Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis
Abstract
1. Introduction
2. AD Pathogenesis
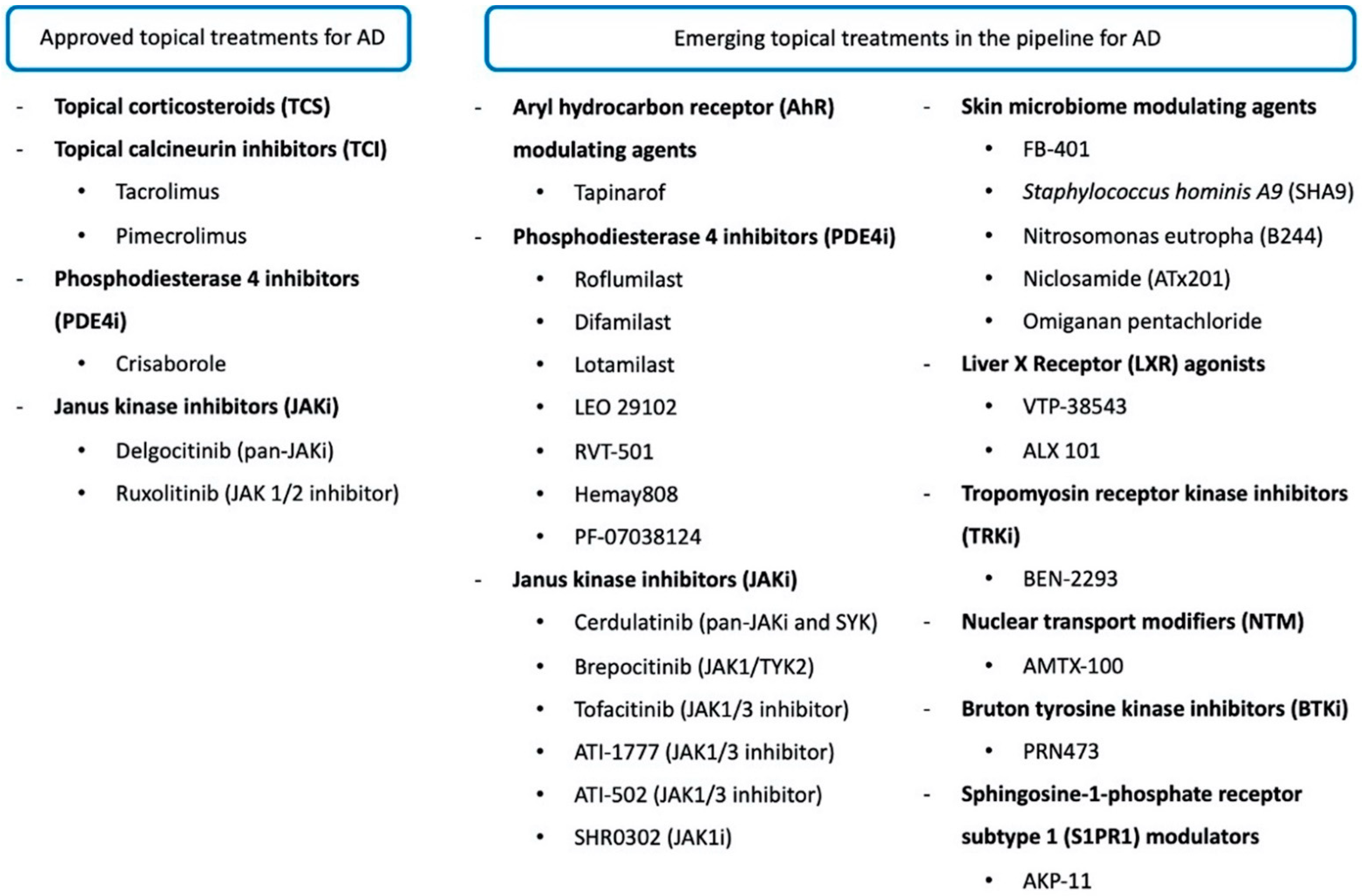
3. Recently Approved Topical Drugs for AD
3.1. Crisaborole
3.2. Delgocitinib
3.3. Ruxolitinib
4. New Emerging Topical Treatments for AD in the Pipeline
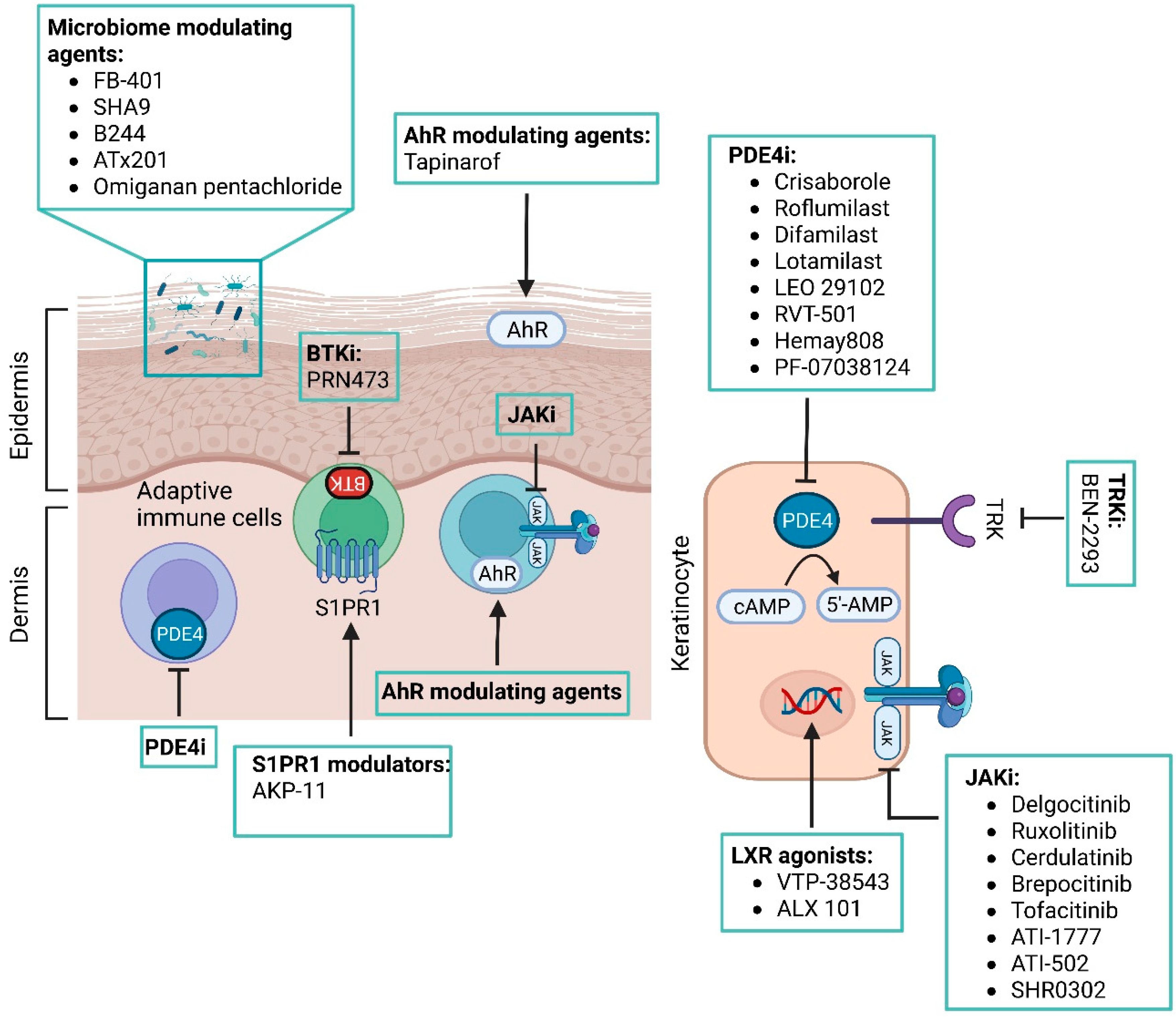
4.1. Aryl Hydrocarbon Receptor Modulating Agents
4.2. Phosphodiesterase 4 Inhibitors
4.3. JAK Inhibitors
4.4. Skin Microbiome Modulating Agents
4.5. Other Novel Therapies in the Pipeline
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Mei-Yen Yong, A.; Tay, Y.K. Atopic dermatitis. Racial and Ethnic differences. Dermatol. Clin. 2017, 35, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990-2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 12, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef]
- Lee, H.H.; Patel, K.R.; Singam, V.; Rastogi, S.; Silverberg, J.I. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 1526–1532.e7. [Google Scholar] [CrossRef]
- Silverberg, N.B. Typical and atypical clinical appearance of atopic dermatitis. Clin. Dermatol. 2017, 35, 354–359. [Google Scholar] [CrossRef]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 2, 606–613. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Chiesa Fuxench, Z.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Drucker, A.M.; Wang, A.R.; Qureshi, A.A. Research gaps in quality of life and economic burden of atopic dermatitis: The National Eczema Association burden of disease audit. JAMA Derm. 2016, 15, 873–887a. [Google Scholar] [CrossRef] [PubMed]
- Ungar, B.; Garcet, S.; Gonzalez, J.; Dhingra, N.; Correa da Rosa, J.; Shemer, A.; Krueger, J.G.; Suarez-Farinas, M.; Guttman-Yassky, E. An Integrated Model of Atopic Dermatitis Biomarkers Highlights the Systemic Nature of the Disease. J. Investig. Dermatol. 2017, 137, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E. Racial differences in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 449–455. [Google Scholar] [CrossRef]
