Development and Pharmacokinetics of a Novel Acetylsalicylic Acid Dry Powder for Pulmonary Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Respirable ASA–Leu Dry Powder Preparation
2.2. Experimental Design
2.3. ASA Quantification
2.4. Morphology and Particle Size
2.5. Powder Flowability and Yield
2.6. Thermal Analysis
2.7. Aerodynamic Assessment
2.8. Pharmacokinetic Study
2.8.1. Animal Model
2.8.2. Administration and Sampling Protocol
2.8.3. Salicylic Acid Quantification
2.8.4. Blood and Lung Sample Treatment
2.8.5. Noncompartmental PK Analysis
2.9. Statistical Analysis
3. Results and Discussion
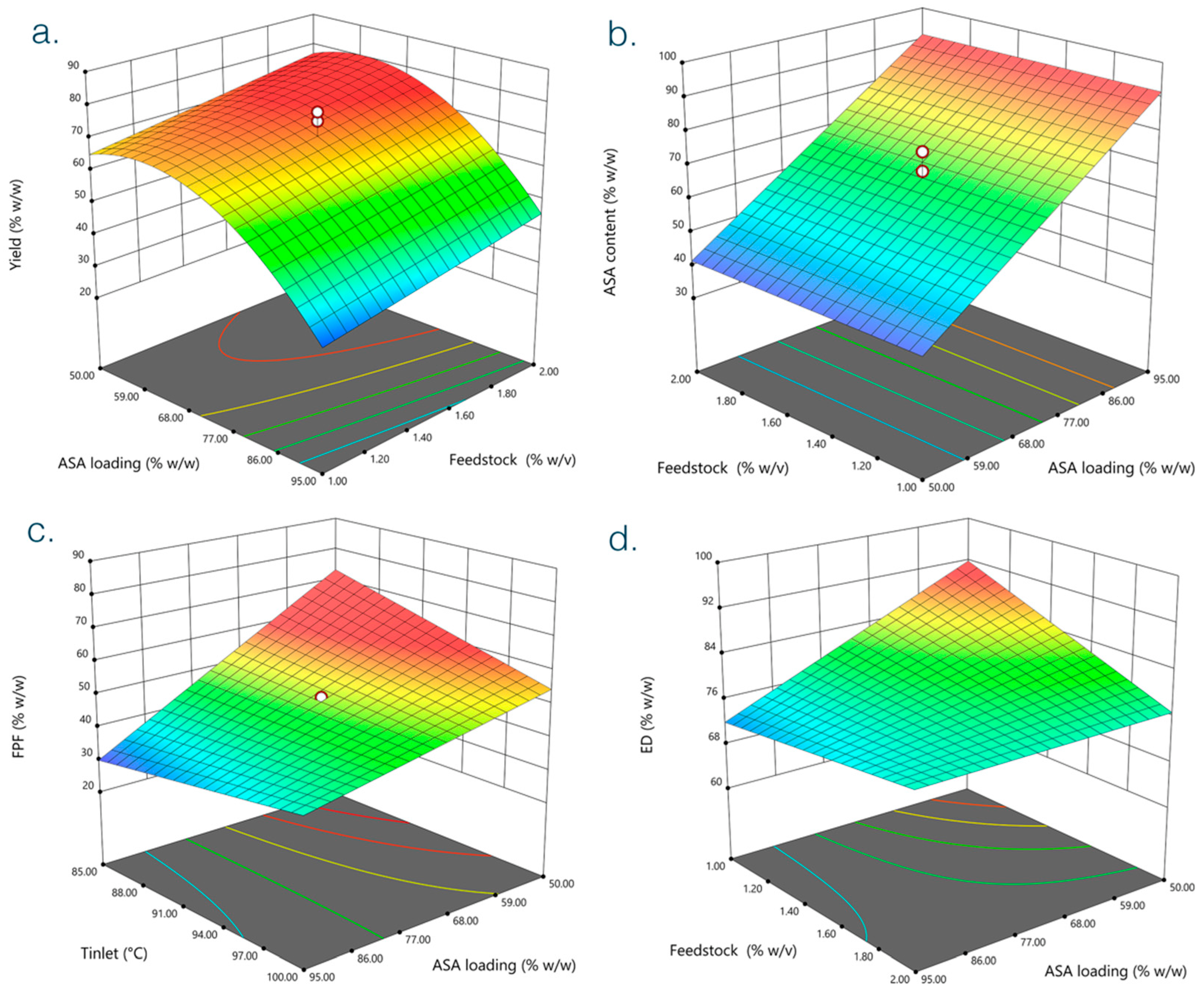
3.1. Determination of Optimal Formulation Conditions
3.2. Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Memorial Sloan Kettering Cancer Center. Common Medications Containing Aspirin, Other Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), or Vitamin E. Available online: https://www.mskcc.org/cancer-care/patient-education/common-medications-containing-aspirin-and-other-nonsteroidal-anti-inflammatory-drugs-nsaids (accessed on 1 December 2022).
