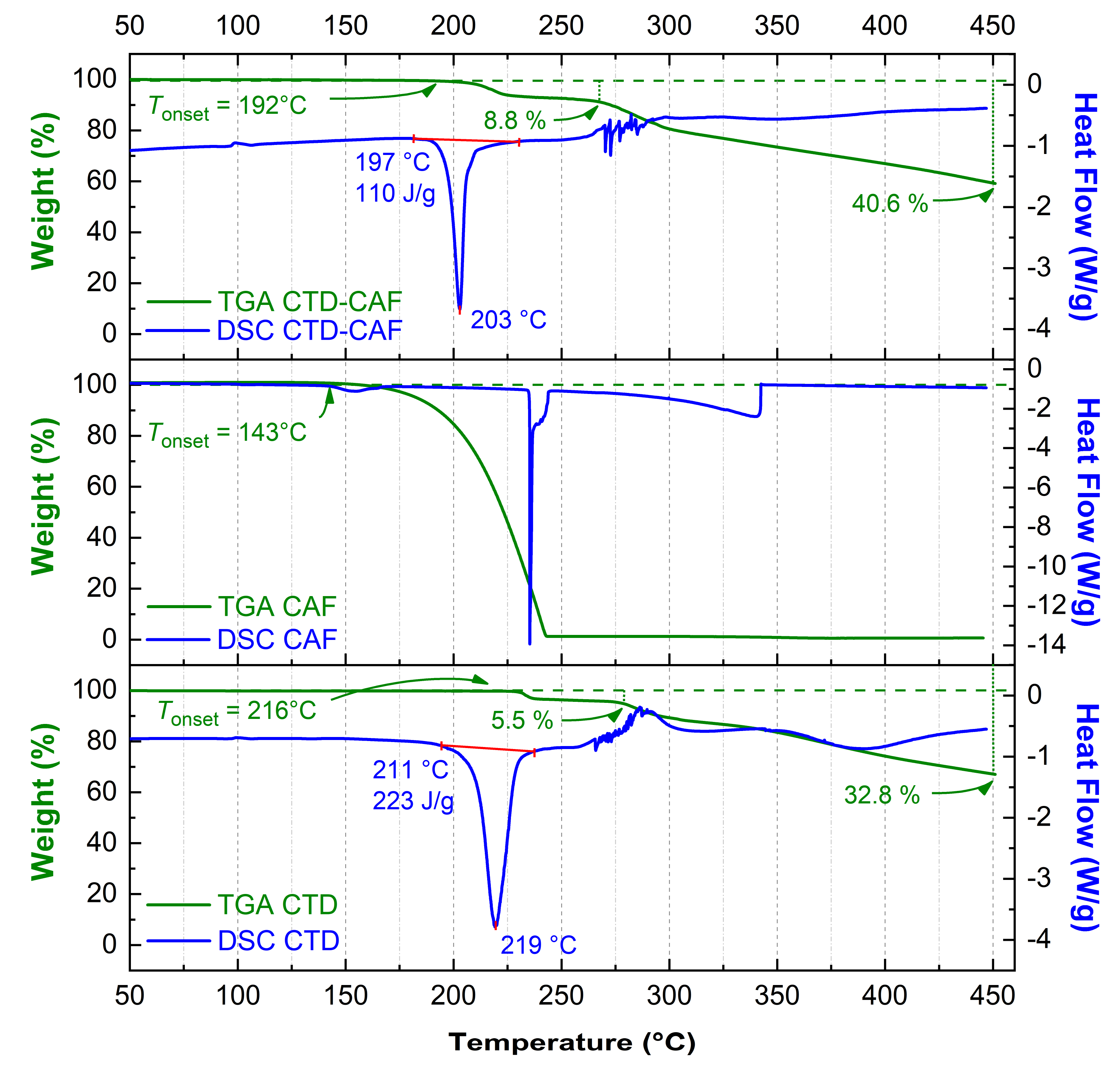
3.4. TG-DSC Analysis
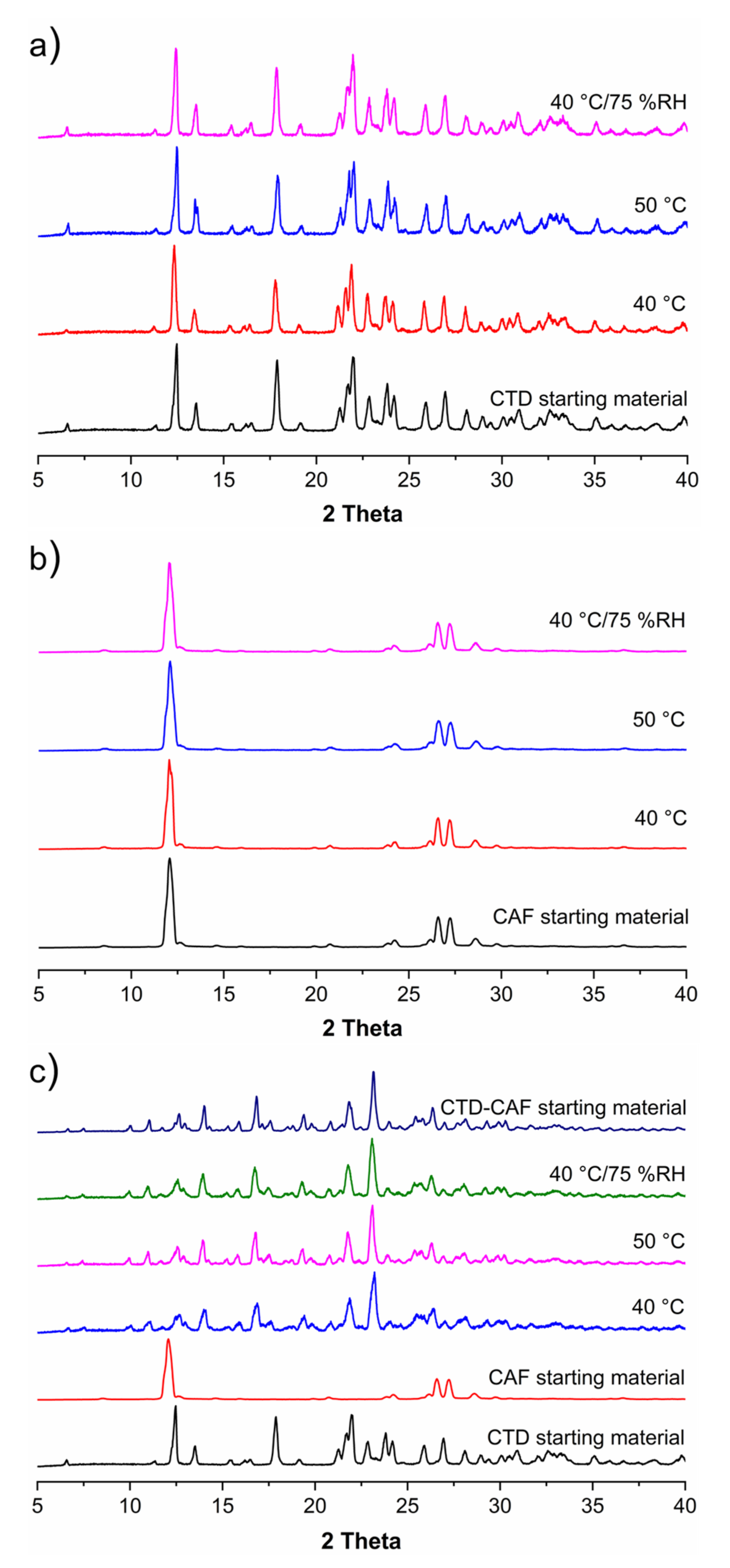
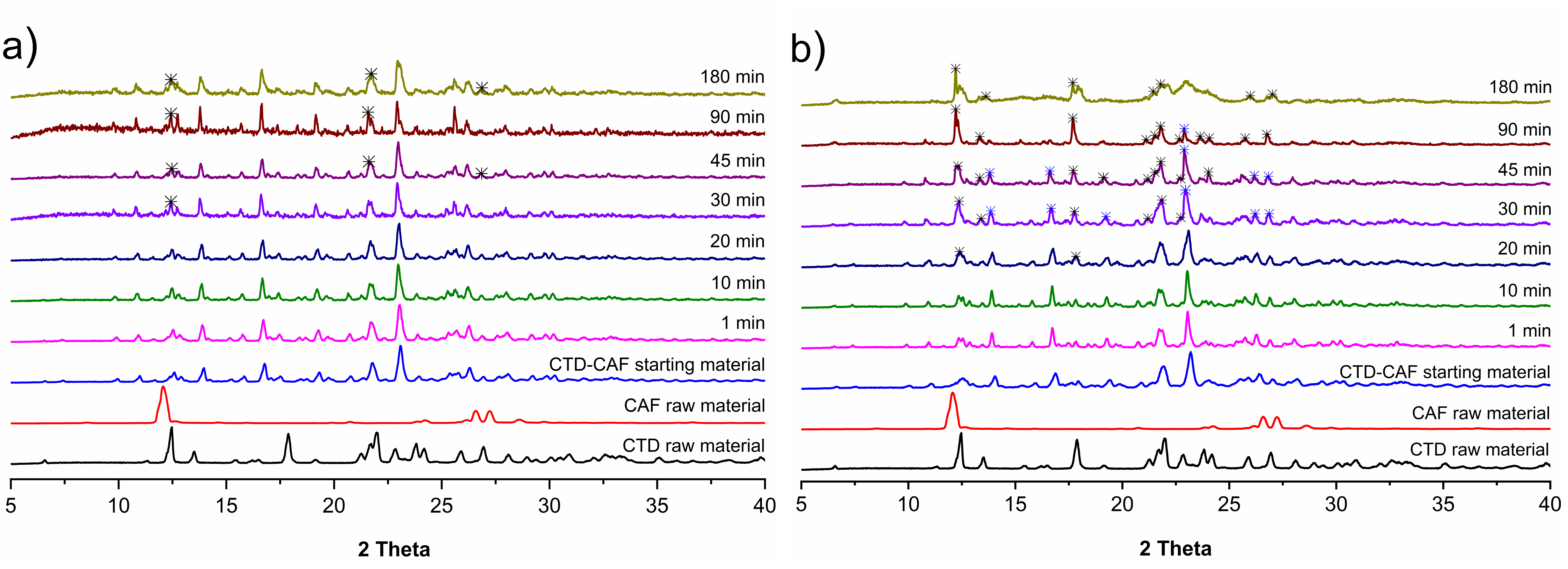
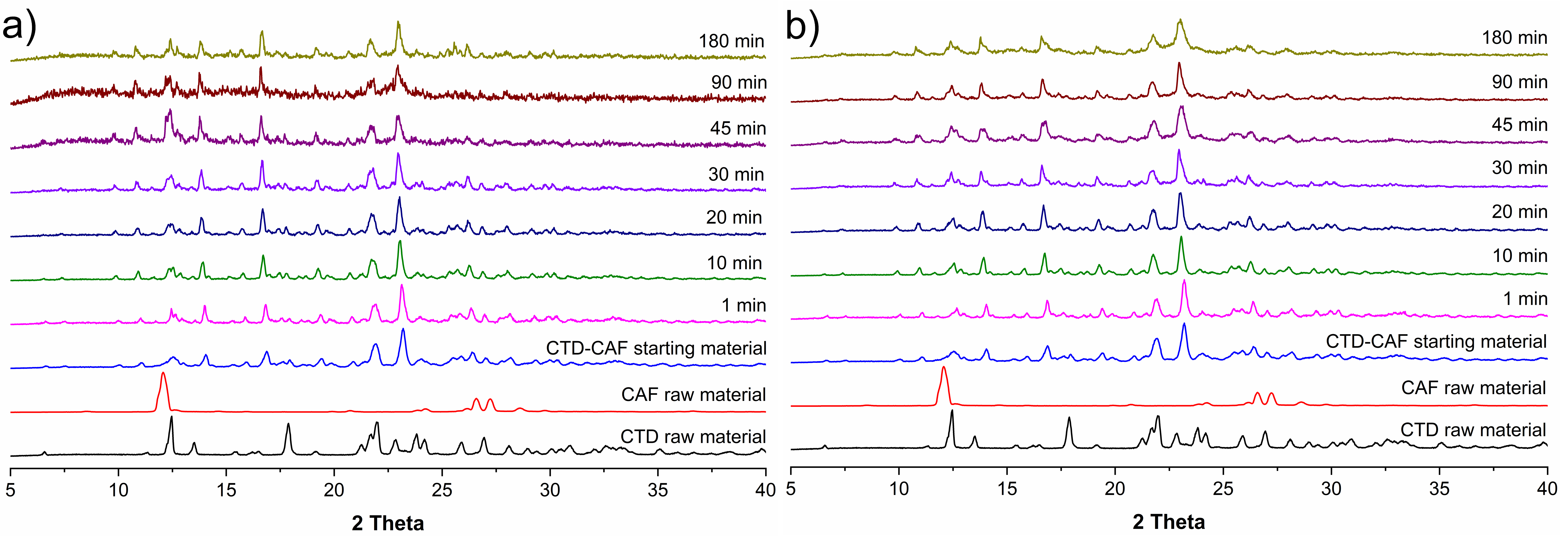
3.5. Solid-State Stability Tests
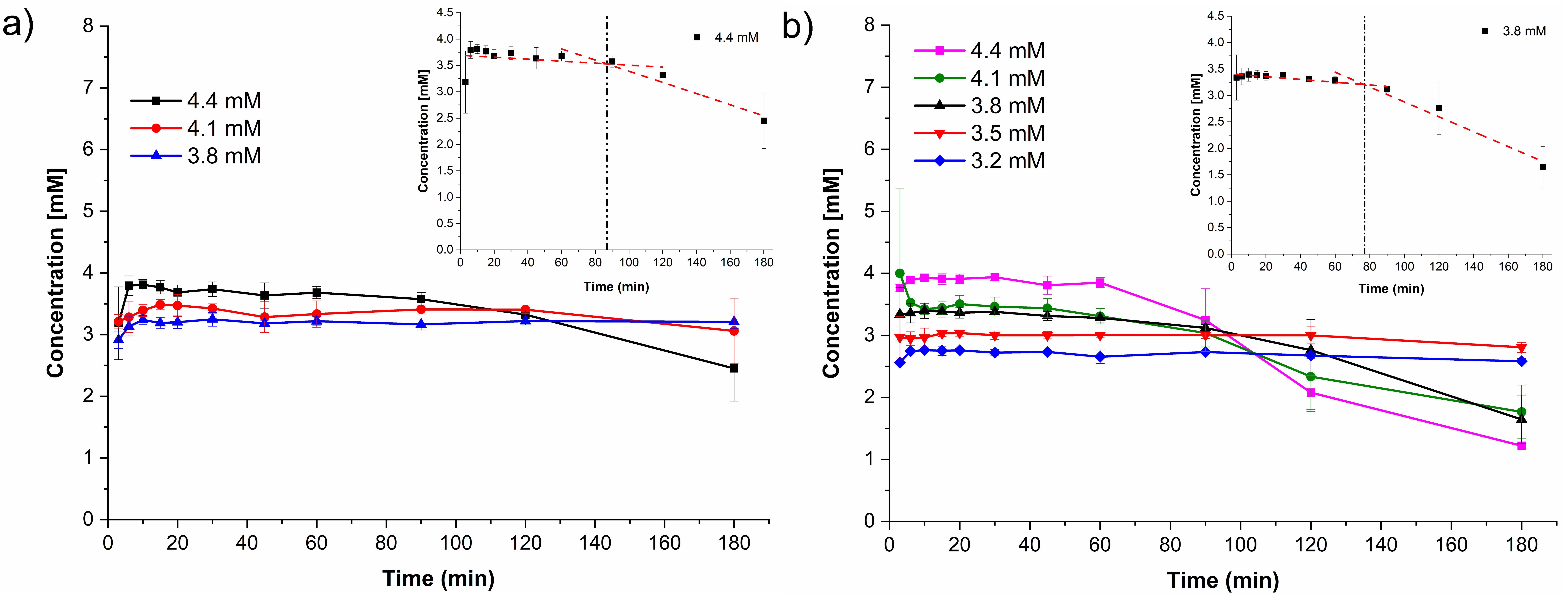
3.6. Solubility Studies
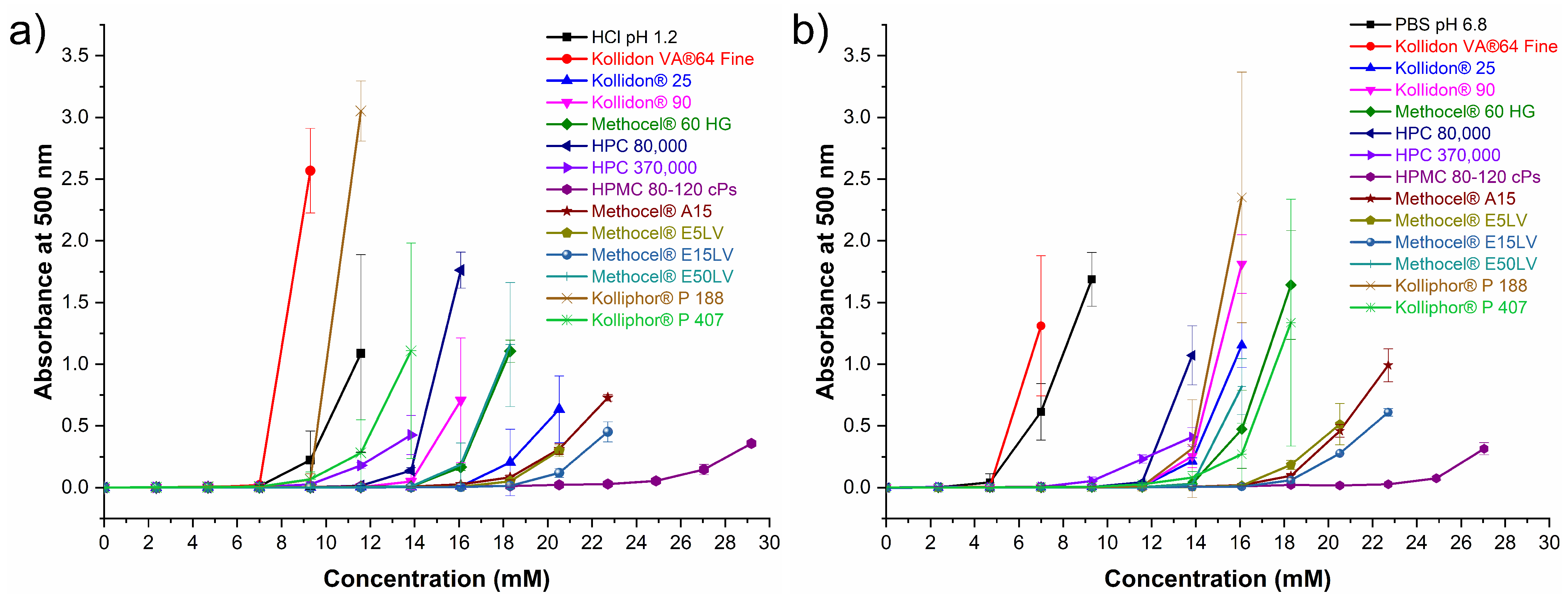
3.7. Polymer Selection for Dissolution Studies
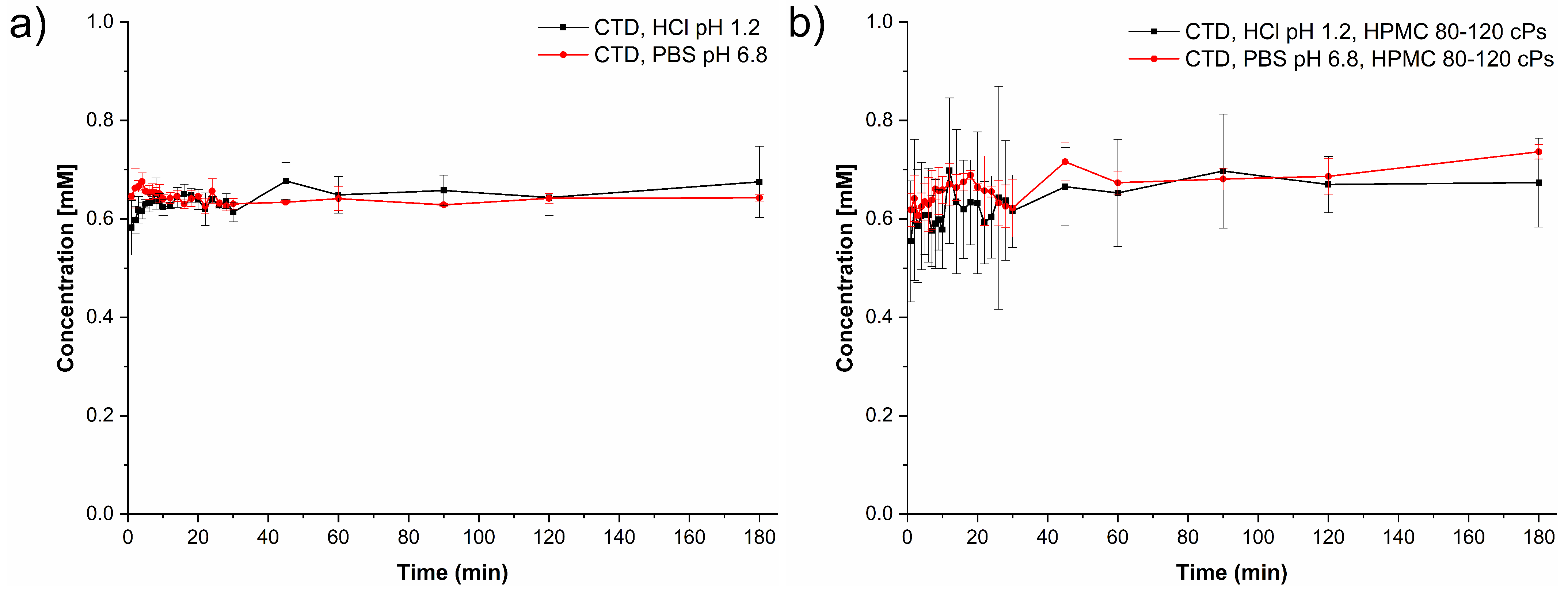
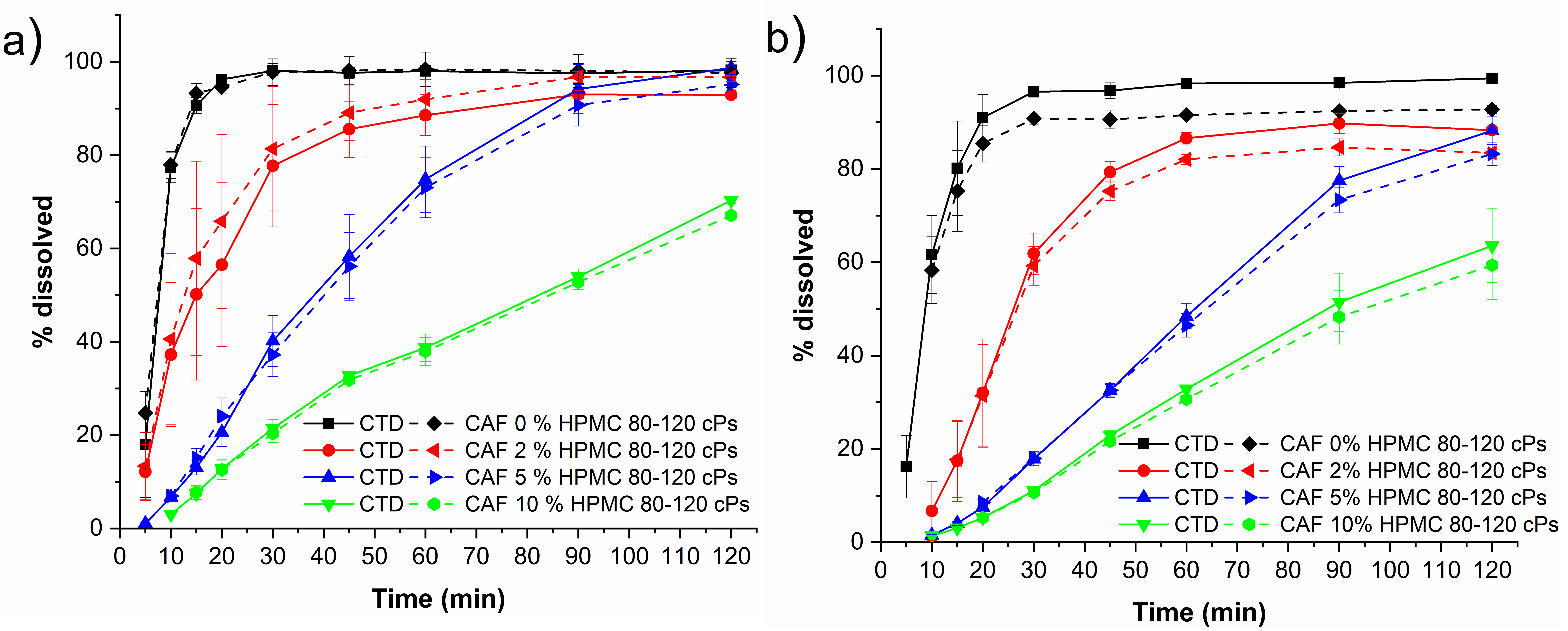