- Shreberk-Hassidim, R.; Ramot, Y.; Zlotogorski, A. Janus kinase inhibitors in dermatology: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 745–753.e19. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of JAK-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013, 72 (Suppl 2), ii111–ii115. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Egbuniwe, I.; Karagiannis, S.N.; Nestle, F.O.; Lacy, K.E. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 2015, 36, 102–111. [Google Scholar] [CrossRef] [PubMed]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.P.; Ma, E.G.M.; Aranda, C.J.; De Lafaille, M.A.C. Non-classical B Cell Memory of Allergic IgE Responses. Front. Immunol. 2019, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sato, S. Il-10–producing regulatory b cells in skin diseases. J. Cutan. Immunol. Allergy 2019, 2, 68–74. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Ishiuji, Y.; Yoshizaki, A.; Kurita, M.; Hayashi, M.; Ishiji, T.; Nakagawa, H.; Asahina, A.; Yanaba, K. IL-10–Producing Regulatory B Cells Are Decreased in Patients with Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 475–478. [Google Scholar] [CrossRef]
- Simon, D.; Hösli, S.; Kostylina, G.; Yawalkar, N.; Simon, H.-U. Anti-CD20 (rituximab) treatment improves atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 122–128. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Punzón, C.; Navarro, J.; Muñoz-Fernández, M.A.; Fresno, M. PDE4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J. Pharmacol. Exp. Ther. 2001, 299, 753–759. [Google Scholar]
