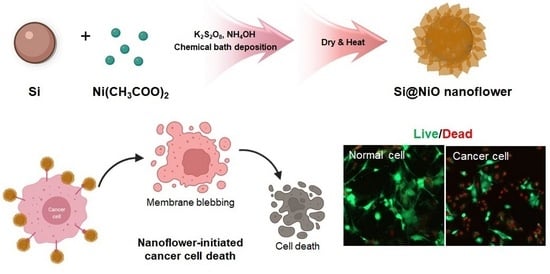
Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity
Abstract
:1. Introduction
2. Materials and Methods
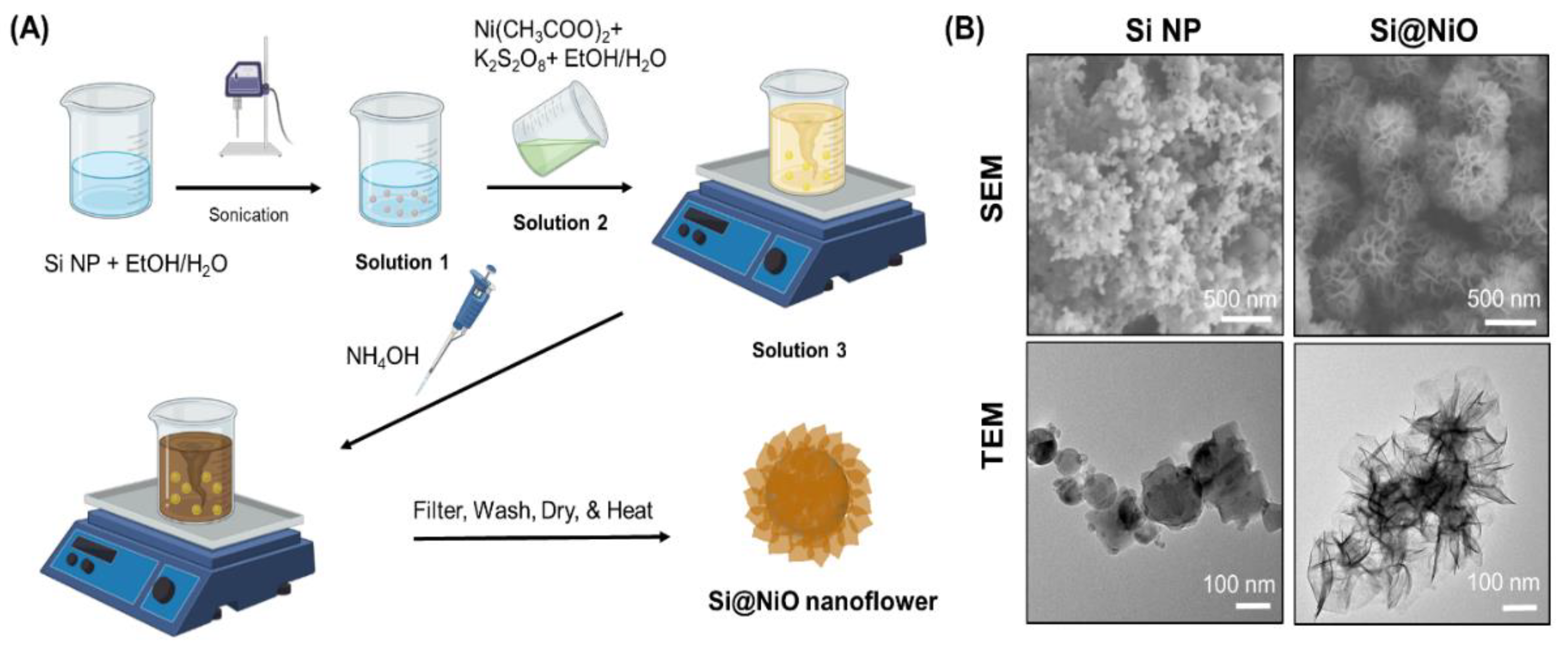
2.1. Synthesis of Si@NiO Nanoflowers
2.2. Characterization of Si@NiO Nanoflowers
2.3. Electrochemical Properties of Si@NiO Nanoflowers
2.4. Metal Ion Release from Si@NiO Nanoflowers
2.5. Cell Culture
2.6. In Vitro Anticancer Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Si@NiO Nanoflowers
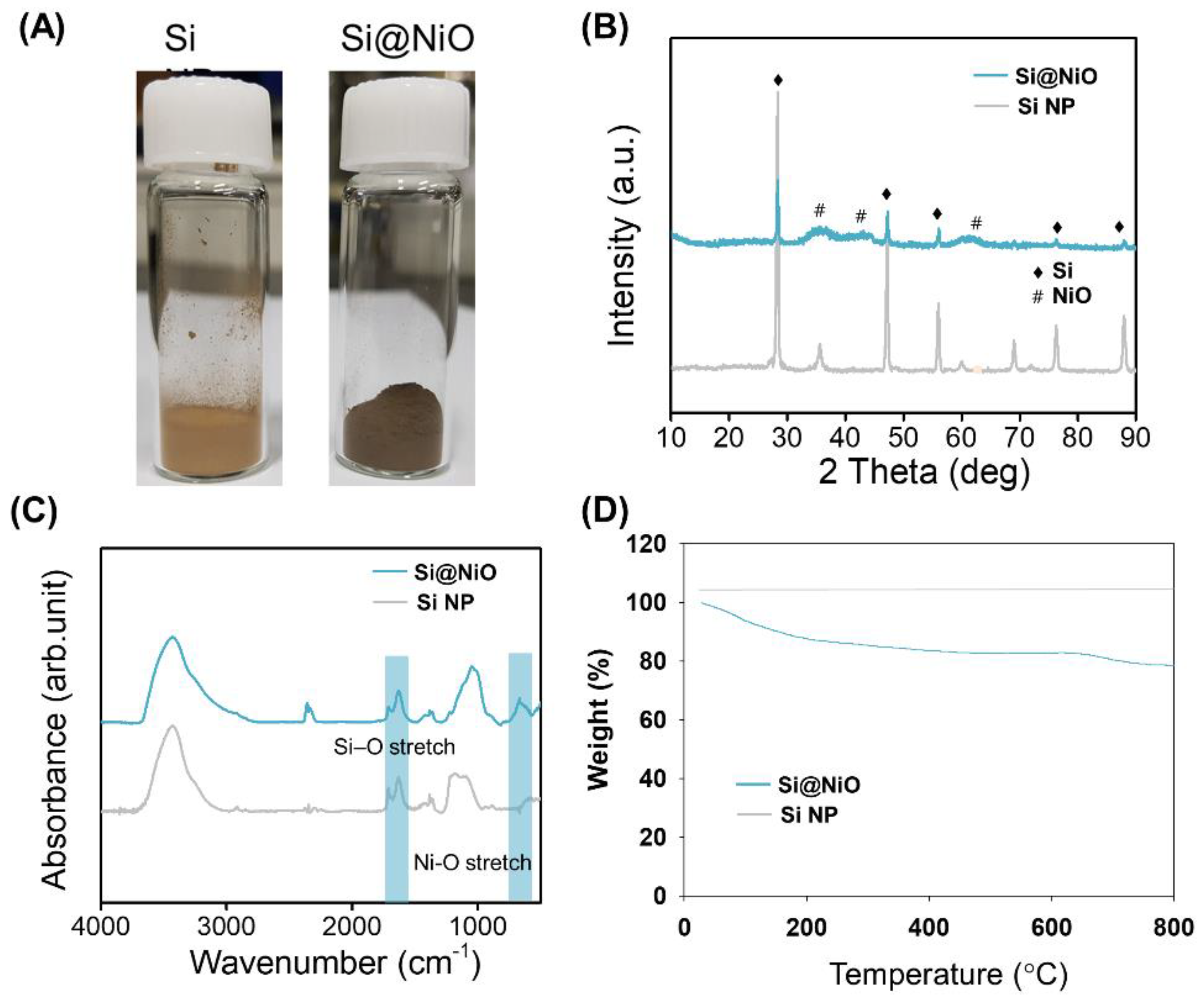
3.2. Characterization of Si@NiO Nanoflowers
3.3. Pore Structure Characterization
3.4. Electrochemical Properties
3.5. Ion Release Test
3.6. Anticancer Properties and Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Landesman-Milo, D.; Goldsmith, M.; Leviatan Ben-Arye, S.; Witenberg, B.; Brown, E.; Leibovitch, S.; Azriel, S.; Tabak, S.; Morad, V.; Peer, D. Hyaluronan grafted lipid-based nanoparticles as RNAi carriers for cancer cells. Cancer Lett. 2013, 334, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chapoy-Villanueva, H.; Martinez-Carlin, I.; Lopez-Berestein, G.; Chavez-Reyes, A. Therapeutic silencing of HPV 16 E7 by systemic administration of siRNA-neutral DOPC nanoliposome in a murine cervical cancer model with obesity. J. BU ON 2015, 20, 1471–1479. [Google Scholar]
- Fan, Y.; Chen, C.; Huang, Y.; Zhang, F.; Lin, G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surf. B Biointerfaces 2017, 151, 19–25. [Google Scholar] [CrossRef]