3.8. Powder Dissolution Studies under Non-Sink Conditions
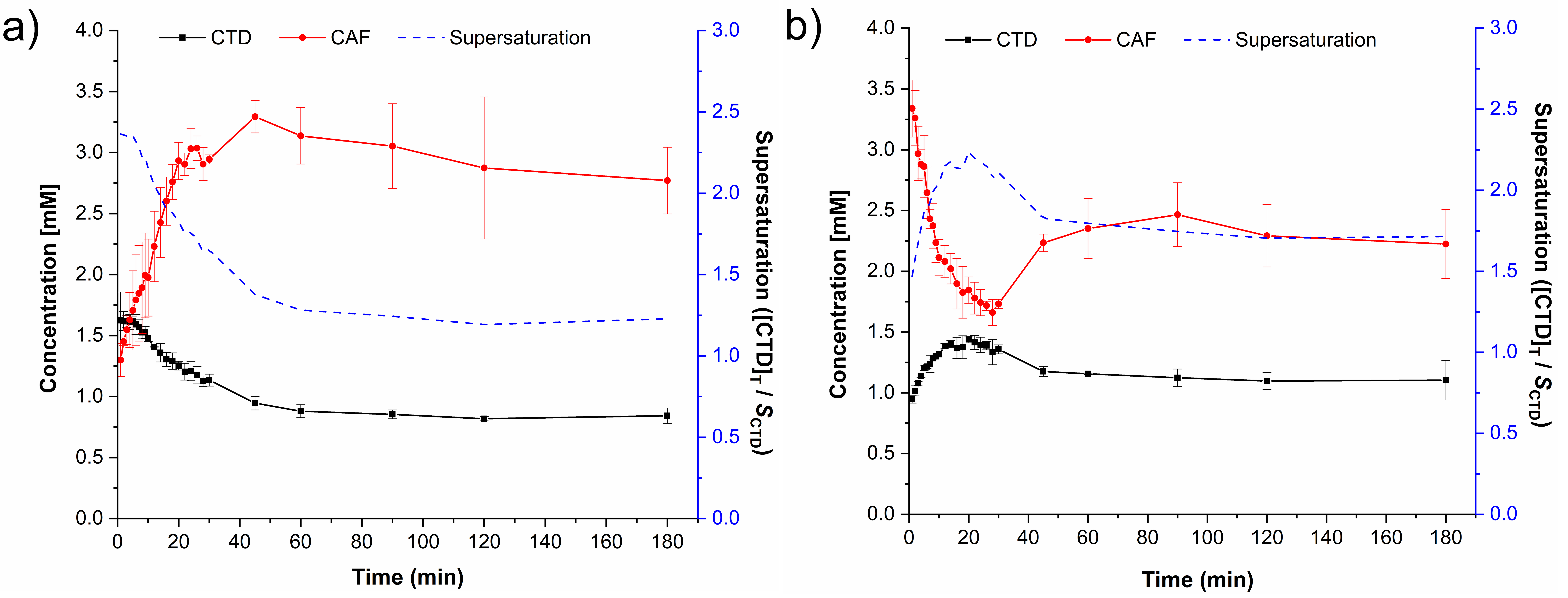
3.9. Induced Precipitation Experiments
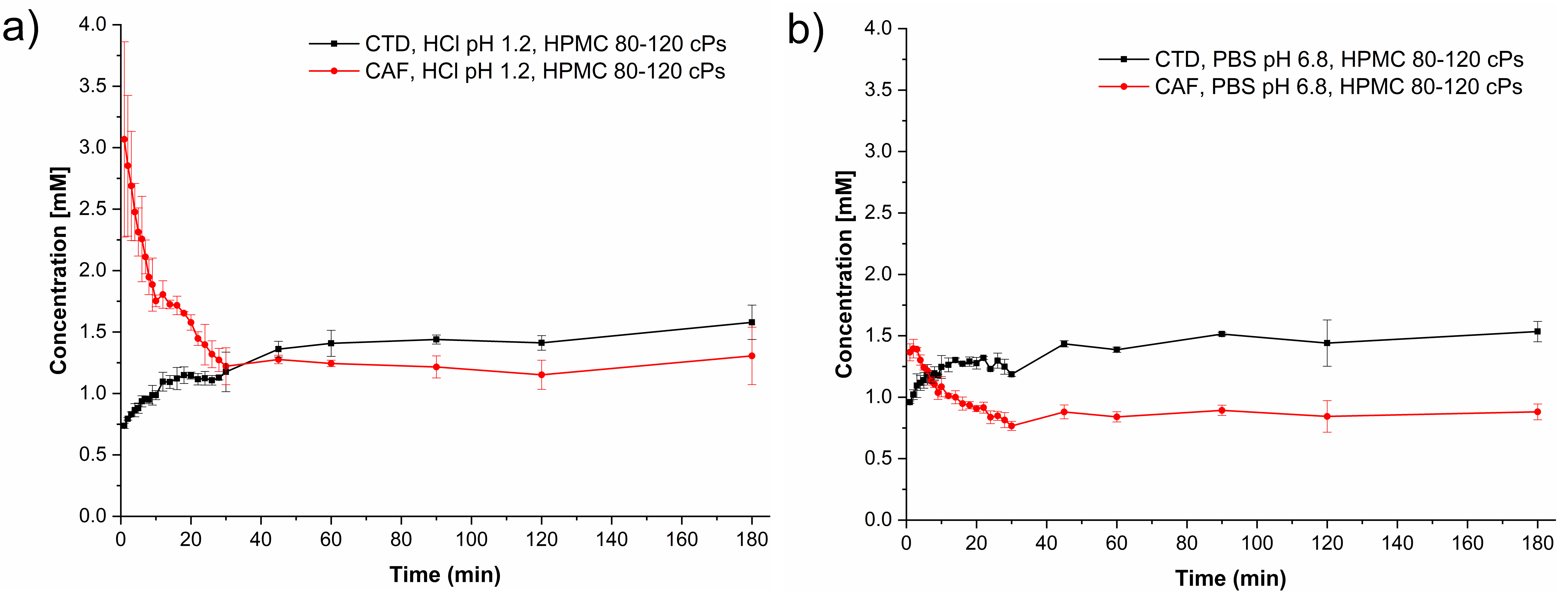
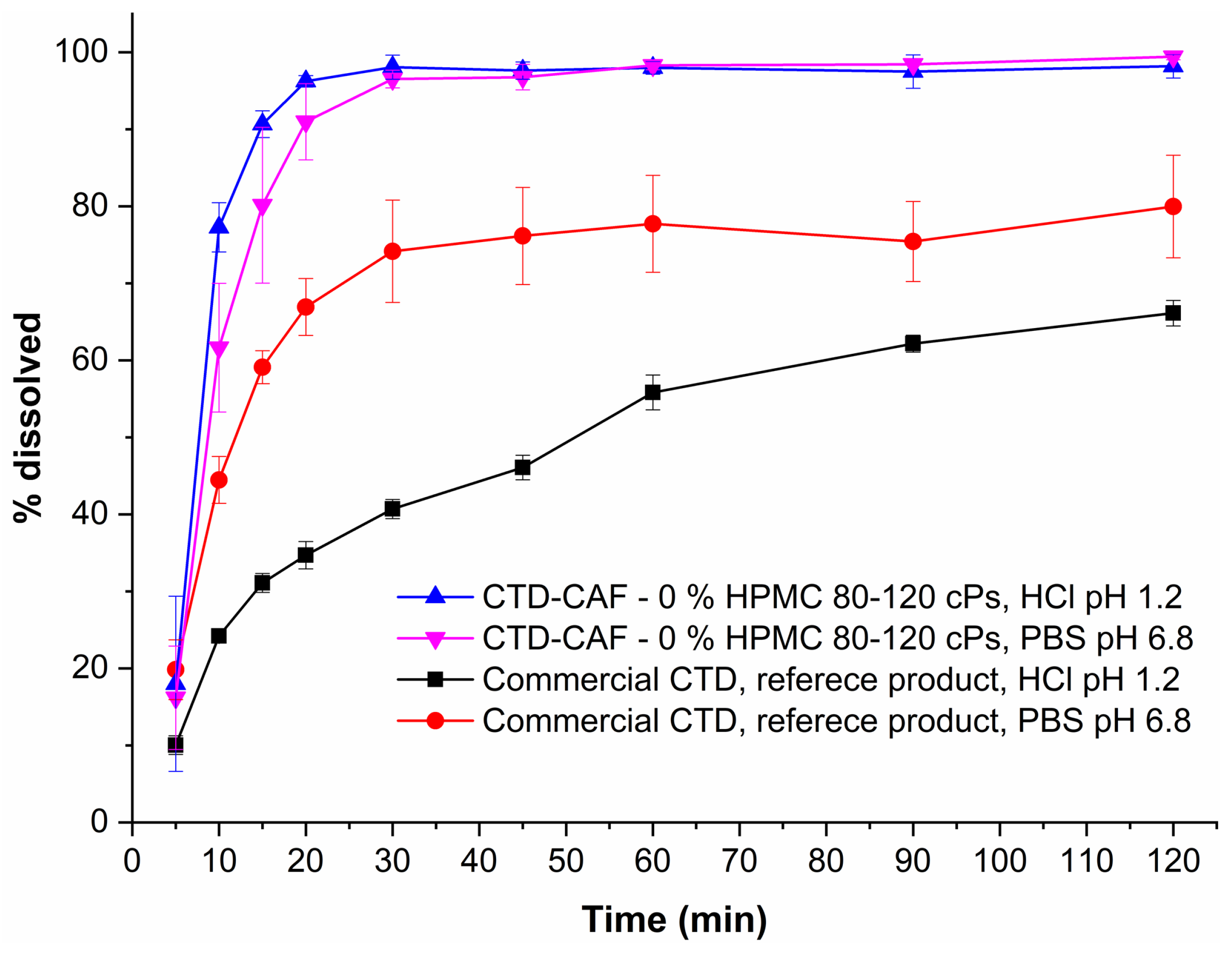
3.10. Dissolution Studies under Sink Conditions
4. Discussion
4.1. Solubility of CTD-CAF Cocrystal and Performance under Non-Sink Conditions
4.2. Study of the Solution-Mediated Phase Transformation (SMPT) Mechanism