- Woo, T.E.; Kuzel, P. Crisaborole 2% Ointment (Eucrisa) for Atopic Dermatitis. Skin Ther. Lett. 2019, 24, 4–6. [Google Scholar]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e6. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Gold, L.F.; Lebwohl, M.G.; Silverberg, J.I.; Tallman, A.M.; Zane, L.T. Early Relief of Pruritus in Atopic Dermatitis with Crisaborole Ointment, A Non-steroidal, Phosphodiesterase 4 Inhibitor. Acta Derm. Venereol. 2018, 98, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J.; Shepard, J.S.; Gower, R.; Su, J.C.; Lynde, C.; Cha, A.; Ports, W.C.; Purohit, V.; Takiya, L.; Werth, J.L.; et al. Safety, Effectiveness, and Pharmacokinetics of Crisaborole in Infants Aged 3 to <24 Months with Mild-to-Moderate Atopic Dermatitis: A Phase IV Open-Label Study (CrisADe CARE 1). Am. J Clin. Dermatol. 2021, 21, 275–284. [Google Scholar] [CrossRef]
- Dhillon, S. Delgocitinib: First approval. Drugs 2020, 80, 609–661. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020, 47, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Delgocitinib ointment in pediatric patients with atopic dermatitis: A phase 3, randomized, double-blind, vehicle- controlled study and a subsequent open-label, long-term study. J. Am. Acad. Dermatol. 2021, 85, 854–862. [Google Scholar] [CrossRef]
- Worm, M.; Bauer, A.; Elsner, P.; Mahler, V.; Molin, S.; Nielsen, T.S.S. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: Data from a randomized, double-blind, vehicle-controlled phase IIa study. Br. J. Dermatol. 2020, 182, 1103–1110. [Google Scholar] [CrossRef]
- Incyte Announces US FDA Approval of Opzelura (Ruxolitinib) Cream, a Topical Jak Inhibitor, for the Treatment of Atopic Dermatitis (Ad). Published 21 September 2021. Available online: https://www.opzelura.com/prescribing-information.pdf (accessed on 13 September 2022).