- Shi, C.; He, Y.; Ding, M.; Wang, Y.; Zhong, J. Nanoimaging of food proteins by atomic force microscopy. Part I: Components, imaging modes, observation ways, and research types. Trends Food Sci. Technol. 2019, 87, 3–13. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gajbhiye, V.; Jain, N.K. A Review of nanocarriers for the delivery of small interfering RNA. Biomaterials 2012, 33, 7138–7150. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: Inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin. Drug Deliv. 2017, 14, 783–796. [Google Scholar] [CrossRef]
- Pietro, P.D.; Strano, G.; Zuccarello, L.; Satriano, C. Gold and silver nanoparticles for applications in theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef] [PubMed]
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic nanomaterials for chemo/photothermal therapy: A promising horizon on effective cancer treatment. Biophys. Rev. 2019, 11, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Pagano, C.; Ceccarini, M.R. Current highlights about the safety of inorganic nanomaterials in healthcare. Curr. Med. Chem. 2019, 26, 2147–2165. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, N.; Esfahani, M.K.M.; Torabi, S.; Alavi, S.E.; Akbarzadeh, A. Effects of nanoliposomal and pegylated nanoliposomal artemisinin in treatment of breast cancer. Ind. J. Clin. Biochem. 2014, 29, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 1–26. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, J.; Hasan, N.; Manikandan, M.; Wu, H.-F. Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci. Rep. 2013, 3, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Su, M.; Ma, L.; Ma, L.; Liu, D.; Wang, Z. Preparation of graphene oxide–silver nanoparticle nanohybrids with highly antibacterial capability. Talanta 2013, 117, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Jin, L.; Sue, H.-J.; Sumi, Y.; Nishimura, R. Facile Decoration of Au nanoparticles on reduced graphene oxide surfaces via a one-step chemical functionalization approach. J. Mater. Chem. A 2013, 1, 10783–10789. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zeng, G.M.; Zhang, P.; Song, B.; Zhang, X.G.; Liu, H.Y.; Huan, S.Y. Graphene sponge decorated with copper nanoparticles as a novel bactericidal filter for inactivation of Escherichia coli. Chemosphere 2017, 184, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Qureshi, M.S.; Haque, F.Z. Biosynthesis of flower-shaped CuO nanostructures and their photocatalytic and antibacterial activities. Nanomicro Lett. 2020, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Ananth, A.; Dharaneedharan, S.; Seo, H.J.; Heo, M.S.; Boo, J.H. Soft Jet plasma-assisted synthesis of zinc oxide nanomaterials: Morphology controls and antibacterial activity of ZnO. Chem. Eng. J. 2017, 322, 742–751. [Google Scholar] [CrossRef]
