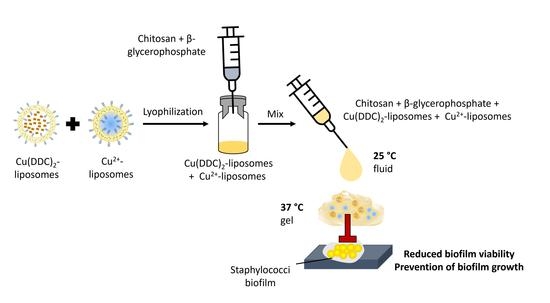
A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Cell Cultures, Materials, and Chemicals
2.2. Preparation of Liposomes
2.3. Liposome Characterization
2.3.1. Size and Polydispersity Index
2.3.2. Quantification of Encapsulated Cu2+
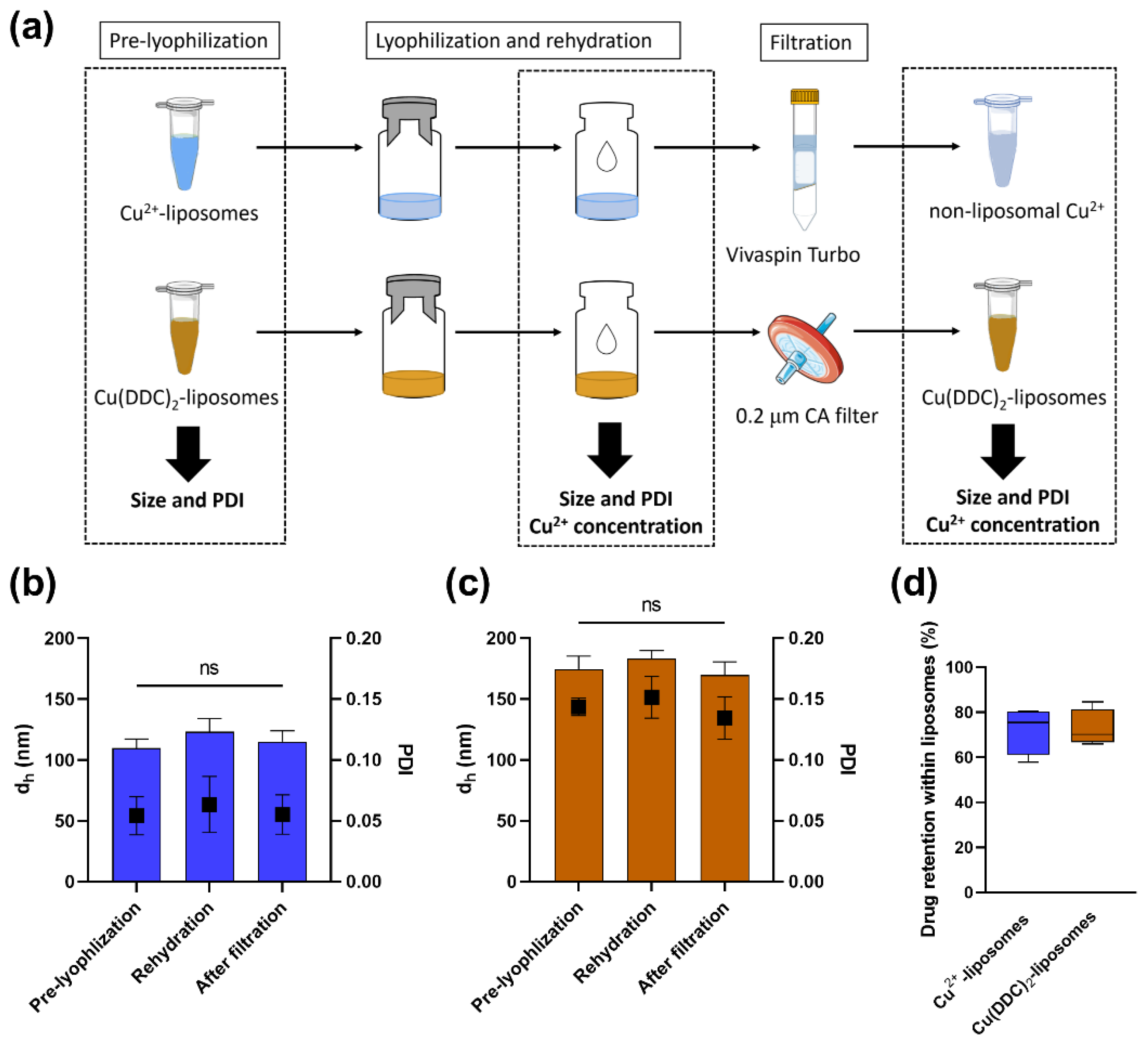
2.4. Lyophilization of Liposomes