- Braunstein, M.; Hickey, A.J.; Ekins, S. Why Wait? The Case for Treating Tuberculosis with Inhaled Drugs. Pharm. Res. 2019, 36, 166. [Google Scholar] [CrossRef] [PubMed]
- Salva, O.; Doreski, P.A.; Giler, C.S.; Quinodoz, D.C.; Guzmán, L.G.; Muñoz, S.E.; Carrillo, M.N.; Porta, D.J.; Ambasch, G.; Coscia, E.; et al. Reversal of SARS-CoV2-Induced Hypoxia by Nebulized Sodium Ibuprofenate in a Compassionate Use Program. Infect. Dis. 2021, 10, 2511–2524. [Google Scholar] [CrossRef] [PubMed]
- Onischuk, A.A.; Tolstikova, T.G.; An’kov, S.V.; Baklanov, A.M.; Valiulin, S.V.; Khvostov, M.V.; Sorokina, I.V.; Dultseva, G.G.; Zhukova, N.A. Ibuprofen, Indomethacin and Diclofenac Sodium Nanoaerosol: Generation, Inhalation Delivery and Biological Effects in Mice and Rats. J. Aerosol. Sci. 2016, 100, 164–177. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Tolstikova, T.G.; Sorokina, I.V.; Zhukova, N.A.; Baklanov, A.M.; Karasev, V.V.; Borovkova, O.V.; Dultseva, G.G.; Boldyrev, V.V.; Fomin, V.M. Analgesic Effect from Ibuprofen Nanoparticles Inhaled by Male Mice. J. Aerosol. Med. Pulm. Drug Deliv. 2009, 22, 245–253. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Tolstikova, T.G.; Sorokina, I.V.; Zhukova, N.A.; Baklanov, A.M.; Karasev, V.V.; Dultseva, G.G.; Boldyrev, V.V.; Fomin, V.M. Anti-Inflammatory Effect from Indomethacin Nanoparticles Inhaled by Male Mice. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 231–243. [Google Scholar] [CrossRef]
- An’kov, S.V.; Tolstikova, T.G.; Onishchuk, A.A.; Khvostov, M.V.; Sorokina, I.V.; Baklanov, A.M.; Fomin, V.M.; Boldyrev, V.V. Analgesic Effect of Several Nonsteroidal Anti-Inflammatory Drug Nanoaerosols. Pharm. Chem. J. 2016, 49, 680–682. [Google Scholar] [CrossRef]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin and Ibuprofen Enhance Pyrazinamide Treatment of Murine Tuberculosis. J. Antimicrob. Chemother. 2007, 59, 313–316. [Google Scholar] [CrossRef]
- Wangberg, H.; White, A.A. Aspirin-Exacerbated Respiratory Disease. Curr. Opin. Immunol. 2020, 66, 9–13. [Google Scholar] [CrossRef]
- Fawzy, A.; Putcha, N.; Aaron, C.P.; Bowler, R.P.; Comellas, A.P.; Cooper, C.B.; Dransfield, M.T.; Han, M.K.; Hoffman, E.A.; Kanner, R.E.; et al. Aspirin Use and Respiratory Morbidity in COPD. Chest 2019, 155, 519–527. [Google Scholar] [CrossRef]
- Goto, T.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. The Association of Aspirin Use with Severity of Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Retrospective Cohort Study. NPJ Prim. Care Respir. Med. 2018, 28, 7. [Google Scholar] [CrossRef]