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.M.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E.; et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double- blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef]
- Blauvelt, A.; Szepietowski, J.C.; Papp, K.; Simpson, E.L.; Silverberg, J.I.; Kim, B.S.; Kwatra, S.G.; Kuligowski, M.E.; Venturanza, M.E.; Sun, K.; et al. Ruxolitinib cream rapidly decreases pruritus in atopic dermatitis: Pooled results from two phase 3 studies. In Proceedings of the AAD VMX 2021, Online, 23–26 April 2021. Poster 26884. [Google Scholar]
- Eichenfield, L.F.; Simpson, E.L.; Siegfried, E.C.; Kuligowski, M.E.; Venturanza, M.E.; Sun, K.; Paller, A.S. Efficacy and safety of ruxolitinib cream among adolescents with atopic dermatitis: Pooled results from two phase 3 studies. In Proceedings of the AAD VMX 2021, Online, 23–26 April 2021. Poster 27633. [Google Scholar]
- Eichenfield, L.F.; Stein Gold, L.F.; Chiesa Fuxench, Z.C.; Venturanza, M.E.; Brar, K.K. Safety and efficacy over 8 weeks and disease control over 52 weeks with ruxolitinib cream among Black or African American patients with atopic dermatitis: Pooled results from two phase 3 studies. In Proceedings of the 2022 American Academy of Dermatology Annual Meeting, Boston, MA, USA, 25–29 March 2022. Abstract: 34794. [Google Scholar]
- Simpson, E.L.; Bissonnette, R.; Stein Gold, L.F.; Chiesa Fuxench, Z.C.; Venturanza, M.E.; Silverberg, J.I. Efficacy of ruxolitinib cream for the treatment of atopic dermatitis by anatomic region: Pooled analysis from two randomized phase 3 studies. In Proceedings of the 2022 American Academy of Dermatology Annual Meeting, Boston, MA, USA, 25–29 March 2022. Abstract: 34587. [Google Scholar]
- Trikha, P.; Lee, D.A. The role of AhR in transcriptional regulation of immune cell development and function. Biochim Biophys Acta Rev. Cancer 2020, 1873, 188335. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural AhR agonist that resolved skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 89–98.e3. [Google Scholar] [CrossRef]
- Dermavant Announces First Patient Dosed in ADORING, Its Pivotal Phase 3 Clinical Program for Tapinarof for the Topical Treatment of Atopic Dermatitis—Dermavant. Published 9 September 2021. Available online: https://www.dermavant.com/dermavant-a. (accessed on 14 September 2022).
- Gooderham, M.J.; Kircik, L.H.; Zirwas, M.; Lee, M.; Kempers, S.E.; Draelos, Z.D.; Ferris, L.; Jones, T.M.; Proulx, E.S.; Bissonnette, R.; et al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in atopic dermatitis: Phase 2 proof-of-concept study. Br. J. Dermatol. 2021, 184, e56–e87. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04773587, Trial of PDE4 Inhibition with Roflumilast for the Management of Atopic Dermatitis (INTEGUMENT-I). Available online: https://clinicaltrials.gov/ct2/show/NCT04773587 (accessed on 14 September 2022).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04773600, Trial of PDE4 Inhibition with Roflumilast for the Management of Atopic Dermatitis (INTEGUMENT-II). Available online: https://clinicaltrials.gov/ct2/show/NCT04773600 (accessed on 14 September 2022).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04845620, Trial of PDE4 Inhibition with Roflumilast for the Management of Atopic Dermatitis (INTEGUMENT-PED). Available online: https://clinicaltrials.gov/ct2/show/NCT04845620 (accessed on 14 September 2022).