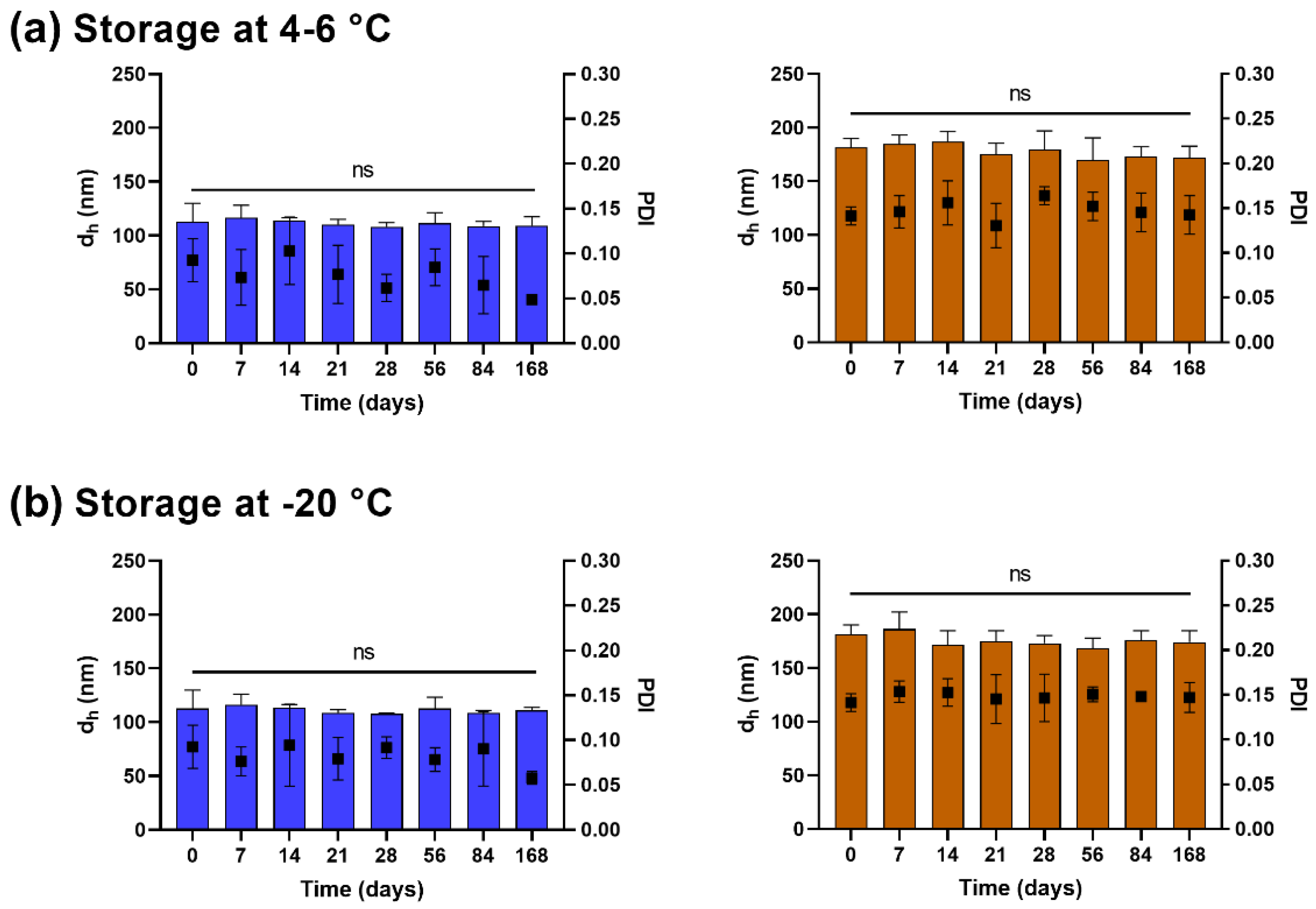
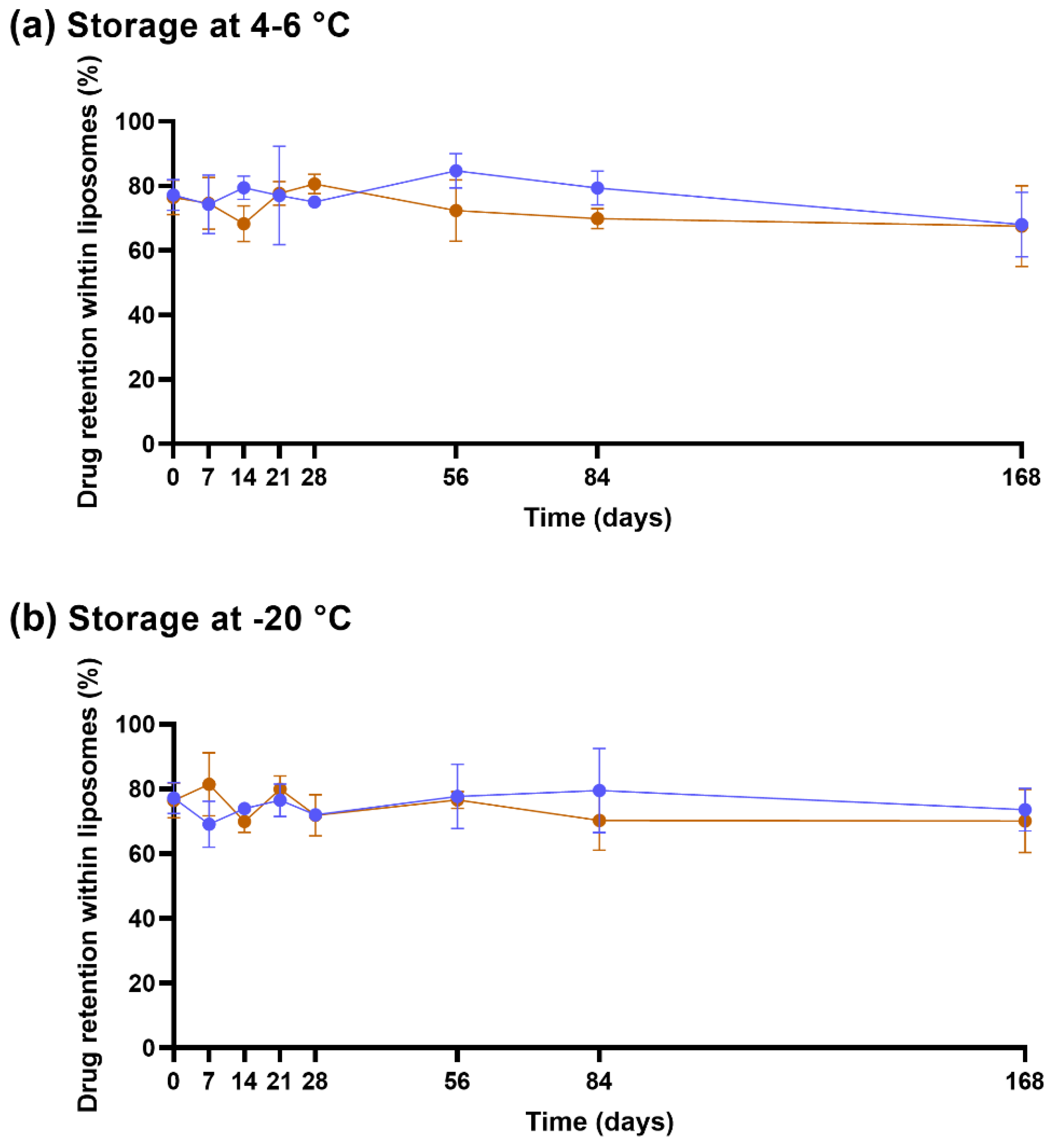
2.5. Stability of Lyophilized Liposomes
2.6. Preparation of Hydrogel
2.7. Rheological Measurements
2.8. Cytotoxicity of Gel
2.8.1. CS-βGP Gel Covering Fibroblast Cells
2.8.2. Fibroblast Cells Exposed to Released Components of CS-βGP Gel
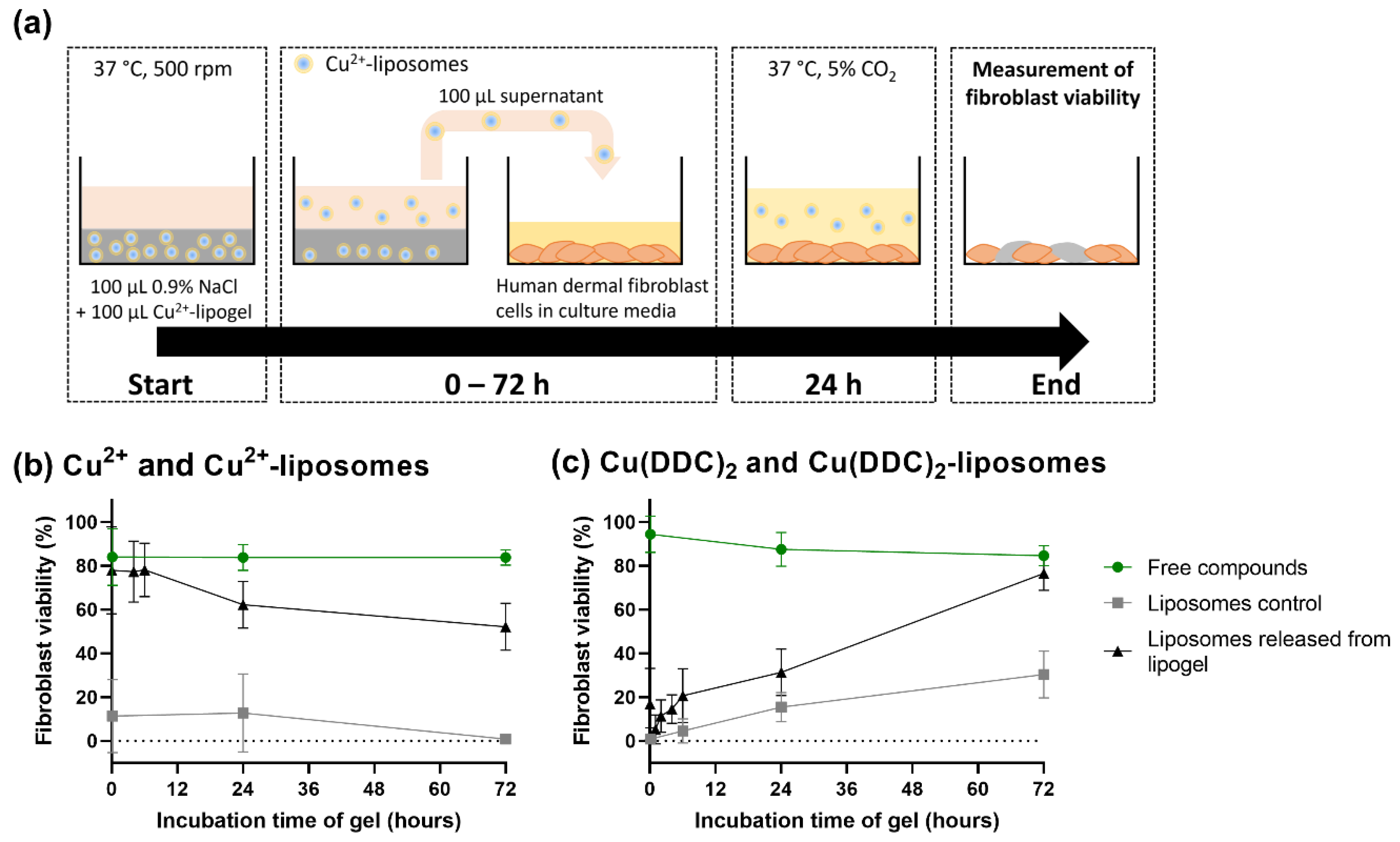