4.3. Performance of CTD-CAF Cocrystal Pre-Formulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sica, D.A.; Carter, B.; Cushman, W.; Hamm, L. Thiazide and Loop Diuretics. J. Clin. Hypertens. 2011, 13, 639–643. [Google Scholar] [CrossRef] [PubMed]
- The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J. Am. Med. Assoc. 2002, 288, 2981–2997. [Google Scholar] [CrossRef]
- O’Brien, J.G.; Chennubhotla, S.A.; Chennubhotla, R.V. Treatment of Edema. Am. Fam. Physician 2005, 71, 2111–2117. [Google Scholar] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Bonfilio, R.; Leal, J.S.; Santos, O.M.M.; Pereira, G.R.; Doriguetto, A.C.; de Araújo, M.B. Analysis of chlorthalidone polymorphs in raw materials and tablets and the effect of forms I and II on the dissolution properties of drug products. J. Pharm. Biomed. Anal. 2014, 88, 562–570. [Google Scholar] [CrossRef]
- Beermann, B.; Groschinsky-Grind, M. Clinical Pharmacokinetics of Diuretics. Clin. Pharmacokinet. 1980, 5, 221–245. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Benet, L.Z. Predicting Drug Disposition via Application of BCS: Transport/Absorption/Elimination Interplay and Development of a Biopharmaceutics Drug Disposition Classification System. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: M9 Biopharmaceutics Classification System Based Biowaivers; U.S. Food and Drug Administration, Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2021.