- Boyle, A.J.; di Gangi, S.; Hamid, U.I.; Mottram, L.-J.; McNamee, L.; White, G.; Cross, L.M.; McNamee, J.J.; O’Kane, C.M.; McAuley, D.F. Aspirin Therapy in Patients with Acute Respiratory Distress Syndrome (ARDS) Is Associated with Reduced Intensive Care Unit Mortality: A Prospective Analysis. Crit. Care 2015, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Nair, A.; Meldrum, K.T.; Hopkinson, P.; Lipworth, B.J. Effect of Aspirin on Airway Inflammation and Pulmonary Function in Patients with Persistent Asthma. J. Allergy Clin. Immunol. 2008, 121, 1184–1189.e4. [Google Scholar] [CrossRef] [PubMed]
- Aaron, C.P.; Schwartz, J.E.; Hoffman, E.A.; Angelini, E.; Austin, J.H.M.; Cushman, M.; Jacobs, D.R.; Kaufman, J.D.; Laine, A.; Smith, L.J.; et al. A Longitudinal Cohort Study of Aspirin Use and Progression of Emphysema-like Lung Characteristics on CT Imaging. Chest 2018, 154, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.D.; Foord, R.; Holgate, S.T. Inhaled Lysine-Aspirin as a Bronchoprovocation Procedure in Aspirin-Sensitive Asthma: Its Repeatability, Absence of a Late-Phase Reaction, and the Role of Histamine. J. Allergy Clin. Immunol. 1989, 84, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Droebner, K.; Haasbach, E.; Dudek, S.E.; Scheuch, G.; Nocker, K.; Canisius, S.; Ehrhardt, C.; von Degenfeld, G.; Ludwig, S.; Planz, O. Pharmacodynamics, Pharmacokinetics, and Antiviral Activity of BAY 81-8781, a Novel NF-ΚB Inhibiting Anti-Influenza Drug. Front. Microbiol. 2017, 8, 2130. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Schror, K.; Navarese, E.P.; Jeong, Y.-H.; Kubica, J.; Bliden, K.P.; Gurbel, P.A. Aspirin as an Adjunctive Pharmacologic Therapy Option for COVID-19: Anti-Inflammatory, Antithrombotic, and Antiviral Effects All in One Agent. J. Exp. Pharm. 2021, 13, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Gurbel, P.A.; Bliden, K.P.; Chaudhary, R.; Tantry, U.S. First In-Human Experience With Inhaled Acetylsalicylic Acid for Immediate Platelet Inhibition. Circulation 2020, 142, 1305–1307. [Google Scholar] [CrossRef]
- Schmid, K.; Arpagaus, C.; Friess, W. Evaluation of the Nano Spray Dryer B-90 for Pharmaceutical Applications. Pharm. Dev. Technol. 2011, 16, 287–294. [Google Scholar] [CrossRef]
- Rita Patrizia, A.; Mariateresa, S.; Pasquale, D.G.; Teresa, M.; Francesca, S.; Paola, R. Nanospray Drying as a Novel Technique for the Manufacturing of Inhalable NSAID Powders. Sci. World J. 2014, 2014, 838410. [Google Scholar] [CrossRef]
- Almansour, K.; Alfagih, I.M.; Ali, R.; Elsayed, M.M.A. Inhalable Microparticles Containing Terbinafine for Management of Pulmonary Fungal Infections: Spray Drying Process Engineering Using Lactose vs. Mannitol as Excipients. J. Drug Deliv. Sci. Technol. 2020, 60, 101991. [Google Scholar] [CrossRef]