2.9. Effect of Released Liposomes from CS-βGP Gel on Fibroblast Viability
2.10. Weight Loss over Time
2.11. Antibiofilm Activity of Gel
2.12. Statistical Analysis
3. Results and Discussion
3.1. Cu2+-Liposomes and Cu(DDC)2-Liposomes Are Stable following Lyophilization
3.2. Lyophilized Liposomes Are Stable over 6 Months
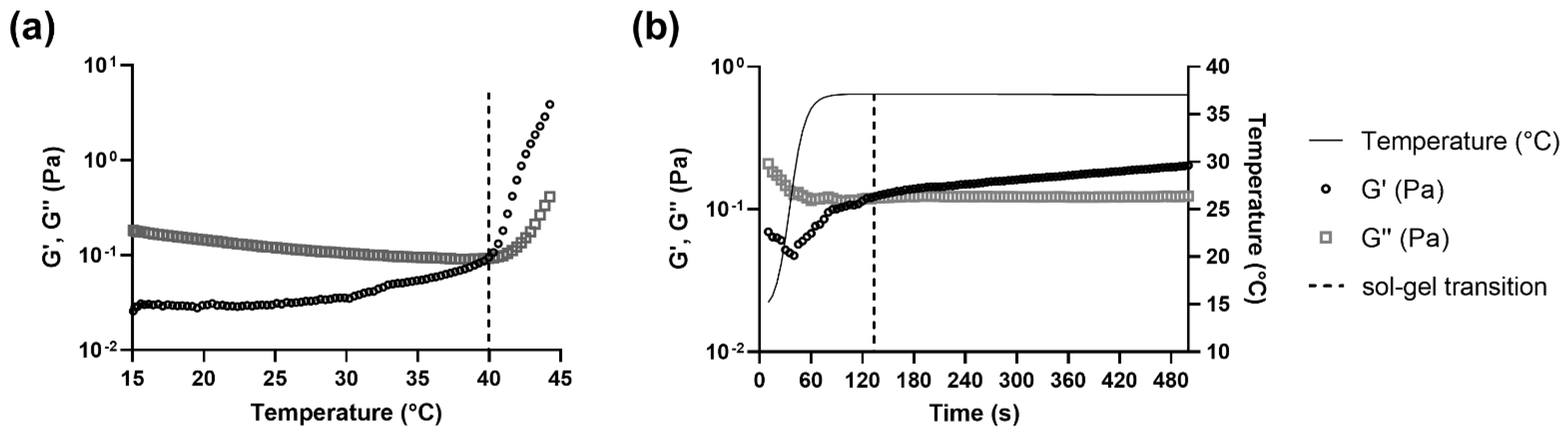
