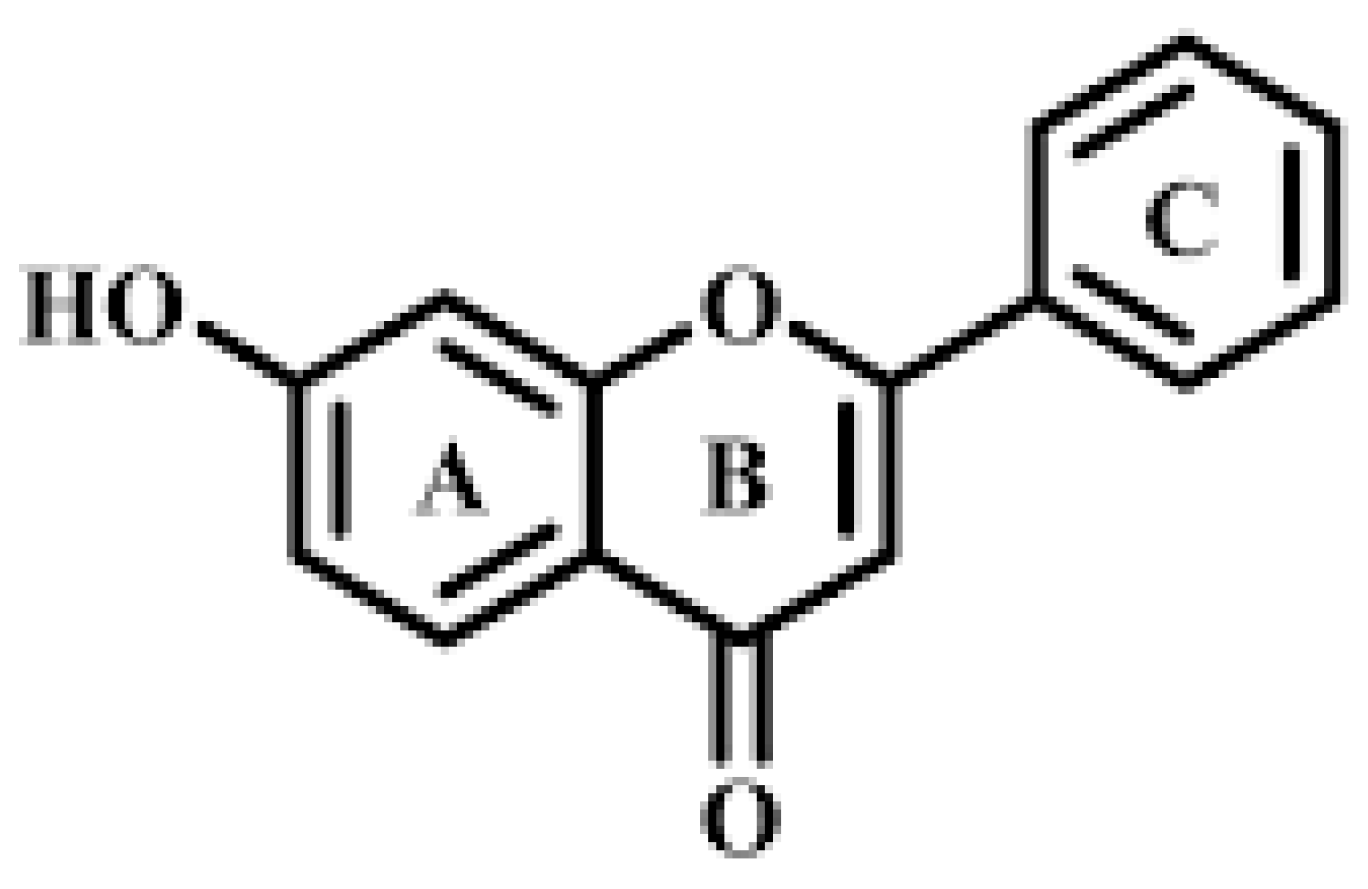
Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone
Abstract
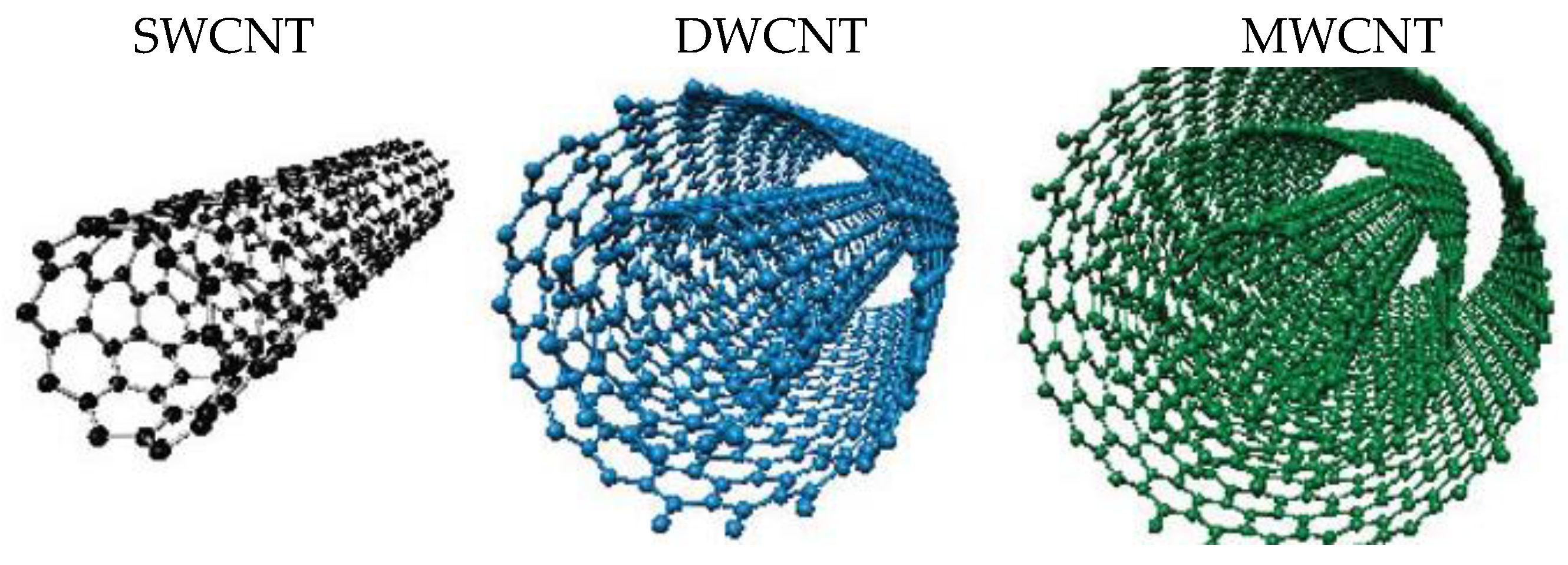
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Buffer Solutions
2.3. In vitro Cytotoxicity Assays
2.4. Fluorescence Measurements
2.4.1. Fluorescence Emission Intensity Calibration Curves
2.5. Encapsulation of 7-HF in the CNTs
2.6. Encapsulation Efficiency
2.7. Zeta Potential Measurements
2.8. In Vitro 7-HF Release
2.9. Statistical Analysis
3. Results and Discussion
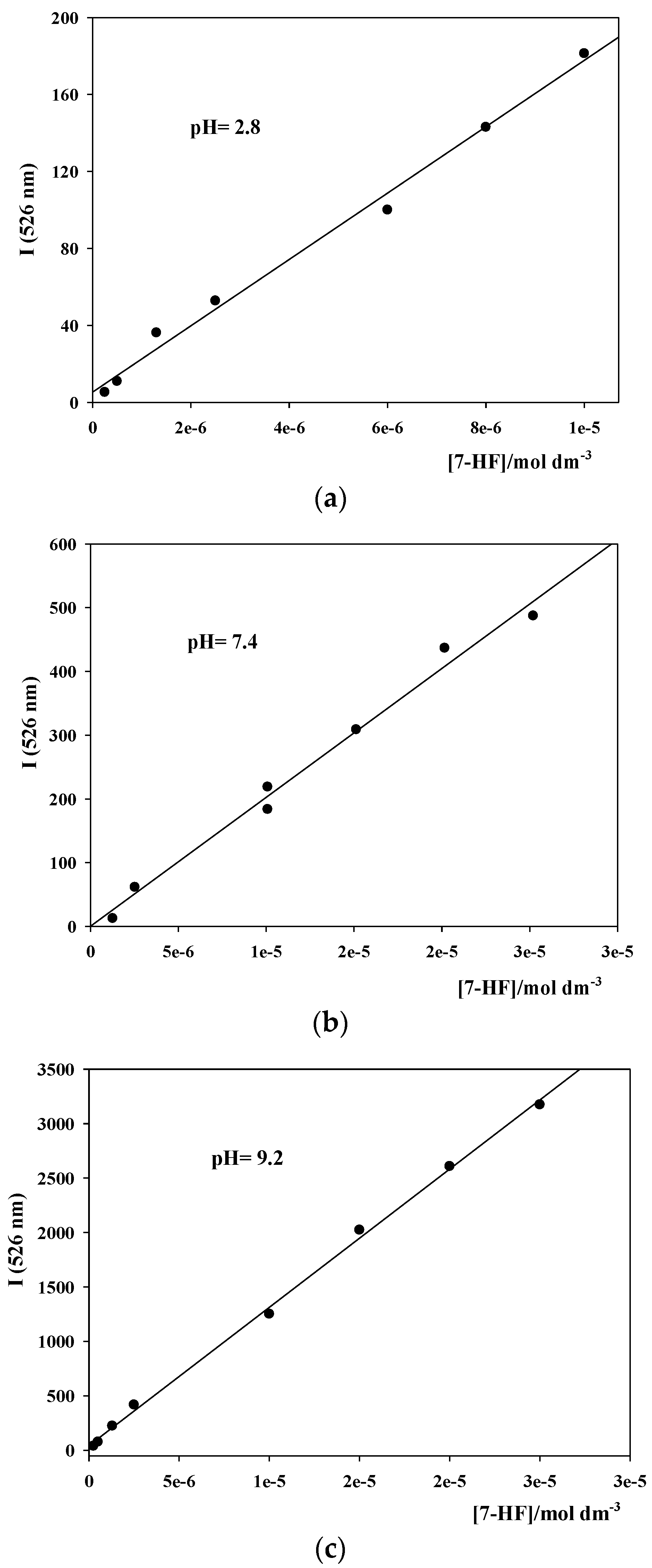