- Schoubben, A.; Giovagnoli, S.; Tiralti, M.C.; Blasi, P.; Ricci, M. Capreomycin Inhalable Powders Prepared with an Innovative Spray-Drying Technique. Int. J. Pharm. 2014, 469, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kaewjan, K.; Srichana, T. Nano Spray-Dried Pyrazinamide-l-Leucine Dry Powders, Physical Properties and Feasibility Used as Dry Powder Aerosols. Pharm. Dev. Technol. 2016, 21, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Dimer, F.A.; Ortiz, M.; Pohlmann, A.R.; Guterres, S.S. Inhalable Resveratrol Microparticles Produced by Vibrational Atomization Spray Drying for Treating Pulmonary Arterial Hypertension. J. Drug. Deliv. Sci. Technol. 2015, 29, 152–158. [Google Scholar] [CrossRef]
- Council of Europe. 2.9.18 Preparations for Inhalation: Aerodynamic Assessment of Fine Particles. In European Pharmacopoeia 7.0; Council of Europe: Strasbourg, France, 2002. [Google Scholar]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Boyer, A.; Bouajila, J.; Couderc, F.; Nepveu, F. Determination of Non-Steroidal Anti-Inflammatory Drugs in Pharmaceuticals and Human Serum by Dual-Mode Gradient HPLC and Fluorescence Detection. J. Chromatogr. B 2007, 857, 59–66. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The Nano Spray Dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van Der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an Excipient in Spray Dried Powder for Inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef]
- Zillen, D.; Beugeling, M.; Hinrichs, W.L.J.; Frijlink, H.W.; Grasmeijer, F. Natural and Bioinspired Excipients for Dry Powder Inhalation Formulations. Curr. Opin. Colloid Interface Sci. 2021, 56, 101497. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A.V. Relationship between Surface Concentration of L-Leucine and Bulk Powder Properties in Spray Dried Formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef]
- Feng, A.L.; Boraey, M.A.; Gwin, M.A.; Finlay, P.R.; Kuehl, P.J.; Vehring, R. Mechanistic Models Facilitate Efficient Development of Leucine Containing Microparticles for Pulmonary Drug Delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, D.; Huck, B.; Nothdurft, B.; Koch, M.; Rudolph, D.; Rutschmann, M.; Feldmann, C.; Hozsa, C.; Furch, M.; Besecke, K.F.W.; et al. Spray-Dried Lactose-Leucine Microparticles for Pulmonary Delivery of Antimycobacterial Nanopharmaceuticals. Drug Deliv. Transl. Res. 2021, 11, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Serrano, D.R.; O’Connell, P.; Couet, W.; Marchand, S.; Healy, A.M.; Tewes, F. Use of Leucine to Improve Aerodynamic Properties of Ciprofloxacin-Loaded Maltose Microparticles for Inhalation. Eur. J. Pharm. Res. 2019, 1, 2–11. [Google Scholar] [CrossRef]
- Seville, P.C.; Learoyd, T.P.; Li, H.-Y.; Williamson, I.J.; Birchall, J.C. Amino Acid-Modified Spray-Dried Powders with Enhanced Aerosolisation Properties for Pulmonary Drug Delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Molina, C.; Kaialy, W.; Nokhodchi, A. The Crucial Role of Leucine Concentration on Spray Dried Mannitol-Leucine as a Single Carrier to Enhance the Aerosolization Performance of Albuterol Sulfate. J. Drug Deliv. Sci. Technol. 2019, 49, 97–106. [Google Scholar] [CrossRef]