3.3. The Sol-Gel Transition of CS-βGP Is Temperature Sensitive
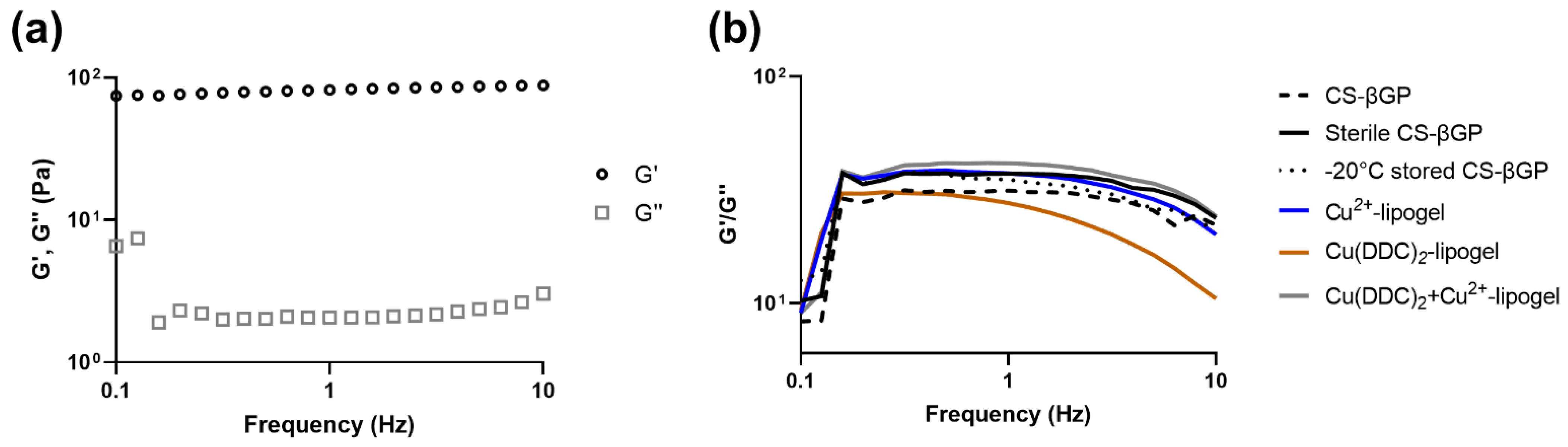
3.4. Mechanical Strength of CS-βGP Gel
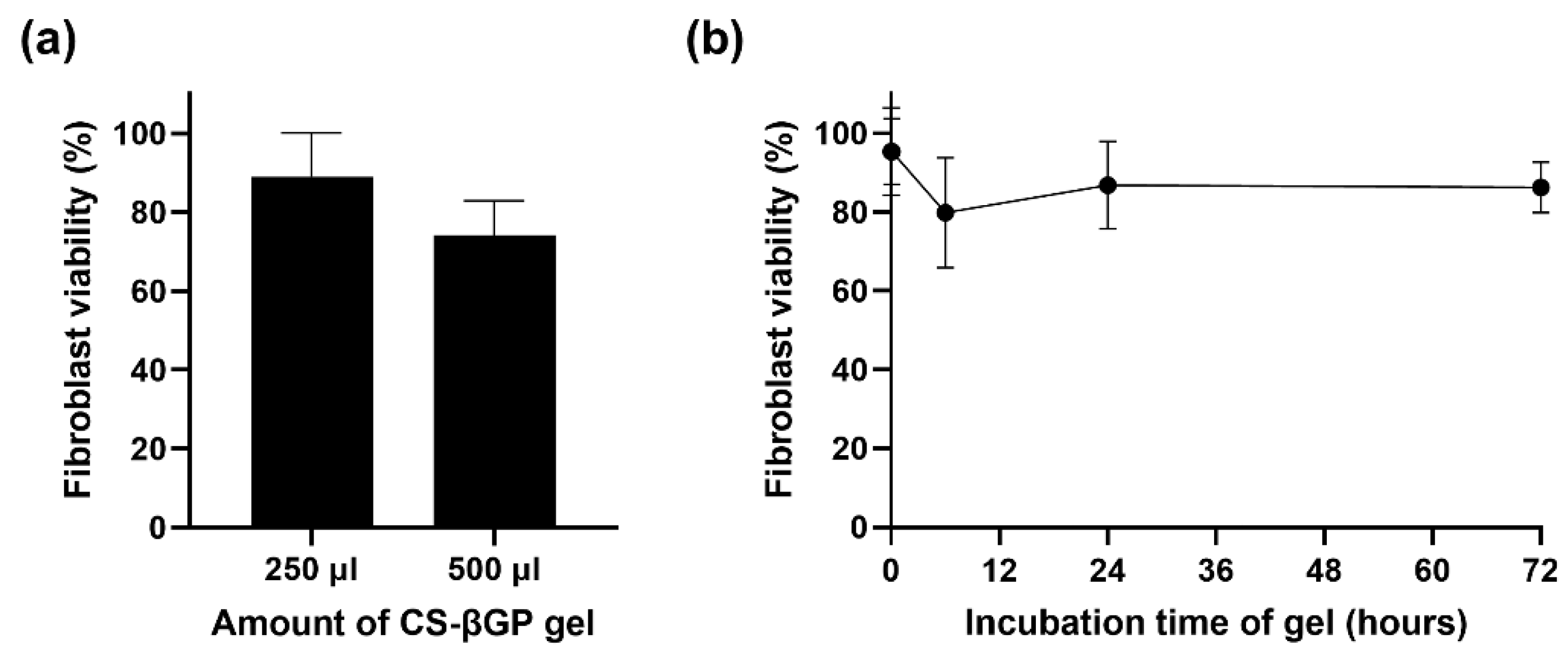
3.5. CS-βGP Is Biocompatible
3.6. Released Liposomes from CS-βGP Gel Affect Fibroblast Viability
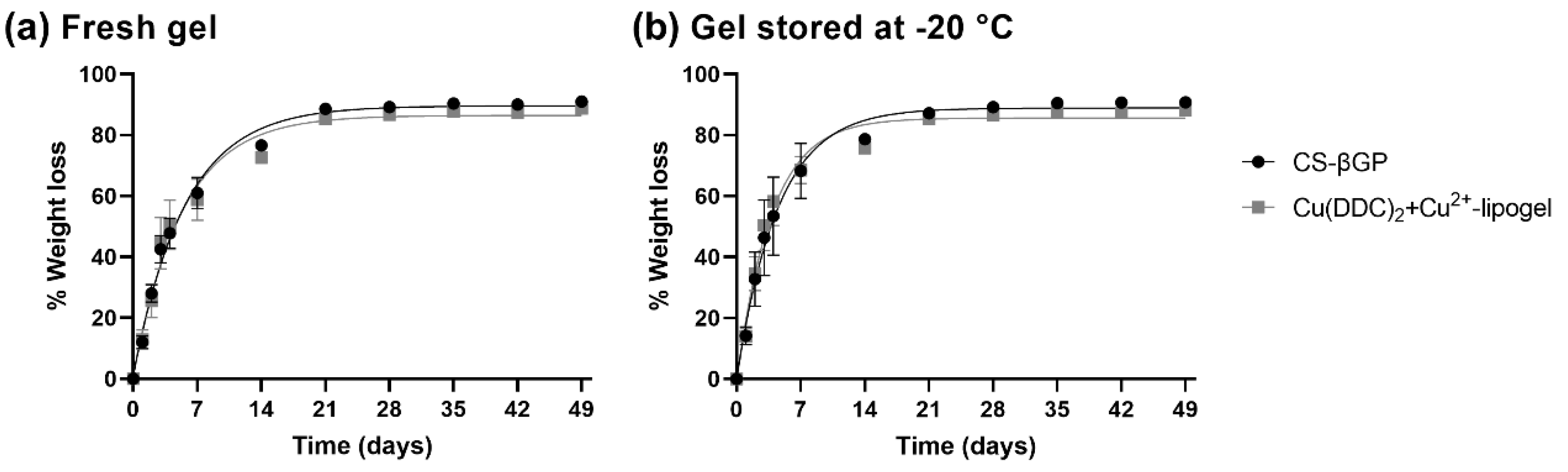
3.7. Weight Loss of CS-βGP Gel and Cu(DDC)2+Cu2+-Lipogel over Time