- Kleinman, E.; Laborada, J.; Metterle, L.; Eichenfield, L.F. What’s New in Topicals for Atopic Dermatitis? Am. J. Clin. Dermatol. 2022, 23, 595–603. [Google Scholar] [CrossRef]
- Saeki, H.; Ito, K.; Yokota, D.; Tsubouchi, H. Difamilast ointment in adult patients with atopic dermatitis: A phase 3 randomized, double-blind, vehicle-controlled trial. J. Am. Acad. Dermatol. 2022, 86, 607–614. [Google Scholar] [CrossRef]
- Saeki, H.; Ito, K.; Yokota, D.; Tsubouchi, H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in pediatric patients with atopic dermatitis: A phase III randomized double-blind, vehicle-controlled trial. Br. J. Dermatol. 2021, 186, 40–49. [Google Scholar] [CrossRef]
- Otsuka Pharmaceutical Co., Ltd. Otsuka’s Moizerto® Ointment Granted Approval in Japan as a Treatment for Atopic Dermatitis. 2021. Available online: https://www.otsuka.co.jp/en/company/newsreleases/2021/20210927_1.html (accessed on 14 September 2022).
- Felding, J.; Sørensen, M.D.; Poulsen, T.D.; Larsen, J.; Andersson, C.; Refer, P.; Engell, K.; Ladefoged, L.G.; Thormann, T.; Vinggaard, A.M.; et al. Discovery and early clinical development of2-{6-[2-(3,5-dichloro-4-pyridyl)acetyl]- 2,3-dimethoxyphenoxy}-N-propylacetamide (LEO 29102), a soft-drug inhibitor of phosphodiesterase 4 for topical treatment of atopic dermatitis. J. Med. Chem. 2014, 57, 5893–5903. [Google Scholar] [CrossRef]
- LEO 29102 Cream in the Treatment of Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT01037881?term=NCT01037881&draw=2&rank=1 (accessed on 15 September 2022).
- Furue, M.; Kitahara, Y.; Akama, H.; Hojo, S.; Hayashi, N.; Nakagawa, H.; JAPANESE E6005 Study Investigators. Safety and efficacy of topical E6005, a phosphodiesterase 4 inhibitor, in Japanese adult patients with atopic dermatitis: Results of a randomized, vehicle-controlled, multicenter clinical trial. J. Dermatol. 2014, 41, 577–585. [Google Scholar] [CrossRef]
- Nemoto, O.; Hayashi, N.; Kitahara, Y.; Furue, M.; Hojo, S.; Nomoto, M.; Shima, S.; Japanese E6005 Study Investigators. Effect of topical phosphodiesterase 4 inhibitor E6005 on Japanese children with atopic dermatitis: Results from a randomized, vehicle-controlled exploratory trial. J. Dermatol. 2016, 43, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Study of RVT-501 in Adult and Adolescent Subjects with Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT02950922?term=RVT-501&cond=Atopic+Dermatitis&draw=2&rank=3 (accessed on 15 September 2022).
- Guo, A.; Lu, P.; Coffey, G.; Conley, P.; Pandey, A.; Wang, Y.L. Dual SYK/JAK inhibition overcomes ibrutinib resistance in chronic lymphocytic leukemia: Cerdulatinib, but not ibrutinib, induces apoptosis of tumor cells protected by the microenvironment. Oncotarget 2017, 8, 12953–12967. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, S.C.; Pavel, A.B.; McHale, K.; Jett, J.E.; Collins, J.; Gillmor, D.; Tabolt, G.; Li, R.; Song, T.; Zhang, N.; et al. A phase 1b, randomized, single-center trial of topical cerdulatinib (DMVT-502) in patients with mild-to-moderate atopic dermatitis. J. Investig. Dermatol. 2021, 141, 1847. [Google Scholar] [CrossRef]
- Aclaris Therapeutics Announces Positive Preliminary Topline Data from Phase 2a Trial of ATI-1777 for Moderate to Severe Atopic Dermatitis. Available online: https://www.globenewswire.com/en/news-release/2021/06/08/2243460/37216/en/Aclaris-Therapeutics-Announces-Positive-Preliminary-Topline-Data-from-Phase-2a-Trial-of-ATI-1777-for-Moderate-to-Severe-Atopic-Dermatitis.html (accessed on 12 September 2022).