- Rattanupatam, T.; Srichana, T. Budesonide Dry Powder for Inhalation: Effects of Leucine and Mannitol on the Efficiency of Delivery. Drug Deliv. 2014, 21, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Rades, T.; Rantanen, J.; Chan, H.-K.; Yang, M. Amino Acids as Stabilizers for Spray-Dried Simvastatin Powder for Inhalation. Int. J. Pharm. 2019, 572, 118724. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Taniguchi, Y.; Tamura, Y.; Ochiai, K.; Makino, K. Effects of L-Leucine on PLGA Microparticles for Pulmonary Administration Prepared Using Spray Drying: Fine Particle Fraction and Phagocytotic Ratio of Alveolar Macrophages. Colloids Surf. A Phys. Eng. Asp. 2018, 537, 411–417. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Yang, F.-F.; Wen, H.; Fu, T.-T.; Liu, C.-Y.; Quan, L.-H.; Liao, Y.-H. The Effect of l -Leucine on the Stabilization and Inhalability of Spray-Dried Solid Lipid Nanoparticles for Pulmonary Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 46, 474–481. [Google Scholar] [CrossRef]
- Luinstra, M.; Rutgers, W.; van Laar, T.; Grasmeijer, F.; Begeman, A.; Isufi, V.; Steenhuis, L.; Hagedoorn, P.; de Boer, A.; Frijlink, H.W. Pharmacokinetics and Tolerability of Inhaled Levodopa from a New Dry-Powder Inhaler in Patients with Parkinson’s Disease. Adv. Chronic. Dis. 2019, 10, 204062231985761. [Google Scholar] [CrossRef]
- Savara Inc. Phase I, Reference-Controlled, Dose Escalating Study to Examine the Pharmacokinetics and Safety of AeroVanc Inhalation Powder. (November 2011–March 2012); NCT01537666; Savara Inc.: Austin, TX, USA, 2014. [Google Scholar]
- Savara Inc. A Phase 2, Randomized, Double Blind, Placebo-Controlled Study of AeroVanc for the Treatment of Persistent Methicillin-Resistant Staphylococcus Aureus Lung Infection in Cystic Fibrosis Patients. (March 2013–November 2014); NCT01746095; Savara Inc.: Austin, TX, USA, 2020. [Google Scholar]
- Savara Inc. A Phase III, Randomized, Double-Blind, Placebo-Controlled Study of AeroVanc for the Treatment of Persistent Methicillin-Resistant Staphylococcus Aureus Lung Infection in Cystic Fibrosis Patients (September 20, 2017–January 15, 2021); NCT03181932; Savara Inc.: Austin, TX, USA, 2021. [Google Scholar]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A Roadmap to Pulmonary Delivery Strategies for the Treatment of Infectious Lung Diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.A.; Llop, J.; Sousa, C.; Kreutzer, B.; Cossío, U.; Forbes, B.; Vieira, I.; Gil, N.; Silva-Lima, B. Pulmonary Administration: Strengthening the Value of Therapeutic Proximity. Front. Med. 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.-R.; Chang, R.Y.K.; Kwok, P.C.L.; Tang, P.; Chen, L.; Chen, D.; Chan, H.-K. Administration of Dry Powders during Respiratory Supports. Ann. Transl. Med. 2021, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Marathe, G.K.; Hartiala, J.; Hazen, S.L.; Allayee, H.; Tang, W.H.W.; McIntyre, T.M. Aspirin Hydrolysis in Plasma Is a Variable Function of Butyrylcholinesterase and Platelet-Activating Factor Acetylhydrolase 1b2 (PAFAH1b2). J. Biol. Chem. 2013, 288, 11940–11948. [Google Scholar] [CrossRef]