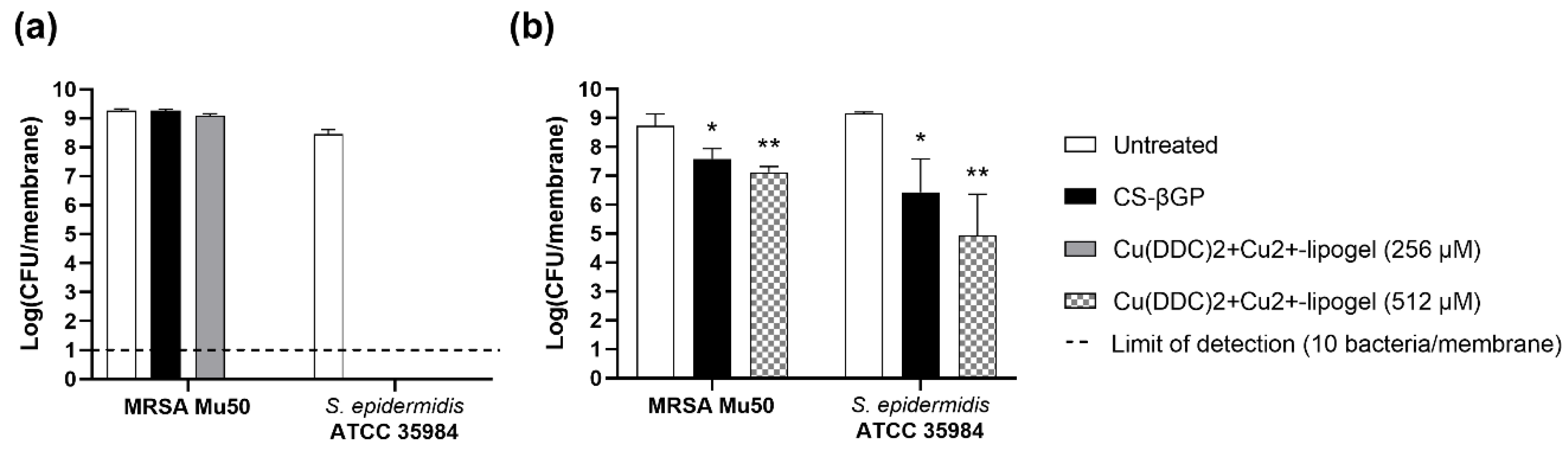
3.8. Antibiofilm Activity of Cu(DDC)2+Cu2+-Lipogel
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, A.C.D.; Santa-Cruz, F.; Ferraz, Á.B. What’s new in infection on surgical site and antibioticoprophylaxis in surgery? Arq. Bras. Cir. Dig. 2021, 33, e1558. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018.