- Singer, J.M.; O’Hare, M.J.; Rehm, C.R.; Zarembo, J.E. Chlorthalidone. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Orlando, FL, USA, 1985; Volume 14, pp. 1–36. [Google Scholar]
- Materson, B.J.; Oster, J.R.; Michael, U.F.; Bolton, S.M.; Burton, Z.C.; Stambaugh, J.E.; Morledge, J. Dose response to chlorthalidone in patients with mild hypertension; Efficacy of a lower dose. Clin. Pharmacol. Ther. 1978, 24, 192–198. [Google Scholar] [CrossRef]
- Sumiye, L.; Vivian, A.; Frisof, K.; Podany, E. Potassium loss associated with hydrochlorothiazide versus chlorthalidone. Clin. Ther. 1981, 4, 308–320. [Google Scholar]
- Dhalla, I.A.; Gomes, T.; Yao, Z.; Nagge, J.; Persaud, N.; Hellings, C.; Mamdani, M.M.; Juurlink, D.N. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: A population-based cohort study. Ann. Intern. Med. 2013, 158, 447–455. [Google Scholar] [CrossRef]
- Mishra, S.K.; Panda, A. A comparative analysis of hydrochlorothiazide and chlorthalidone induced hyponatremia at the dose commonly prescribed in clinical practice. Int. J. Basic Clin. Pharmacol. 2018, 7, 935–940. [Google Scholar] [CrossRef][Green Version]
- Childs, S.L.; Kandi, P.; Lingireddy, S.R. Formulation of a Danazol Cocrystal with Controlled Supersaturation Plays an Essential Role in Improving Bioavailability. Mol. Pharm. 2013, 10, 3112–3127. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Narurkar, A.; Sheen, P.-C.; Hurwitz, E.L.; Augustine, M.A. Effect of Particle Size on the Dissolution Characteristics of Chlorthalidone. Drug Dev. Ind. Pharm. 1987, 13, 319–328. [Google Scholar] [CrossRef]
- Lötter, J.; Krieg, H.M.; Keizer, K.; Breytenbach, J.C. The influence of beta-cyclodextrin on the solubility of chlorthalidone and its enantiomers. Drug Dev. Ind. Pharm. 1999, 25, 879–884. [Google Scholar] [CrossRef]
- Salazar-Miranda, M.A.; Cruz-Sosa, F.; Rodríguez-Huezo, M.E.; Jiménez-Alvarado, R.; Pérez-Alonso, C. Microencapsulation of chlorthalidone by spray-drying of double emulsion and melt granulation coating. Dry. Technol. 2016, 34, 1118–1128. [Google Scholar] [CrossRef]
- Dangre, P.V.; Gilhotra, R.M.; Dhole, S.N. Formulation and development of solid self micro-emulsifying drug delivery system (S-SMEDDS) containing chlorthalidone for improvement of dissolution. J. Pharm. Investig. 2016, 46, 633–644. [Google Scholar] [CrossRef]
- França, M.T.; Nicolay Pereira, R.; Klüppel Riekes, M.; Munari Oliveira Pinto, J.; Stulzer, H.K. Investigation of novel supersaturating drug delivery systems of chlorthalidone: The use of polymer-surfactant complex as an effective carrier in solid dispersions. Eur. J. Pharm. Sci. 2018, 111, 142–152. [Google Scholar] [CrossRef]
- França, M.T.; O’Reilly Beringhs, A.; Nicolay Pereira, R.; Martins Marcos, T.; Bazzo, G.C.; Stulzer, H.K. The role of sodium alginate on the supersaturation state of the poorly soluble drug chlorthalidone. Carbohydr. Polym. 2019, 209, 207–214. [Google Scholar] [CrossRef]
- Martins, F.T.; Bocelli, M.D.; Bonfilio, R.; de Araújo, M.B.; Lima, P.V.d.; Neves, P.P.; Veloso, M.P.; Ellena, J.; Doriguetto, A.C. Conformational Polymorphism in Racemic Crystals of the Diuretic Drug Chlortalidone. Cryst. Growth Des. 2009, 9, 3235–3244. [Google Scholar] [CrossRef]
- Martins, F.T.; Bonfilio, R.; Rosa, I.M.L.; Santos, L.M.; Santos, O.M.M.; Araújo, M.B.; Doriguetto, A.C. The form II of the antihypertensive drug chlorthalidone. CrystEngComm 2013, 15, 3767–3771. [Google Scholar] [CrossRef]
- Martins, F.T.; de abreu, P.J.; Azarias, L.C.; Villis, P.C.M.; de Campos Melo, A.C.; Ellena, J.; Doriguetto, A.C. Form III-like conformation and Form I-like packing in a chloroform channel solvate of the diuretic drug chlortalidone. CrystEngComm 2012, 14, 6173–6177. [Google Scholar] [CrossRef]
- Mohammed, K.; Mohammed, A.A.K.; Abdel Hakiem, A.F.; Mahfouz, R.M. Computational evaluation on the molecular conformation, vibrational spectroscopy, NBO analysis and molecular docking of betaxolol and betaxolol-chlorthalidone cocrystals. J. Mol. Struct. 2020, 1209, 127744. [Google Scholar] [CrossRef]
- Steed, J.W. The role of co-crystals in pharmaceutical design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Multi-component Crystals. In Crystal Engineering: A Textbook; World Scientific Publishing: Hackensack, NJ, USA, 2011; pp. 131–153. [Google Scholar]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen bonds as design elements in organic chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Cocrystal Eutectic Constants and Prediction of Solubility Behavior. Cryst. Growth Des. 2010, 10, 1028–1032. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Supersaturation Potential of Salt, Co-Crystal, and Amorphous Forms of a Model Weak Base. Cryst. Growth Des. 2016, 16, 737–748. [Google Scholar] [CrossRef]
- Salas-Zúñiga, R.; Rodríguez-Ruiz, C.; Höpfl, H.; Morales-Rojas, H.; Sánchez-Guadarrama, O.; Rodríguez-Cuamatzi, P.; Herrera-Ruiz, D. Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers. Pharmaceutics 2020, 12, 23. [Google Scholar] [CrossRef]
- Cavanagh, K.L.; Kuminek, G.; Rodríguez-Hornedo, N. Cocrystal Solubility Advantage and Dose/Solubility Ratio Diagrams: A Mechanistic Approach To Selecting Additives and Controlling Dissolution–Supersaturation–Precipitation Behavior. Mol. Pharm. 2020, 17, 4286–4301. [Google Scholar] [CrossRef]
- Sanchez, J.M. Methylxanthine Content in Commonly Consumed Foods in Spain and Determination of Its Intake during Consumption. Foods 2017, 6, 109. [Google Scholar] [CrossRef]
- Hartley, T.R.; Lovallo, W.R.; Whitsett, T.L.; Sung, B.H.; Wilson, M.F. Caffeine and Stress: Implications for Risk, Assessment, and Management of Hypertension. J. Clin. Hypertens. 2001, 3, 354–382. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef]
- Devogelaer, J.-J.; Meekes, H.; Vlieg, E.; de Gelder, R. Cocrystals in the Cambridge Structural Database: A network approach. Acta Crystallogr. Sect. B 2019, 75, 371–383. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef]
- Zhang, G.G.Z.; Henry, R.F.; Borchardt, T.B.; Lou, X. Efficient Co-crystal Screening Using Solution-Mediated Phase Transformation. J. Pharm. Sci. 2007, 96, 990–995. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Kuminek, G.; Rodríguez-Hornedo, N.; Siedler, S.; Rocha, H.V.A.; Cuffini, S.L.; Cardoso, S.G. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem. Commun. 2016, 52, 5832–5835. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ozaki, S.; Kushida, I. Solvent shift method for anti-precipitant screening of poorly soluble drugs using biorelevant medium and dimethyl sulfoxide. Int. J. Pharm. 2011, 419, 170–174. [Google Scholar] [CrossRef]
- Omori, M.; Uekusa, T.; Oki, J.; Inoue, D.; Sugano, K. Solution-mediated phase transformation at particle surface during cocrystal dissolution. J. Drug Deliv. Sci. Technol. 2020, 56, 101566. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. DIAMOND—Crystal and Molecular Structure Visualization V3. 2; Crystal Impact GbR: Bonn, Germany, 2017. [Google Scholar]
- FDA. Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics; U.S. Food and Drug Administration, Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2015.