3.1. Fluorescence Emission Intensity Calibration Curves for 7-HF at Different pHs
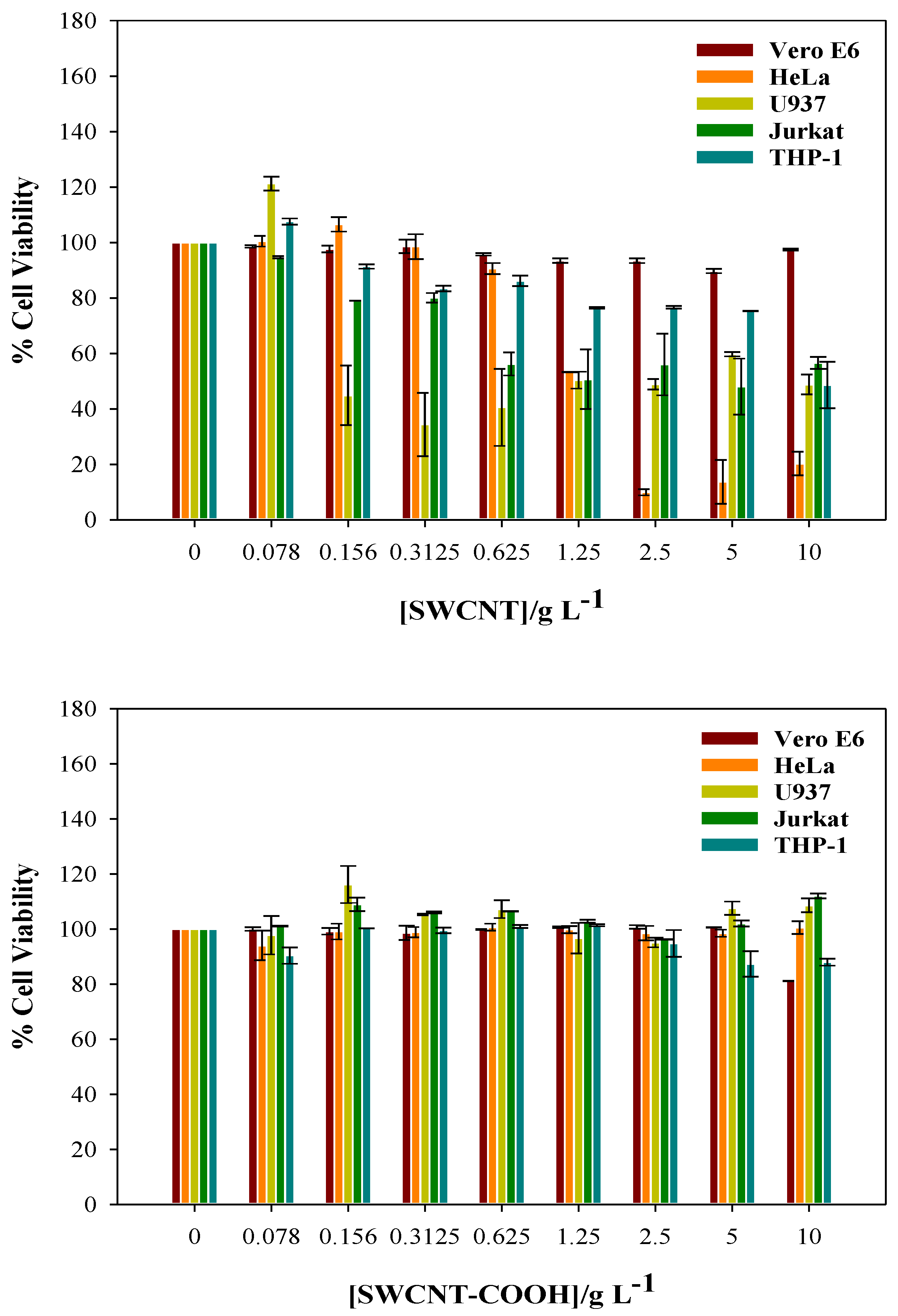
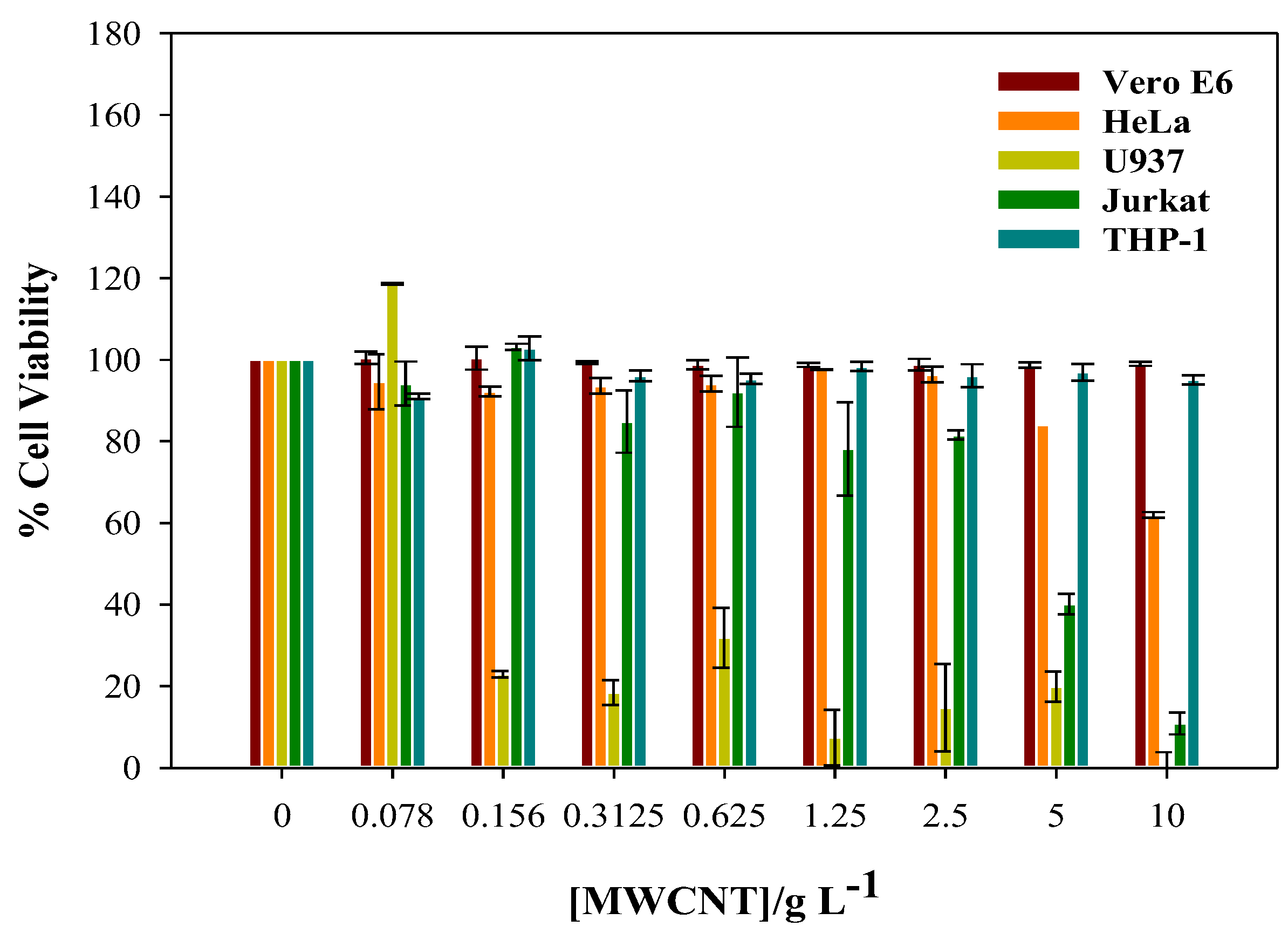
3.2. Cytotoxicity of CNTs
3.3. Encapsulation of 7-HF in CNTs
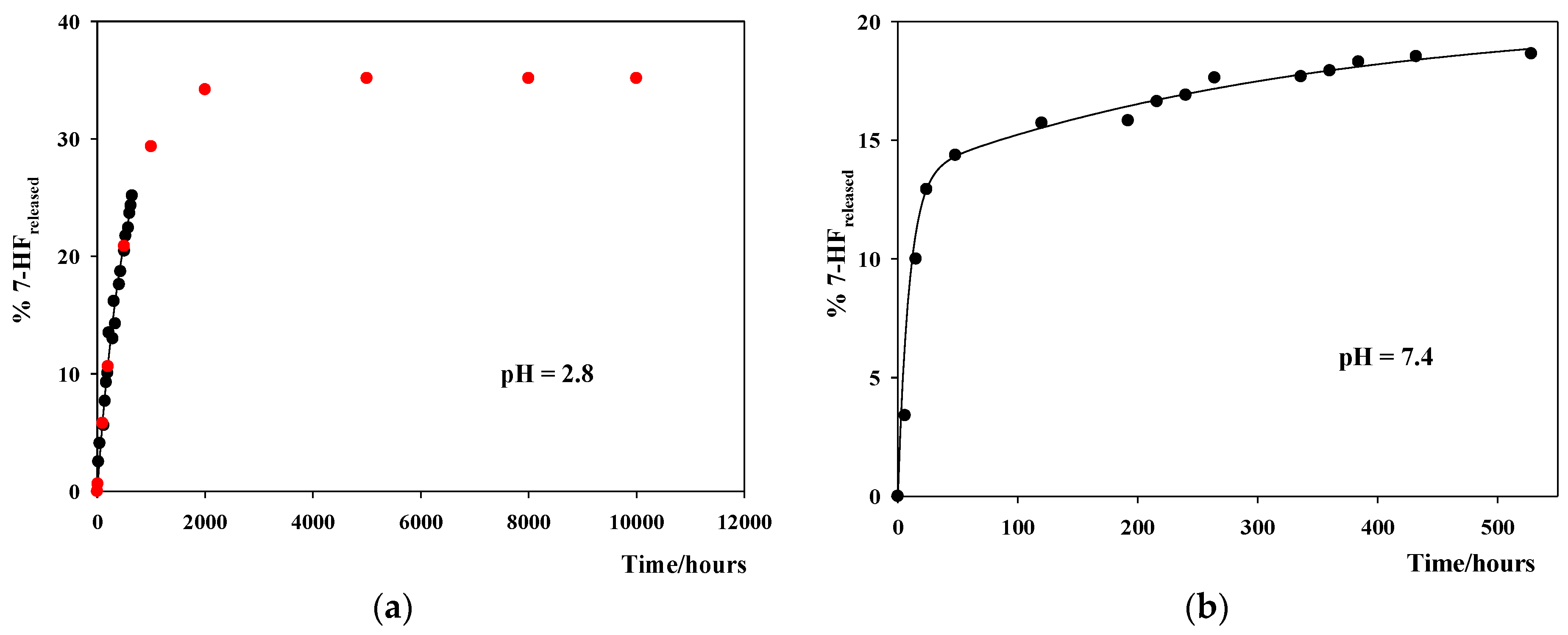
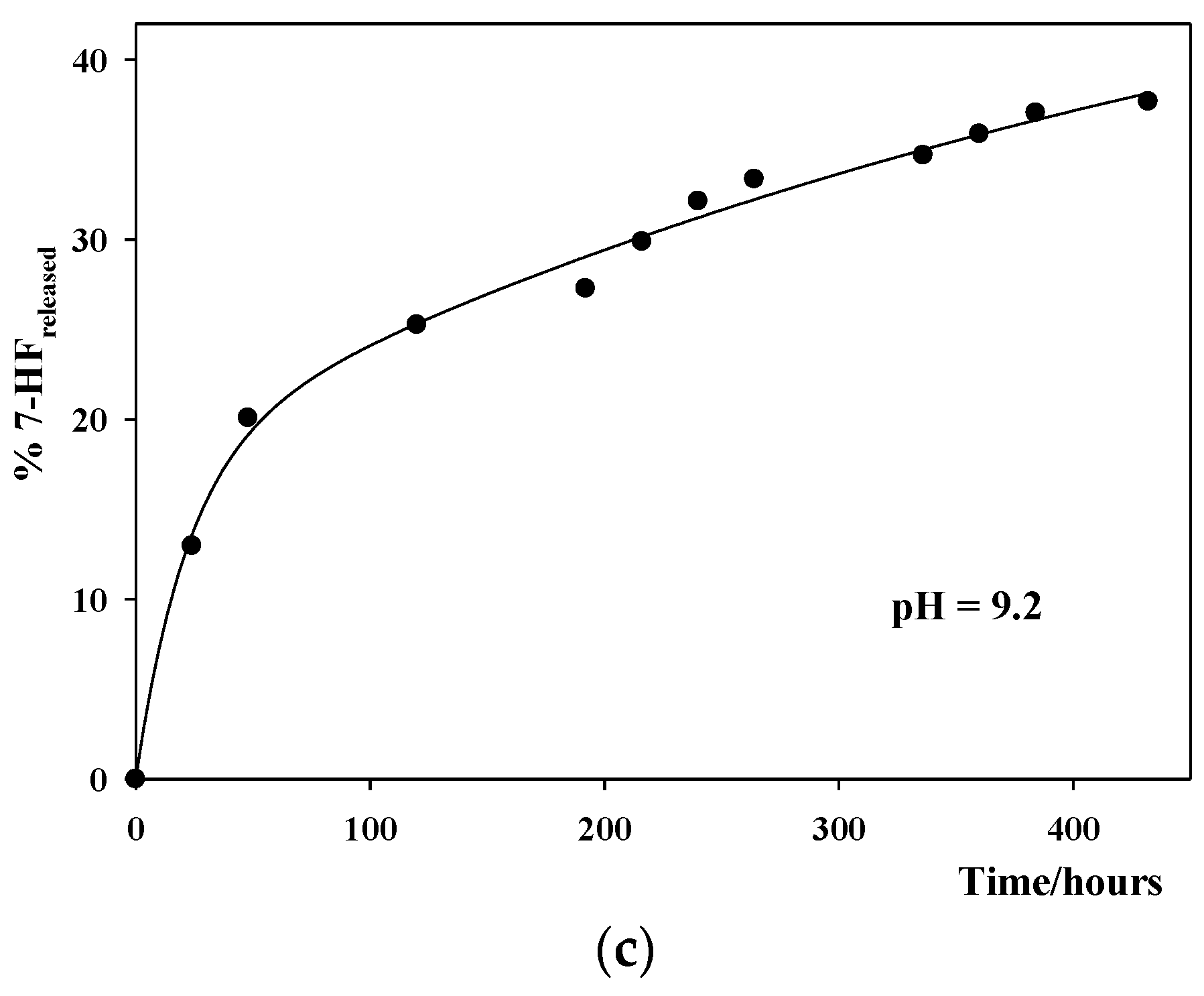
3.4. Study of the In Vitro 7-HF Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemysinthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.K.; Ranjit, A.; Sharma, K.; Prada’s, P.; Shang, X. Bio active compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoida, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Singh, N.; Kaufman, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 12, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.E.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Middleton, E.; Chithan, K. The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. In The Flavonoids: Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman and Hall: London, UK, 1993; pp. 145–166. [Google Scholar]
- Liang, Y.; Xie, M.; Li, J.; Liu, L.; Cao, Y. Influence of 3-Hydroxyflavone on Colloidal Stability and Internationalization of Ag Nanomaterials Into THP-1 Macrophages. Dose Response 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Se Gupta, N.; Sahihi, M.; Dehkhodaei, M.; Kelly, D.; Arany, I. Differential role of 3-hydroxyflavone and 7-hydroxyflavone against nicotine-induced oxidative stress in rat renal proximal tubule cells. PLoS ONE 2017, 12, e0179777. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, Y.Z.; Chen, J.X.; Tang, Y.Z. Inhibition of pro-inflammatory mediators in RAW264.7 cells by 7-hydroxyflavone and 7,8-dihydroxyflavone. J. Pharm. Pharmacol. 2017, 69, 865–894. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Atero, N.A.; Rásquele-Oliveira, F.S.; Fattori, V.; Casageande, R.; Verri, W.A. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinicall and clonical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Ishikawa, Y.; Yoshizawa, Y. Relationship between the structure of methoxylated and hydroxylated flavones and their antiproliferative activitty in HL60 cells. Anticancer Res. 2018, 38, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, H.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Inhibition of enterovirus 71 replication by 7-hydroxyflavone and diidopropyl-flavones-7-yl phosphate. PLoS ONE 2014, 9, e92565. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as prospective neuroprotectant and their therapeutic propensity in aging associated neurological disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Margel, S. Containers for drug delivery. In Composite Sciences and Technology; Book Series; Available online: https://link.springer.com/chapter/10.1007/978-981-16-8146-2_6 (accessed on 11 February 2022).