- Smith, S.; Bhatia, N.; Shanler, S.D.; DeMoor, R.; Schnyder, J. Safety of ATI-502, a novel topical JAK1/3 inhibitor, in adults with moderate to severe atopic dermatitis (AD): Results from phase 2a, open-label trial. J. Am. Acad. Dermatol. 2020, 83, AB170. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Ding, Y.; Tao, X.; Ji, C.; Dong, X.; Lu, J.; Wu, L.; Wang, R.; Lu, Q.; et al. Efficacy and Safety of SHR0302, a Highly Selective Janus Kinase 1 Inhibitor, in Patients with Moderate to Severe Atopic Dermatitis: A Phase II Randomized Clinical Trial. Am J Clin. Dermatol. 2021, 22, 877–889. [Google Scholar] [CrossRef]
- Landis, M.N.; Arya, M.; Smith, S.; Draelos, Z.; Usdan, L.; Tarabar, S.; Pradhan, V.; Aggarwal, S.; Banfield, C.; Peeva, E.; et al. Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: A phase IIb, randomised, double-blind, vehicle-controlled, dose-ranging, and parallel-group study. Br. J. Dermatol. 2022, 187, 878–887. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M.; et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Myles, I.A.; Castillo, C.R.; Barbian, K.D.; Kanakabandi, K.; Virtaneva, K.; Fitzmeyer, E.; Paneru, M.; Otaizo-Carrasquero, F.; Myers, T.G.; Markowitz, T.E.; et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid- mediated TNF-related epithelial repair. Sci. Transl. Med. 2020, 12, eaaz8631. [Google Scholar] [CrossRef] [PubMed]
- Forte Biosciences, Inc. Announces First Patient Dosed in the Clinical Trial of FB-401 for the Treatment of Children and Adults with Atopic Dermatitis. Available online: https://www.fortebiorx.com/investor-relations/news/news-details/2020/Forte-Biosciences-Inc.-Announces-First-Patient-Dosed-in-the-Clinical-Trial-of-FB-401-for-the-Treatment-of-Children-and-Adults-with-Atopic-Dermatitis/default.aspx (accessed on 12 September 2022).
- Nakatsuji, T.; Hata, T.R.; Tong, Y.; Cheng, J.Y.; Shafiq, F.; Butcher, A.M.; Salem, S.S.; Brinton, S.L.; Rudman Spergel, A.K.; Johnson, K.; et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat. Med. 2021, 27, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Targeted Investigation of Microbiome Elimination (TIME-1). Available online: https://clinicaltrials.gov/ct2/show/NCT05177328?term=ShA9&cond=atopic+dermatitis&draw=2&rank=1 (accessed on 14 September 2022).
- Maura, D.; Elmekki, N.; Goddard, C.A. The ammonia oxidizing bacterium Nitrosomonas eutropha blocks T helper 2 cell polarization via the anti-inflammatory cytokine IL-10. Sci. Rep. 2021, 11, 14162. [Google Scholar] [CrossRef] [PubMed]
- Todd Krueger Discusses AOBiomes’ Positive Results from Phase 2b Trial for Both Pruritus (Itch) and Appearance of Atopic Dermatitis (Eczema). Available online: https://www.ceocfointerviews.com/pdfs/AOBiomeTherapeutics22-CEOCFOMagazine-Interview.pdf (accessed on 14 September 2022).
- Weiss, A.; Delavenne, E.; Matias, C.; Lagler, H.; Simon, D.; Li, P.; Hansen, J.U.; Dos Santos, T.P.; Jan, B.; Priemel, P.; et al. Topical niclosamide (ATx201) reduces Staphylococcus aureus colonization and increases Shannon diversity of the skin microbiome in atopic dermatitis patients in a randomized, double-blind, placebo-controlled Phase 2 trial. Clin. Transl. Med. 2022, 12, e790. [Google Scholar] [CrossRef]
- Javia, A.; Misra, A.; Thakkar, H. Liposomes encapsulating novel antimicrobial peptide Omiganan: Characterization and its pharmacodynamic evaluation in atopic dermatitis and psoriasis mice model. Int. J. Pharm. 2022, 624, 12204. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; Buters, T.P.; Krouwels, L.; Boltjes, J.; de Kam, M.L.; van der Wall, H.; van Alewijk, D.C.J.G.; van den Munckhof, E.H.A.; Becker, M.J.; Feiss, G.; et al. Topical antimicrobial peptide omiganan recovers cutaneous dysbiosis but does not improve clinical symptoms in patients with mild to moderate atopic dermatitis in a phase 2 randomized controlled trial. J. Am. Acad. Dermatol. 2022, 86, 854–862. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Dohlman, A.B.; Malik, K.; Antonini, D.; Bissonnette, R.; Chan, T.C.; Zhou, L.; Wen, H.C.; Estrada, Y.; Xu, H.; et al. Effect of short-term liver X receptor activation on epidermal barrier features in mild to moderate atopic dermatitis: A randomized controlled trial. Ann. Allergy Asthma Immunol. 2018, 120, 631–640.e11. [Google Scholar] [CrossRef]
- A Study in Subjects with Moderate Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT03175354?term=ALX+101&cond=Atopic+Dermatitis&draw=2&rank=2 (accessed on 14 September 2022).