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.W.; Stowasser, F. The Crystal Structure of Anhydrous β-Caffeine as Determined from X-ray Powder-Diffraction Data. Chem.—Eur. J. 2007, 13, 2908–2911. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Q3C-Tables and List—ICH (Revision 3); U.S. Food and Drug Administration, Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2017.
- Adsmond, D.A.; Grant, D.J.W. Hydrogen bonding in sulfonamides. J. Pharm. Sci. 2001, 90, 2058–2077. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Mittapalli, S.; Nangia, A. Modularity and three-dimensional isostructurality of novel synthons in sulfonamide-lactam cocrystals. IUCrJ 2015, 2, 389–401. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Supramolecular synthon hierarchy in sulfonamide cocrystals with syn-amides and N-oxides. IUCrJ 2019, 6, 751–760. [Google Scholar] [CrossRef]
- Bolla, G.; Mittapalli, S.; Nangia, A. Celecoxib cocrystal polymorphs with cyclic amides: Synthons of a sulfonamide drug with carboxamide coformers. CrystEngComm 2014, 16, 24–27. [Google Scholar] [CrossRef]
- Mandelcorn, L. Clathrates. Chem. Rev. 1959, 59, 827–839. [Google Scholar] [CrossRef]
- Córdova-Villanueva, E.N.; Rodríguez-Ruiz, C.; Sánchez-Guadarrama, O.; Rivera-Islas, J.; Herrera-Ruiz, D.; Morales-Rojas, H.; Höpfl, H. Diastereomeric Salt Formation by the γ-Amino Acid RS-Baclofen and L-Malic Acid: Stabilization by Strong Heterosynthons Based on Hydrogen Bonds between RNH3+ and COOH/COO− Groups. Cryst. Growth Des. 2018, 18, 7356–7367. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, A practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; Wiley: New York, NY, USA, 2006; Volume 2, pp. 1–23. [Google Scholar]
- Zhang, H.; Zhu, Y.; Qiao, N.; Chen, Y.; Gao, L. Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution. Pharmaceutics 2017, 9, 54. [Google Scholar] [CrossRef]
- Zubair, M.U.; Hassan, M.M.A.; Al-Meshal, I.A. Caffeine. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1986; Volume 15, pp. 71–150. [Google Scholar]
- Bauer, J.; Quick, J.; Krogh, S.; Shada, D. Stability-Indicating Assay for Chlorthalidone Formulation: Evaluation of the USP Analysis and a High-Performance Liquid Chromatographic Analysis. J. Pharm. Sci. 1983, 72, 924–928. [Google Scholar] [CrossRef]
- Martínez-Alejo, J.M.; Domínguez-Chávez, J.G.; Rivera-Islas, J.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H.; Senosiain, J.P. A Twist in Cocrystals of Salts: Changes in Packing and Chloride Coordination Lead to Opposite Trends in the Biopharmaceutical Performance of Fluoroquinolone Hydrochloride Cocrystals. Cryst. Growth Des. 2014, 14, 3078–3095. [Google Scholar] [CrossRef]
- Arenas-García, J.I.; Herrera-Ruiz, D.; Morales-Rojas, H.; Höpfl, H. Interrelation of the dissolution behavior and solid-state features of acetazolamide cocrystals. Eur. J. Pharm. Sci. 2017, 96, 299–308. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Lim, L.-Y.; Go, M.-L. Caffeine and nicotinamide enhances the aqueous solubility of the antimalarial agent halofantrine. Eur. J. Pharm. Sci. 2000, 10, 17–28. [Google Scholar] [CrossRef]
- Omori, M.; Watanabe, T.; Uekusa, T.; Oki, J.; Inoue, D.; Sugano, K. Effects of Coformer and Polymer on Particle Surface Solution-Mediated Phase Transformation of Cocrystals in Aqueous Media. Mol. Pharm. 2020, 17, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Minamisono, T.; Yamashita, T.; Kato, T.; Kushida, I. Supersaturation–Nucleation Behavior of Poorly Soluble Drugs and its Impact on the Oral Absorption of Drugs in Thermodynamically High-Energy Forms. J. Pharm. Sci. 2012, 101, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.-K.; Henry, R.F.; Lou, X.; Duerst, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of Caffeine and Hydroxybenzoic Acids Composed of Multiple Supramolecular Heterosynthons: Screening via Solution-Mediated Phase Transformation and Structural Characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Synthon Hierarchies in Crystal Forms Composed of Theophylline and Hydroxybenzoic Acids: Cocrystal Screening via Solution-Mediated Phase Transformation. Cryst. Growth Des. 2014, 14, 5318–5328. [Google Scholar] [CrossRef]
- Takata, N.; Shiraki, K.; Takano, R.; Hayashi, Y.; Terada, K. Cocrystal Screening of Stanolone and Mestanolone Using Slurry Crystallization. Cryst. Growth Des. 2008, 8, 3032–3037. [Google Scholar] [CrossRef]
- Gagnière, E.; Mangin, D.; Puel, F.; Rivoire, A.; Monnier, O.; Garcia, E.; Klein, J.P. Formation of co-crystals: Kinetic and thermodynamic aspects. J. Cryst. Growth 2009, 311, 2689–2695. [Google Scholar] [CrossRef]
- Ratih, H.; Pamudji, J.S.; Alatas, F.; Soewandhi, S.N. Improving telmisartan mechanical properties through the formation oftelmisartan and oxalic acid co-crystal by slow evaporation andultrasound assisted co-crystallization from solution methods. Songklanakarin J. Sci. Technol. 2020, 42, 188–195. [Google Scholar] [CrossRef]