- Lee, T.C.; Abdullah, H.Z.; Koshy, P.; Idris, M.I. Deposition of novel bioactive nanoflower-like sodium titanate on tio2 coating via anodic oxidation for biomedical applications. Mater. Lett. 2018, 216, 256–260. [Google Scholar] [CrossRef]
- Li, N.; Zeng, C.; Qin, Q.; Zhang, B.; Chen, L.; Luo, Z. Powerful antibacterial activity of graphene/nanoflower-like nickelous hydroxide nanocomposites. Nanomedicine 2018, 13, 2901–2916. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ansari, A.A.; Malik, A.; Chaudhary, A.A.; Syed, J.B.; Khan, A.A. Preparation, antibacterial activity of graphene/nanoflower-like nickelous hydroxide nanocomposites. J. Trace Elem. Med. Biol. 2019, 52, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Manikandan, E.; Kennedy, J.; Jayachandran, M.; Ladchumananandasiivam, R.; De Gomes, U.U.; Maaza, M. Synthesis and characterization studies of NiO nanorods for enhancing solar cell efficiency using photon upconversion materials. Ceram. Int. 2016, 42, 8385–8394. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Raja, A.; Devarajan, P.A. Structural elucidation and spectral characterizations of Co3O4 nanoflakes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 586–591. [Google Scholar] [CrossRef]
- Lokesh, K.; Kavitha, G.; Manikandan, E.; Mani, G.K.; Kaviyarasu, K.; Rayappan, J.B.B.; Ladchumananandasivam, R.; Sundeep Aanand, J.; Jayachandran, M.; Maaza, M. Effective ammonia detection using n-ZnO/p-NiO heterostructured nanofibers. IEEE Sens. J. 2016, 16, 2477–2483. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.G.; Manoj, P.K.; Koshy, P.; Prabhakara Rao, P.; Vaidyan, V.K. Preparation of transparent and semiconducting NiO films. Vacuum 2002, 68, 149–154. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanoparticles in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Costa, M.; Heck, J.D. Perspectives on the mechanism of nickel carcinogenesis. Adv. Inorg. Biochem. 1984, 6, 285–309. [Google Scholar] [PubMed]
- Cai, X.; Lin, M.; Tan, S.; Mai, W.; Zhang, Y.; Liang, Z.; Lin, Z.; Zhang, X. The use of polyethyleneimine-modified reduced graphene oxide as a substrate for silver nanoparticles to produce a material with lower cytotoxicity and long-term antibacterial activity. Carbon 2012, 50, 3407–3415. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, S.V.; Brownlow, W.J.; Xu, X.H. Using nanoparticle optics assay for direct observation of the function of antimicrobial agents in single live bacterial cells. Biochemistry 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B 2016, 164, 352–360. [Google Scholar] [CrossRef]
- Saghatforoush, L.A.; Mehdizadeh, R.; Chalabian, F. Hydrothermal and sonochemical synthesis of a nano-sized nickel(II) schiff base complex as a precursor for nano-sized nickel(II) oxide; spectroscopic, catalytic and antibacterial properties. Transit. Met. Chem. 2010, 35, 903–910. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, T.-H.; Yu, J.-S. Three-dimensional spongy nanographene-functionalized silicon anodes for lithium ion batteries with superior cycling stability. Nano Res. 2018, 11, 233–245. [Google Scholar] [CrossRef]