- A First-in-Human PoC Study with BEN2293 in Patients with Mild to Moderate Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT04737304?term=BEN-2293&draw=2&rank=1 (accessed on 14 September 2022).
- Amytrx Therapeutics Emerges from Stealth to Develop Novel Therapies for Inflammatory Diseases with Lead Program AMTX-100 Currently in Clinical Development for Dermatologic Indications. Available online: https://www.prnewswire.com/news-releases/amytrx-therapeutics-emerges-from-stealth-to-develop-novel-therapies-for-inflammatory-diseases-with-lead-program-amtx-100-currently-in-clinical-development-for-dermatologic-indications-301133549.html (accessed on 14 September 2022).
- Owens, T.D.; Brameld, K.A.; Verner, E.J.; Ton, T.; Li, X.; Zhu, J.; Masjedizadeh, M.R.; Bradshaw, J.M.; Hill, R.J.; Tam, D.; et al. Discovery of Reversible Covalent Bruton’s Tyrosine Kinase Inhibitors PRN473 and PRN1008 (Rilzabrutinib). J. Med. Chem. 2022, 65, 5300–5316. [Google Scholar] [CrossRef]
- Xing, Y.; Chu, K.A.; Wadhwa, J.; Chen, W.; Zhu, J.; Bradshaw, J.M.; Shu, J.; Foulke, M.C.; Loewenstein, N.; Nunn, P.; et al. Preclinical Mechanisms of Topical PRN473, a Bruton Tyrosine Kinase Inhibitor, in Immune-Mediated Skin Disease Models. Immunohorizons 2021, 5, 581–589. [Google Scholar] [CrossRef]
- Igawa, S.; Ohzono, A.; Pham, P.; Wang, Z.; Nakatsuji, T.; Dokoshi, T.; Di Nardo, A. Sphingosine 1-Phosphate Receptor 2 Is Central to Maintaining Epidermal Barrier Homeostasis. J. Investig. Dermatol. 2021, 141, 1188–1197.e5. [Google Scholar] [CrossRef]
- Akaal Pharma Pty Ltd to Develop First-in-Class Topical Treatment for Pruritus in Inflammatory Skin Diseases. Available online: https://akaalpharma.com/akaal-pharma-pty-ltd-to-develop-first-in-class-topical-treatment-for-pruritus-in-inflammatory-skin-diseases/ (accessed on 16 September 2022).
| Agent (Acronym) | Inhibition Target/Activity | Clinical Development Phase | Clinical Trial Identifier | |
|---|---|---|---|---|
| Aryl Hydrocarbon Receptor Modulating Agents | Tapinarof/benvitimod | AhR agonist | III | NCT05142774, NCT05014568, NCT05032859 |
| Phosphodiesterase 4 Inhibitors | Roflumilast (ARQ-151) | PDE4 | III | NCT04804605, NCT04773600, NCT04845620, NCT04773587 |
| Difamilast (OPA-15406/MM36) | PDE4 | III | NCT05372653, NCT03908970, NCT03911401 | |
| Lotamilast (RVT-501/E6005) | PDE4 | III | NCT03394677, NCT02950922 | |
| PF-07038124 | PDE4 | III | NCT05375955 | |
| Topical JAK inhibitors | Delgocitinib | Pan-JAK | Approved in Japan; IIb in EU | NCT03725722 |
| Ruxolitinib | JAK1, JAK2 | III | NCT04921969, NCT05456529, NCT03745638, NCT03745651 |
| Agent (Acronym) | Inhibition Target/Activity | Study Phase | Clinical Trial Identifier | AD Severity | Study Duration | Age (Years) | Primary Endpoint | Status | |
|---|---|---|---|---|---|---|---|---|---|
| Skin microbiome modulating agents | FB-401 | Bacterial replacement | IIb | NCT04504279 | Mild to moderate | 16 weeks | ≥2 | EASI50 | Completed |
| ShA9 | Targeted microbiome transplant | I | NCT05177328 | Moderate to severe | 24 days | 18–80 | Duration of ShA9 survival on the lesional ventral arm skin | Recruting | |
| Nitrosomonas eutropha (B244) | Nitric oxide donor | IIb | NCT04490109 | Mild to moderate | 4 weeks | 18–65 | Mean change in WI-NRS | Completed | |