- Childs, S.L.; Rodríguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. CrystEngComm 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; Bahia de Oliveira da Rocha, A.; Gonçalves Cardoso, S.; Rodríguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y. Handbook of Aqueous Solubility Data, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Jasani, M.S.; Kale, D.P.; Singh, I.P.; Bansal, A.K. Influence of Drug–Polymer Interactions on Dissolution of Thermodynamically Highly Unstable Cocrystal. Mol. Pharm. 2019, 16, 151–164. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Ali, H.R.H.; Velaga, S.P. Effects of Polymer and Surfactant on the Dissolution and Transformation Profiles of Cocrystals in Aqueous Media. Cryst. Growth Des. 2014, 14, 643–648. [Google Scholar] [CrossRef]
- Yoshimura, M.; Miyake, M.; Kawato, T.; Bando, M.; Toda, M.; Kato, Y.; Fukami, T.; Ozeki, T. Impact of the Dissolution Profile of the Cilostazol Cocrystal with Supersaturation on the Oral Bioavailability. Cryst. Growth Des. 2017, 17, 550–557. [Google Scholar] [CrossRef]
- Xu, S.; Dai, W.-G. Drug precipitation inhibitors in supersaturable formulations. Int. J. Pharm. 2013, 453, 36–43. [Google Scholar] [CrossRef]
- Greco, K.; Bogner, R. Solution-Mediated Phase Transformation: Significance During Dissolution and Implications for Bioavailability. J. Pharm. Sci. 2012, 101, 2996–3018. [Google Scholar] [CrossRef]
- Rathi, S.; Chavan, R.B.; Shastri, N.R. Classification of the crystallization tendency of active pharmaceutical ingredients (APIs) and nutraceuticals based on their nucleation and crystal growth behaviour in solution state. Drug Deliv. Transl. Res. 2020, 10, 70–82. [Google Scholar] [CrossRef]


| Formulation | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| CTD-CAF a | 64.3 | 64.3 | 64.3 | 64.3 |
| HPMC 80–120 cPs b | - | 2.8 | 7.1 | 14.2 |
| Parameter | Value |
|---|---|
| Formula | C36H32Cl2N8O10S2 |
| MW (g mol−1) | 871.71 |
| T (K) | 298 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 8.3218(5) |
| b (Å) | 14.6139(9) |
| c (Å) | 16.2923(10) |
| α (deg) | 72.529(3) |
| β (deg) | 81.128(3) |
| γ (deg) | 86.070(3) |
| Volume (Å3) | 1866.8(2) |
| Z | 2 |
| ρcalc (g cm−3) | 1.551 |
| μ (mm−1) | 3.225 |
| R1 (I ≥ 2σ(I)) | 0.0550 |
| wR2 (all data) | 0.1568 |
| GOF | 1.029 |
| Compound | Motif | ||||
|---|---|---|---|---|---|
| I | II | III | IV | Reference | |
| CTD-CAF | ✓ | ✓ | ✓ | ✓ | This work |
| CTD polymorph I | ✓ | ✓ | ✓ | ✓ | [23] |
| CTD polymorph II | ✓ | - | - | - | [24] |
| CTD polymorph III | ✓ | - | ✓ | - | [23] |
| CTD chloroform solvate | - | ✓ | - | - | [25] |
| Dissolution Medium | Sdrug [mM] | SCC [mM] | Keu | SA | D0D | D0CC |
|---|---|---|---|---|---|---|
| HCl pH 1.2 | 0.687 ± 0.005 | 2.00 ± 0.03 | 12.3 ± 0.6 | 2.91 ± 0.05 | 0.859 ± 0.006 | 0.295 ± 0.004 |
| PBS pH 6.8 | 0.643 ± 0.007 | 2.05 ± 0.05 | 16 ± 1 | 3.19 ± 0.09 | 0.92 ± 0.01 | 0.288 ± 0.007 |
| Solid Form (Dissolution Media) a | AUC0–45 b | AUC45–180 b | AUCTotal b | (AUCTotal, PM or CC/AUCTotal, CTD) |
|---|---|---|---|---|
| CTD (1.2) | 28.0 | 88.6 | 116.6 | - |
| CTD (6.8) | 28.2 | 86.2 | 114.4 | - |
| CTD (1.2, HPMC) | 27.5 | 91.0 | 118.5 | - |
| CTD (6.8, HPMC) | 28.9 | 94.0 | 122.9 | - |
| PM (1.2) | 32.9 | 98.8 | 131.7 | 1.12 |
| PM (6.8) | 32.3 | 99.3 | 131.6 | 1.15 |
| PM (1.2, HPMC) | 34.4 | 98.5 | 132.9 | 1.12 |
| PM (6.8, HPMC) | 31.5 | 108.6 | 140.1 | 1.13 |
| CC (1.2) | 55.1 | 114.7 | 169.8 | 1.45 |
| CC (6.8) | 57.2 | 151.0 | 208.2 | 1.81 |
| CC (1.2, HPMC) | 49.4 | 196.0 | 245.4 | 2.07 |
| CC (6.8, HPMC) | 55.3 | 198.2 | 253.5 | 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Ruiz, C.; Montes-Tolentino, P.; Domínguez-Chávez, J.G.; Morales-Rojas, H.; Höpfl, H.; Herrera-Ruiz, D. Tailoring Chlorthalidone Aqueous Solubility by Cocrystallization: Stability and Dissolution Behavior of a Novel Chlorthalidone-Caffeine Cocrystal. Pharmaceutics 2022, 14, 334. https://doi.org/10.3390/pharmaceutics14020334
Rodríguez-Ruiz C, Montes-Tolentino P, Domínguez-Chávez JG, Morales-Rojas H, Höpfl H, Herrera-Ruiz D. Tailoring Chlorthalidone Aqueous Solubility by Cocrystallization: Stability and Dissolution Behavior of a Novel Chlorthalidone-Caffeine Cocrystal. Pharmaceutics. 2022; 14(2):334. https://doi.org/10.3390/pharmaceutics14020334
Chicago/Turabian StyleRodríguez-Ruiz, Christian, Pedro Montes-Tolentino, Jorge Guillermo Domínguez-Chávez, Hugo Morales-Rojas, Herbert Höpfl, and Dea Herrera-Ruiz. 2022. "Tailoring Chlorthalidone Aqueous Solubility by Cocrystallization: Stability and Dissolution Behavior of a Novel Chlorthalidone-Caffeine Cocrystal" Pharmaceutics 14, no. 2: 334. https://doi.org/10.3390/pharmaceutics14020334
APA StyleRodríguez-Ruiz, C., Montes-Tolentino, P., Domínguez-Chávez, J. G., Morales-Rojas, H., Höpfl, H., & Herrera-Ruiz, D. (2022). Tailoring Chlorthalidone Aqueous Solubility by Cocrystallization: Stability and Dissolution Behavior of a Novel Chlorthalidone-Caffeine Cocrystal. Pharmaceutics, 14(2), 334. https://doi.org/10.3390/pharmaceutics14020334