- Xia, C.; Yanjun, X.; Ning, W. Facile synthesis of NiO nanoflowers and their electrocatalytic performance. Sens. Actuators B 2011, 153, 434–438. [Google Scholar] [CrossRef]
- Jo, J.H.; Kim, H.C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, Y.; Cho, H.; Lee, S.; Yang, S.H.; Kim, S.J.; Lee, D.N. Robust copper metal–organic framework-embedded polysiloxanes for biomedical applications: Its antibacterial effects on mrsa and in vitro cytotoxicity. Nanomaterials 2021, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.N.; Gwon, K.; Kim, Y.; Cho, H.; Lee, S. Immobilization of antibacterial copper metal–organic framework containing glutarate and 1,2-bis(4-pyridyl)ethylene ligands on polydimethylsiloxane and its low cytotoxicity. J. Ind. Eng. Chem. 2021, 102, 135–145. [Google Scholar] [CrossRef]
- Gwon, K.; Jo, E.J.; Sahu, A.; Lee, J.Y.; Kim, M.G.; Tae, G. Improved near infrared-mediated hydrogel formation using diacrylated pluronic f127-coated upconversion nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Favors, Z.; Li, C.; Liu, C.; Ye, R.; Fu, C.; Bozhilov, K.; Guo, J.; Ozkan, M.; Ozkan, C.S. Silicon and carbon nanocomposite spheres with enhanced electrochemical performance for full cell lithium ion batteries. Sci. Rep. 2017, 7, 