- Fu, C.J.; Melethil, S.; Mason, W.D. The Pharmacokinetics of Aspirin in Rats and the Effect of Buffer. J. Pharm. Biopharm. 1991, 19, 157–173. [Google Scholar] [CrossRef]
- Higgs, G.A.; Salmon, J.A.; Henderson, B.; Vane, J.R. Pharmacokinetics of Aspirin and Salicylate in Relation to Inhibition of Arachidonate Cyclooxygenase and Antiinflammatory Activity. Proc. Natl. Acad. Sci. USA 1987, 84, 1417–1420. [Google Scholar] [CrossRef]
- Tandel, H.; Florence, K.; Misra, A. Protein and Peptide Delivery through Respiratory Pathway. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: London, UK, 2011; pp. 429–479. [Google Scholar]





| Factor | Low Level | High Level | |
|---|---|---|---|
| A | ASA loading (%w/w) | 50 | 95 |
| B | Feedstock (%w/v) | 1 | 2 |
| C | Ethanol (%v/v)) | 30 | 40 |
| D | Inlet temperature (°C) | 85 | 100 |
| E | Air flow rate (L/min) | 95 | 110 |
| F | Back pressure (mbar) | 50 | 65 |
| Factor | A | B | C | D | E | F | Responses | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ASA Loading | Feedstock | Ethanol | Tinlet | Air Flow Rate | Back Pressure | Yield | ASA Content | FPF | ED | |
| Run# | % w/w | % w/v | % v/v | °C | L/min | mbar | % w/w | % w/w | % w/w | % w/w |
| 1 | 50.0 | 2.0 | 40.0 | 85.0 | 95.0 | 50.0 | 79.8 | 53.6 | 64.9 | 77.0 |
| 2 | 95.0 | 1.0 | 30.0 | 85.0 | 110.0 | 50.0 | 37.1 | 94.7 | 27.4 | 71.4 |
| 3 | 50.0 | 1.0 | 30.0 | 100.0 | 95.0 | 65.0 | 63.4 | 39.9 | 58.4 | 98.7 |
| 4 | 95.0 | 2.0 | 40.0 | 100.0 | 110.0 | 65.0 | 40.4 | 93.0 | 39.3 | 83.7 |
| 5 | 50.0 | 2.0 | 40.0 | 100.0 | 95.0 | 65.0 | 72.6 | 45.3 | 51.4 | 73.6 |
| 6 | 50.0 | 2.0 | 30.0 | 100.0 | 110.0 | 50.0 | 73.8 | 39.3 | 55.0 | 79.7 |
| 7 | 50.0 | 1.0 | 30.0 | 85.0 | 95.0 | 50.0 | 76.7 | 42.9 | 85.8 | 98.5 |
| 8 | 72.5 | 1.5 | 35.0 | 92.5 | 102.5 | 57.5 | 59.8 | 62.7 | 50.8 | 77.5 |
| 9 | 50.0 | 1.0 | 40.0 | 85.0 | 110.0 | 65.0 | 65.7 | 36.7 | 76.5 | 103.2 |
| 10 | 50.0 | 2.0 | 30.0 | 85.0 | 110.0 | 65.0 | 79.5 | 36.0 | 68.7 | 82.0 |
| 11 | 95.0 | 1.0 | 30.0 | 100.0 | 110.0 | 65.0 | 30.5 | 88.0 | 50.1 | 84.0 |
| 12 | 72.5 | 1.5 | 35.0 | 92.5 | 102.5 | 57.5 | 74.9 | 68.5 | 45.3 | 69.8 |
| 13 | 95.0 | 1.0 | 40.0 | 100.0 | 95.0 | 50.0 | 25.7 | 81.6 | 36.0 | 69.6 |
| 14 | 95.0 | 2.0 | 30.0 | 100.0 | 95.0 | 50.0 | 47.3 | 89.9 | 37.1 | 74.9 |
| 15 | 72.5 | 1.5 | 35.0 | 92.5 | 102.5 | 57.5 | 77.6 | 74.3 | 40.4 | 72.8 |
| 16 | 50.0 | 1.0 | 40.0 | 100.0 | 110.0 | 50.0 | 57.3 | 35.6 | 56.4 | 72.4 |