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Kragh, K.N.; Richter, K. Introduction: Biofilms 101. In Antibiofilm Strategies: Current and Future Applications to Prevent, Control and Eradicate Biofilms; Richter, K., Kragh, K.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 11, pp. 3–15. [Google Scholar]
- Li, Y.; Li, G.; Sha, X.; Li, L.; Zhang, K.; Liu, D.; Hao, Y.; Cui, X.; Wang, L.; Wang, H. An intelligent vancomycin release system for preventing surgical site infections of bone tissues. Biomater. Sci. 2020, 8, 3202–3211. [Google Scholar] [CrossRef]
- Percival, S.L. Importance of biofilm formation in surgical infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Sexton, D.J.; Kanafani, Z.A.; Auten, G.; Kaye, K.S. Severe surgical site infection in community hospitals: Epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2007, 28, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front. Microbiol. 2022, 13, 999893. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Backstrom, I.; Leung, A.; Chen, K.; Malhotra, A.; Edwards, K.; Bally, M.B. Nanoscale reaction vessels designed for synthesis of copper-drug complexes suitable for preclinical development. PLoS ONE 2016, 11, e0153416. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Shi, M.; Leung, A.W.; Dragowska, W.H.; Sanche, L.; Bally, M.B. Development and optimization of an injectable formulation of copper diethyldithiocarbamate, an active anticancer agent. Int. J. Nanomed. 2017, 12, 4129–4146. [Google Scholar] [CrossRef]
- Ren, L.; Feng, W.; Shao, J.; Ma, J.; Xu, M.; Zhu, B.Z.; Zheng, N.; Liu, S. Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting. Theranostics 2020, 10, 6384–6398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Jiang, M.; Wu, X.; Zhang, M.; Guan, X.; Ma, J.; Zhang, W. A nanosystem of copper(II)-disulfiram for cancer treatment with high efficacy and few side effects. Front. Mater. Sci. 2021, 15, 553–566. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, H.; Fang, X.; Liu, Z.; Yang, Z.; Yong, J.; Yang, Q.; Bai, Y.; Ren, H.; Xu, H.; et al. Surface decoration via physical interaction of cupric diethyldithiocarbamate nanocrystals and its impact on biodistribution and tumor targeting. ACS Appl. Mater. Interfaces 2021, 13, 36894–36908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, K.; Sun, J.; Feng, F. Apoferritin as a carrier of Cu(II) diethyldithiocarbamate and biomedical application for glutathione-responsive combination chemotherapy. ACS Appl. Bio Mater. 2020, 3, 654–663. [Google Scholar] [CrossRef]
- Hartwig, F.; Köll-Weber, M.; Süss, R. Preclinical in vitro studies with 3D spheroids to evaluate Cu(DDC)2 containing liposomes for the treatment of neuroblastoma. Pharmaceutics 2021, 13, 894. [Google Scholar] [CrossRef]
- Marengo, A.; Forciniti, S.; Dando, I.; Dalla Pozza, E.; Stella, B.; Tsapis, N.; Yagoubi, N.; Fanelli, G.; Fattal, E.; Heeschen, C.; et al. Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 61–72. [Google Scholar] [CrossRef]
- Paun, R.A.; Dumut, D.C.; Centorame, A.; Thuraisingam, T.; Hajduch, M.; Mistrik, M.; Dzubak, P.; De Sanctis, J.B.; Radzioch, D.; Tabrizian, M. One-step synthesis of nanoliposomal copper diethyldithiocarbamate and its assessment for cancer therapy. Pharmaceutics 2022, 14, 640. [Google Scholar] [CrossRef]
- Kaul, L.; Süss, R.; Zannettino, A.; Richter, K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience 2021, 24, 102092. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Askari, V.R. Thermosensitive chitosan-β-glycerophosphate hydrogels as targeted drug delivery systems: An overview on preparation and their applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6640893. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Tafaghodi, M.; Ganji, F.; Abnoos, K.; Naghizadeh, H. In vitro insulin release from thermosensitive chitosan hydrogel. AAPS PharmSciTech 2012, 13, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Qi, X.; Jiang, Y.; Zhu, X.; Zhang, Z.; Qin, X.; Wu, Z. Topotecan hydrochloride liposomes incorporated into thermosensitive hydrogel for sustained and efficient in situ therapy of H22 tumor in Kunming mice. Pharm. Dev. Technol. 2015, 20, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Shan, W.; Gao, J.; Liang, W. A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J. Biomater. Sci. Polym. Ed. 2013, 24, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2019, 9, 434–443. [Google Scholar] [CrossRef]
- Tucker, L.J.; Grant, C.S.; Gautreaux, M.A.; Amarasekara, D.L.; Fitzkee, N.C.; Janorkar, A.V.; Varadarajan, A.; Kundu, S.; Priddy, L.B. Physicochemical and antimicrobial properties of thermosensitive chitosan hydrogel loaded with fosfomycin. Mar. Drugs 2021, 19, 144. [Google Scholar] [CrossRef]