- Shah, A.; Aftab, S.; Anisad, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102426. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of magnetic hydrogels for hyperthermia and drug delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent biomedical approaches.for quitosano based materials as drug delivery nanocarriersBiomedical. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Ijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Sabino, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the 4 functionalized carbon nanotubes as smart nanocarriers for drug delivery applications. In Fullernenes, Graphemes and Nanotubes: A Pharmaceutical Approach; Frumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 4; pp. 105–133. [Google Scholar] [CrossRef]
- Gholozadeh, H.; Ghorbani-HasanSaraei, A.; Tahermansouri, H.; Shaidi, S.A. The simultaneous adsorption and desorption of flavonoids from bitter orange peel by the carboxylared multi-walled carbon nanotubes. Carbon Lett. 2019, 29, 273–279. [Google Scholar] [CrossRef]
- Daneshmehr, S. Carbon nanotubes for delivery of quercetin as anticancer drug: Theoretical study. Procedia Mater. Sci. 2015, 11, 131–136. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaisya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Hassani, M.; Tahghighi, A.; Rohani, M.; Ekmati, M.; Ahmadian, M.; Ahmavvand, H. Robust antibacterial activity of functionalized carbon nanotube-levofloxacine conjugate based on in vitro and in vivo studies. Sci. Rep. 2022, 12, 10064. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Kang, E.; Chan-Park, M.B. Smart nanomicelles with bacterial infection-responsive disassembly for selective antimicrobial applications. Biomater. Sci. 2021, 9, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Molina-Velázquez, S. Final Master’s Project. 2021. [Google Scholar]
- Moyá, M.L.; Ostos, F.J.; Moreno, I.; García, D.; Moreno-Gordillo, P.; Rosado, I.V.; López-Cornejo, P. Metallo-Liposomes Derived from the [Ru(bpy)3]2+ complex as nanocarriers of therapeutic agents. Chemosensors 2021, 9, 90. [Google Scholar] [CrossRef]