| 17 | 72.5 | 1.5 | 35.0 | 92.5 | 102.5 | 57.5 | 68.9 | 65.5 | 49.3 | 76.8 |
| 18 | 95.0 | 2.0 | 30.0 | 85.0 | 95.0 | 65.0 | 56.1 | 95.7 | 34.5 | 75.7 |
| 19 | 95.0 | 2.0 | 40.0 | 85.0 | 110.0 | 50.0 | 42.2 | 91.9 | 33.8 | 76.4 |
| 20 | 95.0 | 1.0 | 40.0 | 85.0 | 95.0 | 65.0 | 40.0 | 95.6 | 27.0 | 68.1 |
| Serum | |||
|---|---|---|---|
| Parameter | Estimate (±SE) | ||
| ASA DPI Suspension | ASA DPI | Oral | |
| kel (h−1) | 0.154 | 0.176 | 0.317 |
| t1/2 (h) | 4.49 | 3.93 | 2.19 |
| tmax (h) | 0.333 | 1 | 1 |
| Cmax (±SE) (nmol/mL) | 71.0 (8.6) | 166.5 (27.1) | 281.7 (22.9) |
| AUClast (±SE) (h nmol/mL) | 281.9 (29.8) | 644.1 (53.2) | 1115 (50) |
| AUCinf (h nmol/mL) | 449.1 | 990.2 | 1342 |
| AUC% extrapolated * | 37.2 | 34.9 | 16.9 |
| Relative bioavailability (F) ** | 1.33 | 3.36 | - |
| LUNG | |||
| Parameter | Estimate (±SE) | ||
| ASA DPI suspension | ASA DPI | Oral | |
| kel (h−1) | 1.06 | 0.23 | 0.176 |
| t1/2 (h) | 0.654 | 3.02 | 3.93 |
| tmax (h) | 1 | 1 | 2 |
| Cmax (±SE) (nmol/mL) | 68.2 (44.3) | 68.6 (6.4) | 85.6 (3.5) |
| AUClast (±SE) (h nmol/mL) | 146.0 (4.7) | 206.9 (21.3) | 374.6 (12.0) |
| AUCinf (h nmol/mL) | 149.0 | 284.5 | 614.6 |
| AUC% extrapolated * | 2.0 | 27.3 | 39.0 |
| Relative bioavailability (F) ** | 0.97 | 3.21 | - |
| AUClung/AUCserum # | 0.52 | 0.32 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacławski, A.; Politis, S.; Balafas, E.; Mina, E.; Papakyriakopoulou, P.; Christodoulou, E.; Kostomitsopoulos, N.; Rekkas, D.M.; Valsami, G.; Giovagnoli, S. Development and Pharmacokinetics of a Novel Acetylsalicylic Acid Dry Powder for Pulmonary Administration. Pharmaceutics 2022, 14, 2819. https://doi.org/10.3390/pharmaceutics14122819
Pacławski A, Politis S, Balafas E, Mina E, Papakyriakopoulou P, Christodoulou E, Kostomitsopoulos N, Rekkas DM, Valsami G, Giovagnoli S. Development and Pharmacokinetics of a Novel Acetylsalicylic Acid Dry Powder for Pulmonary Administration. Pharmaceutics. 2022; 14(12):2819. https://doi.org/10.3390/pharmaceutics14122819
Chicago/Turabian StylePacławski, Adam, Stavros Politis, Evangelos Balafas, Ekaterini Mina, Paraskevi Papakyriakopoulou, Eirini Christodoulou, Nikolaos Kostomitsopoulos, Dimitrios M. Rekkas, Georgia Valsami, and Stefano Giovagnoli. 2022. "Development and Pharmacokinetics of a Novel Acetylsalicylic Acid Dry Powder for Pulmonary Administration" Pharmaceutics 14, no. 12: 2819. https://doi.org/10.3390/pharmaceutics14122819
APA StylePacławski, A., Politis, S., Balafas, E., Mina, E., Papakyriakopoulou, P., Christodoulou, E., Kostomitsopoulos, N., Rekkas, D. M., Valsami, G., & Giovagnoli, S. (2022). Development and Pharmacokinetics of a Novel Acetylsalicylic Acid Dry Powder for Pulmonary Administration. Pharmaceutics, 14(12), 2819. https://doi.org/10.3390/pharmaceutics14122819