- Kaya, G.; Oytun, F. Rheological properties of İnjectable hyaluronic acid hydrogels for soft tissue engineering applications. Biointerface Res. Appl. Chem. 2020, 11, 8424–8430. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leclair, G.; Hildgen, P.; Gupta, A.; Leroux, J.C. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J. Control Release 2002, 82, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, A.; Rouini, M.R.; Johari Daha, F.; Moghimi, H.R. Hydrogel-embeded vesicles, as a novel approach for prolonged release and delivery of liposome, in vitro and in vivo. J. Liposome Res. 2013, 23, 235–243. [Google Scholar] [CrossRef]
- Rassouli, A.; Kiani, K.; Hosseinzadeh Ardakani, Y.; Akbari Javar, H.; Khanamani Falahatipour, S. A comparative pharmacokinetic study of a novel sustained release danofloxacin formulation and the conventional product in rabbits. Vet. Res. Forum 2021, 12, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Dai, Y.; Li, C.; Qiu, Z.; Wang, X.; Tian, F.; Zhou, S.; Liu, Q.; Xing, H.; Lu, Y.; et al. Pharmacokinetics and pharmacodynamics evaluation of a thermosensitive chitosan based hydrogel containing liposomal doxorubicin. Eur. J. Pharm. Sci. 2016, 92, 137–145. [Google Scholar] [CrossRef]
- Huang, P.; Su, W.; Han, R.; Lin, H.; Yang, J.; Xu, L.; Ma, L. Physicochemical, antibacterial properties, and compatibility of ZnO-NP/chitosan/β-glycerophosphate composite hydrogels. J. Microbiol. Biotechnol. 2022, 32, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Chua, S.L.; Yam, J.K.H.; Givskov, M.; Yang, L.; Tolker-Nielsen, T. Gauging and visualizing c-di-GMP levels in Pseudomonas aeruginosa using fluorescence-based biosensors. In Methods in Molecular Biology, 11th ed.; Sauer, K., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1657, pp. 87–98. [Google Scholar]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological study of chitosan/polyol-phosphate systems: Influence of the polyol part on the thermo-induced gelation mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Richter, K.; Thomas, N.; Claeys, J.; McGuane, J.; Prestidge, C.A.; Coenye, T.; Wormald, P.-J.; Vreugde, S. A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob. Agents Chemother. 2017, 61, e00481-17. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of liposomal formulations: Still necessary, still challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Kannan, V.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J. Liposome Res. 2015, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv. Drug Deliv. Rev. 2019, 151–152, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Wessman, P.; Edwards, K.; Mahlin, D. Structural effects caused by spray- and freeze-drying of liposomes and bilayer disks. J. Pharm. Sci. 2010, 99, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Bindu, B.; Bindra, A.; Rath, G. Temperature management under general anesthesia: Compulsion or option. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Ghasemi Tahrir, F.; Ganji, F.; Mani, A.R.; Khodaverdi, E. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Drug Deliv. 2016, 23, 1038–1046. [Google Scholar] [CrossRef]
- Zingel, C.; Sachse, A.; Röβling, G.L.; Müller, R.H. Lyophilization and rehydration of iopromide-carrying liposomes. Int. J. Pharm. 1996, 140, 13–24. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-coating effect on the characteristics of liposomes: A focus on bioactive compounds and essential oils: A review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Nugraheni, P.S.; Soeriyadi, A.H.; Sediawan, W.B.; Ustadi; Budhijanto, W. Influence of salt addition and freezing-thawing on particle size and zeta potential of nano-chitosan. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012052. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the freeze-thaw technique affect the properties of the alginate/chitosan glutamate gels with posaconazole as a model antifungal drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of beta-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Hung, C.C.; Chang, C.W.; Chao, J.H.; Hsieh, B.T. Evaluation of injectable chitosan-based co-cross-linking hydrogel for local delivery of (188)Re-LIPO-DOX to breast-tumor-bearing mouse model. Anticancer Res. 2018, 38, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures-A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.F.; Zou, S.H.; Chen, X.G.; Liu, C.S.; Li, J.J.; Zhou, X.; Liu, Y.; Cheng, X.J. Characterizations of chitosan-based highly porous hydrogel—The effects of the solvent. J. Appl. Polym. Sci. 2012, 125, E88–E98. [Google Scholar] [CrossRef]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J. Biomed. Mater. Res. A 2008, 86, 824–832. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/beta-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef]
- Jeong, S.; Jeong, S.; Chung, S.; Kim, A. Revisiting in vitro release test for topical gel formulations: The effect of osmotic pressure explored for better bio-relevance. Eur. J. Pharm. Sci. 2018, 112, 102–111. [Google Scholar] [CrossRef]