- López-López, M.; Bernal, E.; Moyá, M.L.; Sánchez, F.; López-Cornejo, P. Study of ionic surfactants interactions with carboxylated single-walled carbon nanotubes by using ion-selective electrodes? Electrochem. Commun. 2016, 67, 31–34. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Varenikov, A.S.; Roshal, A.D. 7-Hydroxyflavone revisited: Spectral, acid−base properties, and interplay of the protolytic forms in the ground and excited states. J. Phys. Chem. A 2014, 118, 3068–3080. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
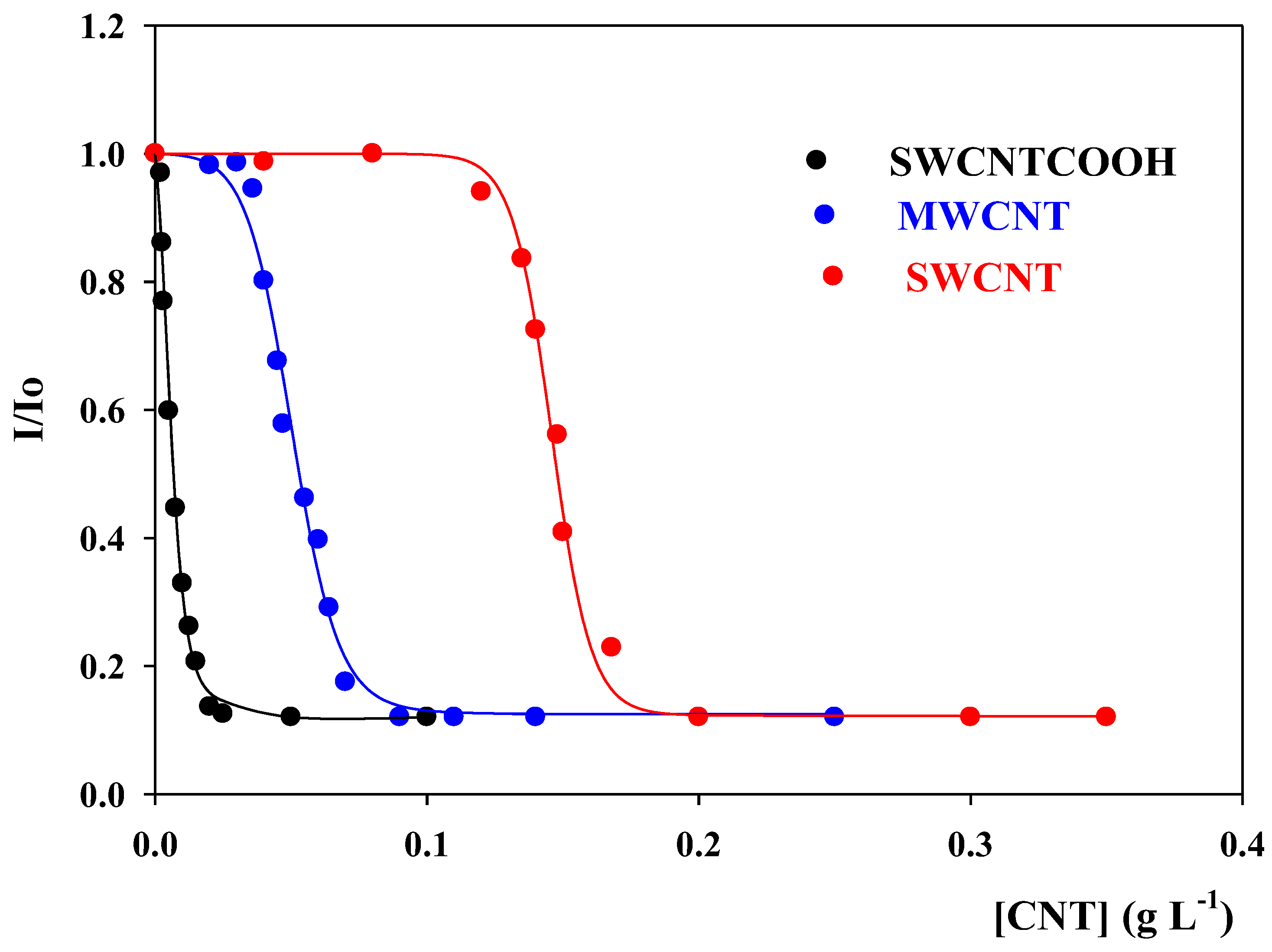
| 100% 7-HFencapsulated | |||
|---|---|---|---|
| pH = 7.4 | [SWNCTs]/g·L−1 | [SWCNT-COOH]/g·L−1 | [MWNCTs]/g·L−1 |
| [7-HF] = 1.51·10−5 M | 0.20 | 0.025 | 0.090 |
| CNTs | Kmax/g−1 L |
|---|---|
| SWCNT | (1.5 ± 0.3)·103 |
| MWCNT | (1.2 ± 0.2)·103 |
| SWCNT-COOH | (1.5 ± 0.3)·103 |
| 7-HF/SWCNT | ||
| pH | 103 kn/h−1 | |
| 2.0 | 1.7 ± 0.4 | |
| pH | 103 kn/h−1 | 103 ka/h−1 |
| 7.4 | 1.8 ± 0.5 | 68 ± 10 |
| 9.2 | 1.9 ± 0.7 | 50 ± 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. https://doi.org/10.3390/pharmaceutics14122806
Espíndola C, Correa AJ, López-López M, López-Cornejo P, Bernal E, Lebrón JA, Ostos FJ, Benhnia MR-E-I, Moyá ML. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics. 2022; 14(12):2806. https://doi.org/10.3390/pharmaceutics14122806
Chicago/Turabian StyleEspíndola, Cecilia, Alejandro Javier Correa, Manuel López-López, Pilar López-Cornejo, Eva Bernal, José Antonio Lebrón, Francisco José Ostos, Mohammed Rafii-El-Idrissi Benhnia, and María Luisa Moyá. 2022. "Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone" Pharmaceutics 14, no. 12: 2806. https://doi.org/10.3390/pharmaceutics14122806
APA StyleEspíndola, C., Correa, A. J., López-López, M., López-Cornejo, P., Bernal, E., Lebrón, J. A., Ostos, F. J., Benhnia, M. R.-E.-I., & Moyá, M. L. (2022). Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics, 14(12), 2806. https://doi.org/10.3390/pharmaceutics14122806