| Niclosamide (ATx201) | Decolonization of S. aureus | II | NCT04339985 | Mild to moderate | 2 weeks | 12–60 | Mean change in EASI score | Completed | |
| Omiganan pentachloride | Antimicrobial cationic peptide | II | NCT03091426 | Mild to moderate | 7 weeks | 18–65 | Clinical evaluation [oSCORAD] | Completed | |
| Others | VTP-38543 | LXR-β agonist | I/II | NCT02655679 | Mild to moderate | 28 days | 18–65 | Number of AEs | Completed |
| ALX 101 | LXR-β agonist | II | NCT03859986 | Moderate | 56 days | ≥12 | Mean change in EASI score at week 8 | Unknown | |
| BEN-2293 | Pan-TRK | I/II | NCT04737304 | Mild to moderate | NA | 18–65 | Safety and tolerability | Recruiting | |
| HY209 | GPCR19 | II | NCT04530643 | Mild to moderate | 28 days | 18–65 | Safety and tolerability | Recruiting | |
| AMTX-100 | Nuclear transport modifier | I/IIb | NCT04313400 | Mild to moderate | NA | ≥18 | Maximum tolerable dose | Active not recruiting | |
| PRN473 | BTK inhibitor | IIa | NCT04992546 | Mild to moderate | 42 days | 18–70 | Number of AEs | Recruiting | |
| AKP-11 | S1PR1 | II | NA | ||||||
| Phosphodiesterase 4 Inhibitors | Hemay-808 | PDE4 | II | NCT04352595 | Mild to moderate | 29 days | 18–65 | Change in the EASI score relative to the baseline | NA |
| LEO 29102 | PDE4 | II | NCT01037881 | Mild to moderate | 28 days | 18–65 | Mean change in EASI score at week 8 | Completed | |
| Topical JAK inhibitors | Tofacitinib | JAK1, JAK3 | II (discontinued) | NCT02001181 | Mild to moderate | 28 days | 18–60 | Change in the EASI score relative to the baseline | Completed |
| ATI-1777 | JAK1, JAK3 | IIb | NCT05432596 | Moderate to severe | 28 days | 12–65 | Percentage change from baseline in EASI score at Week 4 | Recruiting | |
| ATI-502 | JAK1, JAK3 | II (discontinued) | NCT03585296 | Moderate to severe | 56 days | >18 | Number of AEs | Completed | |
| Brepocitinib (PF-06700841) | JAK1/TYK2 | IIb | NCT03903822 | Moderate to severe | 42 days | 12–75 | Percentage change from baseline in EASI score at Week 8 | Completed | |
| Jaktinib | Pan-JAK | I/II | NCT04435392 | Mild to moderate | NA | 18–65 | Proportion of participants achieving PGA response of 0/1 | Recruting | |
| CEE321 | Pan-JAK | I | NCT04612062 | Mild to moderate | NA | 18–65 | Number of AEs | Completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, L.M.; Chiricozzi, A.; Calabrese, L.; Mannino, M.; Peris, K. Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2767. https://doi.org/10.3390/pharmaceutics14122767
Pinto LM, Chiricozzi A, Calabrese L, Mannino M, Peris K. Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis. Pharmaceutics. 2022; 14(12):2767. https://doi.org/10.3390/pharmaceutics14122767
Chicago/Turabian StylePinto, Lorenzo Maria, Andrea Chiricozzi, Laura Calabrese, Maria Mannino, and Ketty Peris. 2022. "Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis" Pharmaceutics 14, no. 12: 2767. https://doi.org/10.3390/pharmaceutics14122767
APA StylePinto, L. M., Chiricozzi, A., Calabrese, L., Mannino, M., & Peris, K. (2022). Novel Therapeutic Strategies in the Topical Treatment of Atopic Dermatitis. Pharmaceutics, 14(12), 2767. https://doi.org/10.3390/pharmaceutics14122767