44838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasiri, K.W.D.K.; Nguyen, C.C.; Parimalam, B.S.; Jurng, S.Y.; Lucht, B.L. Citric acid based pre-sei for improvement of silicon electrodes in lithium ion batteries. J. Electrochem. Soc. 2018, 165, A1991–A1996. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Cui, B.; Zhang, X.; Zhang, D.; Xu, X. Xu, Preparation of MoS2 nanoflowers with rich active sites as an efficient adsorbent for aqueous organic dyes. New J. Chem. 2020, 44, 4558–4567. [Google Scholar] [CrossRef]
- Mali, S.S.; Betty, C.A.; Bhosale, P.N.; Patil, P.S.; Hong, C.K. From nanocorals to nanorods to nanoflowers nanoarchitecture for efficient dye-sensitized solar cells at relatively low film thickness: All Hydrothermal Process. Sci. Rep. 2014, 4, 5451. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.J.; Yang, I.J.; Tettey, C.O.; Kim, K.M.; Shin, H.M. In-vitro anticancer activity of green synthesized silver nanoparticles on MCF-7 human breast cancer cells. Mater. Sci. Eng. C 2016, 68, 430–435. [Google Scholar] [CrossRef]
- Whitten, D.L.; Myers, S.P.; Hawrelak, J.A.; Wohlmuth, H. The effect of St John’s wort extracts on CYP3A: A systematic review of prospective clinical trials. Br. J. Clin. Pharmacol. 2006, 62, 512–526. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Karimi, F.; Xie, K.; Fu, Q.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. MOF-mediated destruction of cancer using the cell’s own hydrogen peroxide. ACS Appl. Mater. Interfaces 2017, 9, 33599–33608. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Son, Y.-O.; Pratheeshkumar, P.; Shi, X. Oxidative stress and metal carcinogenesis. Free Radic. Biol. Med. 2012, 53, 743–757. [Google Scholar] [CrossRef] [PubMed]
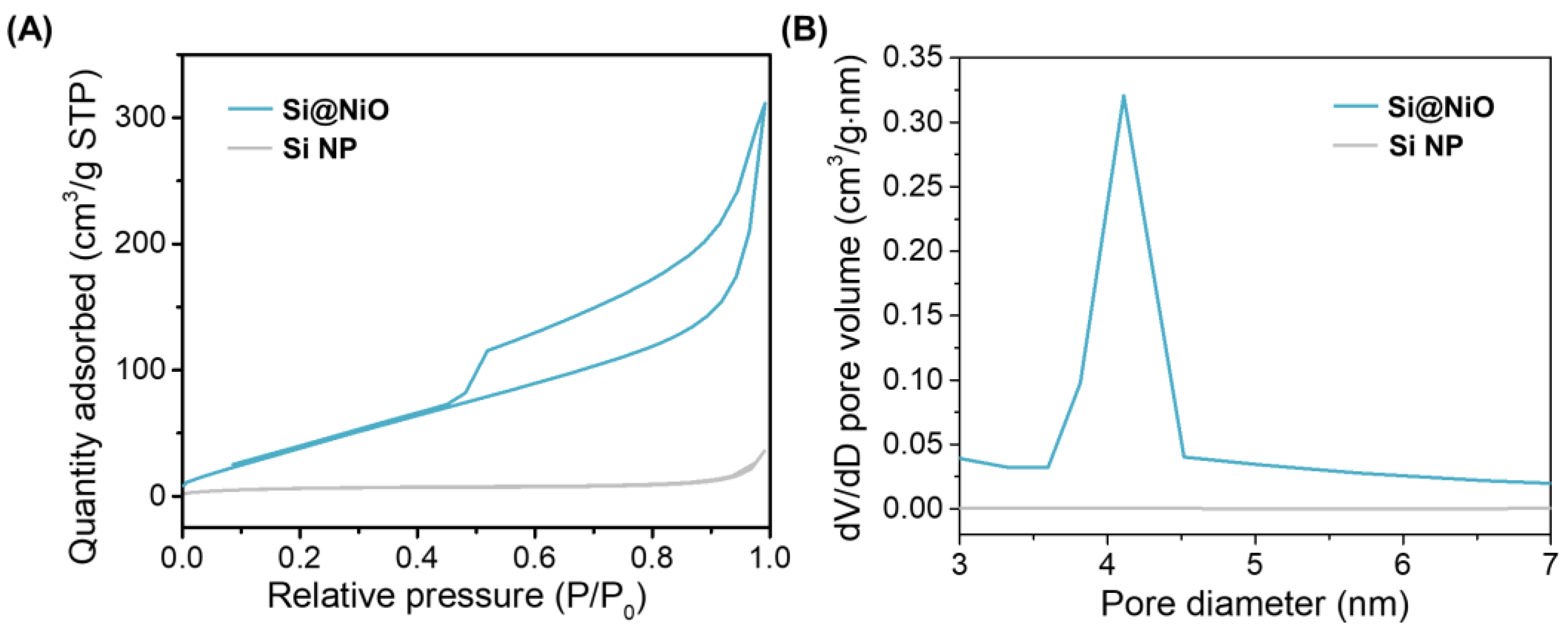
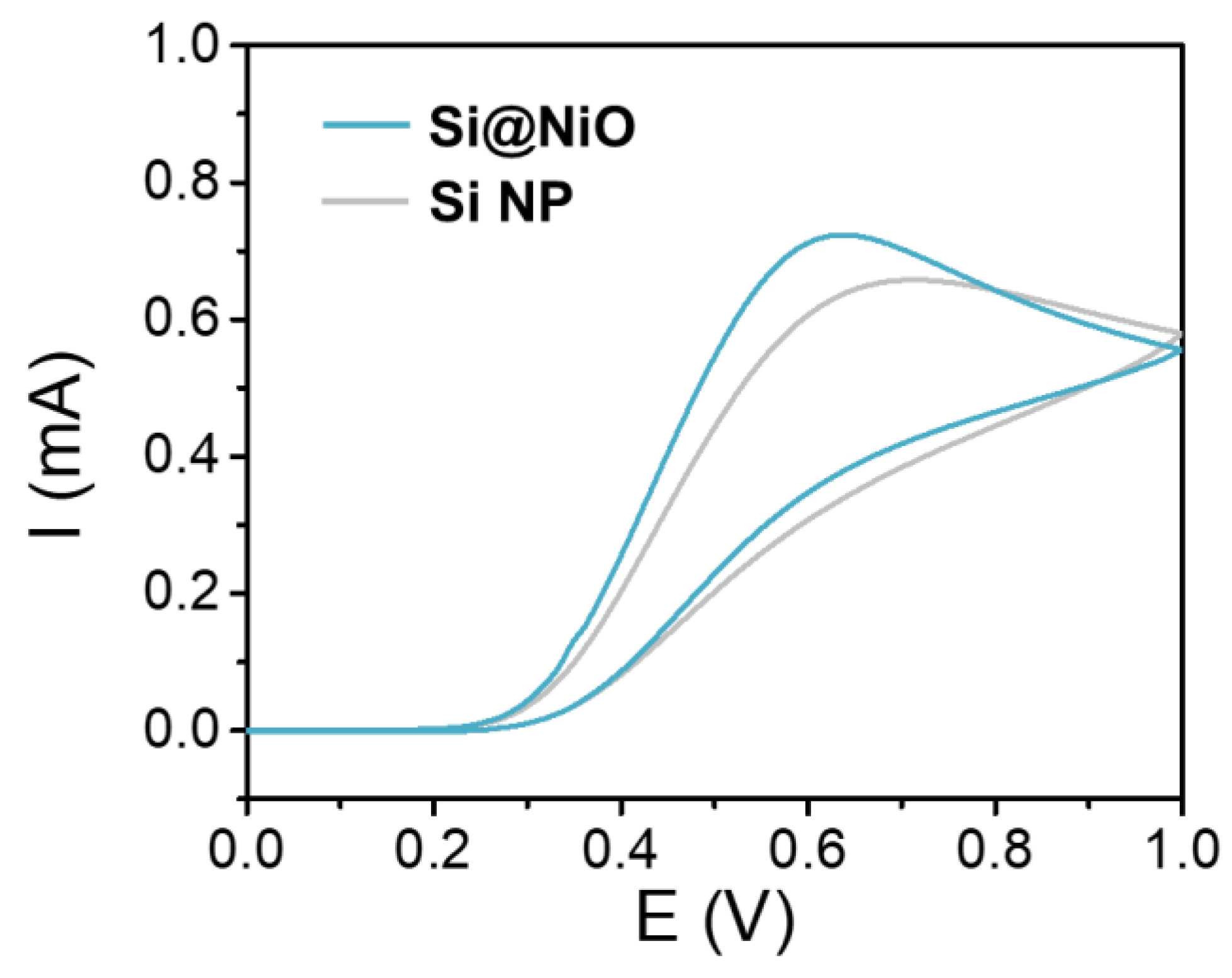
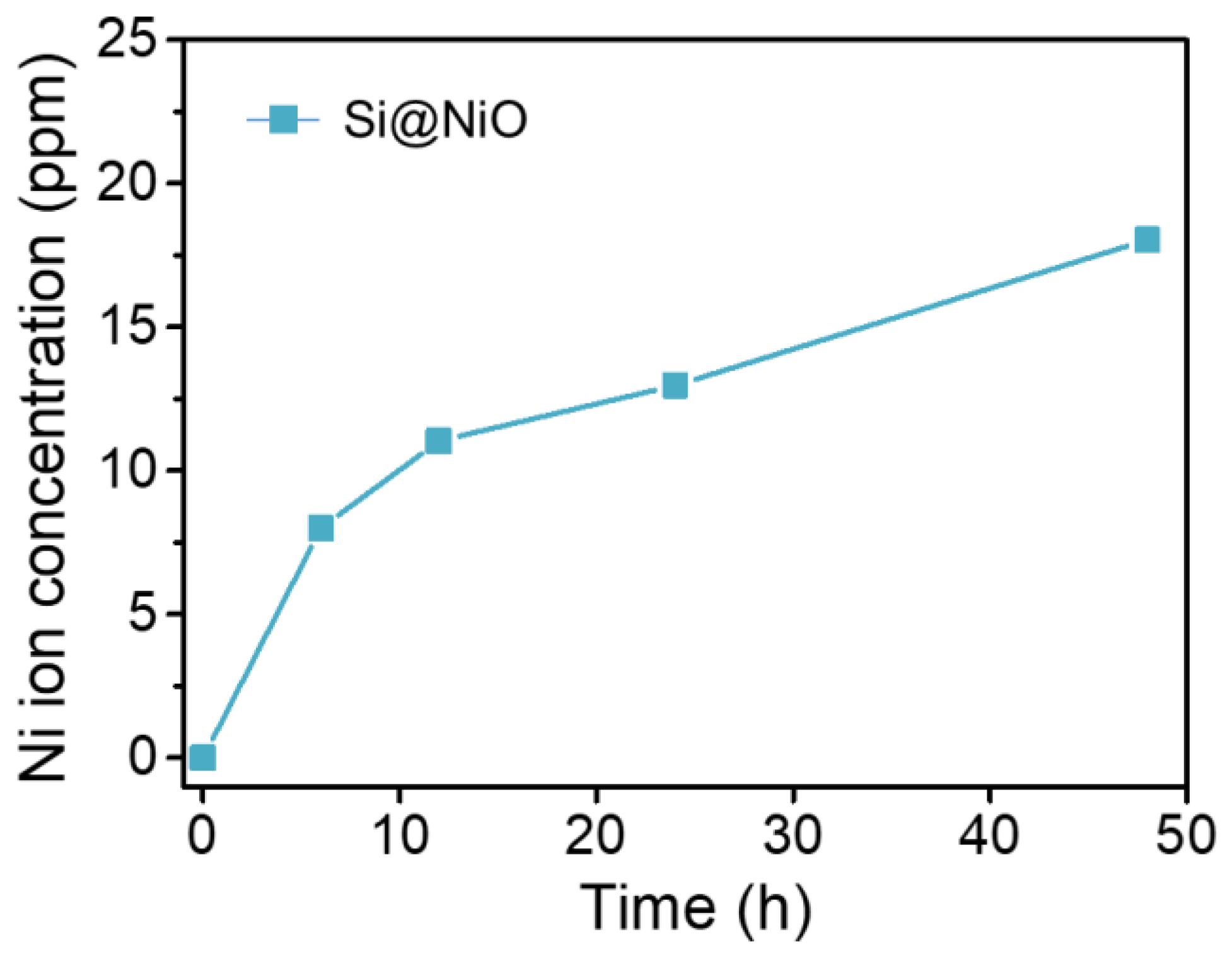
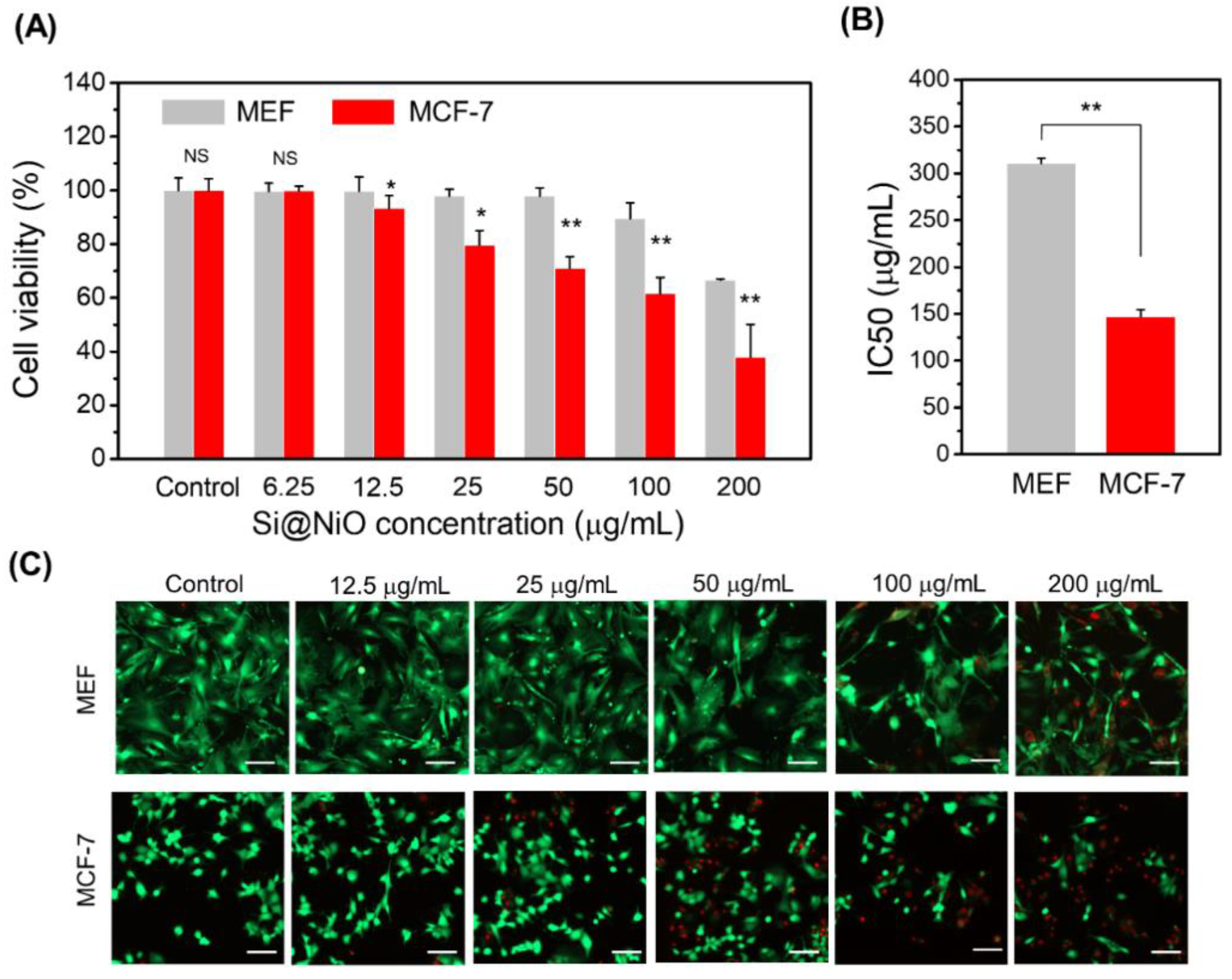
| Sample | Vmicro (cm3/g) | Vmeso (cm3/g) | SBET (m2/g) | Average pore size (nm) |
|---|---|---|---|---|
| Si NP | 0.006 | 0.05 | 23.50 | 0.56 |
| Si@NiO | 0.01 | 0.59 | 206.08 | 6.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, K.; Park, J.-D.; Lee, S.; Yu, J.-S.; Lee, D.N. Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity. Pharmaceutics 2022, 14, 268. https://doi.org/10.3390/pharmaceutics14020268
Gwon K, Park J-D, Lee S, Yu J-S, Lee DN. Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity. Pharmaceutics. 2022; 14(2):268. https://doi.org/10.3390/pharmaceutics14020268
Chicago/Turabian StyleGwon, Kihak, Jong-Deok Park, Seonhwa Lee, Jong-Sung Yu, and Do Nam Lee. 2022. "Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity" Pharmaceutics 14, no. 2: 268. https://doi.org/10.3390/pharmaceutics14020268
APA StyleGwon, K., Park, J.-D., Lee, S., Yu, J.-S., & Lee, D. N. (2022). Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity. Pharmaceutics, 14(2), 268. https://doi.org/10.3390/pharmaceutics14020268