- Duffy, C.; O’Riordan, D.; O’Sullivan, M.; Jacquier, J.C. In vitro evaluation of chitosan copper chelate gels as a multimicronutrient feed additive for cattle. J. Sci. Food Agric. 2018, 98, 4177–4183. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Litou, C.; Box, K.J.; Dressman, J.B.; Kostewicz, E.S.; Kuentz, M.; Reppas, C. In vitro methods to assess drug precipitation in the fasted small intestine-A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 536–556. [Google Scholar] [CrossRef] [PubMed]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable thermosensitive chitosan/pullulan-based hydrogels with improved mechanical properties and swelling capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Alvarez, C.; Chiquete-Félix, N.; Contreras-Zentella, M.; Guerrero-Castillo, S.; Peña, A.; Uribe-Carvajal, S. Staphylococcus epidermidis: Metabolic adaptation and biofilm formation in response to different oxygen concentrations. Pathog. Dis. 2016, 74, ftv111. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.X.; Chen, X.G.; Zhao, Q.S.; Liu, C.S.; Cheng, X.J.; Wang, L.C. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J. Mater. Sci. Mater. Med. 2009, 20, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Lišková, J.; Douglas, T.E.; Beranová, J.; Skwarczyńska, A.; Božič, M.; Samal, S.K.; Modrzejewska, Z.; Gorgieva, S.; Kokol, V.; Bačáková, L. Chitosan hydrogels enriched with polyphenols: Antibacterial activity, cell adhesion and growth and mineralization. Carbohydr. Polym. 2015, 129, 135–142. [Google Scholar] [CrossRef]
- Pakzad, Y.; Ganji, F. Thermosensitive hydrogel for periodontal application: In vitro drug release, antibacterial activity and toxicity evaluation. J. Biomater. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef]
- Cobrado, L.; Azevedo, M.M.; Silva-Dias, A.; Ramos, J.P.; Pina-Vaz, C.; Rodrigues, A.G. Cerium, chitosan and hamamelitannin as novel biofilm inhibitors? J. Antimicrob. Chemother. 2012, 67, 1159–1162. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Peterson, S.B.; Irie, Y.; Borlee, B.R.; Murakami, K.; Harrison, J.J.; Colvin, K.M.; Parsek, M.R. Different methods for culturing biofilms in vitro. In Biofilm Infections; Bjarnsholt, T., Jensen, P.Ø., Moser, C., Høiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 251–266. [Google Scholar]
- Dong, D.; Thomas, N.; Thierry, B.; Vreugde, S.; Prestidge, C.A.; Wormald, P.-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS ONE 2015, 10, e0131806. [Google Scholar] [CrossRef] [PubMed]
- Thaarup, I.C.; Bjarnsholt, T. Current in vitro biofilm-infected chronic wound models for developing new treatment possibilities. Adv. Wound Care 2020, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in surgical site infections: Recent advances and novel prevention and eradication strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections: Surgical Site Infections; ECDC: Stockholm, Sweden, 2019.
| Parameters | Freezing | Primary Drying | Secondary Drying | |
|---|---|---|---|---|
| First Step | Second Step | |||
| Temperature (°C) | −80 | −45 | 0 | 25 |
| Pressure (mbar) | - | 0.07 | 0.001 | 0.001 |
| Time (h) | 12 | 42 | 3 | 3 |
| CS-βGP Mix | Sterile | + Cu2+-Liposomes | +Cu(DDC)2-Liposomes | Temperature (°C) ± SD | Total Time (s) ± SD | Time (s) at 37 °C |
|---|---|---|---|---|---|---|
| Freshly prepared | - | - | - | 35.3 ± 3.1 | ND | ND |
| + | - | - | 34.2 ± 2.9 | 68 ± 16 | NR | |
| + | + | - | 34.8 ± 0.5 | 70 ± 4 | NR | |
| + | - | + | 38.8 ± 1.5 | 330 ± 144 | 255 | |
| + | + | + | 33.3 ± 2.6 | 75 ± 14 | NR | |
| stored at −20 °C | + | - | - | 39.2 ± 1.0 | 90 ± 25 | 15 |
| + | + | + | 37.9 ± 3.3 | 118 ± 50 | 43 |
| CS-βGP Gel | + Cu(DDC)2-Liposomes + Cu2+-Liposomes | Mean Time until 50% Weight Loss [95% CI] (Days) | Rate Constant [95% CI] (1/Days) | R2 |
|---|---|---|---|---|
| fresh | − | 3.9 [3.5 to 4.4] | 0.18 [0.16 to 0.20] | 0.988 |
| + | 3.7 [3.1 to 4.5] | 0.19 [0.15 to 0.22] | 0.972 | |
| −20 °C | − | 3.1 [2.6 to 3.8] | 0.22 [0.18 to 0.26] | 0.966 |
| + | 2.6 [2.3 to 3.1] | 0.26 [0.23 to 0.30] | 0.975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaul, L.; Grundmann, C.E.; Köll-Weber, M.; Löffler, H.; Weiz, A.; Zannettino, A.C.W.; Richter, K.; Süss, R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics 2022, 14, 2841. https://doi.org/10.3390/pharmaceutics14122841
Kaul L, Grundmann CE, Köll-Weber M, Löffler H, Weiz A, Zannettino ACW, Richter K, Süss R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics. 2022; 14(12):2841. https://doi.org/10.3390/pharmaceutics14122841
Chicago/Turabian StyleKaul, Laurine, Clara E. Grundmann, Monika Köll-Weber, Hanna Löffler, Artur Weiz, Andrew C. W. Zannettino, Katharina Richter, and Regine Süss. 2022. "A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections" Pharmaceutics 14, no. 12: 2841. https://doi.org/10.3390/pharmaceutics14122841
APA StyleKaul, L., Grundmann, C. E., Köll-Weber, M., Löffler, H., Weiz, A., Zannettino, A. C. W., Richter, K., & Süss, R. (2022). A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics, 14(12), 2841. https://doi.org/10.3390/pharmaceutics14122841